Incorporation of Antimony Ions in Heptaisobutyl Polyhedral Oligomeric Silsesquioxanes
Abstract
:1. Introduction
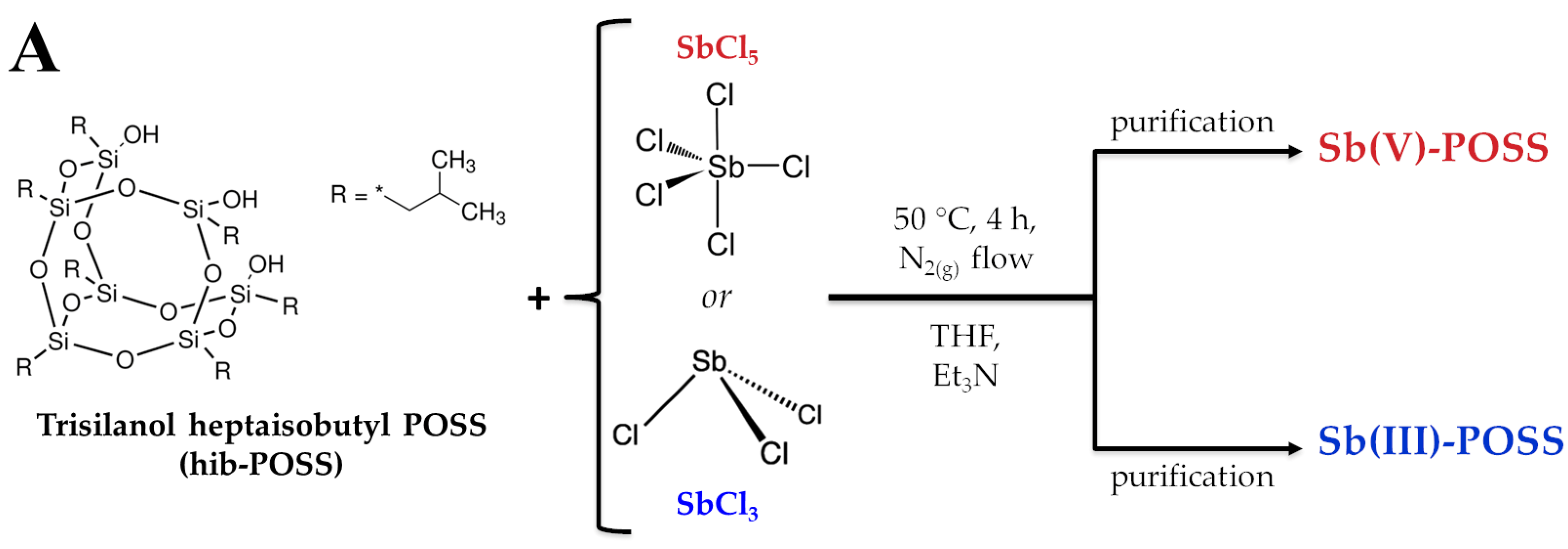
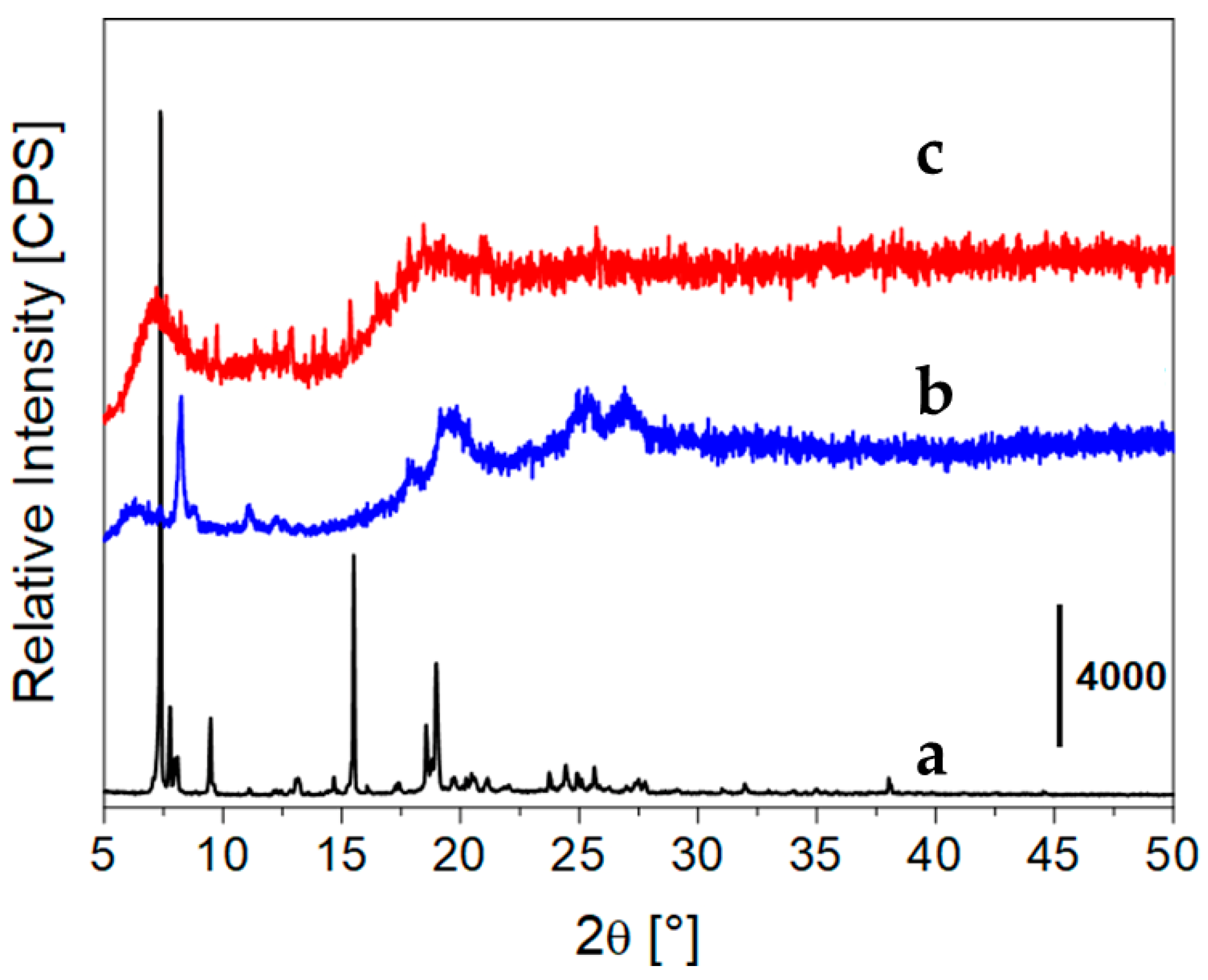
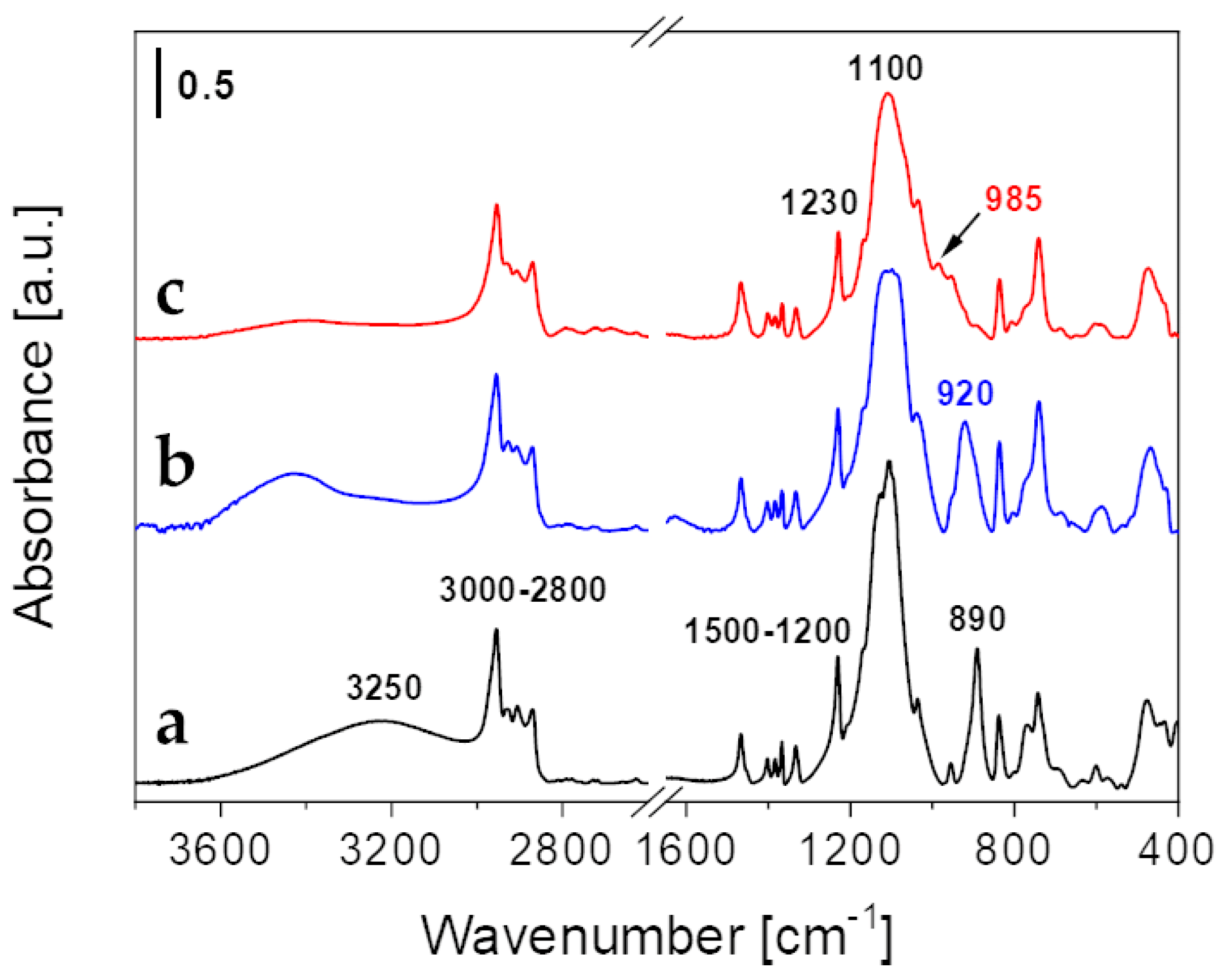
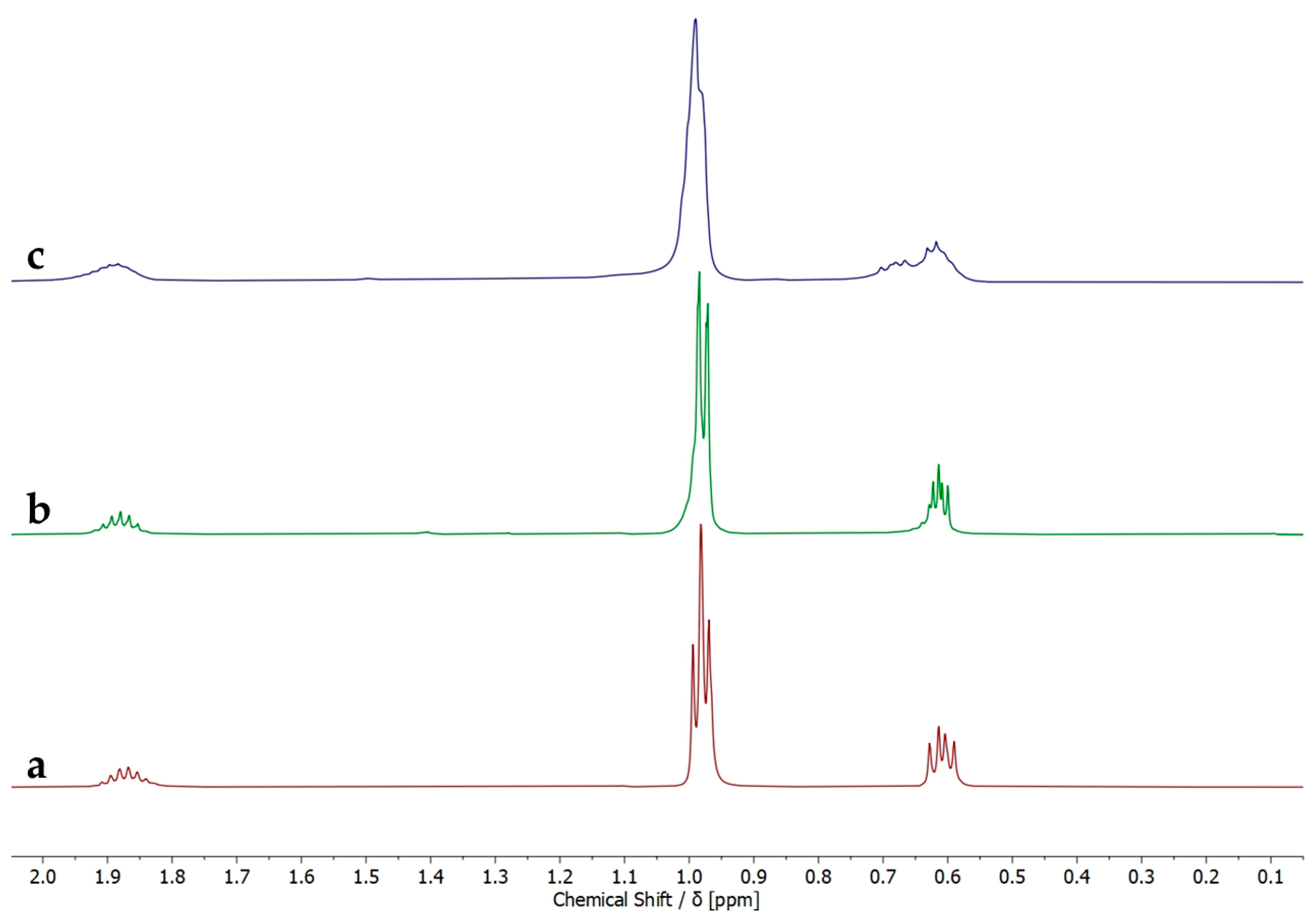
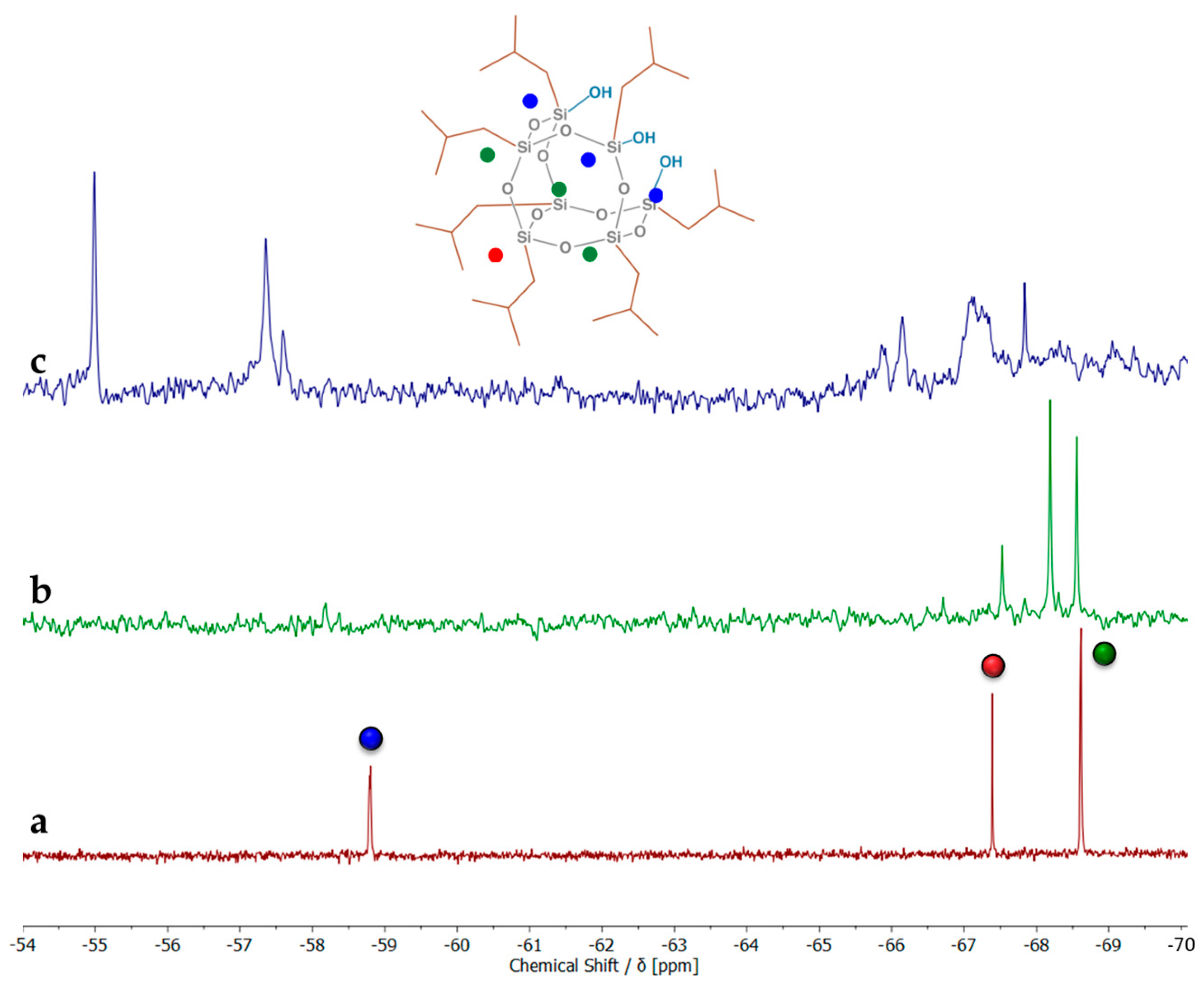
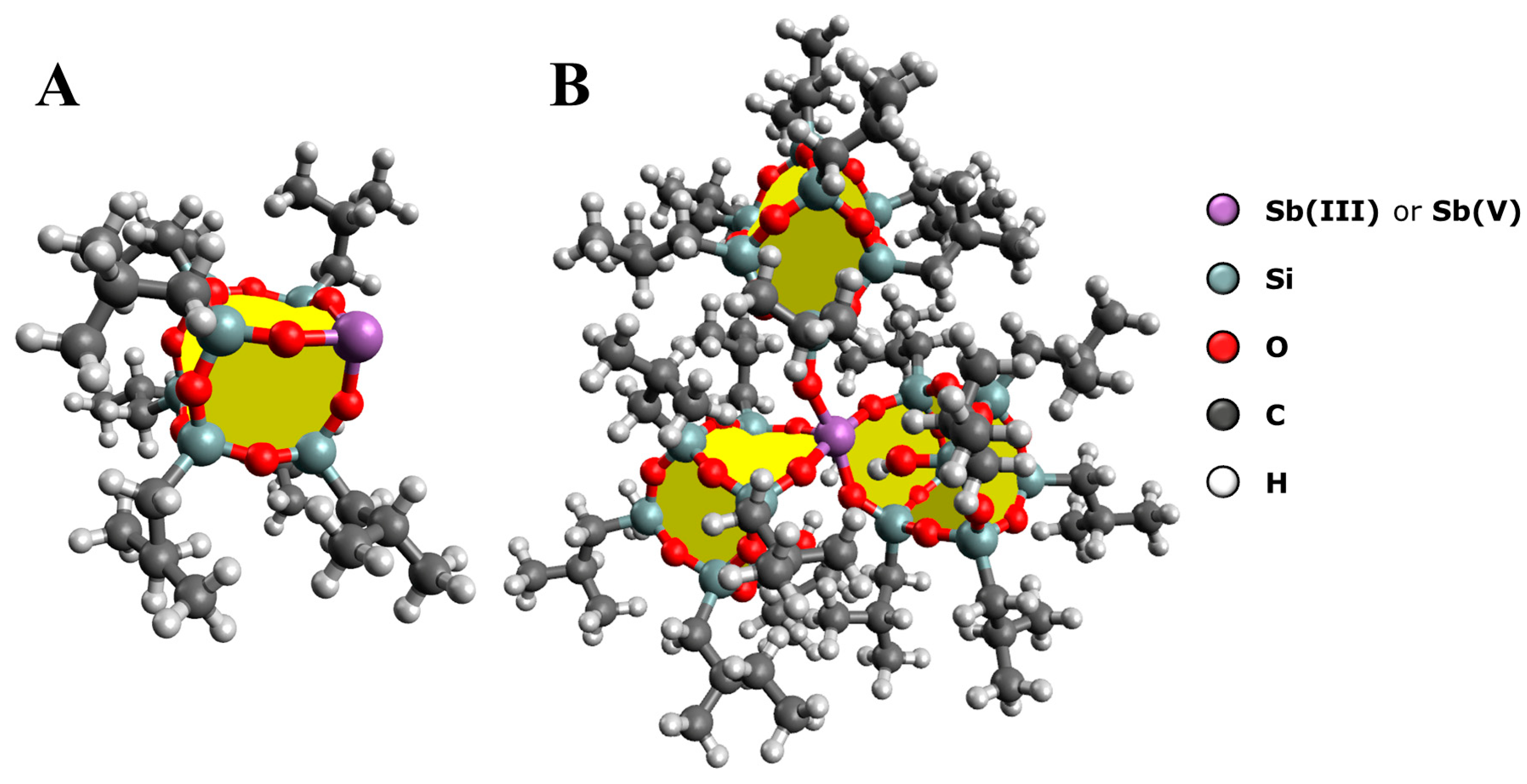
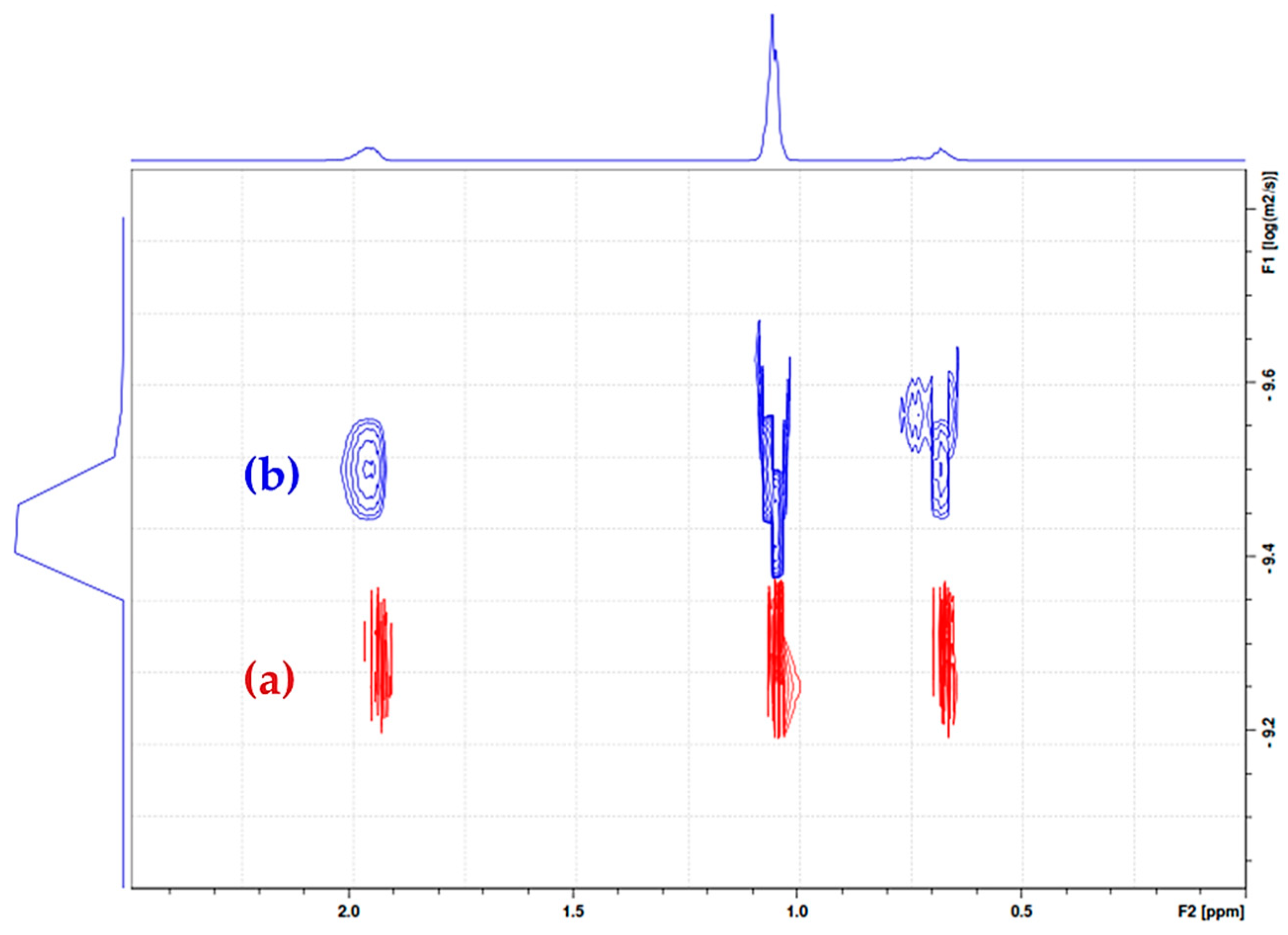
2. Results and Discussion
3. Materials and Methods
3.1. Reactants
3.2. Materials
3.2.1. Synthesis of Sb(III)-POSS
3.2.2. Synthesis of Sb(V)-POSS
3.3. Analytical Methods
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pescarmona, P.P.; Maschmeyer, T. Review: Oligomeric Silsesquioxanes: Synthesis, Characterization and Selected Applications. Aust. J. Chem. 2001, 54, 583–596. [Google Scholar] [CrossRef]
- Cordes, D.B.; Lickiss, P.D.; Rataboul, F. Recent Developments in the Chemistry of Cubic Polyhedral Oligosilsesquioxanes. Chem. Rev. 2010, 110, 2081–2173. [Google Scholar] [CrossRef] [PubMed]
- Kalia, S.; Pielichowski, K. (Eds.) Polymer/POSS Nanocomposites and Hybrid Materials: Preparation, Properties, Applications; Springer Series on Polymer and Composite Materials; Springer International Publishing: Cham, Switzerland, 2018; ISBN 978-3-030-02326-3. [Google Scholar]
- Laird, M.; Herrmann, N.; Ramsahye, N.; Totée, C.; Carcel, C.; Unno, M.; Bartlett, J.R.; Wong Chi Man, M. Large Polyhedral Oligomeric Silsesquioxane Cages: The Isolation of Functionalized POSS with an Unprecedented Si18O27 Core. Angew. Chem. Int. Ed. 2021, 60, 3022–3027. [Google Scholar] [CrossRef] [PubMed]
- Quadrelli, E.A.; Basset, J.-M. On Silsesquioxanes’ Accuracy as Molecular Models for Silica-Grafted Complexes in Heterogeneous Catalysis. Coord. Chem. Rev. 2010, 254, 707–728. [Google Scholar] [CrossRef]
- Feher, F.J.; Newman, D.A.; Walzer, J.F. Silsesquioxanes as Models for Silica Surfaces. J. Am. Chem. Soc. 1989, 111, 1741–1748. [Google Scholar] [CrossRef]
- Zhou, H.; Ye, Q.; Xu, J. Polyhedral Oligomeric Silsesquioxane-Based Hybrid Materials and Their Applications. Mater. Chem. Front. 2017, 1, 212–230. [Google Scholar] [CrossRef]
