Metal-Organic Cages Based on Phosphorescent Organometallics
Abstract
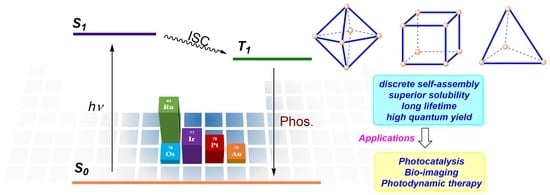
:1. Introduction
2. Metal-Organic Cages Based on Phosphorescent Organometallics
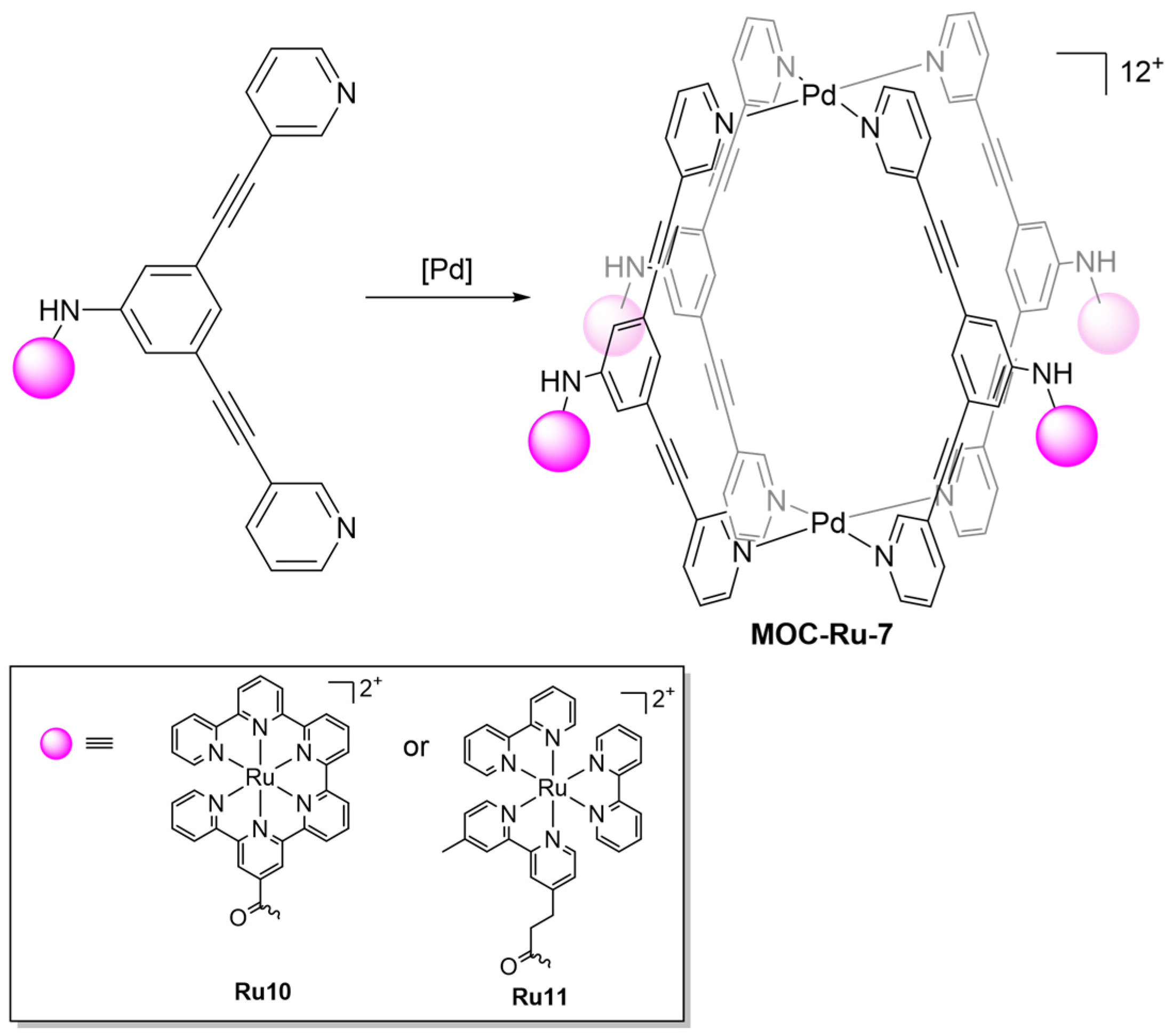
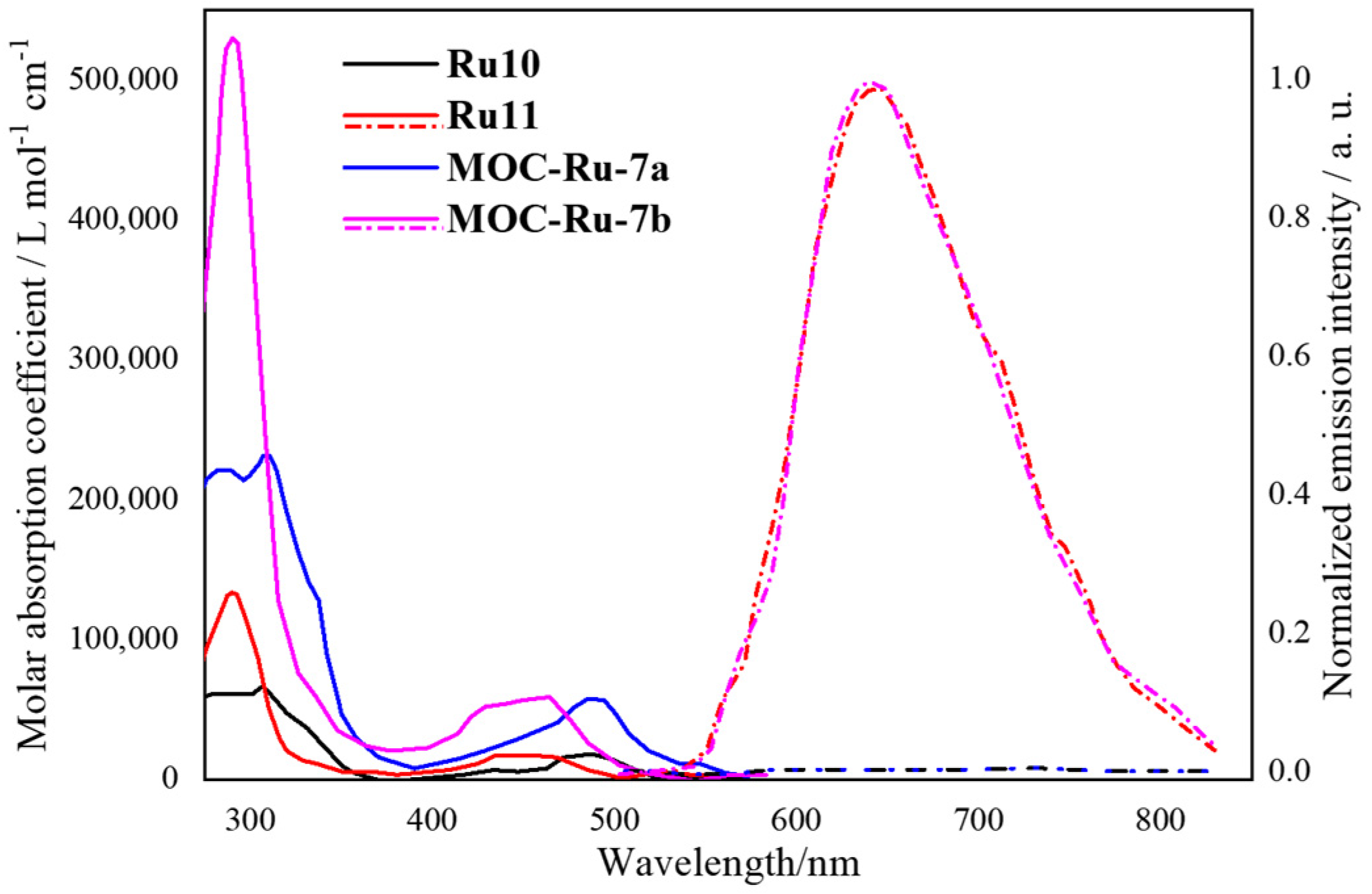
2.1. Phosphorescent MOCs Incorporating d6 Metal Ion
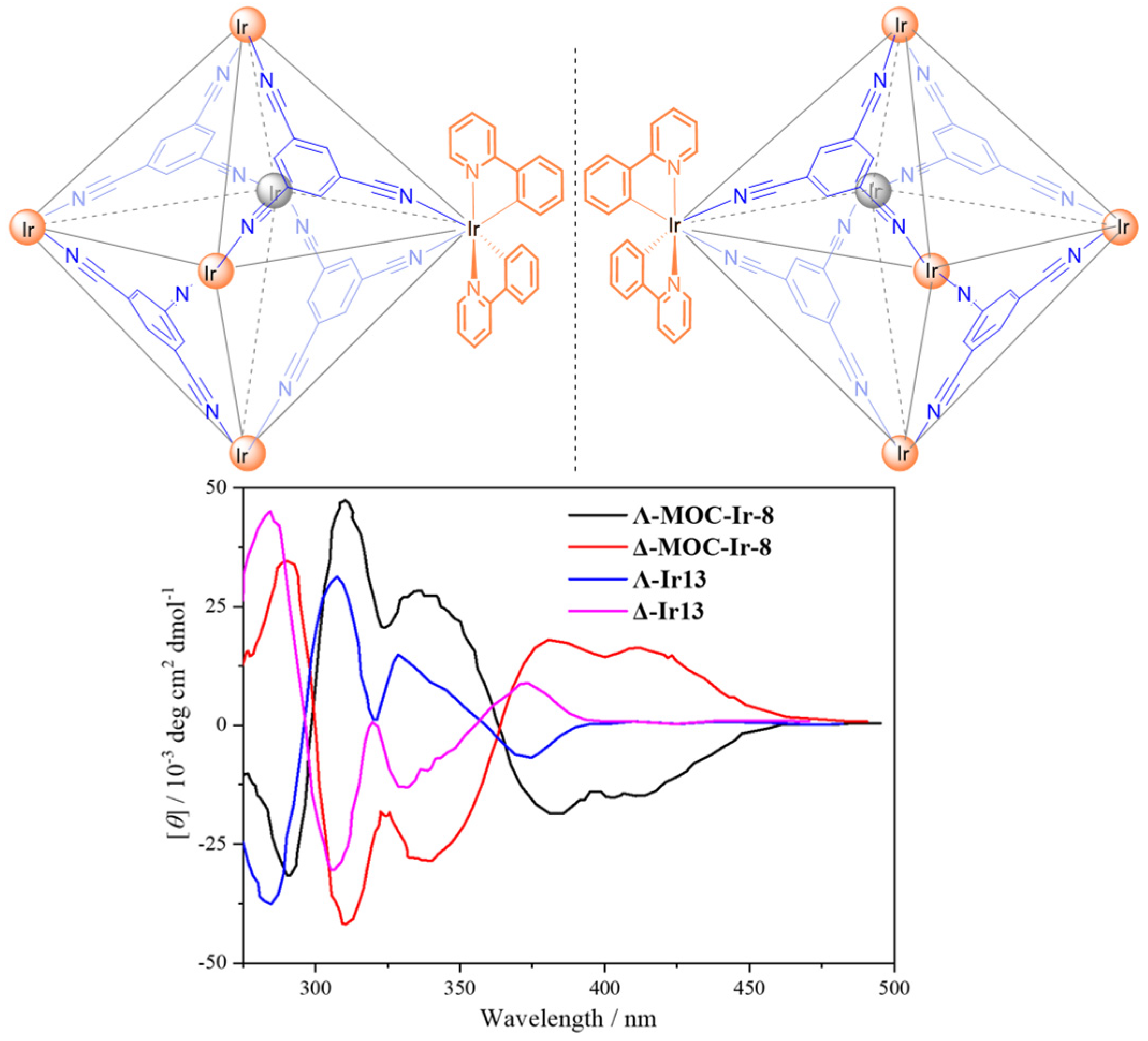
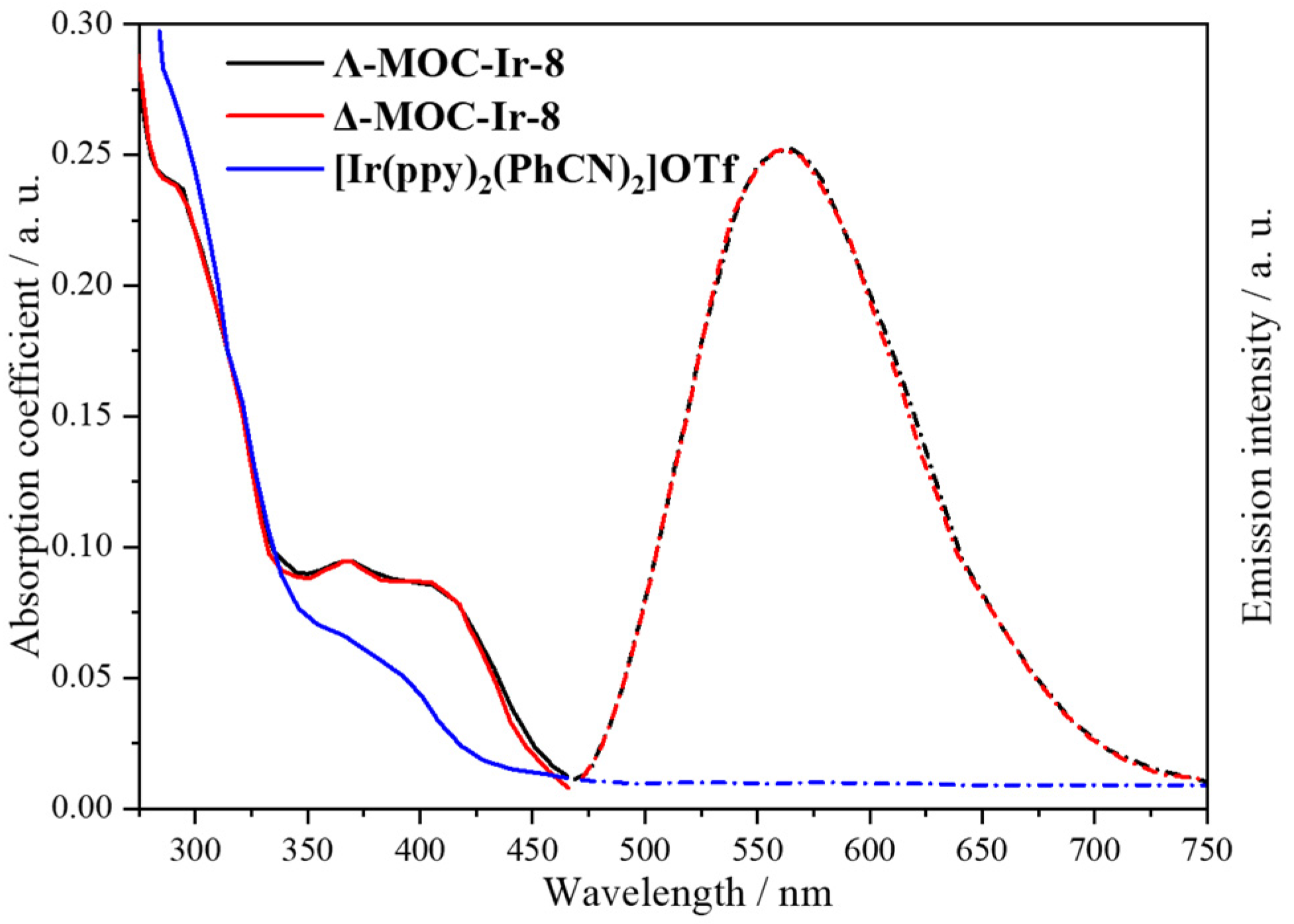
2.2. Phosphorescent MOCs Incorporating d8 Metal Ion
2.3. Phosphorescent MOCs Incorporating d10 Metal Ion
3. Conclusions and Prospect
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Furukawa, H.; Cordova, K.E.; O’Keeffe, M.; Yaghi, O.M. The chemistry and applications of metal-organic frameworks. Science 2013, 341, 1230444. [Google Scholar] [CrossRef]
- Gong, W.; Chen, Z.; Dong, J.; Liu, Y.; Cui, Y. Chiral Metal-Organic Frameworks. Chem. Rev. 2022, 122, 9078–9144. [Google Scholar] [CrossRef] [PubMed]
- Pan, M.; Wu, K.; Zhang, J.-H.; Su, C.-Y. Chiral metal–organic cages/containers (MOCs): From structural and stereochemical design to applications. Coord. Chem. Rev. 2019, 378, 333–349. [Google Scholar] [CrossRef]
- El-Sayed, E.M.; Yuan, Y.D.; Zhao, D.; Yuan, D. Zirconium Metal-Organic Cages: Synthesis and Applications. Acc. Chem. Res. 2022, 55, 1546–1560. [Google Scholar] [CrossRef] [PubMed]
- Vardhan, H.; Yusubov, M.; Verpoort, F. Self-assembled metal–organic polyhedra: An overview of various applications. Coord. Chem. Rev. 2016, 306, 171–194. [Google Scholar] [CrossRef]
- Lee, S.; Jeong, H.; Nam, D.; Lah, M.S.; Choe, W. The rise of metal-organic polyhedra. Chem. Soc. Rev. 2021, 50, 528–555. [Google Scholar] [CrossRef]
- Gosselin, A.J.; Rowland, C.A.; Bloch, E.D. Permanently Microporous Metal-Organic Polyhedra. Chem. Rev. 2020, 120, 8987–9014. [Google Scholar] [CrossRef]
- Percastegui, E.G.; Ronson, T.K.; Nitschke, J.R. Design and Applications of Water-Soluble Coordination Cages. Chem. Rev. 2020, 120, 13480–13544. [Google Scholar] [CrossRef]
- Saalfrank, R.W.; Stark, A.; Peters, K.; von Schnering, H.G. The First “Adamantoid” Alkaline Earth Metal Chelate Complex: Synthesis, Structure, and Reactivity. Angew. Chem. Int. Ed. 1988, 27, 851–853. [Google Scholar] [CrossRef]
