Hydroxyapatite–Clay Composite for Bone Tissue Engineering: Effective Utilization of Prawn Exoskeleton Biowaste
Abstract
:1. Introduction
2. Results and Discussion
2.1. Structural and Vibrational Analysis
2.2. Thermal Analysis
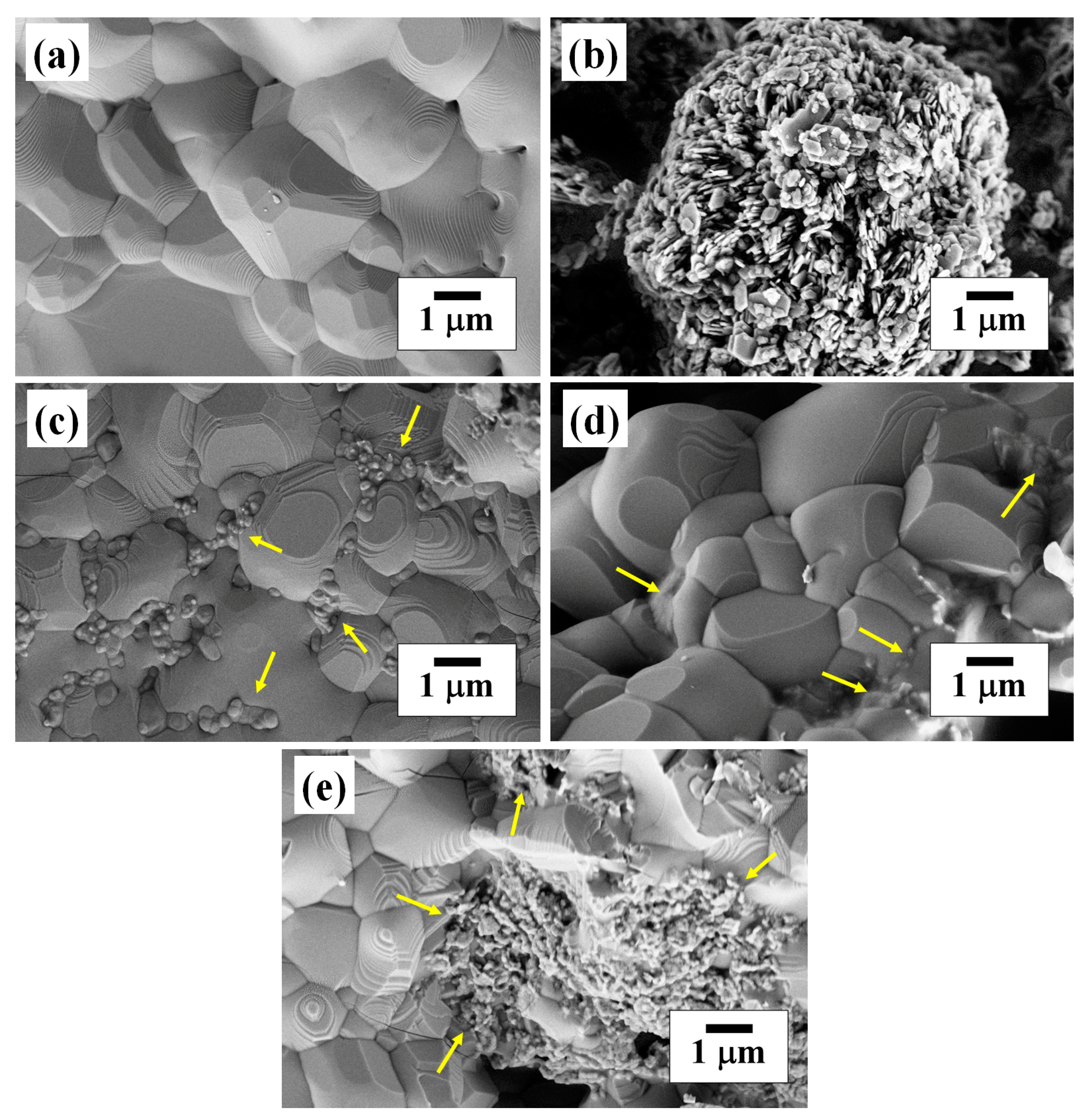
2.3. Morphological Analysis
2.4. Mechanical and Physical Properties
3. Materials and Methods
3.1. Materials
3.2. Preparation of Hydroxyapatite Clay Composite from Prawn Shells
3.3. Characterization Tools
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Satish, P.; Salian, A.; Hadagalli, K.; Mandal, S. Preparation and Structural Characteristics of Biphasic Calcium Phosphates from Prawn Shell Bio-Waste. Adv. Appl. Ceram. 2023, 122, 69–78. [Google Scholar] [CrossRef]
- Bogdanoviciene, I.; Beganskiene, A.; Tõnsuaadu, K.; Glaser, J.; Meyer, H.J.; Kareiva, A. Calcium Hydroxyapatite, Ca10(PO4)6(OH)2 Ceramics Prepared by Aqueous Sol–Gel Processing. Mater. Res. Bull. 2006, 41, 1754–1762. [Google Scholar] [CrossRef]
- Yuan, X.; Xu, Y.; Lu, T.; He, F.; Zhang, L.; He, Q.; Ye, J. Enhancing the Bioactivity of Hydroxyapatite Bioceramic via Encapsulating with Silica-Based Bioactive Glass Sol. J. Mech. Behav. Biomed. Mater. 2022, 128, 105104. [Google Scholar] [CrossRef]
- Venkatesan, J.; Anil, S. Hydroxyapatite Derived from Marine Resources and Their Potential Biomedical Applications. Biotechnol. Bioprocess. Eng. 2021, 26, 312–324. [Google Scholar] [CrossRef]
- Prakasam, M.; Locs, J.; Salma-Ancane, K.; Loca, D.; Largeteau, A.; Berzina-Cimdina, L. Biodegradable Materials and Metallic Implants—A Review. J. Funct. Biomater. 2017, 8, 44. [Google Scholar] [CrossRef] [PubMed]
- Bikramjit Basu Biomaterials Science and Tissue Engineering: Principles and Methods. Available online: https://www.amazon.com/Biomaterials-Science-Tissue-Engineering-Principles/dp/1108415156 (accessed on 20 October 2023).
- Sandomierski, M.; Zielińska, M.; Adamska, K.; Patalas, A.; Voelkel, A. Calcium Montmorillonite as a Potential Carrier in the Release of Bisphosphonates. N. J. Chem. 2022, 46, 3401–3408. [Google Scholar] [CrossRef]