- Dong, F.; Lu, L.; Ha, C.-S. Silsesquioxane-Containing Hybrid Nanomaterials: Fascinating Platforms for Advanced Applications. Macromol. Chem. Phys. 2019, 220, 1800324. [Google Scholar] [CrossRef]
- Tunstall-Garcia, H.; Charles, B.L.; Evans, R.C. The Role of Polyhedral Oligomeric Silsesquioxanes in Optical Applications. Adv. Photonics Res. 2021, 2, 2000196. [Google Scholar] [CrossRef]
- John, Ł.; Ejfler, J. A Brief Review on Selected Applications of Hybrid Materials Based on Functionalized Cage-like Silsesquioxanes. Polymers 2023, 15, 1452. [Google Scholar] [CrossRef]
- Calabrese, C.; Aprile, C.; Gruttadauria, M.; Giacalone, F. POSS Nanostructures in Catalysis. Catal. Sci. Technol. 2020, 10, 7415–7447. [Google Scholar] [CrossRef]
- Blanco, I.; Abate, L.; Bottino, F.A.; Bottino, P. Hepta Isobutyl Polyhedral Oligomeric Silsesquioxanes (Hib-POSS). J. Therm. Anal. Calorim. 2012, 108, 807–815. [Google Scholar] [CrossRef]
- Ye, M.; Wu, Y.; Zhang, W.; Yang, R. Synthesis of Incompletely Caged Silsesquioxane (T7-POSS) Compounds via a Versatile Three-Step Approach. Res. Chem. Intermed. 2018, 44, 4277–4294. [Google Scholar] [CrossRef]
- Imoto, H.; Ueda, Y.; Sato, Y.; Nakamura, M.; Mitamura, K.; Watase, S.; Naka, K. Corner-and Side-Opened Cage Silsesquioxanes: Structural Effects on the Materials Properties. Eur. J. Inorg. Chem. 2020, 2020, 737–742. [Google Scholar] [CrossRef]
- Kaneko, Y.; Coughlin, E.B.; Gunji, T.; Itoh, M.; Matsukawa, K.; Naka, K. Silsesquioxanes: Recent Advancement and Novel Applications. Int. J. Polym. Sci. 2012, 2012, e453821. [Google Scholar] [CrossRef]
- Lorenz, V.; Edelmann, F.T. Dimeric Silsesquioxanes and Metallasilsesquioxanes—En Route to Large, Welldefined Si-O-Assemblies. Z. Anorg. Allg. Chem. 2004, 630, 1147–1157. [Google Scholar] [CrossRef]
- Ye, X.; Li, J.; Zhang, W.; Yang, R.; Li, J. Fabrication of Eco-Friendly and Multifunctional Sodium-Containing Polyhedral Oligomeric Silsesquioxane and Its Flame Retardancy on Epoxy Resin. Compos. B Eng. 2020, 191, 107961. [Google Scholar] [CrossRef]
- Prigyai, N.; Chanmungkalakul, S.; Ervithayasuporn, V.; Yodsin, N.; Jungsuttiwong, S.; Takeda, N.; Unno, M.; Boonmak, J.; Kiatkamjornwong, S. Lithium-Templated Formation of Polyhedral Oligomeric Silsesquioxanes (POSS). Inorg. Chem. 2019, 58, 15110–15117. [Google Scholar] [CrossRef]