- Olenyuk, B.; Whiteford, J.A.; Fechtenkötter, A.; Stang, P.J. Self-assembly of nanoscale cuboctahedra by coordination chemistry. Nature 1999, 398, 769–799. [Google Scholar] [CrossRef]
- Sun, Q.F.; Iwasa, J.; Ogawa, D.; Ishido, Y.; Sato, S.; Ozeki, T.; Sei, Y.; Yamaguchi, K.; Fujita, M. Self-assembled M24L48 polyhedra and their sharp structural switch upon subtle ligand variation. Science 2010, 328, 1144–1147. [Google Scholar] [CrossRef] [PubMed]
- Meyer, M.; Kersting, B.; Powers, R.E.; Raymond, K.N. Rearrangement Reactions in Dinuclear Triple Helicates. Inorg. Chem. 1997, 36, 5179–5191. [Google Scholar] [CrossRef]
- Cotton, F.A.; Lin, C.; Murillo, C.A. Supramolecular Arrays Based on Dimetal Building Units. Acc. Chem. Res. 2001, 34, 759–771. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Crawley, M.R.; Hauke, C.E.; Friedman, A.E.; Cook, T.R. Phosphorescent Decanuclear Bimetallic Pt(6)M(4) (M = Zn, Fe) Tetrahedral Cages. Inorg. Chem. 2017, 56, 4258–4262. [Google Scholar] [CrossRef]
- Oliveri, C.G.; Ulmann, P.A.; Wiester, M.J.; Mirkin, C.A. Heteroligated Supramolecular Coordination Complexes Formed via the Halide-Induced Ligand Rearrangement Reaction. Acc. Chem. Res. 2008, 41, 1618–1629. [Google Scholar] [CrossRef]
- Hosono, N.; Gochomori, M.; Matsuda, R.; Sato, H.; Kitagawa, S. Metal-Organic Polyhedral Core as a Versatile Scaffold for Divergent and Convergent Star polymer Synthesis. J. Am. Chem. Soc. 2016, 138, 6525–6531. [Google Scholar] [CrossRef]
- Percastegui, E.G.; Mosquera, J.; Ronson, T.K.; Plajer, A.J.; Kieffer, M.; Nitschke, J.R. Waterproof Architectures Through Subcomponent Self-Assembly. Chem. Sci. 2019, 10, 2006–2018. [Google Scholar] [CrossRef]
- Rota Martir, D.; Zysman-Colman, E. Photoactive supramolecular cages incorporating Ru(ii) and Ir(iii) metal complexes. Chem. Commun. 2018, 55, 139–158. [Google Scholar] [CrossRef]
- Gutiérrez, M.; Zhang, Y.; Tan, J.-C. Confinement of Luminescent Guests in Metal-Organic Frameworks: Understanding Pathways from Synthesis and Multimodal Characterization to Potential Applications of LG@MOF Systems. Chem. Rev. 2022, 122, 10438–10483. [Google Scholar] [CrossRef]
- Li, Y.-L.; Li, A.-J.; Huang, S.-L.; Vittal, J.J.; Yang, G.-Y. Polypyridyl Ru(II) or cyclometalated Ir(III) functionalized architectures for photocatalysis. Chem. Soc. Rev. 2023, 52, 4725–4754. [Google Scholar] [CrossRef]