- Murugan, R.; Ramakrishna, S. Development of Nanocomposites for Bone Grafting. Compos. Sci. Technol. 2005, 65, 2385–2406. [Google Scholar] [CrossRef]
- Dorozhkin, S.V. Calcium Orthophosphates. Biomatter 2011, 1, 121–164. [Google Scholar] [CrossRef]
- Salgado, A.J.; Coutinho, O.P.; Reis, R.L. Bone Tissue Engineering: State of the Art and Future Trends. Macromol. Biosci. 2004, 4, 743–765. [Google Scholar] [CrossRef]
- Kwaśniewska, A.; Chocyk, D.; Gładyszewski, G.; Borc, J.; Świetlicki, M.; Gładyszewska, B. The Influence of Kaolin Clay on the Mechanical Properties and Structure of Thermoplastic Starch Films. Polymers 2020, 12, 73. [Google Scholar] [CrossRef]
- Frost, R.L.; Kristof, J. Raman and Infrared Spectroscopic Studies of Kaolinite Surfaces Modified by Intercalation. In Clay Surfaces-Fundamental and Applications; Elsevier: Amsterdam, The Netherlands, 2004; pp. 184–215. [Google Scholar]
- Torres-Luna, J.A.; Carriazo, J.G. Porous Aluminosilicic Solids Obtained by Thermal-Acid Modification of a Commercial Kaolinite-Type Natural Clay. Solid State Sci. 2019, 88, 29–35. [Google Scholar] [CrossRef]
- Tunç, S.; Duman, O.; Polat, T.G. Effects of Montmorillonite on Properties of Methyl Cellulose/Carvacrol Based Active Antimicrobial Nanocomposites. Carbohydr. Polym. 2016, 150, 259–268. [Google Scholar] [CrossRef]
- Lemma, R.; Irassar, E.F.; Rahhal, V. Calcined Illitic Clays as Portland Cement Replacements. In Calcined Clays for Sustainable Concrete; Springer: Dordrecht, The Netherlands, 2015; pp. 269–276. [Google Scholar]
- Elmoubarki, R.; Mahjoubi, F.Z.; Tounsadi, H.; Moustadraf, J.; Abdennouri, M.; Zouhri, A.; El Albani, A.; Barka, N. Adsorption of Textile Dyes on Raw and Decanted Moroccan Clays: Kinetics, Equilibrium and Thermodynamics. Water Resour. Ind. 2015, 9, 16–29. [Google Scholar] [CrossRef]
- Murray, H.H. Overview—Clay Mineral Applications. Appl. Clay Sci. 1991, 5, 379–395. [Google Scholar] [CrossRef]