- Gießmann, S.; Lorenz, V.; Liebing, P.; Hilfert, L.; Fischer, A.; Edelmann, F.T. Synthesis and Structural Study of New Metallasilsesquioxanes of Potassium and Uranium. Dalton Trans. 2017, 46, 2415–2419. [Google Scholar] [CrossRef]
- Hanssen, R.W.J.M.; Meetsma, A.; van Santen, R.A.; Abbenhuis, H.C.L. Synthesis, Structural Characterization, and Transmetalation Reactions of a Tetranuclear Magnesium Silsesquioxane Complex. Inorg. Chem. 2001, 40, 4049–4052. [Google Scholar] [CrossRef]
- Lorenz, V.; Fischer, A.; Edelmann, F.T. Silsesquioxane Chemistry, 6: The First Beryllium Silsesquioxane: Synthesis and Structure of [Cy7Si7O12BeLi]2·2THF. Inorg. Chem. Commun. 2000, 3, 292–295. [Google Scholar] [CrossRef]
- Levitsky, M.M.; Zubavichus, Y.V.; Korlyukov, A.A.; Khrustalev, V.N.; Shubina, E.S.; Bilyachenko, A.N. Silicon and Germanium-Based Sesquioxanes as Versatile Building Blocks for Cage Metallacomplexes. A Review. J. Clust. Sci. 2019, 30, 1283–1316. [Google Scholar] [CrossRef]
- Kaźmierczak, J.; Kuciński, K.; Stachowiak, H.; Hreczycho, G. Introduction of Boron Functionalities into Silsesquioxanes: Novel Independent Methodologies. Chem.—Eur. J. 2018, 24, 2509–2514. [Google Scholar] [CrossRef]
- Astakhov, G.S.; Levitsky, M.M.; Zubavichus, Y.V.; Khrustalev, V.N.; Titov, A.A.; Dorovatovskii, P.V.; Smol’yakov, A.F.; Shubina, E.S.; Kirillova, M.V.; Kirillov, A.M.; et al. Cu6- and Cu8-Cage Sil-and Germsesquioxanes: Synthetic and Structural Features, Oxidative Rearrangements, and Catalytic Activity. Inorg. Chem. 2021, 60, 8062–8074. [Google Scholar] [CrossRef] [PubMed]
- Gerritsen, G.; Duchateau, R.; Yap, G.P.A. Boron, Aluminum, and Gallium Silsesquioxane Compounds, Homogeneous Models for Group 13 Element-Containing Silicates and Zeolites. Organometallics 2003, 22, 100–110. [Google Scholar] [CrossRef]
- Besselink, R.; Venkatachalam, S.; van Wüllen, L.; ten Elshof, J.E. Incorporation of Niobium into Bridged Silsesquioxane Based Silica Networks. J. Sol-Gel Sci. Technol. 2014, 70, 473–481. [Google Scholar] [CrossRef]
