Vibrational Coherence in the Metal–Metal-Bonded Excited State of Pt(II) Complexes
Abstract
:1. Introduction
2. 1/3MMLCT and 1/3MC Excited States of d8 Metal Complexes
3. Ultrafast Spectroscopy and CVWP

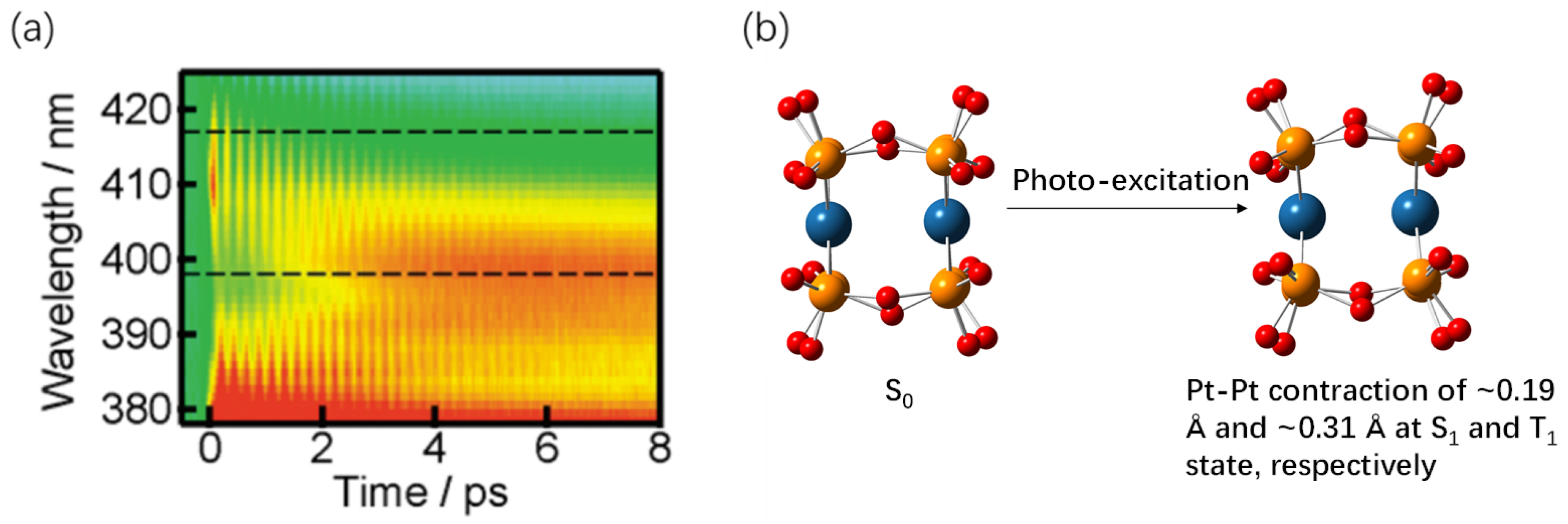
4. Molecular Structural Dynamics and Vibrational Wavepacket
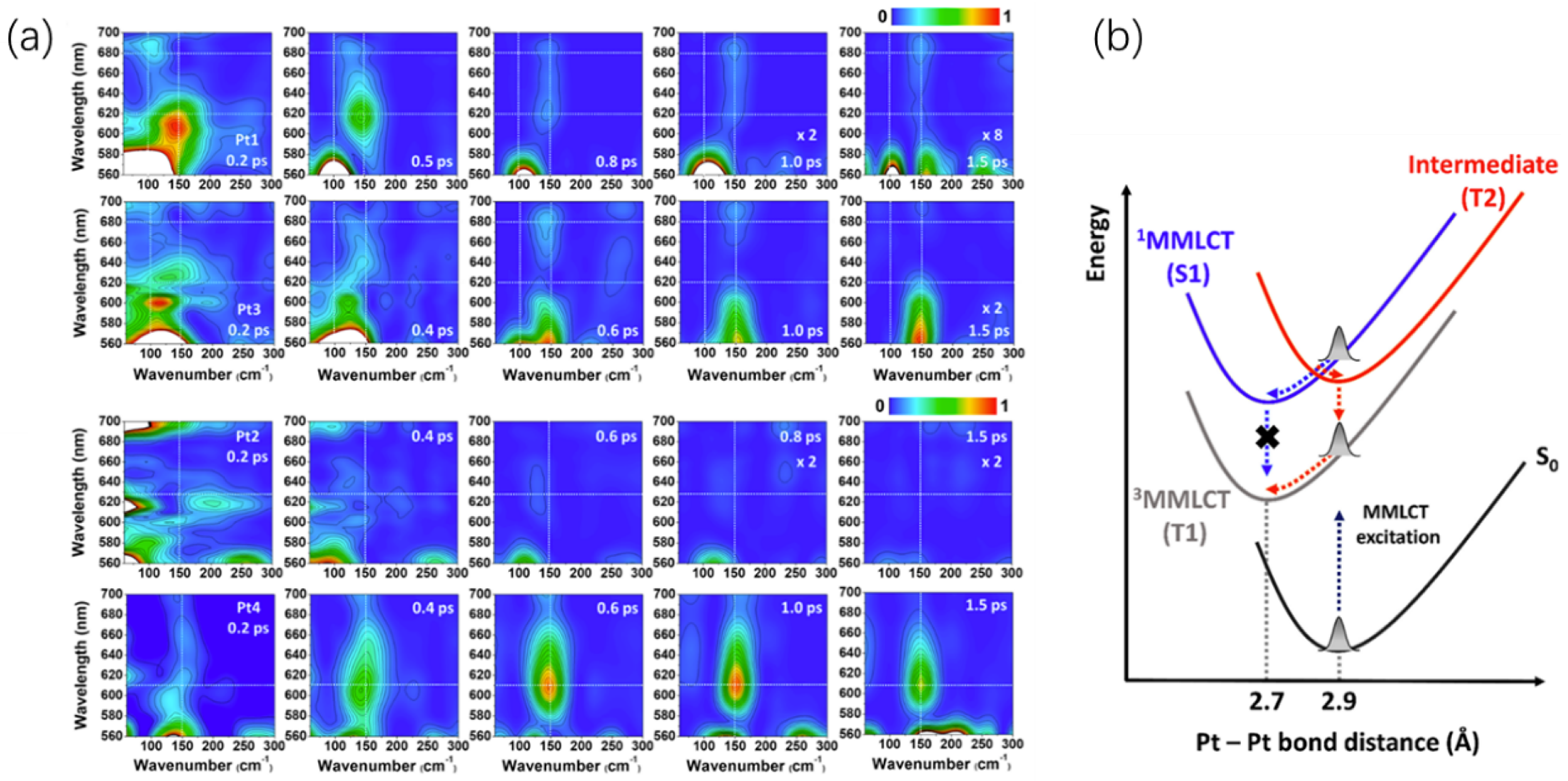
5. Excited State Dynamics and Coherent Time
6. Prospective Future Directions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Brédas, J.-L.; Sargent, E.H.; Scholes, G.D. Photovoltaic concepts inspired by coherence effects in photosynthetic systems. Nat. Mater. 2017, 16, 35–44. [Google Scholar] [CrossRef] [PubMed]
- Collini, E.; Wong, C.Y.; Wilk, K.E.; Curmi, P.M.; Brumer, P.; Scholes, G.D. Coherently wired light-harvesting in photosynthetic marine algae at ambient temperature. Nature 2010, 463, 644–647. [Google Scholar] [CrossRef] [PubMed]
- Nelson, T.R.; Ondarse-Alvarez, D.; Oldani, N.; Rodriguez-Hernandez, B.; Alfonso-Hernandez, L.; Galindo, J.F.; Kleiman, V.D.; Fernandez-Alberti, S.; Roitberg, A.E.; Tretiak, S. Coherent exciton-vibrational dynamics and energy transfer in conjugated organics. Nat. Commun. 2018, 9, 2316. [Google Scholar] [CrossRef]
- Haedler, A.T.; Kreger, K.; Issac, A.; Wittmann, B.; Kivala, M.; Hammer, N.; Köhler, J.; Schmidt, H.-W.; Hildner, R. Long-range energy transport in single supramolecular nanofibres at room temperature. Nature 2015, 523, 196–199. [Google Scholar] [CrossRef] [PubMed]
- Scholes, G.D.; Fleming, G.R.; Olaya-Castro, A.; Van Grondelle, R. Lessons from nature about solar light harvesting. Nat. Chem. 2011, 3, 763–774. [Google Scholar] [CrossRef] [PubMed]
- Sung, J.; Kim, P.; Fimmel, B.; Würthner, F.; Kim, D. Direct observation of ultrafast coherent exciton dynamics in helical π-stacks of self-assembled perylene bisimides. Nat. Commun. 2015, 6, 8646. [Google Scholar] [CrossRef] [PubMed]