- Nasruddin; Agustini, S.; Sholeh, M. Utilization of Kaolin as a Filling Material for Rubber Solid Tire Compounds for Two-Wheeled Electric Scooters. IOP Conf. Ser. Mater. Sci. Eng. 2021, 1143, 012010. [Google Scholar] [CrossRef]
- Luo, W.; Fan, Z.; Wan, J.; Hu, Q.; Dong, H.; Zhang, X.; Zhou, Z. Study on the Reusability of Kaolin as Catalysts for Catalytic Pyrolysis of Low-Density Polyethylene. Fuel 2021, 302, 121164. [Google Scholar] [CrossRef]
- Dondi, M.; Iglesias, C.; Dominguez, E.; Guarini, G.; Raimondo, M. The Effect of Kaolin Properties on Their Behaviour in Ceramic Processing as Illustrated by a Range of Kaolins from the Santa Cruz and Chubut Provinces, Patagonia (Argentina). Appl. Clay Sci. 2008, 40, 143–158. [Google Scholar] [CrossRef]
- Abdullah, M.E.; Jaya, R.P.; Shahafuddin, M.N.A.; Yaacob, H.; Wan Ibrahim, M.H.; Nazri, F.M.; Ramli, N.I.; Mohammed, A.A. Performance of Kaolin Clay on the Concrete Pavement. IOP Conf. Ser. Mater. Sci. Eng. 2018, 358, 012049. [Google Scholar] [CrossRef]
- Murray, H.H. Traditional and New Applications for Kaolin, Smectite, and Palygorskite: A General Overview. Appl. Clay Sci. 2000, 17, 207–221. [Google Scholar] [CrossRef]
- Marsh, G. Electronics—A Major Market for Reinforced Plastics. Reinf. Plast. 2008, 52, 38–41. [Google Scholar] [CrossRef]
- Mareri, P.; Bastide, S.; Binda, N.; Crespy, A. Mechanical Behaviour of Polypropylene Composites Containing Fine Mineral Filler: Effect of Filler Surface Treatment. Compos. Sci. Technol. 1998, 58, 747–752. [Google Scholar] [CrossRef]
- Song, S.; Dong, L.; Zhang, Y.; Chen, S.; Li, Q.; Guo, Y.; Deng, S.; Si, S.; Xiong, C. Lauric Acid/Intercalated Kaolinite as Form-Stable Phase Change Material for Thermal Energy Storage. Energy 2014, 76, 385–389. [Google Scholar] [CrossRef]
- Obada, D.O.; Osseni, S.A.; Sina, H.; Salami, K.A.; Oyedeji, A.N.; Dodoo-Arhin, D.; Bansod, N.D.; Csaki, S.; Atta, A.Y.; Fasanya, O.O.; et al. Fabrication of Novel Kaolin-Reinforced Hydroxyapatite Scaffolds with Robust Compressive Strengths for Bone Regeneration. Appl. Clay Sci. 2021, 215, 106298. [Google Scholar] [CrossRef]