- Cui, Y.; Yue, Y.; Qian, G.; Chen, B. Luminescent functional metal-organic frameworks. Chem. Rev. 2012, 112, 1126–1162. [Google Scholar] [CrossRef]
- Cook, T.R.; Stang, P.J. Recent Developments in the Preparation and Chemistry of Metallacycles and Metallacages via Coordination. Chem. Rev. 2015, 115, 7001–7045. [Google Scholar] [CrossRef]
- Xu, L.; Wang, Y.X.; Yang, H.B. Recent advances in the construction of fluorescent metallocycles and metallocages via coordination-driven self-assembly. Dalton Trans. 2015, 44, 867–890. [Google Scholar] [CrossRef]
- To, W.-P.; Wan, Q.; Tong, G.S.M.; Che, C.-M. Recent Advances in Metal Triplet Emitters with d6, d8, and d10 Electronic Configurations. Trends Chem. 2020, 2, 796–812. [Google Scholar] [CrossRef]
- Laramee-Milette, B.; Nastasi, F.; Puntoriero, F.; Campagna, S.; Hanan, G.S. Photo-Induced Assembly of a Luminescent Tetraruthenium Square. Chemistry 2017, 23, 16497–16504. [Google Scholar] [CrossRef]
- Hauke, C.E.; Oldacre, A.N.; Fulong, C.R.P.; Friedman, A.E.; Cook, T.R. Coordination-Driven Self-Assembly of Ruthenium Polypyridyl Nodes Resulting in Emergent Photophysical and Electrochemical Properties. Inorg. Chem. 2018, 57, 3587–3595. [Google Scholar] [CrossRef] [PubMed]
- Li, K.; Zhang, L.Y.; Yan, C.; Wei, S.C.; Pan, M.; Zhang, L.; Su, C.Y. Stepwise assembly of Pd(6)(RuL(3))(8) nanoscale rhombododecahedral metal-organic cages via metalloligand strategy for guest trapping and protection. J. Am. Chem. Soc. 2014, 136, 4456–4459. [Google Scholar] [CrossRef]
- Yang, J.; Bhadbhade, M.; Donald, W.A.; Iranmanesh, H.; Moore, E.G.; Yan, H.; Beves, J.E. Self-assembled supramolecular cages containing ruthenium(II) polypyridyl complexes. Chem. Commun. 2015, 51, 4465–4468. [Google Scholar] [CrossRef] [PubMed]
- Rota Martir, D.; Cordes, D.B.; Slawin, A.M.Z.; Escudero, D.; Jacquemin, D.; Warriner, S.L.; Zysman-Colman, E. A luminescent [Pd(4)Ru(8)](24+) supramolecular cage. Chem. Commun. 2018, 54, 6016–6019. [Google Scholar] [CrossRef]
- Elliott, A.B.; Lewis, J.E.; van der Salm, H.; McAdam, C.J.; Crowley, J.D.; Gordon, K.C. Luminescent Cages: Pendant Emissive Units on [Pd2L4](4+) “Click“ Cages. Inorg. Chem. 2016, 55, 3440–3447. [Google Scholar] [CrossRef]