- Leshchev, D.; Valentine, A.J.S.; Kim, P.; Mills, A.W.; Roy, S.; Chakraborty, A.; Biasin, E.; Haldrup, K.; Hsu, D.J.; Kirschner, M.S. Revealing Excited-State Trajectories on Potential Energy Surfaces with Atomic Resolution in Real Time. Angew. Chem. Int. Ed. 2023, 62, e202304615. [Google Scholar] [CrossRef]
- Monni, R.; Capano, G.; Auböck, G.; Gray, H.B.; Vlček, A.; Tavernelli, I.; Chergui, M. Vibrational coherence transfer in the ultrafast intersystem crossing of a diplatinum complex in solution. Proc. Natl. Acad. Sci. USA 2018, 115, E6396–E6403. [Google Scholar] [CrossRef]
- Kim, P.; Valentine, A.J.S.; Roy, S.; Mills, A.W.; Chakraborty, A.; Castellano, F.N.; Li, X.; Chen, L.X. Ultrafast Excited-State Dynamics of Photoluminescent Pt(II) Dimers Probed by a Coherent Vibrational Wavepacket. J. Phys. Chem. C 2021, 12, 6794–6803. [Google Scholar] [CrossRef]
- Kim, T.W.; Kim, P.; Mills, A.W.; Chakraborty, A.; Kromer, S.; Valentine, A.J.S.; Castellano, F.N.; Li, X.; Chen, L.X. Ligand-Structure-Dependent Coherent Vibrational Wavepacket Dynamics in Pyrazolate-Bridged Pt(II) Dimers. J. Phys. Chem. C 2022, 126, 11487–11497. [Google Scholar] [CrossRef]
- Kim, P.; Kelley, M.S.; Chakraborty, A.; Wong, N.L.; Van Duyne, R.P.; Schatz, G.C.; Castellano, F.N.; Chen, L.X. Coherent vibrational wavepacket dynamics in platinum (II) dimers and their implications. J. Phys. Chem. C 2018, 122, 14195–14204. [Google Scholar] [CrossRef]
- Iwamura, M.; Fukui, A.; Nozaki, K.; Kuramochi, H.; Takeuchi, S.; Tahara, T. Coherent Vibration and Femtosecond Dynamics of the Platinum Complex Oligomers upon Intermolecular Bond Formation in the Excited State. Angew. Chem. Int. Ed. 2020, 59, 23154–23161. [Google Scholar] [CrossRef] [PubMed]