- Sandomierski, M.; Buchwald, Z.; Voelkel, A. Calcium Montmorillonite and Montmorillonite with Hydroxyapatite Layer as Fillers in Dental Composites with Remineralizing Potential. Appl. Clay Sci. 2020, 198, 105822. [Google Scholar] [CrossRef]
- Katti, K.S.; Katti, D.R.; Dash, R. Synthesis and Characterization of a Novel Chitosan/Montmorillonite/Hydroxyapatite Nanocomposite for Bone Tissue Engineering. Biomed. Mater. 2008, 3, 034122. [Google Scholar] [CrossRef]
- Bhowmick, A.; Jana, P.; Pramanik, N.; Mitra, T.; Banerjee, S.L.; Gnanamani, A.; Das, M.; Kundu, P.P. Multifunctional Zirconium Oxide Doped Chitosan Based Hybrid Nanocomposites as Bone Tissue Engineering Materials. Carbohydr. Polym. 2016, 151, 879–888. [Google Scholar] [CrossRef]
- Morgan, E.F.; Unnikrisnan, G.U.; Hussein, A.I. Bone Mechanical Properties in Healthy and Diseased States. Annu. Rev. Biomed. Eng. 2018, 20, 119–143. [Google Scholar] [CrossRef] [PubMed]
- Kłosek-Wawrzyn, E.; Małolepszy, J.; Murzyn, P. Sintering Behavior of Kaolin with Calcite. Procedia Eng. 2013, 57, 572–582. [Google Scholar] [CrossRef]
- Prekajski, M.; Mirković, M.; Todorović, B.; Matković, A.; Marinović-Cincović, M.; Luković, J.; Matović, B. Ouzo Effect—New Simple Nanoemulsion Method for Synthesis of Strontium Hydroxyapatite Nanospheres. J. Eur. Ceram. Soc. 2016, 36, 1293–1298. [Google Scholar] [CrossRef]
- Hadagalli, K.; Kumar, R.; Mandal, S.; Basu, B. Structural, Compositional and Spectral Investigation of Prawn Exoskeleton Nanocomposite: UV Protection from Mycosporine-like Amino Acids. Mater. Chem. Phys. 2020, 249, 123002. [Google Scholar] [CrossRef]
- Tironi, A.; Trezza, M.A.; Irassar, E.F.; Scian, A.N. Thermal Treatment of Kaolin: Effect on the Pozzolanic Activity. Procedia Mater. Sci. 2012, 1, 343–350. [Google Scholar] [CrossRef]