- Schmidt, A.; Hollering, M.; Han, J.; Casini, A.; Kuhn, F.E. Self-assembly of highly luminescent heteronuclear coordination cages. Dalton Trans. 2016, 45, 12297–12300. [Google Scholar] [CrossRef]
- Chepelin, O.; Ujma, J.; Wu, X.; Slawin, A.M.; Pitak, M.B.; Coles, S.J.; Michel, J.; Jones, A.C.; Barran, P.E.; Lusby, P.J. Luminescent, enantiopure, phenylatopyridine iridium-based coordination capsules. J. Am. Chem. Soc. 2012, 134, 19334–19337. [Google Scholar] [CrossRef] [PubMed]
- Rota Martir, D.; Escudero, D.; Jacquemin, D.; Cordes, D.B.; Slawin, A.M.Z.; Fruchtl, H.A.; Warriner, S.L.; Zysman-Colman, E. Homochiral Emissive Lambda(8) -and Delta(8) -[Ir(8) Pd(4)](16+) Supramolecular Cages. Chemistry 2017, 23, 14358–14366. [Google Scholar] [CrossRef]
- Pritchard, V.E.; Rota Martir, D.; Oldknow, S.; Kai, S.; Hiraoka, S.; Cookson, N.J.; Zysman-Colman, E.; Hardie, M.J. Homochiral Self-Sorted and Emissive Ir(III) Metallo-Cryptophanes. Chemistry 2017, 23, 6290–6294. [Google Scholar] [CrossRef]
- Li, C.; Wang, Y.; Lu, Y.; Guo, J.; Zhu, C.; He, H.; Duan, X.; Pan, M.; Su, C.Y. An iridium(III)-palladium(II) metal-organic cage for efficient mitochondria-targeted photodynamic therapy. Chin. Chem. Lett. 2020, 31, 1183–1187. [Google Scholar] [CrossRef]
- Wragg, A.B.; Metherell, A.J.; Cullen, W.; Ward, M.D. Stepwise assembly of mixed-metal coordination cages containing both kinetically inert and kinetically labile metal ions: Introduction of metal-centred redox and photophysical activity at specific sites. Dalton Trans. 2015, 44, 17939–17949. [Google Scholar] [CrossRef]
- Goeb, S.; Prusakova, V.; Wang, X.; Vezinat, A.; Salle, M.; Castellano, F.N. Phosphorescent self-assembled Pt(II) tetranuclear metallocycles. Chem. Commun. 2011, 47, 4397–4399. [Google Scholar] [CrossRef]
- Li, Y.; Huo, G.F.; Liu, B.; Song, B.; Zhang, Y.; Qian, X.; Wang, H.; Yin, G.Q.; Filosa, A.; Sun, W.; et al. Giant Concentric Metallosupramolecule with Aggregation-Induced Phosphorescent Emission. J. Am. Chem. Soc. 2020, 142, 14638–14648. [Google Scholar] [CrossRef] [PubMed]
- Walters, D.T.; Powers, X.B.; Olmstead, M.M.; Balch, A.L. The Preparation of Luminescent, Mechanochromic Molecular Containers from Non-Emissive Components: The Box Cations, [Au(6) (Triphos)(4) Br](5+) and [Au(6) (Triphos)(4) Br(2)](4). Chemistry 2019, 25, 3849–3857. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Q.; Li, F.; Huang, C. Phosphorescent chemosensors based on heavy-metal complexes. Chem. Soc. Rev. 2010, 39, 3007–3030. [Google Scholar] [CrossRef]