- Radler, J.J.; Lingerfelt, D.B.; Castellano, F.N.; Chen, L.X.; Li, X. Role of vibrational dynamics on excited-state electronic coherence in a binuclear platinum complex. J. Phys. Chem. A 2018, 122, 5071–5077. [Google Scholar] [CrossRef]
- Monni, R.; Auböck, G.; Kinschel, D.; Aziz-Lange, K.M.; Gray, H.B.; Vlček, A.; Chergui, M. Conservation of vibrational coherence in ultrafast electronic relaxation: The case of diplatinum complexes in solution. Chem. Phys. Lett. 2017, 683, 112–120. [Google Scholar] [CrossRef]
- Cho, S.; Mara, M.W.; Wang, X.; Lockard, J.V.; Rachford, A.A.; Castellano, F.N.; Chen, L.X. Coherence in metal-metal-to-ligand-charge-transfer excited states of a dimetallic complex investigated by ultrafast transient absorption anisotropy. J. Phys. Chem. A 2011, 115, 3990–3996. [Google Scholar] [CrossRef] [PubMed]
- Li, K.; Tong, G.S.M.; Wan, Q.; Cheng, G.; Tong, W.-Y.; Ang, W.-H.; Kwong, W.-L.; Che, C.-M. Highly phosphorescent platinum(II) emitters: Photophysics, materials and biological applications. Chem. Sci. 2016, 7, 1653–1673. [Google Scholar] [CrossRef] [PubMed]
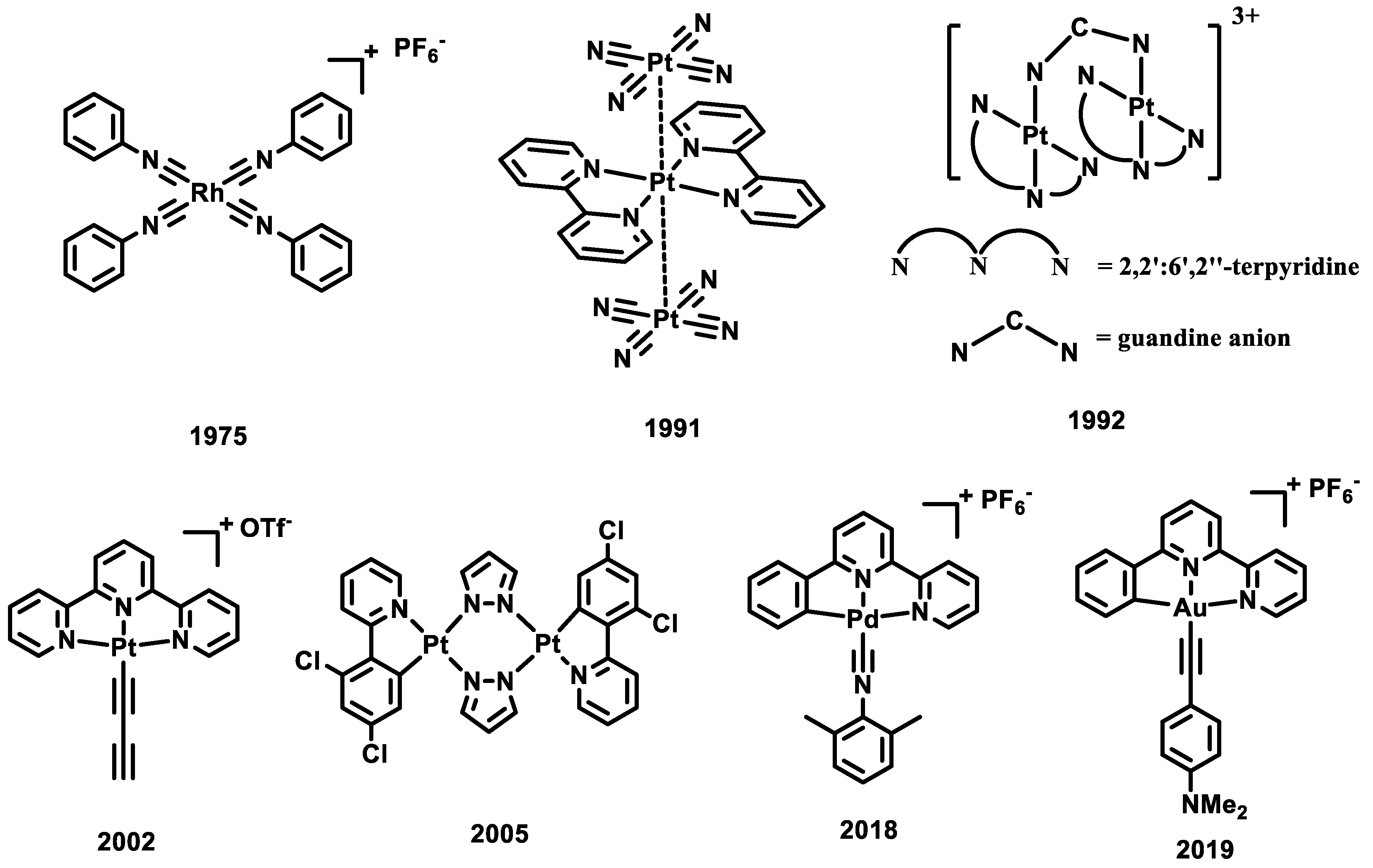
- Mann, K.R.; Gordon, J.; Gray, H.B. Characterization of oligomers of tetrakis (phenyl isocyanide) rhodium (I) in acetonitrile solution. J. Am. Chem. Soc. 1975, 97, 3553–3555. [Google Scholar] [CrossRef]
- Maiuri, M.; Garavelli, M.; Cerullo, G. Ultrafast Spectroscopy: State of the Art and Open Challenges. J. Am. Chem. Soc. 2020, 142, 3–15. [Google Scholar] [CrossRef]
- Zewail, A.H. Femtochemistry: Atomic-Scale Dynamics of the Chemical Bond. J. Phys. Chem. A 2000, 104, 5660–5694. [Google Scholar] [CrossRef]
- Wan, Q.; Yang, J.; To, W.-P.; Che, C.-M. Strong metal-metal Pauli repulsion leads to repulsive metallophilicity in closed-shell d8 and d10 organometallic complexes. Proc. Natl. Acad. Sci. USA 2021, 118, e2019265118. [Google Scholar] [CrossRef]