- Marchesi, S.; Carniato, F.; Boccaleri, E. Synthesis and Characterisation of a Novel Europium(III)-Containing Heptaisobutyl-POSS. New J. Chem. 2014, 38, 2480–2485. [Google Scholar] [CrossRef]
- Willauer, A.R.; Dabrowska, A.M.; Scopelliti, R.; Mazzanti, M. Structure and Small Molecule Activation Reactivity of a Metallasilsesquioxane of Divalent Ytterbium. Chem. Commun. 2020, 56, 8936–8939. [Google Scholar] [CrossRef]
- Lorenz, V.; Fischer, A.; Gießmann, S.; Gilje, J.W.; Gun’ko, Y.; Jacob, K.; Edelmann, F.T. Disiloxanediolates and Polyhedral Metallasilsesquioxanes of the Early Transition Metals and F-Elements. Coord. Chem. Rev. 2000, 206, 321–368. [Google Scholar] [CrossRef]
- Marchesi, S.; Carniato, F.; Marchese, L.; Boccaleri, E. Luminescent Mesoporous Silica Built through Self-Assembly of Polyhedral Oligomeric Silsesquioxane and Europium(III) Ions. ChemPlusChem 2015, 80, 915–918. [Google Scholar] [CrossRef]
- Kumar, B.; Kumar, A.P.; Bindu, P.; Mukherjee, A.; Patra, S. Red Light Emission of POSS Triol Chelated with Europium. Asian J. Nanosci. Mater. 2019, 2, 244–256. [Google Scholar] [CrossRef]
- Marchesi, S.; Bisio, C.; Boccaleri, E.; Carniato, F. A Luminescent Polysilsesquioxane Obtained by Self-Condensation of Anionic Polyhedral Oligomeric Silsequioxanes (POSS) and Europium(III) Ions. ChemPlusChem 2020, 85, 176–182. [Google Scholar] [CrossRef]
- Marchesi, S.; Bisio, C.; Carniato, F. Synthesis of Novel Luminescent Double-Decker Silsesquioxanes Based on Partially Condensed TetraSilanolPhenyl POSS and Tb3+/Eu3+ Lanthanide Ions. Processes 2022, 10, 758. [Google Scholar] [CrossRef]
- Marchesi, S.; Miletto, I.; Bisio, C.; Gianotti, E.; Marchese, L.; Carniato, F. Eu3+ and Tb3+ @ PSQ: Dual Luminescent Polyhedral Oligomeric Polysilsesquioxanes. Materials 2022, 15, 7996. [Google Scholar] [CrossRef] [PubMed]
- Levitsky, M.M.; Yalymov, A.I.; Kulakova, A.N.; Petrov, A.A.; Bilyachenko, A.N. Cage-like Metallasilsesquioxanes in Catalysis: A Review. J. Mol. Catal. A-Chem. 2017, 426, 297–304. [Google Scholar] [CrossRef]
- Lorenz, V.; Edelmann, F.T. Metallasilsesquioxanes. In Advances in Organometallic Chemistry; West, R., Hill, A.F., Stone, F.G.A., Eds.; Academic Press: Cambridge, MA, USA, 2005; Volume 53, pp. 101–153. [Google Scholar]