- Benkacem, S.; Boudeghdegh, K.; Zehani, F.; Hamidouche, M.; Belhocine, Y. Preparation, Microstructure Studies and Mechanical Properties of Glazes Ceramic Sanitary Ware Based on Kaolin. Sci. Sinter. 2021, 53, 209–221. [Google Scholar] [CrossRef]
- Mgbemena, C.O.; Ibekwe, N.O.; Sukumar, R.; Menon, A.R.R. Characterization of Kaolin Intercalates of Oleochemicals Derived from Rubber Seed (Hevea brasiliensis) and Tea Seed (Camelia sinensis) Oils. J. King Saud. Univ. Sci. 2013, 25, 149–155. [Google Scholar] [CrossRef]
- Amenta, E.; King, H.E.; Petermann, H.; Uskoković, V.; Tommasini, S.M.; Macica, C.M. Vibrational Spectroscopic Analysis of Hydroxyapatite in HYP Mice and Individuals with X-Linked Hypophosphatemia. Ther. Adv. Chronic Dis. 2018, 9, 268–281. [Google Scholar] [CrossRef] [PubMed]
- Frost, R.L.; Thu Ha, T.; Kristof, J. FT-Raman Spectroscopy of the Lattice Region of Kaolinite and Its Intercalates. Vib. Spectrosc. 1997, 13, 175–186. [Google Scholar] [CrossRef]
- Horváth, E.; Kristóf, J.; Frost, R.L. Vibrational Spectroscopy of Intercalated Kaolinites. Part I. Appl. Spectrosc. Rev. 2010, 45, 130–147. [Google Scholar] [CrossRef]
- Peng, H.; Vaughan, J.; Vogrin, J. The Effect of Thermal Activation of Kaolinite on Its Dissolution and Re-Precipitation as Zeolites in Alkaline Aluminate Solution. Appl. Clay Sci. 2018, 157, 189–197. [Google Scholar] [CrossRef]
- Garskaite, E.; Gross, K.-A.; Yang, S.-W.; Yang, T.C.-K.; Yang, J.-C.; Kareiva, A. Effect of Processing Conditions on the Crystallinity and Structure of Carbonated Calcium Hydroxyapatite (CHAp). CrystEngComm 2014, 16, 3950. [Google Scholar] [CrossRef]
- Skinner, H.C.W.; Kittelberger, J.S.; Beebe, R.A. Thermal Instability in Synthetic Hydroxyapatites. J. Phys. Chem. 1975, 79, 2017–2019. [Google Scholar] [CrossRef]
- Ondro, T.; Trník, A. Kinetic Behaviour of Thermal Transformations of Kaolinite. In Proceedings of the 23rd International Meeting of Thermophysics 2018, Smolenice, Slovakia, 7–9 November 2018; p. 020033. [Google Scholar]