- Biedermann, J.; Gliemann, G.; Klement, U.; Range, K.-J.; Zabel, M. Spectroscopic studies of cyclo-metallated Pt (lI) complexes: Optical absorption and emission and the structure of single crystal [Pt (bpm)(CN)2]·H2O (bpm = 2, 2′-bipyrimidine). Inorganica Chim. Acta 1990, 169, 63–70. [Google Scholar] [CrossRef]
- Miskowski, V.M.; Houlding, V.H. Electronic spectra and photophysics of platinum(II) complexes with a-diimine ligands solid-state effects. 2. Metal-metal interaction in double salts and linear chains. Inorg. Chem. 1991, 30, 4446–4452. [Google Scholar] [CrossRef]
- Yip, H.-K.; Che, C.-M.; Zhou, Z.-Y.; Mak, T. Photophysical Properties and X-ray Crystal Structure of a Luminescent Platinum (II) Dimer [Pt2(2,2-6,2-terpyridine)2(Gua)](CIO4)3.H2O (Gua = guanidine anion). Chem. Commun. 1992, 1369, 1369–1371. [Google Scholar] [CrossRef]
- Ma, B.; Li, J.; Djurovich, P.I.; Yousufuddin, M.; Bau, R.; Thompson, M.E. Synthetic control of pt…pt separation and photophysics of binuclear platinum complexes. J. Am. Chem. Soc. 2005, 127, 28–29. [Google Scholar] [CrossRef]
- Yam, V.W.-W.; Wong, K.M.-C.; Zhu, N. Solvent-Induced Aggregation through Metal…Metal/π…π Interactions: Large Solvatochromism of Luminescent Organoplatinum (II) Terpyridyl Complexes. J. Am. Chem. Soc. 2002, 124, 6506–6507. [Google Scholar] [CrossRef]
- Wan, Q.; To, W.-P.; Yang, C.; Che, C.-M. The metal-metal-to-ligand charge transfer excited state and supramolecular polymerization of luminescent pincer PdII-isocyanide complexes. Angew. Chem. Int. Ed. 2018, 57, 3089–3093. [Google Scholar] [CrossRef]
- Wan, Q.; Xia, J.; Lu, W.; Yang, J.; Che, C.-M. Kinetically controlled self-assembly of phosphorescent AuIII aggregates and ligand-to-metal–metal charge transfer excited state: A combined spectroscopic and DFT/TDDFT study. J. Am. Chem. Soc. 2019, 141, 11572–11582. [Google Scholar] [CrossRef]