- Levitsky, M.M.; Bilyachenko, A.N. Modern Concepts and Methods in the Chemistry of Polyhedral Metallasiloxanes. Coord. Chem. Rev. 2016, 306, 235–269. [Google Scholar] [CrossRef]
- Pan, G. Polyhedral Oligomeric Silsesquioxane (POSS). In Physical Properties of Polymers Handbook; Mark, J.E., Ed.; Springer: New York, NY, USA, 2007; pp. 577–584. ISBN 978-0-387-69002-5. [Google Scholar]
- Ward, A.J.; Masters, A.F.; Maschmeyer, T. Metallasilsesquioxanes: Molecular Analogues of Heterogeneous Catalysts. In Applications of Polyhedral Oligomeric Silsesquioxanes; Hartmann-Thompson, C., Ed.; Advances in Silicon Science; Springer: Dordrecht, The Netherlands, 2011; pp. 135–166. ISBN 978-90-481-3787-9. [Google Scholar]
- Henig, J.; Tóth, É.; Engelmann, J.; Gottschalk, S.; Mayer, H.A. Macrocyclic Gd3+ Chelates Attached to a Silsesquioxane Core as Potential Magnetic Resonance Imaging Contrast Agents: Synthesis, Physicochemical Characterization, and Stability Studies. Inorg. Chem. 2010, 49, 6124–6138. [Google Scholar] [CrossRef]
- Köytepe, S.; Demirel, M.H.; Gültek, A.; Seçkin, T. Metallo-Supramolecular Materials Based on Terpyridine-Functionalized Polyhedral Silsesquioxane. Polym. Int. 2014, 63, 778–787. [Google Scholar] [CrossRef]
- Li, Y.; Dong, X.-H.; Zou, Y.; Wang, Z.; Yue, K.; Huang, M.; Liu, H.; Feng, X.; Lin, Z.; Zhang, W.; et al. Polyhedral Oligomeric Silsesquioxane Meets “Click” Chemistry: Rational Design and Facile Preparation of Functional Hybrid Materials. Polymer 2017, 125, 303–329. [Google Scholar] [CrossRef]
- Murugavel, R.; Voigt, A.; Walawalkar, M.G.; Roesky, H.W. Hetero- and Metallasiloxanes Derived from Silanediols, Disilanols, Silanetriols, and Trisilanols. Chem. Rev. 1996, 96, 2205–2236. [Google Scholar] [CrossRef]
- Feher, F.J.; Budzichowski, T.A. Heterosilsesquioxanes: Synthesis and Characterization of Group 15 Containing Polyhedral Oligosilsesquioxanes. Organometallics 1991, 10, 812–815. [Google Scholar] [CrossRef]