- Faqir, N.M.; Shawabkeh, R.; Al-Harthi, M.; Wahhab, H.A. Fabrication of Geopolymers from Untreated Kaolin Clay for Construction Purposes. Geotech. Geol. Eng. 2019, 37, 129–137. [Google Scholar] [CrossRef]
- Li, X.; Ouyang, J.; Zhou, Y.; Yang, H. Assembling Strategy to Synthesize Palladium Modified Kaolin Nanocomposites with Different Morphologies. Sci. Rep. 2015, 5, 13763. [Google Scholar] [CrossRef] [PubMed]
- Dwari, R.K.; Mishra, B.K. Evaluation of Flocculation Characteristics of Kaolinite Dispersion System Using Guar Gum: A Green Flocculant. Int. J. Min. Sci. Technol. 2019, 29, 745–755. [Google Scholar] [CrossRef]
- Hadagalli, K.; Panda, A.K.; Mandal, S.; Basu, B. Faster Biomineralization and Tailored Mechanical Properties of Marine-Resource-Derived Hydroxyapatite Scaffolds with Tunable Interconnected Porous Architecture. ACS Appl. Bio Mater. 2019, 2, 2171–2184. [Google Scholar] [CrossRef] [PubMed]
- Pan, Y.; Xiong, D. Study on Compressive Mechanical Properties of Nanohydroxyapatite Reinforced Poly(Vinyl Alcohol) Gel Composites as Biomaterial. J. Mater. Sci. Mater. Med. 2009, 20, 1291–1297. [Google Scholar] [CrossRef]
- Sabree, I.; Gough, J.E.; Derby, B. Mechanical Properties of Porous Ceramic Scaffolds: Influence of Internal Dimensions. Ceram. Int. 2015, 41, 8425–8432. [Google Scholar] [CrossRef]
- Yu, H.; Matthew, H.W.; Wooley, P.H.; Yang, S. Effect of Porosity and Pore Size on Microstructures and Mechanical Properties of Poly-ε-caprolactone-Hydroxyapatite Composites. J. Biomed. Mater. Res. B Appl. Biomater. 2008, 86B, 541–547. [Google Scholar] [CrossRef]
- Akram, M.; Ahmed, R.; Shakir, I.; Ibrahim, W.A.W.; Hussain, R. Extracting Hydroxyapatite and Its Precursors from Natural Resources. J. Mater. Sci. 2014, 49, 1461–1475. [Google Scholar] [CrossRef]
- Komalkrishna, H.; Jyoth, S.T.; Kundu, B.; Mandal, S. Low Temperature Development of Nano-Hydroxyapatite from Austromegabalanus Psittacus, Star Fish and Sea Urchin. Mater. Today Proc. 2017, 4, 11933–11938. [Google Scholar] [CrossRef]
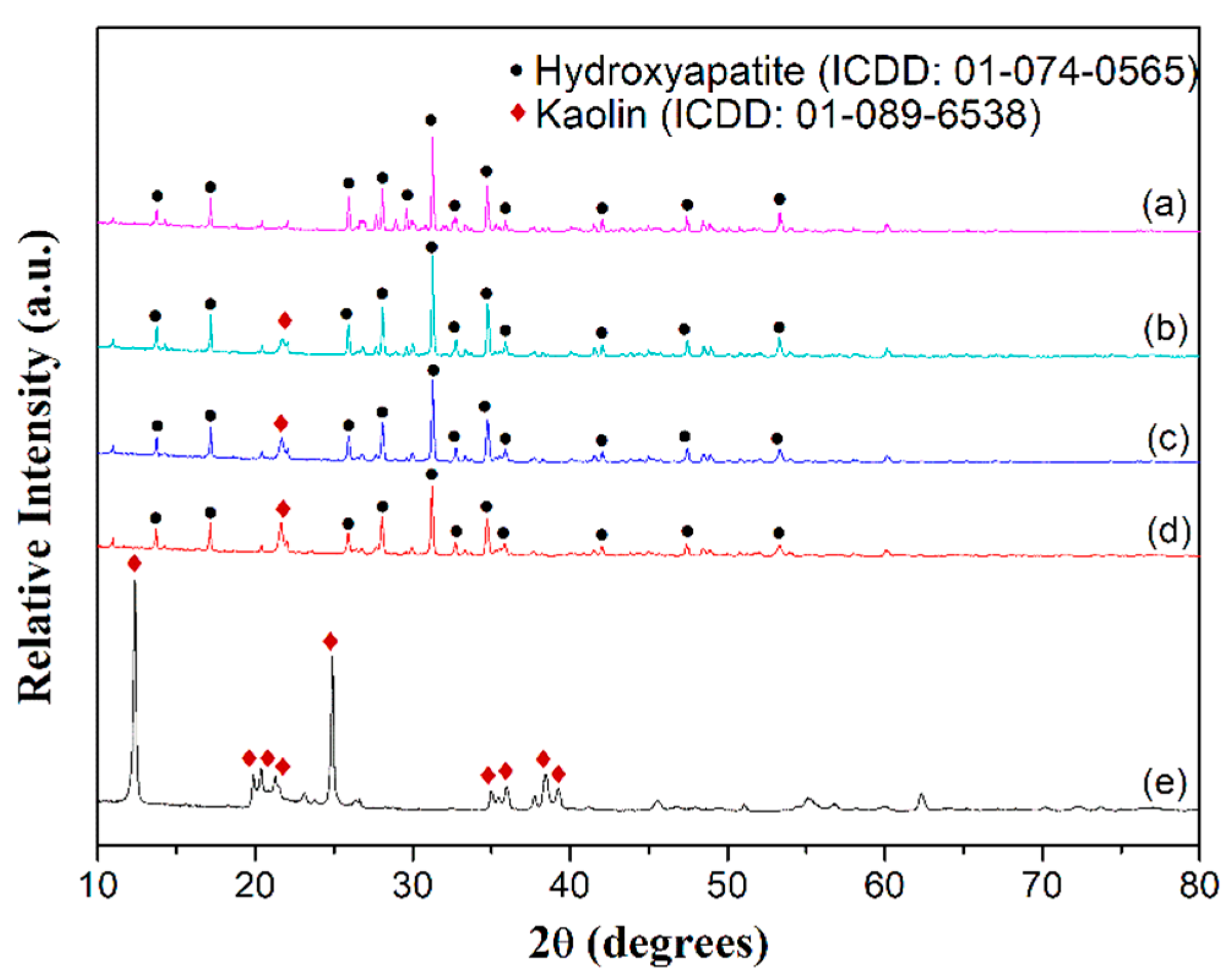