- Wu, K.; Li, K.; Hou, Y.J.; Pan, M.; Zhang, L.Y.; Chen, L.; Su, C.Y. Homochiral D4-symmetric metal-organic cages from stereogenic Ru(II) metalloligands for effective enantioseparation of atropisomeric molecules. Nat. Commun. 2016, 7, 10487. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Li, K.; Zhao, F.; Zhang, L.; Pan, M.; Fan, Y.Z.; Guo, J.; Shi, J.; Su, C.Y. A metal-organic cage incorporating multiple light harvesting and catalytic centres for photochemical hydrogen production. Nat. Commun. 2016, 7, 13169. [Google Scholar] [CrossRef] [PubMed]
- Wu, K.; Li, K.; Chen, S.; Hou, Y.J.; Lu, Y.L.; Wang, J.S.; Wei, M.J.; Pan, M.; Su, C.Y. The Redox Coupling Effect in a Photocatalytic Ru(II) -Pd(II) Cage with TTF Guest as Electron Relay Mediator for Visible-Light Hydrogen-Evolving Promotion. Angew. Chem. Int. Ed. 2020, 59, 2639–2643. [Google Scholar] [CrossRef]
- Wang, Y.P.; Wu, K.; Pan, M.; Li, K.; Mo, J.T.; Duan, X.H.; He, H.Z.; Shen, J.; Su, C.Y. One-/Two-Photon Excited Cell Membrane Imaging and Tracking by a Photoactive Nanocage. ACS Appl. Mater. Interfaces 2020, 12, 35873–35881. [Google Scholar] [CrossRef]
- Guo, J.; Xu, Y.W.; Li, K.; Xiao, L.M.; Chen, S.; Wu, K.; Chen, X.D.; Fan, Y.Z.; Liu, J.M.; Su, C.Y. Regio- and Enantioselective Photodimerization within the Confined Space of a Homochiral Ruthenium/Palladium Heterometallic Coordination Cage. Angew. Chem. Int. Ed. 2017, 56, 3852–3856. [Google Scholar] [CrossRef]
- Wang, J.S.; Wu, K.; Yin, C.; Li, K.; Huang, Y.; Ruan, J.; Feng, X.; Hu, P.; Su, C.Y. Cage-confined photocatalysis for wide-scope unusually selective [2 + 2] cycloaddition through visible-light triplet sensitization. Nat. Commun. 2020, 11, 4675. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Chen, J.; Yang, J.; Jiao, Z.; Su, C.Y. Elaborating E/Z-Geometry of Alkenes via Cage-Confined Arylation Catalysis of Terminal Olefins. Angew. Chem. Int. Ed. 2023, 62, e202303288. [Google Scholar] [CrossRef] [PubMed]
- Kikuchi, T.; Sato, S.; Fujita, M. Well-Defined DNA Nanoparticles Templated by Self-Assembled M12L24 Molecular Spheres and Binding of Complementary Oligonucleotides. J. Am. Chem. Soc. 2010, 132, 15930–15932. [Google Scholar] [CrossRef]