- Ly, K.T.; Chen-Cheng, R.-W.; Lin, H.-W.; Shiau, Y.-J.; Liu, S.-H.; Chou, P.-T.; Tsao, C.-S.; Huang, Y.-C.; Chi, Y. Near-infrared organic light-emitting diodes with very high external quantum efficiency and radiance. Nat. Photonics 2017, 11, 63–68. [Google Scholar]
- Mauro, M.; Aliprandi, A.; Septiadi, D.; Kehr, N.S.; De Cola, L. When self-assembly meets biology: Luminescent platinum complexes for imaging applications. Chem. Soc. Rev. 2014, 43, 4144–4166. [Google Scholar] [CrossRef]
- Roundhill, D.M.; Gray, H.B.; Che, C.-M. Pyrophosphito-bridged diplatinum chemistry. Acc. Chem. Res. 1989, 22, 55–61. [Google Scholar] [CrossRef]
- Chergui, M.; Collet, E. Photoinduced Structural Dynamics of Molecular Systems Mapped by Time-Resolved X-ray Methods. Chem. Rev. 2017, 117, 11025–11065. [Google Scholar] [CrossRef] [PubMed]
- Fayer, M.D. Dynamics of Liquids, Molecules, and Proteins Measured with Ultrafast 2D IR Vibrational Echo Chemical Exchange Spectroscopy. Annu. Rev. Phys. Chem. 2009, 60, 21–38. [Google Scholar] [CrossRef] [PubMed]
- Kraack, J.P.; Hamm, P. Surface-Sensitive and Surface-Specific Ultrafast Two-Dimensional Vibrational Spectroscopy. Chem. Rev. 2017, 117, 10623–10664. [Google Scholar] [CrossRef]
- Brixner, T.; Mančal, T.; Stiopkin, I.V.; Fleming, G.R. Phase-stabilized two-dimensional electronic spectroscopy. J. Chem. Phys. 2004, 121, 4221–4236. [Google Scholar] [CrossRef] [PubMed]
- Dubietis, A.; Couairon, A. Governing Physical Effects. In Ultrafast Supercontinuum Generation in Transparent Solid-State Media; Springer International Publishing: Cham, Switzerland, 2019; pp. 9–26. [Google Scholar]
- Delor, M.; Scattergood, P.A.; Sazanovich, I.V.; Parker, A.W.; Greetham, G.M.; Meijer, A.J.H.M.; Towrie, M.; Weinstein, J.A. Toward control of electron transfer in donor-acceptor molecules by bond-specific infrared excitation. Science 2014, 346, 1492–1495. [Google Scholar] [CrossRef]