- Feher, F.J.; Budzichowski, T.A.; Rahimian, K.; Ziller, J.W. Reactions of Incompletely-Condensed Silsesquioxanes with Pentamethylantimony: A New Synthesis of Metallasilsesquioxanes with Important Implications for the Chemistry of Silica Surfaces. J. Am. Chem. Soc. 1992, 114, 3859–3866. [Google Scholar] [CrossRef]
- Zhang, W.; Camino, G.; Yang, R. Polymer/Polyhedral Oligomeric Silsesquioxane (POSS) Nanocomposites: An Overview of Fire Retardance. Prog. Polym. Sci. 2017, 67, 77–125. [Google Scholar] [CrossRef]
- Glodek, T.; Boyd, S.; Mcaninch, I.; Lascala, J. Properties and Performance of Fire Resistant Eco-Composites Using Polyhedral Oligomeric Silsesquioxane (POSS) Fire Retardants. Compos. Sci. Technol. 2008, 68, 2994–3001. [Google Scholar] [CrossRef]
- Kim, H.-J.; Kwon, Y.; Kim, C.K. Mechanical Property and Thermal Stability of Polyurethane Composites Reinforced with Polyhedral Oligomeric Silsesquioxanes and Inorganic Flame Retardant Filler. J. Nanosci. Nanotechnol. 2014, 14, 6048–6052. [Google Scholar] [CrossRef]
- Palin, L.; Rombolà, G.; Milanesio, M.; Boccaleri, E. The Use of POSS-Based Nanoadditives for Cable-Grade PVC: Effects on Its Thermal Stability. Polymers 2019, 11, 1105. [Google Scholar] [CrossRef] [PubMed]
- Weil, E.D.; Levchik, S.V. Commercial Flame Retardancy of Unsaturated Polyester and Vinyl Resins: Review. J. Fire Sci. 2004, 22, 293–303. [Google Scholar] [CrossRef]
- Li, N.; Xia, Y.; Mao, Z.; Wang, L.; Guan, Y.; Zheng, A. Influence of Antimony Oxide on Flammability of Polypropylene/Intumescent Flame Retardant System. Polym. Degrad. Stab. 2012, 97, 1737–1744. [Google Scholar] [CrossRef]
- Kim, H.; Kim, J.-S.; Jeong, W. Study on the Flame Retardancy and Hazard Evaluation of Poly(Acrylonitrile-Co-Vinylidene Chloride) Fibers by the Addition of Antimony-Based Flame Retardants. Polymers 2022, 14, 42. [Google Scholar] [CrossRef]
- Zanetti, M.; Camino, G.; Canavese, D.; Morgan, A.B.; Lamelas, F.J.; Wilkie, C.A. Fire Retardant Halogen−Antimony−Clay Synergism in Polypropylene Layered Silicate Nanocomposites. Chem. Mater. 2002, 14, 189–193. [Google Scholar] [CrossRef]
- Faryal, S.; Zafar, M.; Nazir, M.S.; Ali, Z.; Hussain, M.; Muhammad Imran, S. The Synergic Effect of Primary and Secondary Flame Retardants on the Improvement in the Flame Retardant and Mechanical Properties of Thermoplastic Polyurethane Nanocomposites. Appl. Sci. 2022, 12, 10866. [Google Scholar] [CrossRef]