- Murase, T.; Sato, S.; Fujita, M. Nanometer-sized shell molecules that confine endohedral polymerizing units. Angew. Chem. Int. Ed. 2007, 46, 1083–1085. [Google Scholar] [CrossRef]
- Lewis, J.E.; McAdam, C.J.; Gardiner, M.G.; Crowley, J.D. A facile “click” approach to functionalised metallosupramolecular architectures. Chem. Commun. 2013, 49, 3398–3400. [Google Scholar] [CrossRef]
- Henwood, A.F.; Zysman-Colman, E. Lessons learned in tuning the optoelectronic properties of phosphorescent iridium(iii) complexes. Chem. Commun. 2017, 53, 807–826. [Google Scholar] [CrossRef] [PubMed]
- Martir, D.R.; Momblona, C.; Pertegas, A.; Cordes, D.B.; Slawin, A.M.; Bolink, H.J.; Zysman-Colman, E. Chiral Iridium(III) Complexes in Light-Emitting Electrochemical Cells: Exploring the Impact of Stereochemistry on the Photophysical Properties and Device Performances. ACS. Appl. Mater. Interfaces 2016, 8, 33907–33915. [Google Scholar] [CrossRef] [PubMed]
- Oldknow, S.; Martir, D.R.; Pritchard, V.E.; Blitz, M.A.; Fishwick, C.W.G.; Zysman-Colman, E.; Hardie, M.J. Structure-switching M(3)L(2) Ir(iii) coordination cages with photo-isomerising azo-aromatic linkers. Chem. Sci. 2018, 9, 8150–8159. [Google Scholar] [CrossRef] [PubMed]
- Yam, V.W.; Wong, K.M. Luminescent metal complexes of d6, d8 and d10 transition metal centres. Chem. Commun. 2011, 47, 11579–11592. [Google Scholar] [CrossRef]
- Yam, V.W.; Au, V.K.; Leung, S.Y. Light-Emitting Self-Assembled Materials Based on d(8) and d(10) Transition Metal Complexes. Chem. Rev. 2015, 115, 7589–7728. [Google Scholar] [CrossRef]
- Liu, Y.; Persson, P.; Sundstrom, V.; Warnmark, K. Fe N-Heterocyclic Carbene Complexes as Promising Photosensitizers. Acc. Chem. Res. 2016, 49, 1477–1485. [Google Scholar] [CrossRef]
- Yu-Lut Leung, S.; Wing-Wah Yam, V. Hierarchical helices of helices directed by Pt⋯Pt and π–π stacking interactions: Reciprocal association of multiple helices of dinuclear alkynylplatinum(ii) complex with luminescence enhancement behavior. Chem. Sci. 2013, 4, 4228–4234. [Google Scholar] [CrossRef]