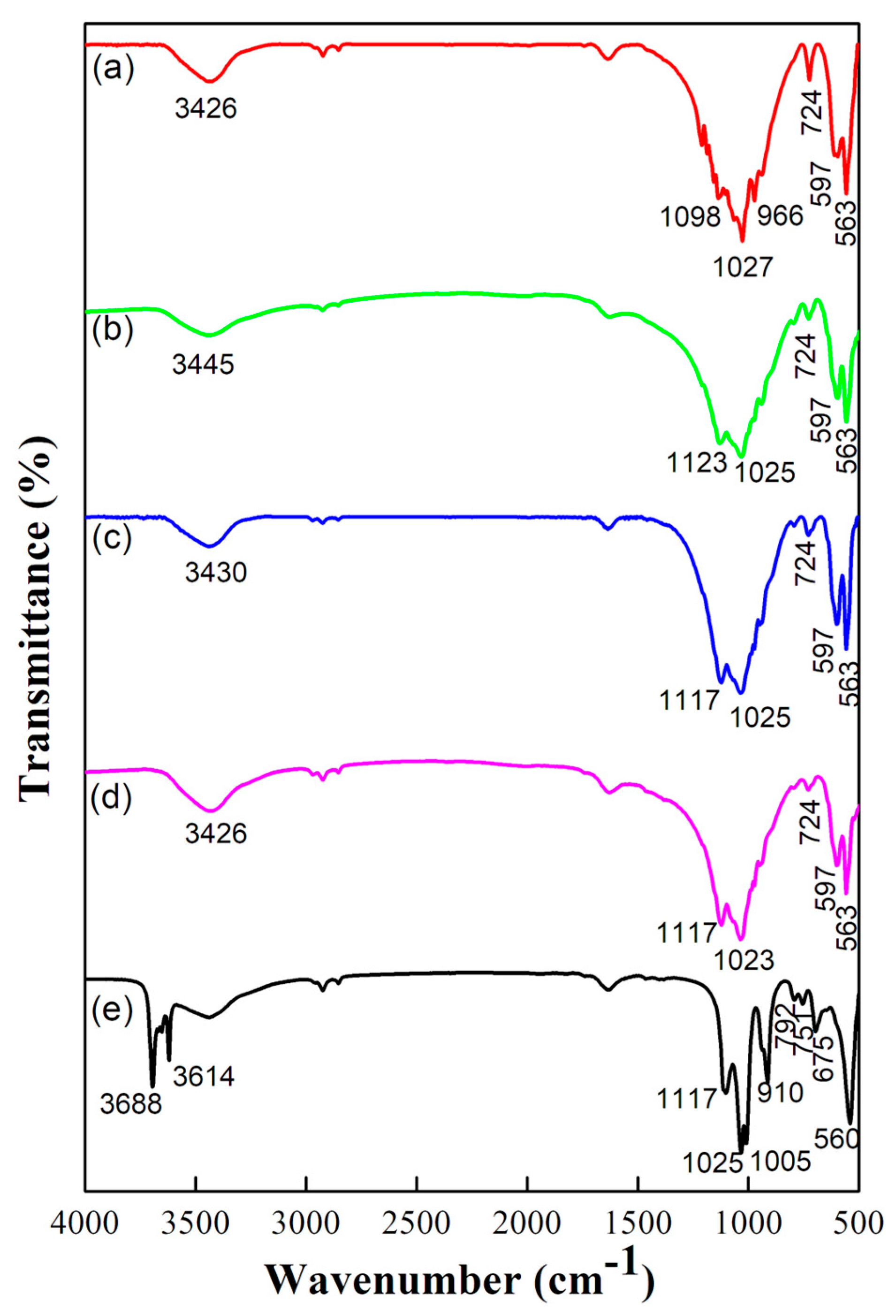







| Wavenumber (cm−1) | Stretching Mode | Functional Group | Source |
|---|---|---|---|
| 560, 751, 792 | - | Si-O-Al | Kaolin |
| 563, 597, 724 | Asymmetric bending | PO43− | HA |
| 966 | Symmetric stretching | PO43− | HA |
| 1023–1027, 1098 | Asymmetric stretching | PO43− | HA |
| 675, 910 | - | Al-OH | Kaolin |
| 1005, 1025 | In-plane stretching | Si-O | Kaolin |
| 1117–1123 | Longitudinal mode | Si-O | Kaolin |
| 1540 | Asymmetric stretching | CO32− | Atmospheric CO2 |
| 3426 | Stretching of OH− | OH− | HA |
| Wavenumber (cm−1) | Assignment | Vibration Groups | Source |
|---|---|---|---|
| 972 | Symmetric vibrational mode of P-O (stretching mode) | ν1 PO43− | HA |
| 412 | Doubly bending mode | ν2 PO43− | HA |
| 1045 | Triply generate antisymmetric stretching mode | ν3 PO43− | HA |
| 737 | Triply degenerate bending mode | ν4 PO43− | HA |
| 615–620 | Si-O-Al translation | Si-O-Al | Kaolin |
| 3620 | O-H stretching | ν5 OH− | Kaolin |
| 3696 | O-H stretching | ν1 OH− | Kaolin |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Satish, P.; Hadagalli, K.; Praveen, L.L.; Nowl, M.S.; Seikh, A.H.; Alnaser, I.A.; Abdo, H.S.; Mandal, S. Hydroxyapatite–Clay Composite for Bone Tissue Engineering: Effective Utilization of Prawn Exoskeleton Biowaste. Inorganics 2023, 11, 427. https://doi.org/10.3390/inorganics11110427
Satish P, Hadagalli K, Praveen LL, Nowl MS, Seikh AH, Alnaser IA, Abdo HS, Mandal S. Hydroxyapatite–Clay Composite for Bone Tissue Engineering: Effective Utilization of Prawn Exoskeleton Biowaste. Inorganics. 2023; 11(11):427. https://doi.org/10.3390/inorganics11110427
Chicago/Turabian StyleSatish, Perabathula, Komalakrushna Hadagalli, Lakkimsetti Lakshmi Praveen, Mahin Saif Nowl, Asiful H. Seikh, Ibrahim A. Alnaser, Hany S. Abdo, and Saumen Mandal. 2023. "Hydroxyapatite–Clay Composite for Bone Tissue Engineering: Effective Utilization of Prawn Exoskeleton Biowaste" Inorganics 11, no. 11: 427. https://doi.org/10.3390/inorganics11110427