- Alphazan, T.; Díaz Álvarez, A.; Martin, F.; Grampeix, H.; Enyedi, V.; Martinez, E.; Rochat, N.; Veillerot, M.; Dewitte, M.; Nys, J.-P.; et al. Shallow Heavily Doped N++ Germanium by Organo-Antimony Monolayer Doping. ACS Appl. Mater. Interfaces 2017, 9, 20179–20187. [Google Scholar] [CrossRef] [PubMed]
- Alphazan, T.; Florian, P.; Thieuleux, C. Ethoxy and Silsesquioxane Derivatives of Antimony as Dopant Precursors: Unravelling the Structure and Thermal Stability of Surface Species on SiO2. Phys. Chem. Chem. Phys. 2017, 19, 8595–8601. [Google Scholar] [CrossRef] [PubMed]
- Khouchaf, L.; Boulahya, K.; Das, P.P.; Nicolopoulos, S.; Kis, V.K.; Lábár, J.L. Study of the Microstructure of Amorphous Silica Nanostructures Using High-Resolution Electron Microscopy, Electron Energy Loss Spectroscopy, X-ray Powder Diffraction, and Electron Pair Distribution Function. Materials 2020, 13, 4393. [Google Scholar] [CrossRef] [PubMed]
- Carniato, F.; Boccaleri, E.; Marchese, L.; Fina, A.; Tabuani, D.; Camino, G. Synthesis and Characterisation of Metal Isobutylsilsesquioxanes and Their Role as Inorganic–Organic Nanoadditives for Enhancing Polymer Thermal Stability. Eur. J. Inorg. Chem. 2007, 2007, 585–591. [Google Scholar] [CrossRef]
- Marchesi, S.; Carniato, F.; Palin, L.; Boccaleri, E. POSS as Building-Blocks for the Preparation of Polysilsesquioxanes through an Innovative Synthetic Approach. Dalton Trans. 2015, 44, 2042–2046. [Google Scholar] [CrossRef] [PubMed]
- Komagata, Y.; Iimura, T.; Shima, N.; Kudo, T. A Theoretical Study of the Insertion of Atoms and Ions into Titanosilsequioxane (Ti-POSS) in Comparison with POSS. Int. J. Polym. Sci. 2012, 2012, e391325. [Google Scholar] [CrossRef]
- Jin, L.; Hong, C.; Li, X.; Sun, Z.; Feng, F.; Liu, H. Corner-Opening and Corner-Capping of Mono-Substituted T8 POSS: Product Distribution and Isomerization. Chem. Commun. 2022, 58, 1573–1576. [Google Scholar] [CrossRef]