- Liu, S.; Sun, H.; Ma, Y.; Ye, S.; Liu, X.; Zhou, X.; Mou, X.; Wang, L.; Zhao, Q.; Huang, W. Rational design of metallophosphors with tunable aggregation-induced phosphorescent emission and their promising applications in time-resolved luminescence assay and targeted luminescence imaging of cancer cells. J. Mater. Chem. 2012, 22, 22167–22173. [Google Scholar] [CrossRef]
- Schmidbaur, H. Ludwig Mond Lecture. High-carat gold compounds. Chem. Soc. Rev. 1995, 24, 391–400. [Google Scholar] [CrossRef]
- King, C.; Khan, M.N.I.; Staples, R.J.; Fackler, J.P. Luminescent Mononuclear Gold(I) Phosphines. Inorg. Chem. 1992, 31, 3236–3238. [Google Scholar] [CrossRef]
- Yang, Y.; Chen, J.S.; Liu, J.Y.; Zhao, G.J.; Liu, L.; Han, K.L.; Cook, T.R.; Stang, P.J. Photophysical Properties of a Post-Self-Assembly Host/Guest Coordination Cage: Visible Light Driven Core-to-Cage Charge Transfer. J. Phys. Chem. Lett. 2015, 6, 1942–1947. [Google Scholar] [CrossRef] [PubMed]
- Wegeberg, C.; Wenger, O.S. Luminescent First-Row Transition Metal Complexes. JACS Au 2021, 1, 1860–1876. [Google Scholar] [CrossRef] [PubMed]








| Entry | MOCs | (Metallo) Ligand | Node Precursor | λem (nm) a | ΦPL (%) | τPL (ns) | Applications |
|---|---|---|---|---|---|---|---|
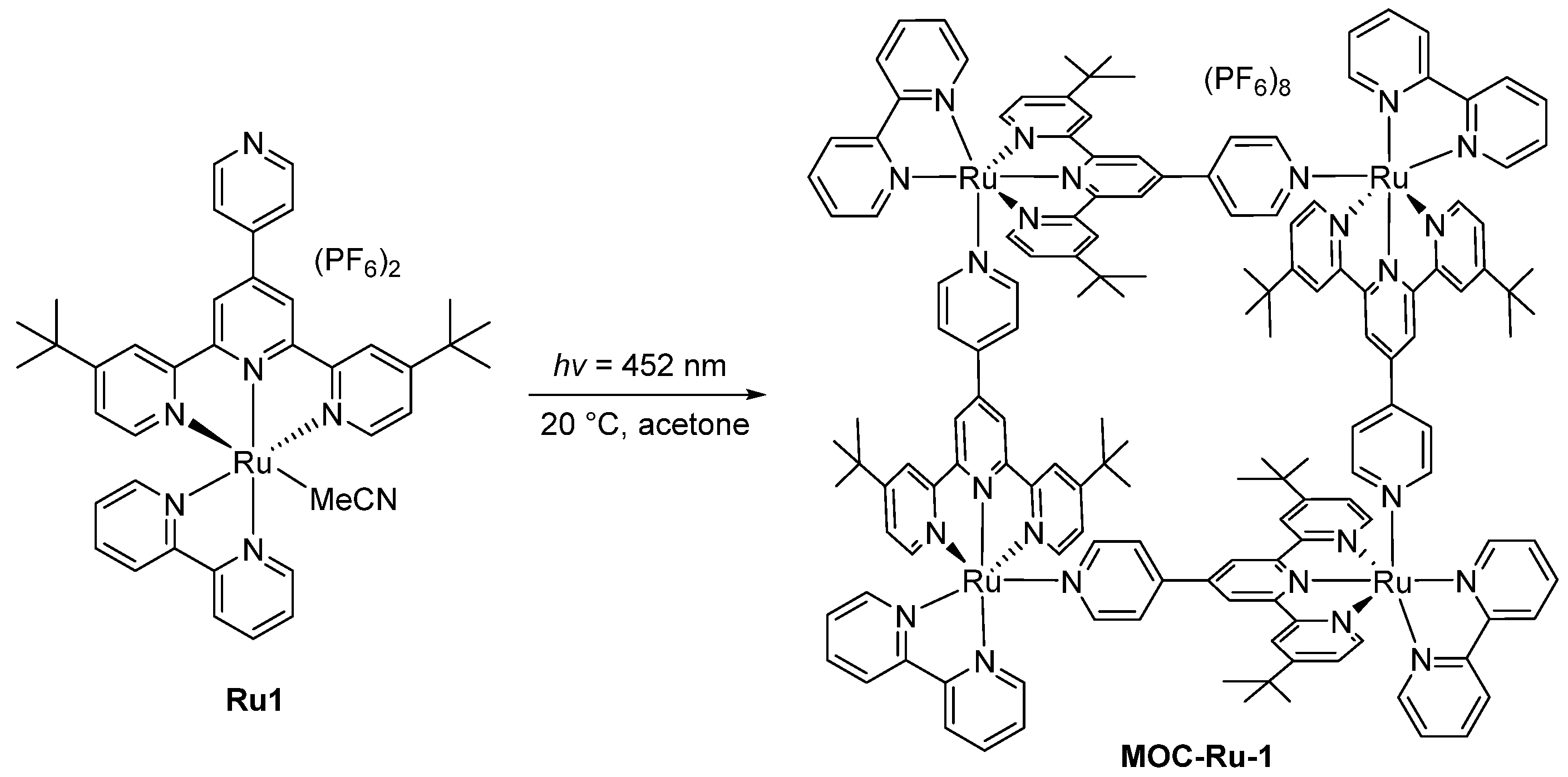
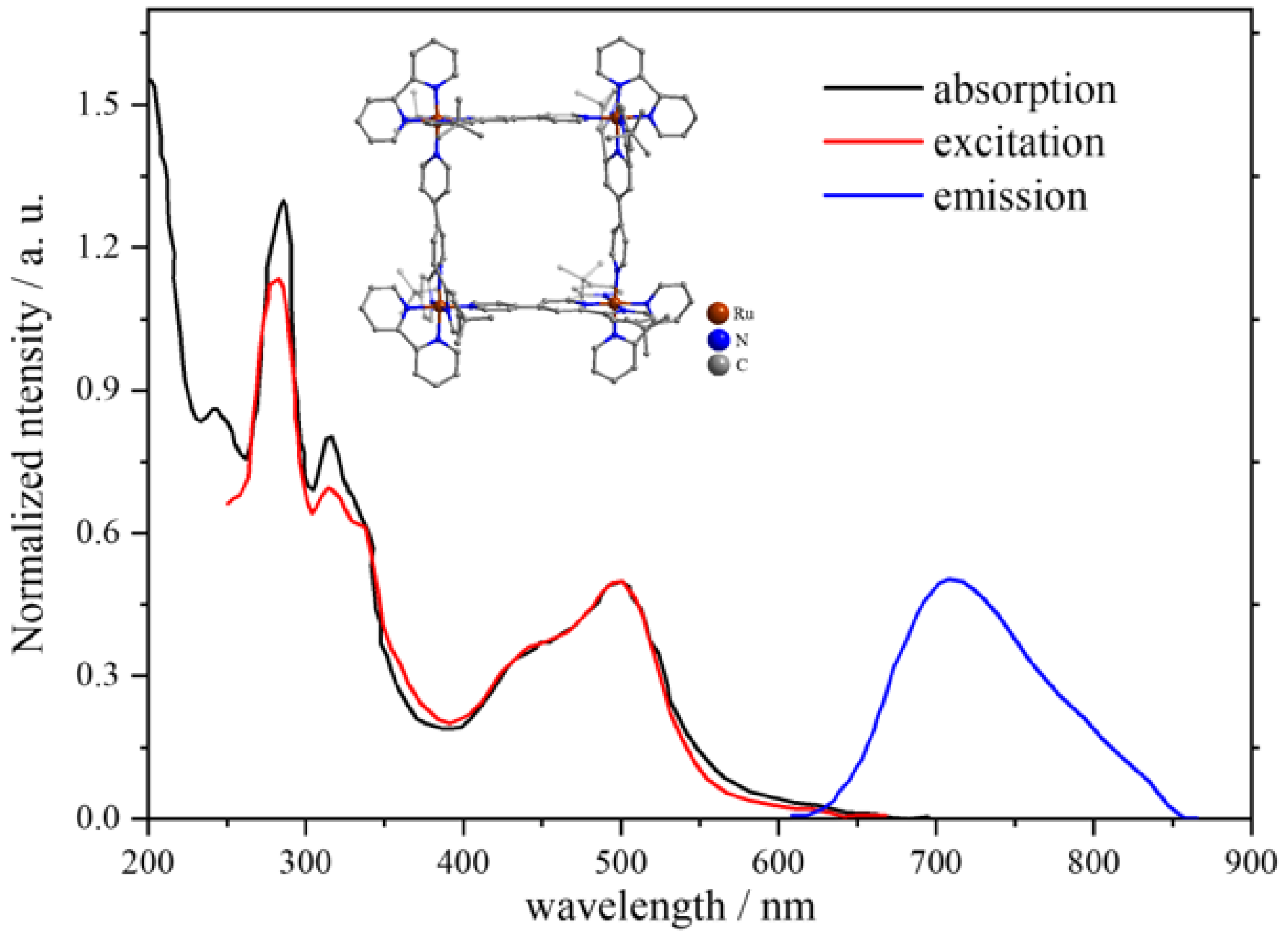
| 1 | MOC-Ru-1 [25] [Ru4] (PF6)6 | - |  Ru1 | 745 | 1 | 135 | - g |
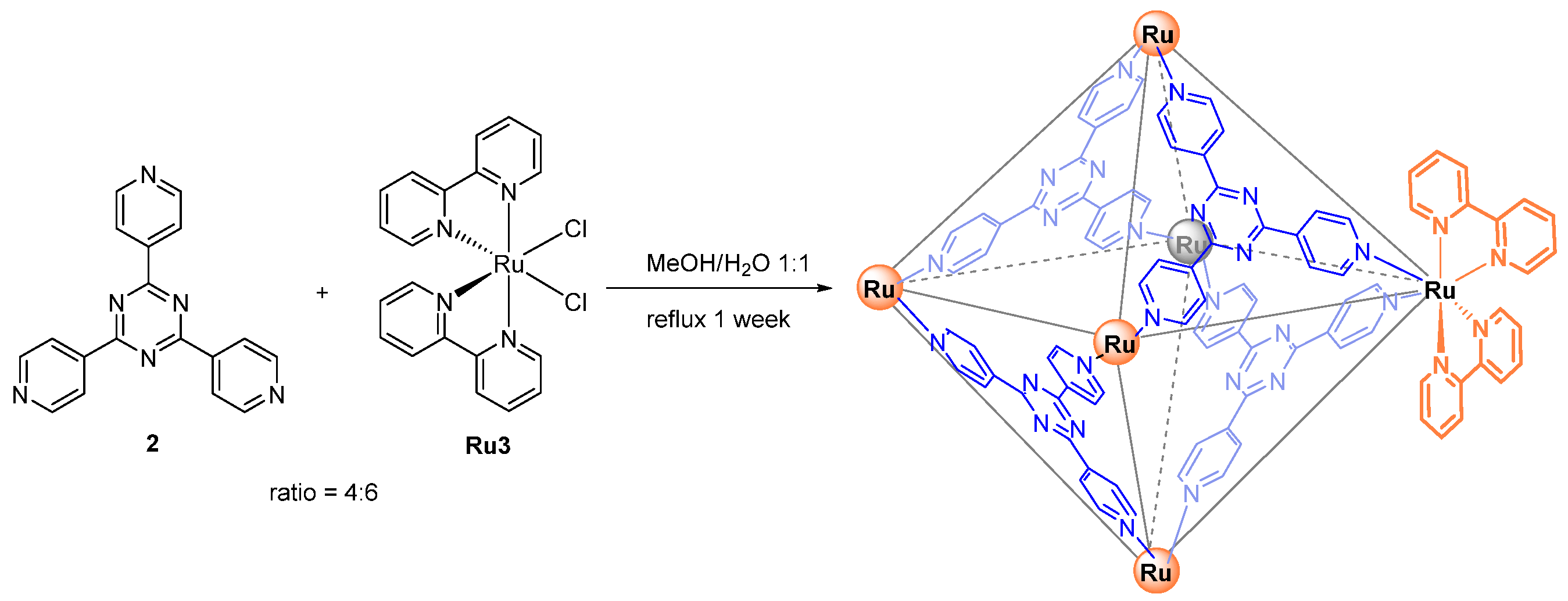
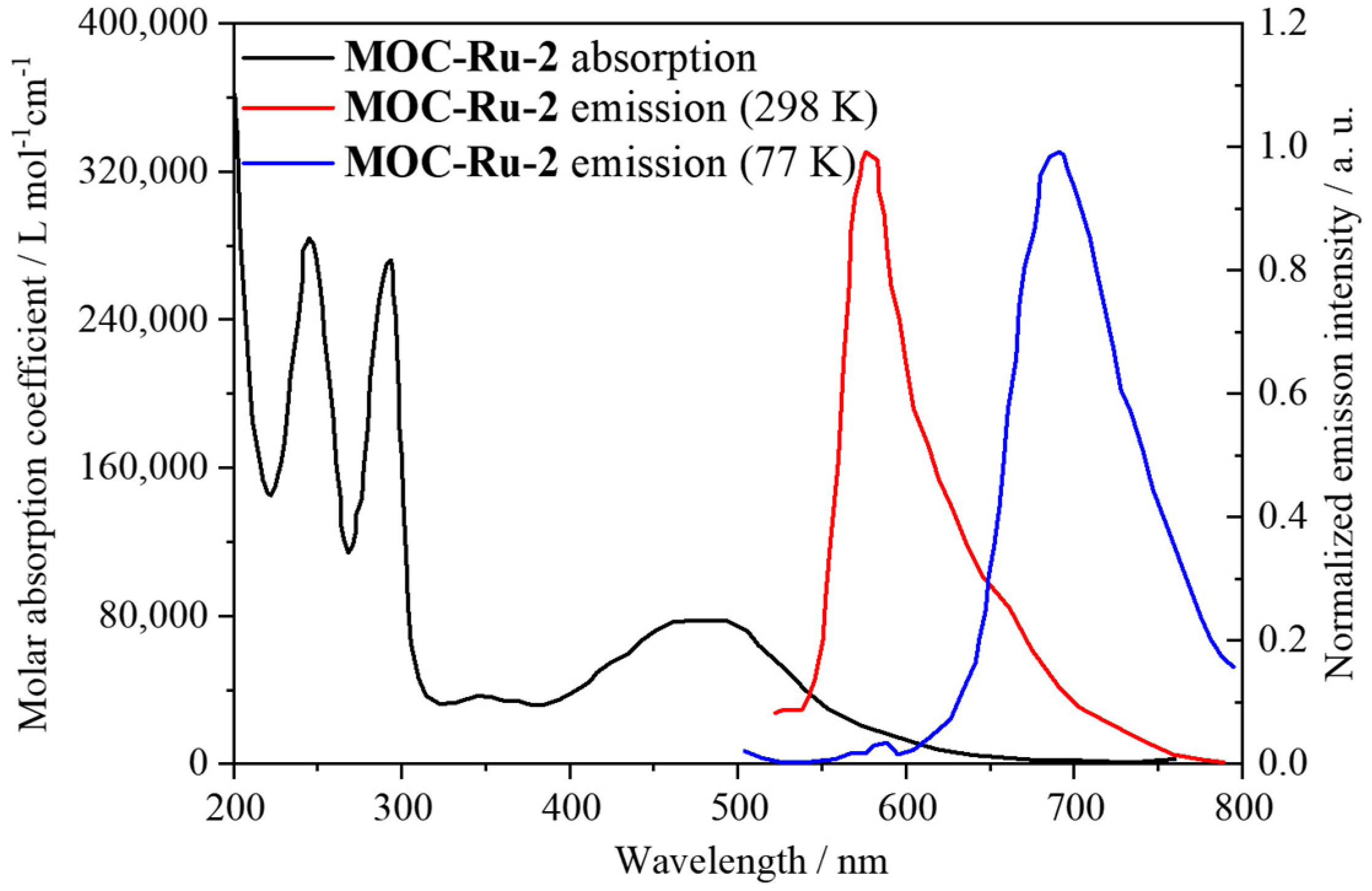
| 2 | MOC-Ru-2 [26] [L4 Ru6]Cl12 |  2 |  Ru3 | 577 689 b | <0.1 | 2, 790 | - g |
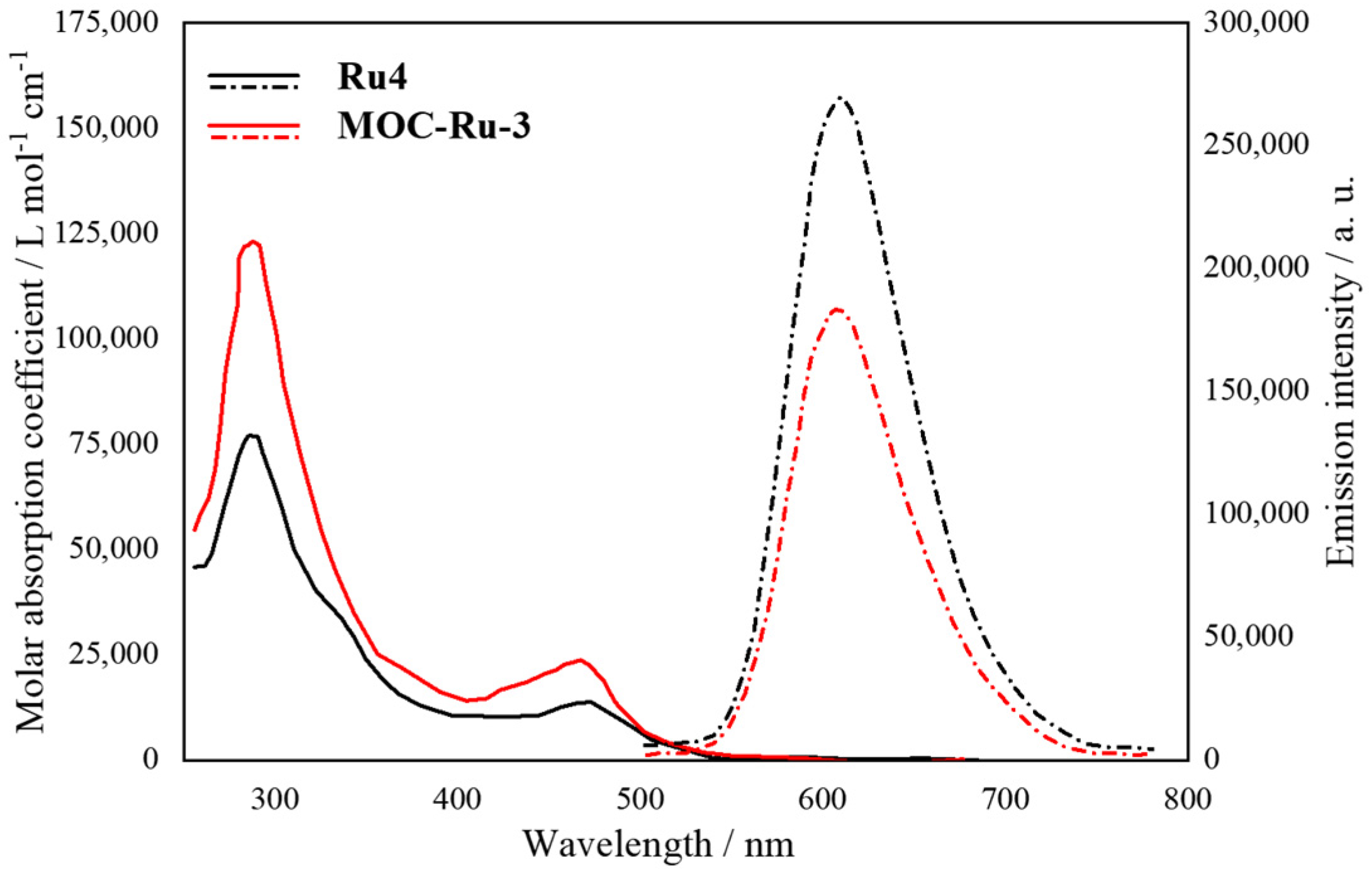
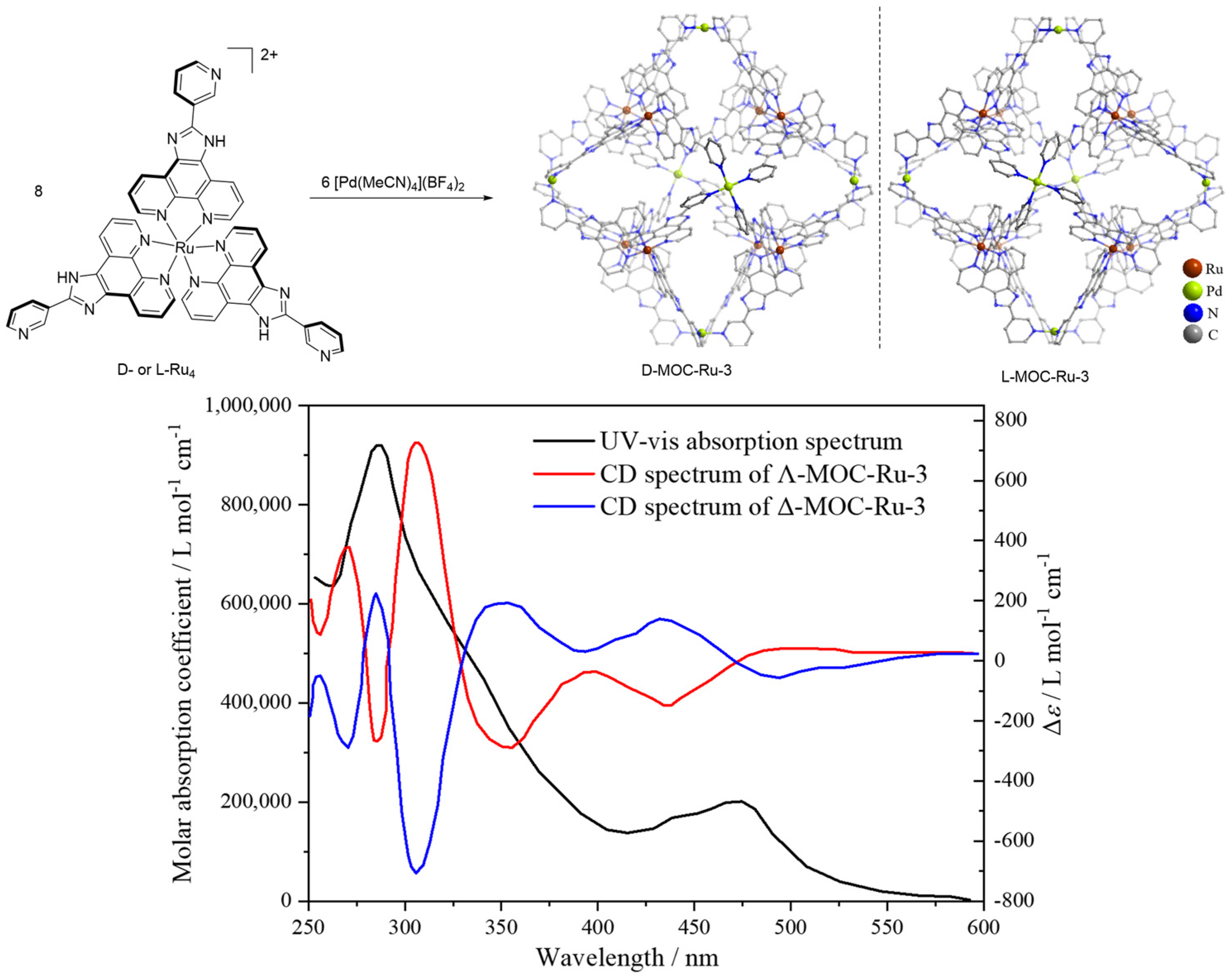
| 3 | MOC-Ru-3 [27] [Ru8Pd6] (BF4)28 | 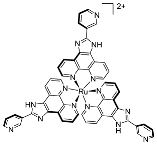 Ru4 | [Pd(MeCN)4](BF4)2 | 610 c | - g | 484 | Photocatalysis and bio-imaging |
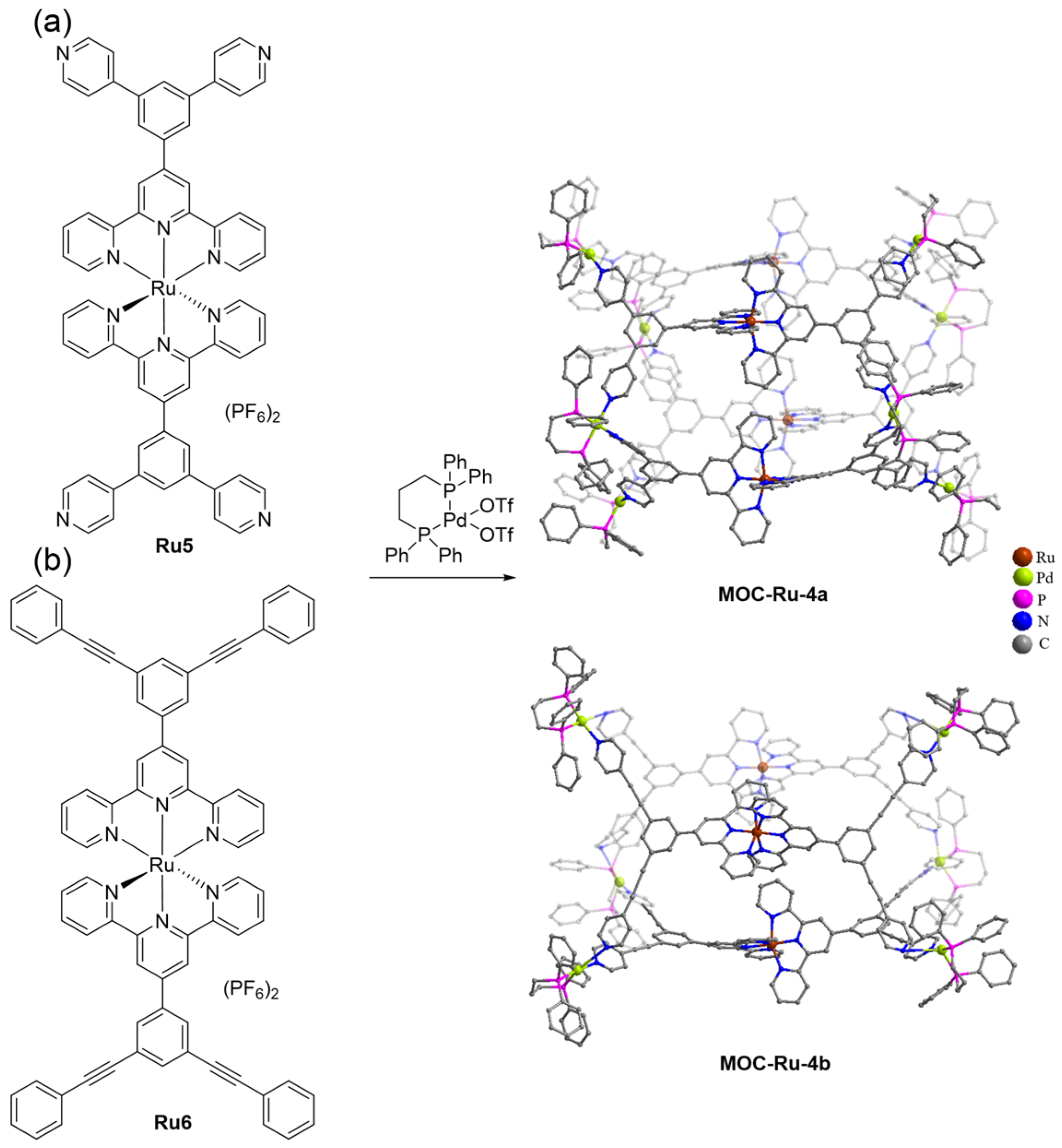
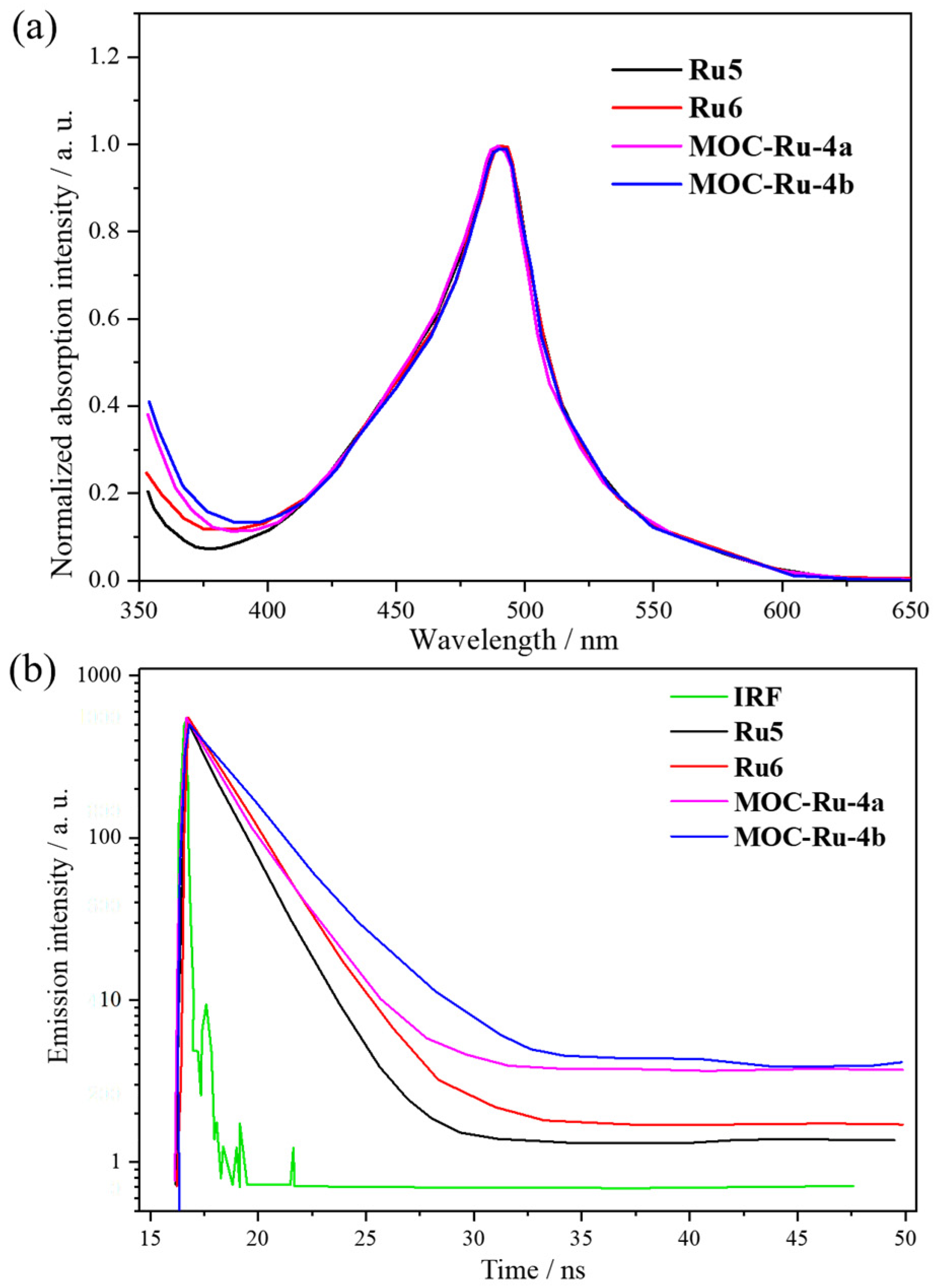
| 4 | MOC-Ru-4a [28] [Ru4Pd8] (PF6)24 | 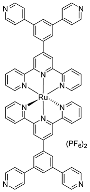 Ru5 | 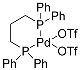 | 640 | - g | 1.21 | - g |
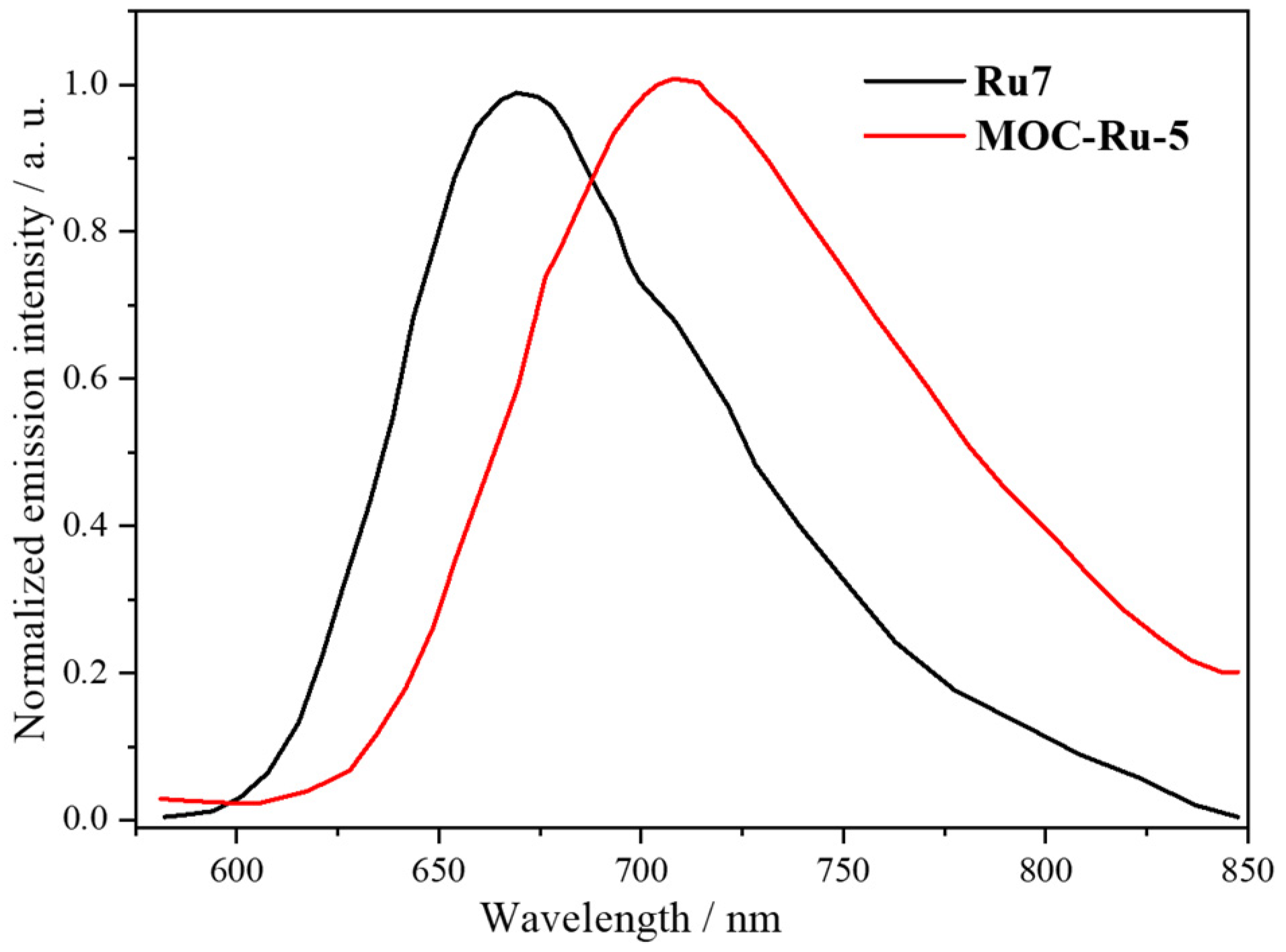
| 5 | MOC-Ru-5 [29] [Ru8Pd4] (BF4)24 |  Ru7 | [Pd(MeCN)4] (BF4)2 | 710 d | 6.9 | 151 and 700 h | - g |
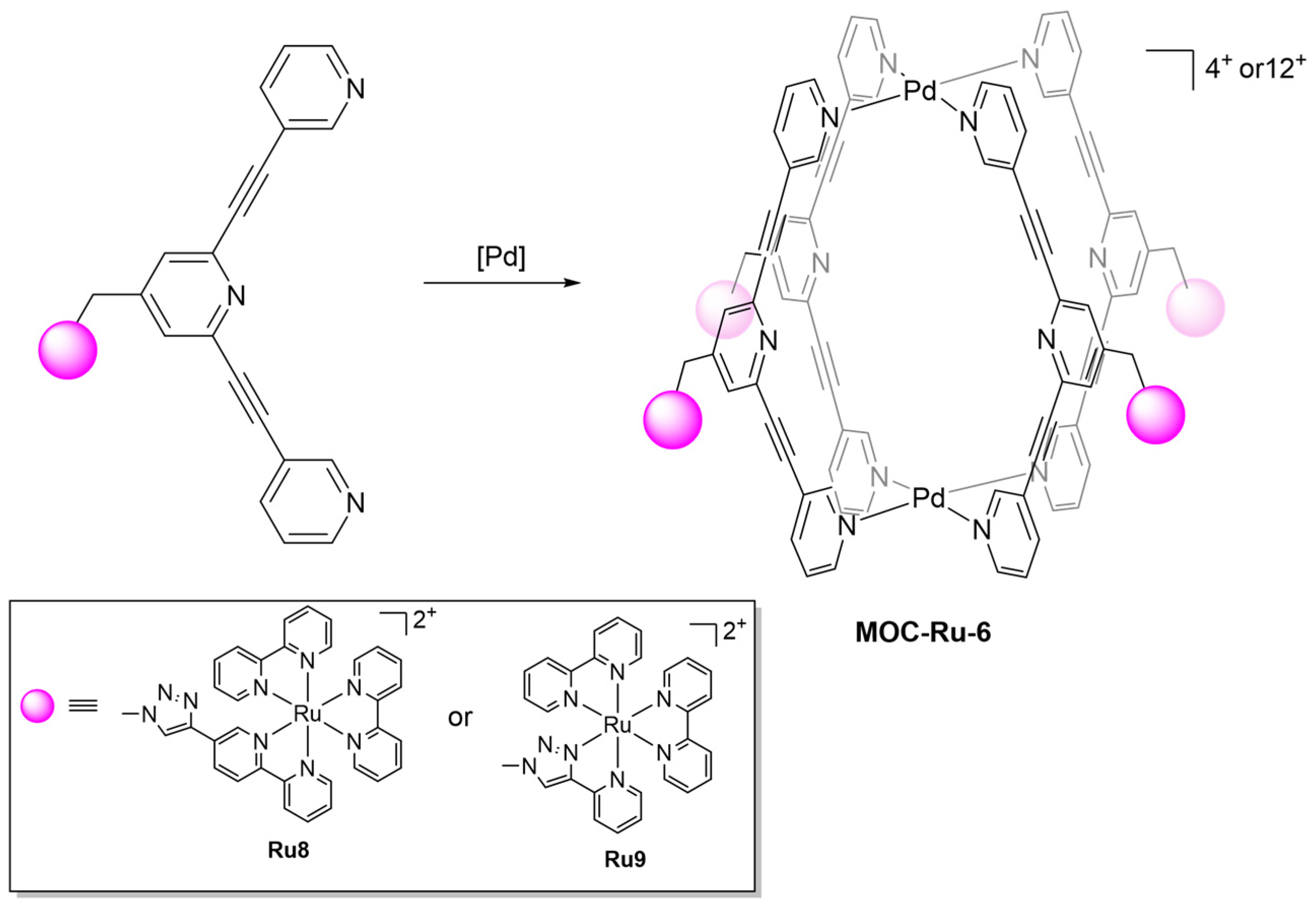
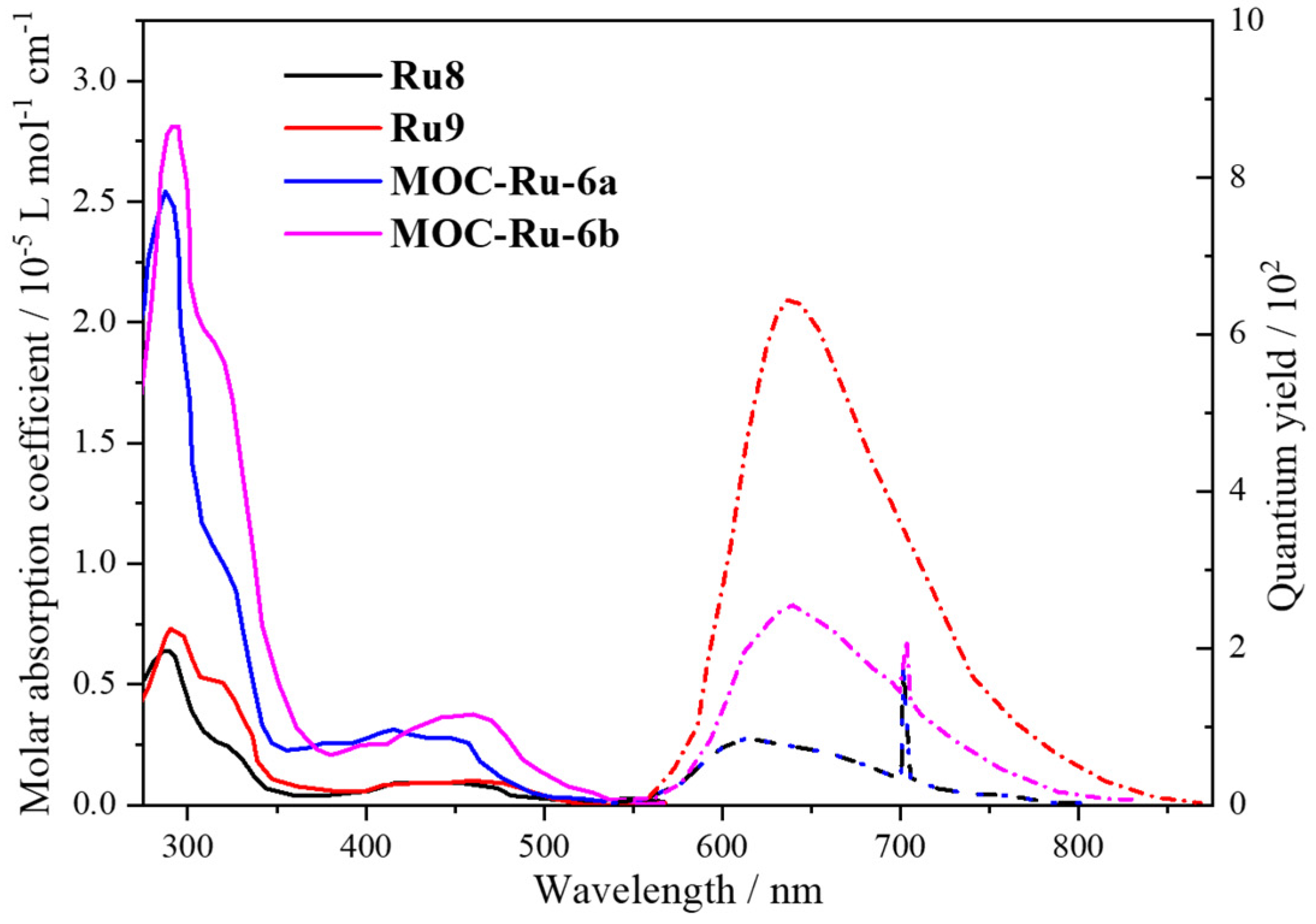
| 6 | MOC-Ru-6a [30] [Ru4Pd2] (BF4)12 | 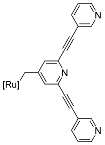 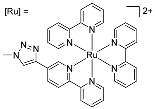 Ru8 | [Pd(MeCN)4] (BF4)2 | 638 e | 2.6 | 659 | - g |