- Loman-Cortes, P.; Binte Huq, T.; Vivero-Escoto, J.L. Use of Polyhedral Oligomeric Silsesquioxane (POSS) in Drug Delivery, Photodynamic Therapy and Bioimaging. Molecules 2021, 26, 6453. [Google Scholar] [CrossRef]
- Alphazan, T.; Mathey, L.; Schwarzwälder, M.; Lin, T.-H.; Rossini, A.J.; Wischert, R.; Enyedi, V.; Fontaine, H.; Veillerot, M.; Lesage, A.; et al. Monolayer Doping of Silicon through Grafting a Tailored Molecular Phosphorus Precursor onto Oxide-Passivated Silicon Surfaces. Chem. Mater. 2016, 28, 3634–3640. [Google Scholar] [CrossRef]
- Carniato, F.; Boccaleri, E.; Marchese, L. A Versatile Route to Bifunctionalized Silsesquioxane (POSS): Synthesis and Characterisation of Ti-Containing Aminopropylisobutyl-POSS. Dalton Trans. 2007, 1, 36–39. [Google Scholar] [CrossRef]
- Klein, R.A.; Marinus, C. Synthesis and use of metallized polyhedral oligomeric silsequioxane catalyst compositions. WO2015062759A1, 7 May 2015. [Google Scholar]
- Evans, R. The Interpretation of Small Molecule Diffusion Coefficients: Quantitative Use of Diffusion-Ordered NMR Spectroscopy. Prog. Nucl. Magn. Reson. Spectrosc. 2020, 117, 33–69. [Google Scholar] [CrossRef] [PubMed]
- Dill, K.A.; Bromberg, S. Molecular Driving Forces: Statistical Thermodynamics in Chemistry and Biology; Garland Science: New York, NY, USA, 2003; ISBN 978-0-8153-2051-7. [Google Scholar]
- Einstein, A. Über Die von Der Molekularkinetischen Theorie Der Wärme Geforderte Bewegung von in Ruhenden Flüssigkeiten Suspendierten Teilchen. Ann. Phys. 1905, 322, 549–560. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Marchesi, S.; Bisio, C.; Carniato, F.; Boccaleri, E. Incorporation of Antimony Ions in Heptaisobutyl Polyhedral Oligomeric Silsesquioxanes. Inorganics 2023, 11, 426. https://doi.org/10.3390/inorganics11110426
Marchesi S, Bisio C, Carniato F, Boccaleri E. Incorporation of Antimony Ions in Heptaisobutyl Polyhedral Oligomeric Silsesquioxanes. Inorganics. 2023; 11(11):426. https://doi.org/10.3390/inorganics11110426
Chicago/Turabian StyleMarchesi, Stefano, Chiara Bisio, Fabio Carniato, and Enrico Boccaleri. 2023. "Incorporation of Antimony Ions in Heptaisobutyl Polyhedral Oligomeric Silsesquioxanes" Inorganics 11, no. 11: 426. https://doi.org/10.3390/inorganics11110426