| 7 | MOC-Ru-7b [31] [Ru4Pd2] (BF4)12 |   Ru11 | [Pd(MeCN)4] (BF4)2 | 640 c | 66 | - g | - g |
| 8 | MOC-Ir-8 [32] [L4Ir6] (OTF)6 | 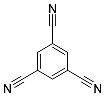 12 12 | 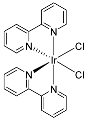 Ir13 | 575 f | 4 | - g | - g |
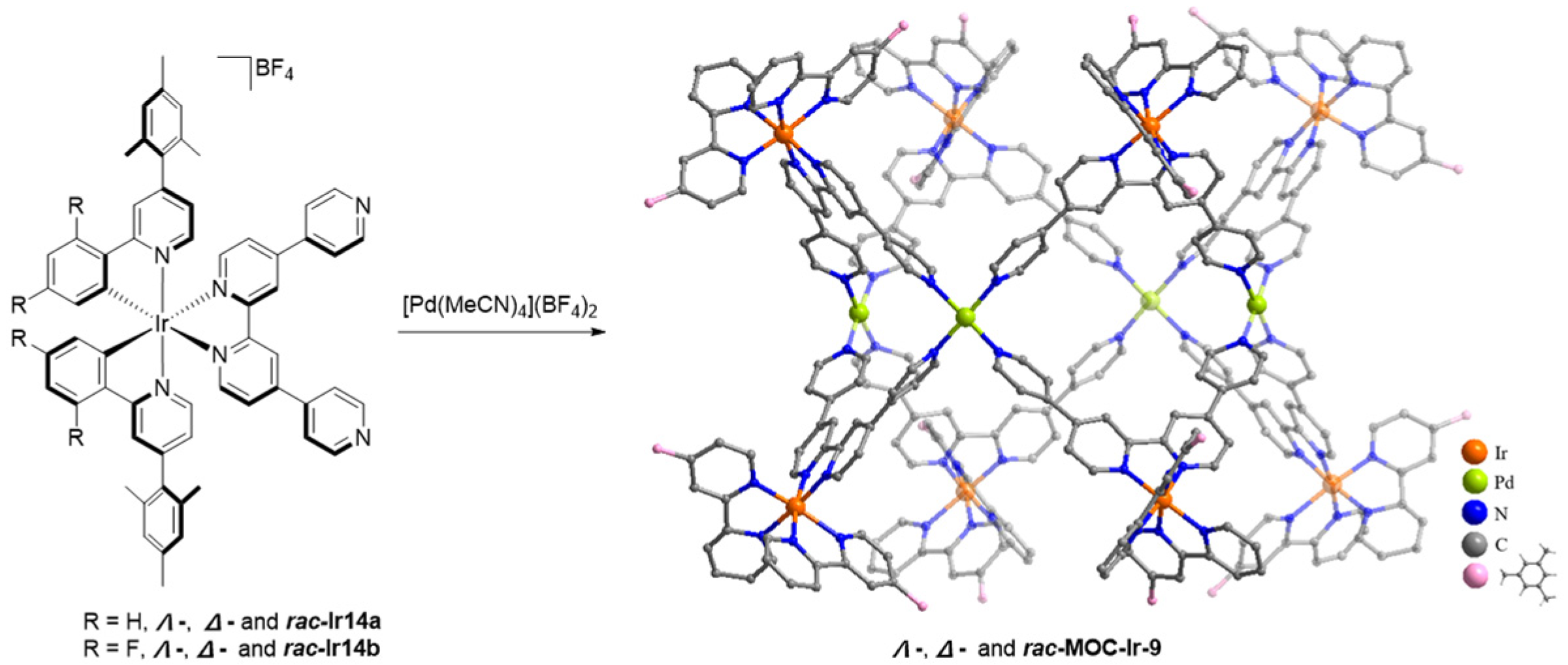
| 9 | MOC-Ir-9 [33] [Ir8Pd4] (BF4)16 |  Ir14 | [Pd(MeCN)4](BF4)2 | 9a 655, d 9b 569 d | 9a 5, 9b 14 | 9a 202 9b 825 | - g |
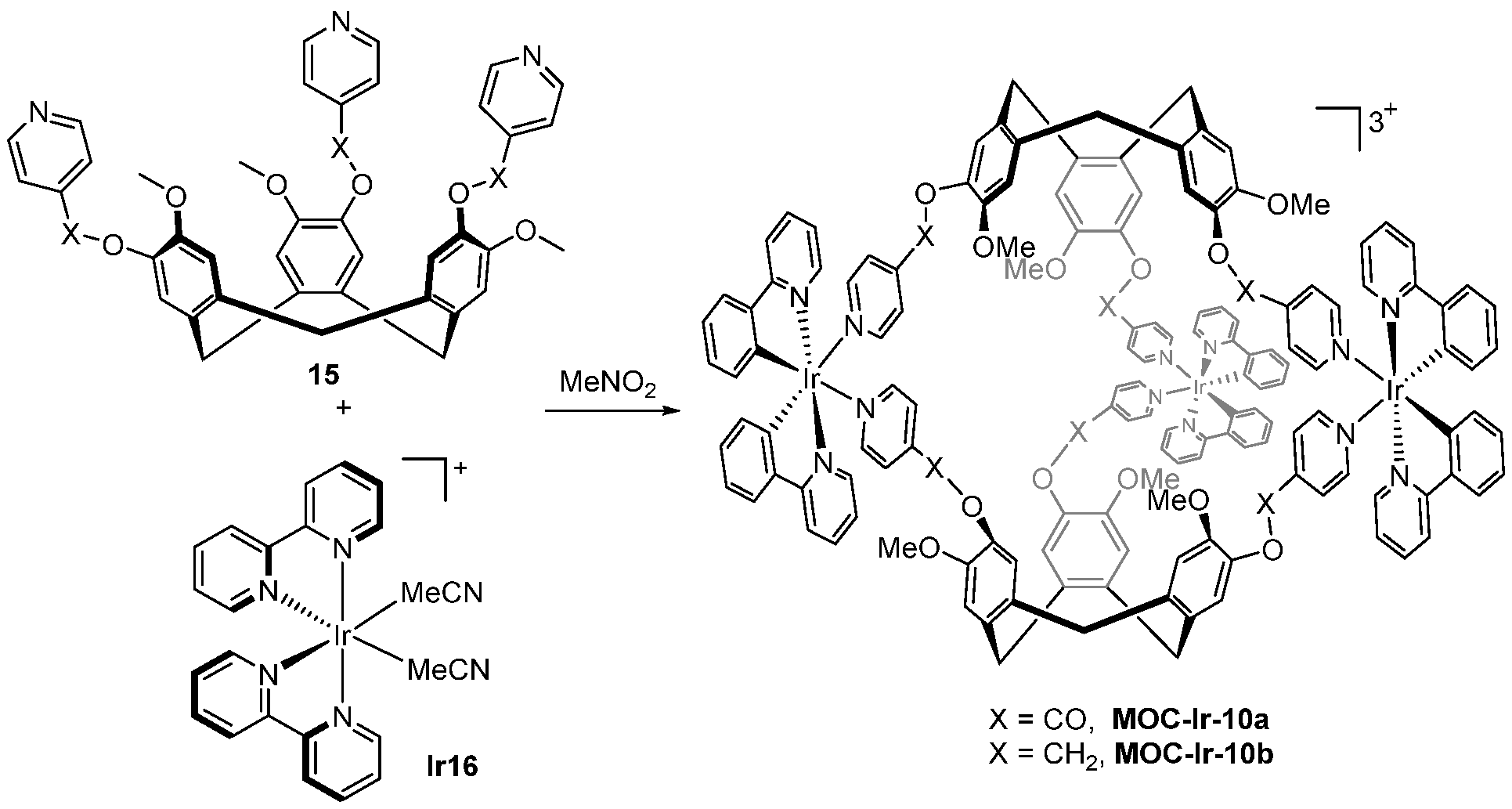
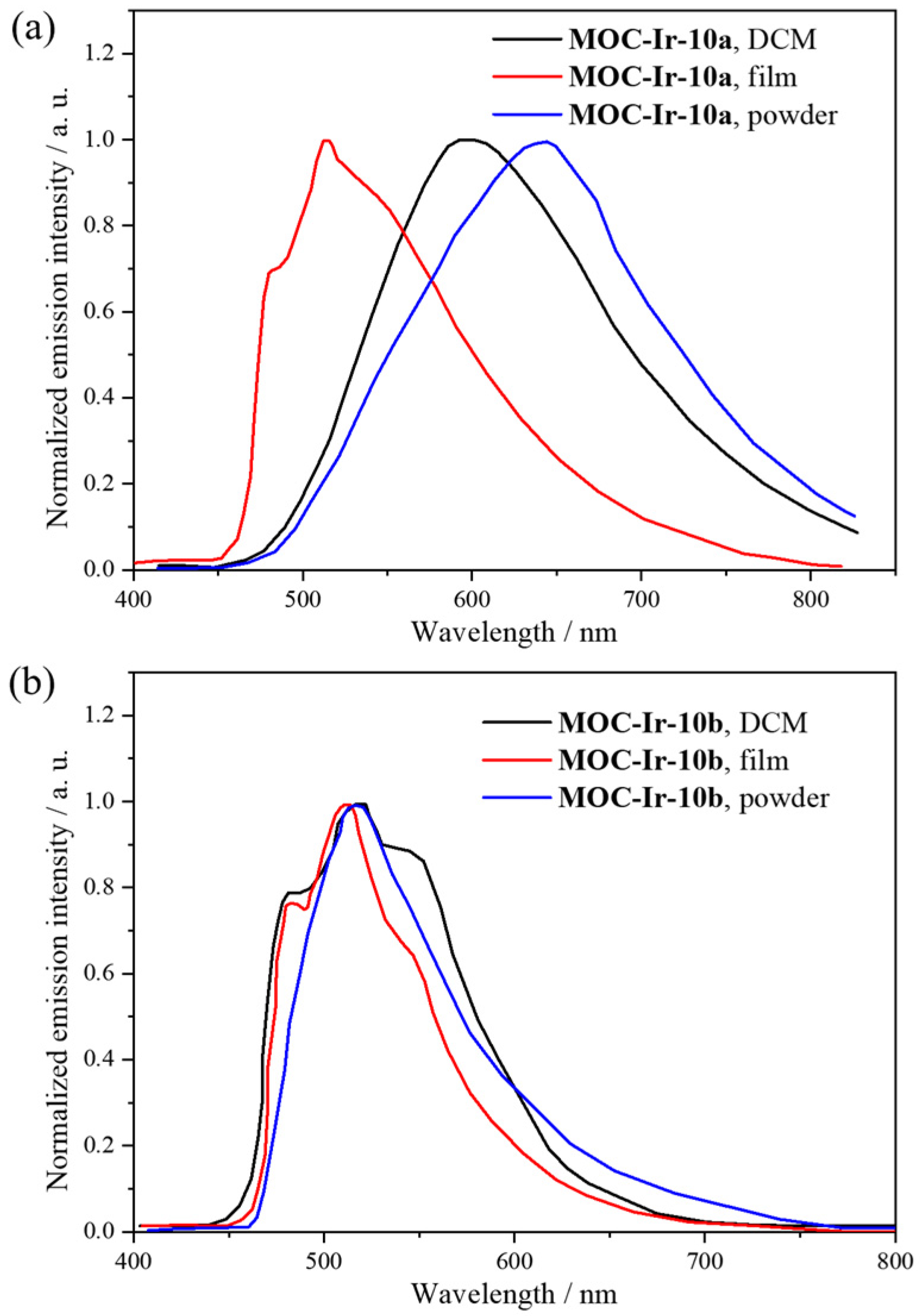
| 10 | MOC-Ir-10 [34] [L2Ir3] (PF6)3 | 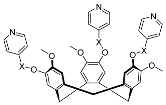 15 |  Ir16 | 10a 604, d 10b 485 d | 10a 1, 10b 15 | 10a 59 and 129, h 10b 523 and 887 h | - g |
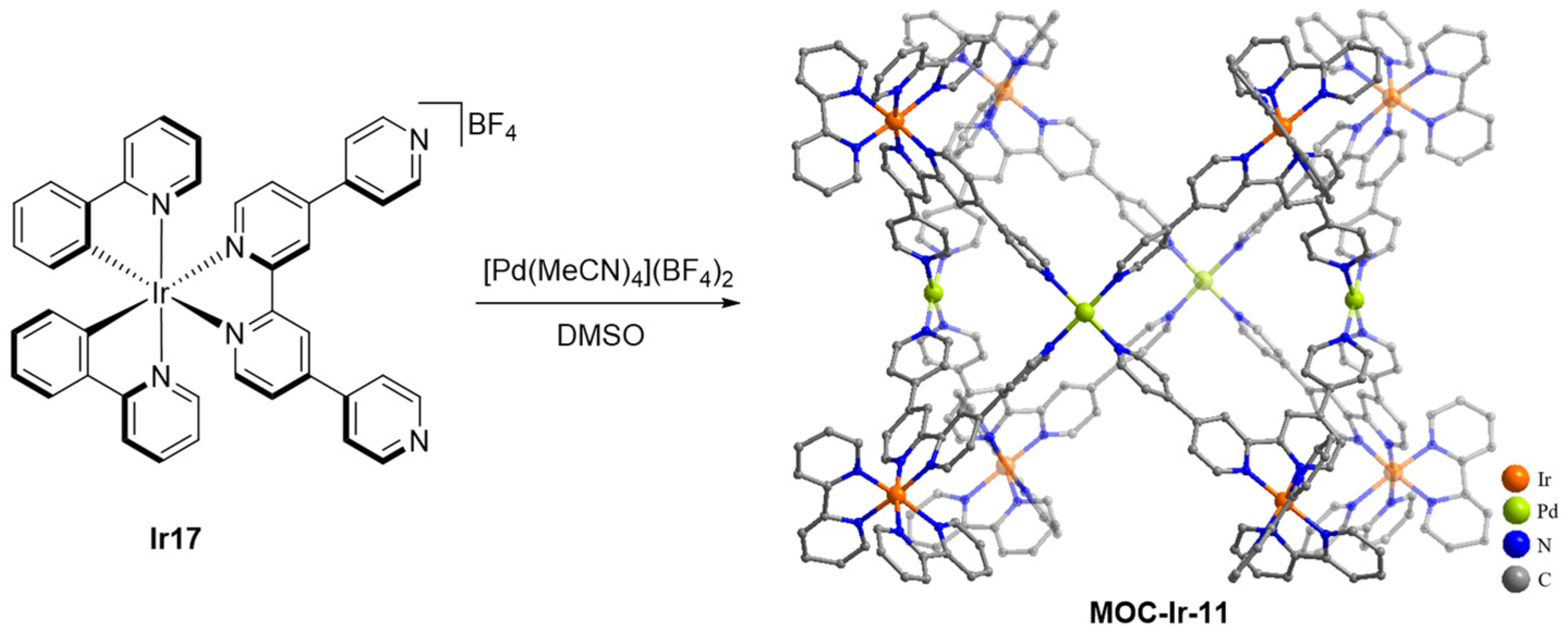
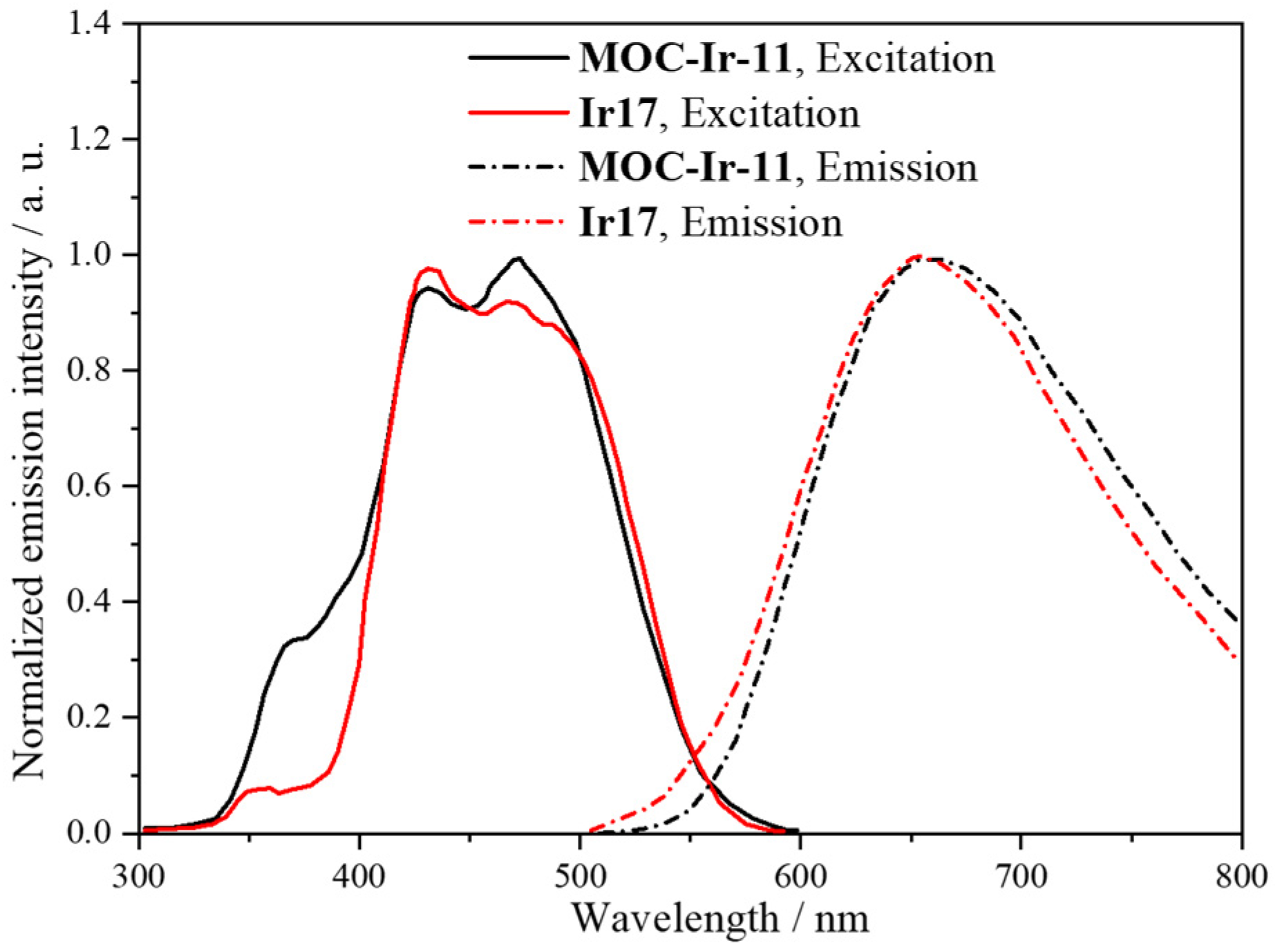
| 11 | MOC-Ir-11 [35] [Ir8Pd4] (BF4)16 | 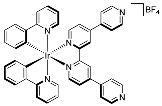 Ir17 | [Pd(MeCN)4](BF4)2 | 658 c | 3.5 | 141 | Bio-imaging and photodynamic therapy |
| 12 | MOC-Os-12 [36] [L12(Os)4(Cd)4] (ClO4)16 |  | OsCl3·3H2O Cd(ClO4)2·6H2O | 625 | 2.5 | 156 and 73 h | - g |
| 13 | MOC-Pt-13b [37] [Pt2Pt2] (OTf)4 | 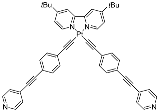 Pt18b |  | 527 | 16.7 | 15,500 | - g |
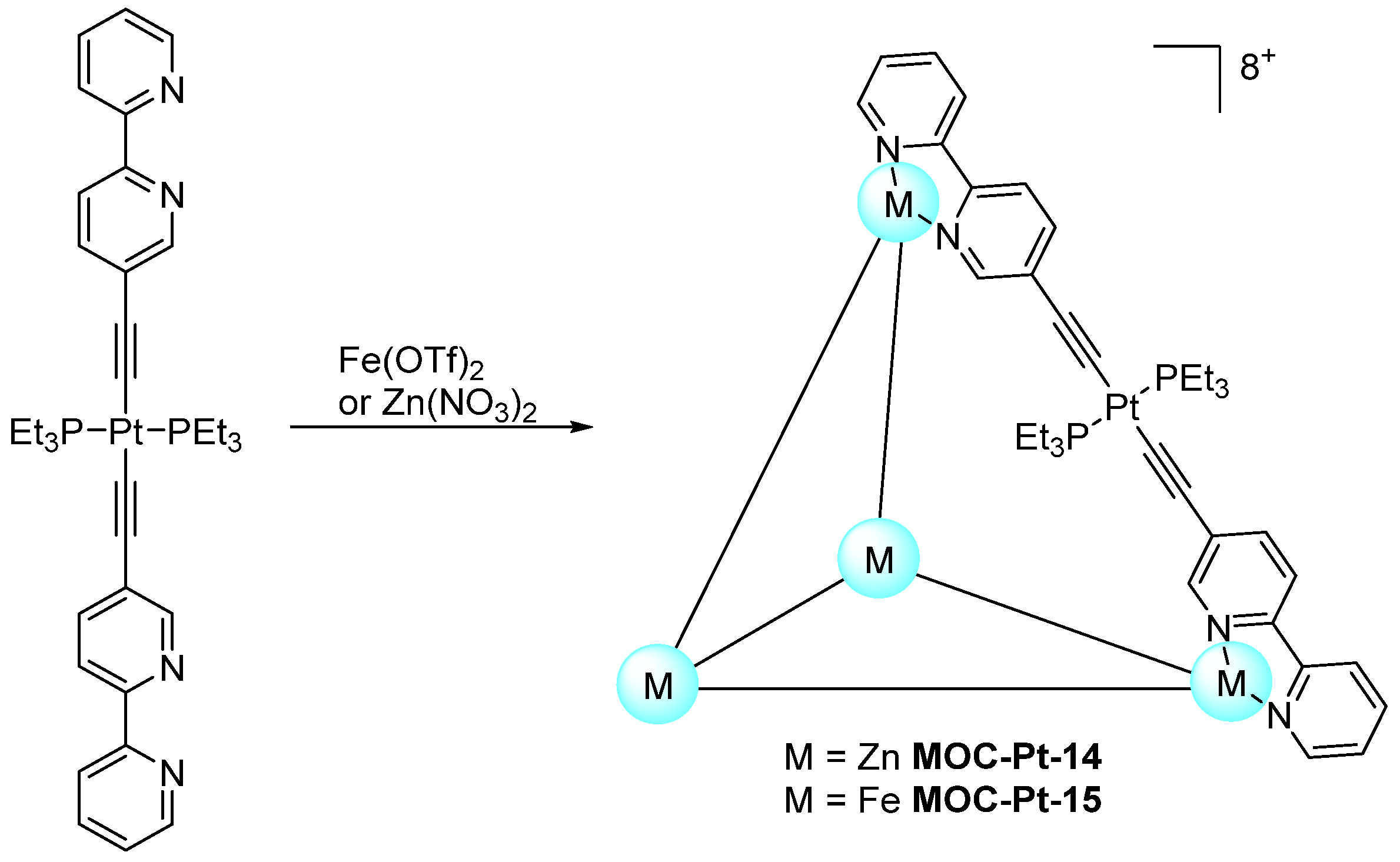
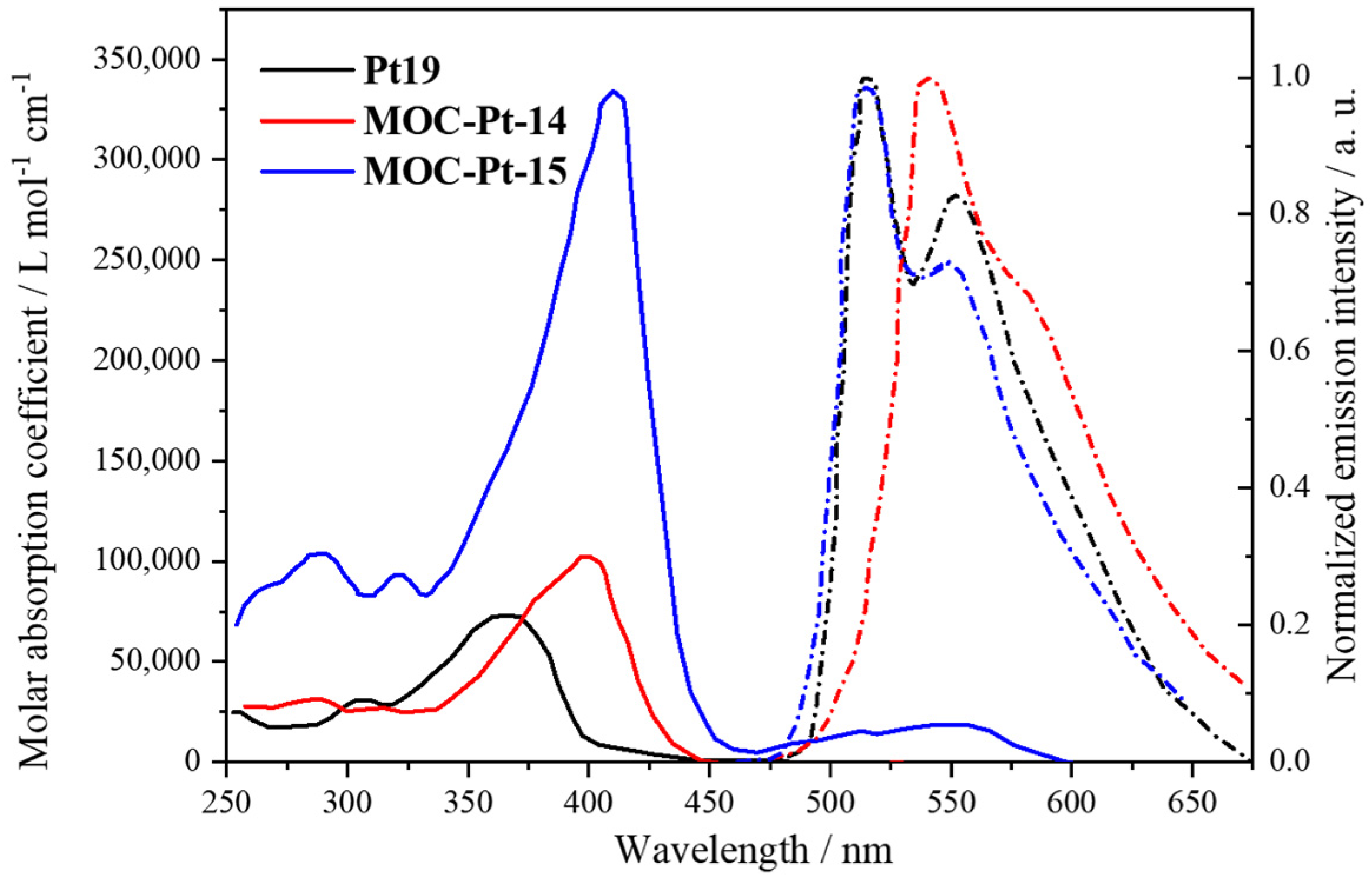
| 14 | MOC-Pt-14 [14] [Pt6Zn4] (PF6)8 |  Pt19 | Zn(NO3)2·6H2O | 545 | 10 | 95,000 | - g |
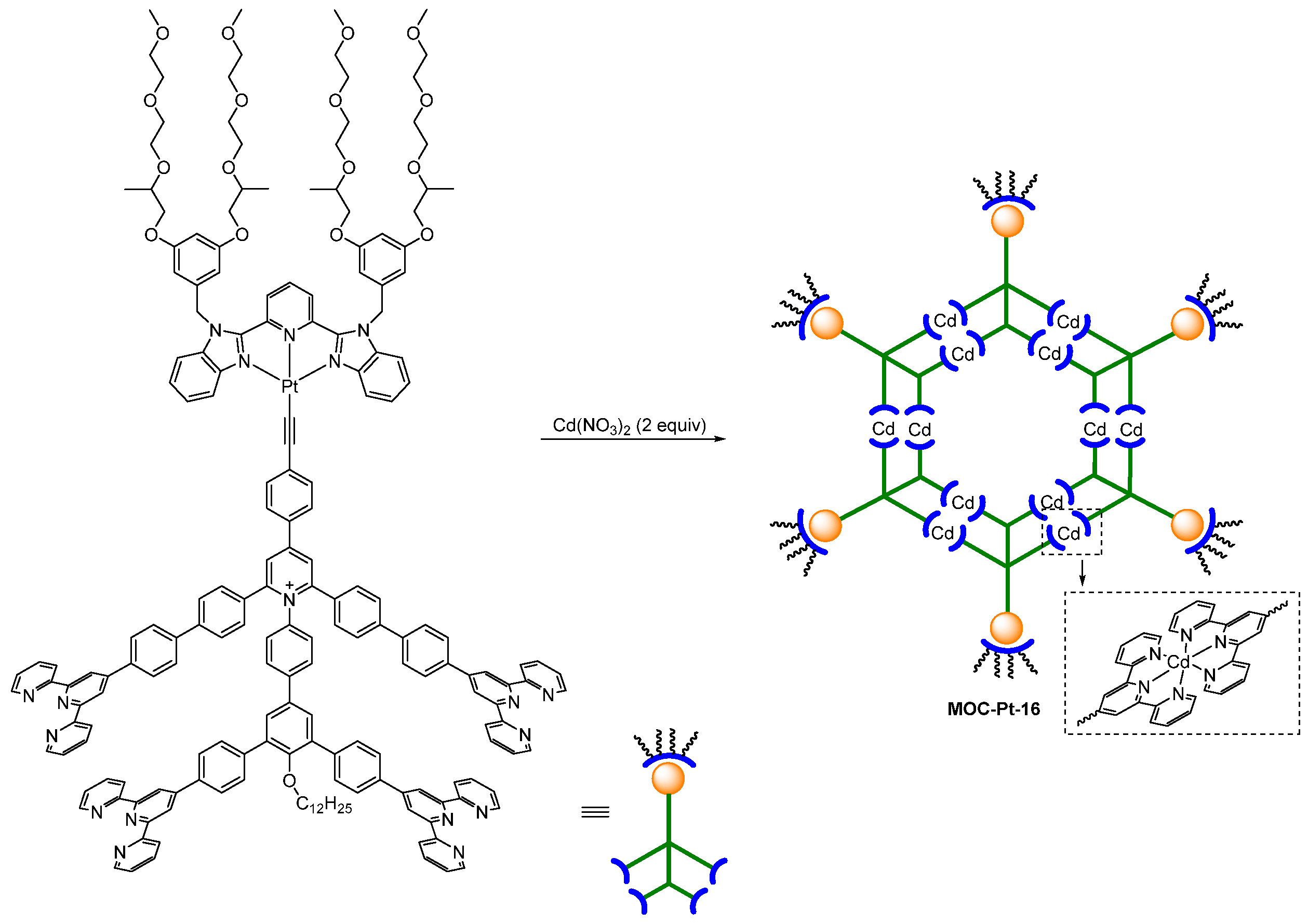
| 15 | MOC-Pt-16 [38] [Pt6Cd12] (PF6)36 | 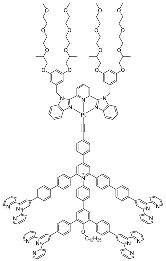 Pt20 | Cd(NO3)2 | 567 | - g | 218 | - g |
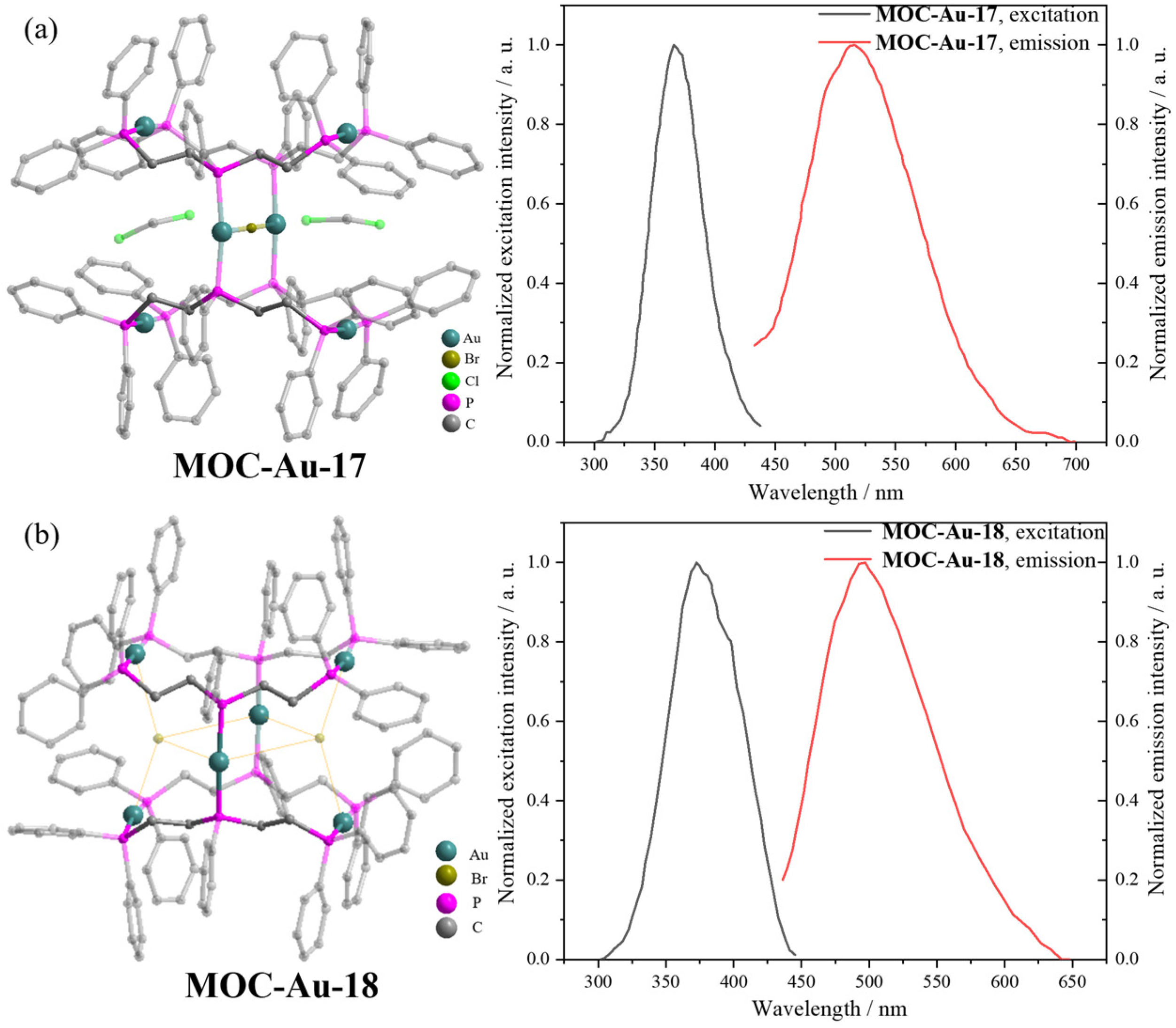
| 16 | MOC-Au-17 [39] [L4Au6Br] (SbF6)5 | 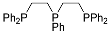 | Chloro(tetrahydrothiophene) gold KBr | 485 | - g | 10 | - g |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yu, Y.; Wang, X.; Liu, Y.; Zou, C. Metal-Organic Cages Based on Phosphorescent Organometallics. Inorganics 2023, 11, 436. https://doi.org/10.3390/inorganics11110436
Yu Y, Wang X, Liu Y, Zou C. Metal-Organic Cages Based on Phosphorescent Organometallics. Inorganics. 2023; 11(11):436. https://doi.org/10.3390/inorganics11110436
Chicago/Turabian StyleYu, Yunliang, Xiaoxia Wang, Yuliang Liu, and Chao Zou. 2023. "Metal-Organic Cages Based on Phosphorescent Organometallics" Inorganics 11, no. 11: 436. https://doi.org/10.3390/inorganics11110436