- Leong, T.X.; Collins, B.K.; Dey Baksi, S.; Mackin, R.T.; Sribnyi, A.; Burin, A.L.; Gladysz, J.A.; Rubtsov, I.V. Tracking Energy Transfer across a Platinum Center. J. Phys. Chem. A 2022, 126, 4915–4930. [Google Scholar] [CrossRef]
- Wang, P.; Koo, Y.H.; Kim, W.; Yang, W.; Cui, X.; Ji, W.; Zhao, J.; Kim, D. Broadband Visible Light Harvesting N^N Pt(II) Bisacetylide Complex with Bodipy and Naphthalene Diimide Ligands: Förster Resonance Energy Transfer and Intersystem Crossing. J. Phys. Chem. C 2017, 121, 11117–11128. [Google Scholar] [CrossRef]
- Consani, C.; Prémont-Schwarz, M.; ElNahhas, A.; Bressler, C.; van Mourik, F.; Cannizzo, A.; Chergui, M. Vibrational Coherences and Relaxation in the High-Spin State of Aqueous [FeII(bpy)3]2+ Angew. Chem. Int. Ed. Engl. 2009, 48, 7184–7187. [Google Scholar] [CrossRef]
- Auböck, G.; Chergui, M. Sub-50-fs photoinduced spin crossover in [Fe(bpy)3]2+. Nat. Chem. 2015, 7, 629–633. [Google Scholar] [CrossRef]
- Iwamura, M.; Nozaki, K.; Takeuchi, S.; Tahara, T. Real-Time Observation of Tight Au–Au Bond Formation and Relevant Coherent Motion upon Photoexcitation of [Au(CN)2–] Oligomers. J. Am. Chem. Soc. 2013, 135, 538–541. [Google Scholar] [CrossRef]
- Iwamura, M.; Wakabayashi, R.; Maeba, J.; Nozaki, K.; Takeuchi, S.; Tahara, T. Coherent vibration and ultrafast dynamics upon bond formation in excited dimers of an Au(I) complex. Phys. Chem. Chem. Phys. 2016, 18, 5103–5107. [Google Scholar] [CrossRef]
- van der Veen, R.M.; Cannizzo, A.; van Mourik, F.; Vlček, A., Jr.; Chergui, M. Vibrational Relaxation and Intersystem Crossing of Binuclear Metal Complexes in Solution. J. Am. Chem. Soc. 2011, 133, 305–315. [Google Scholar] [CrossRef] [PubMed]
- Haldrup, K.; Dohn, A.O.; Shelby, M.L.; Mara, M.W.; Stickrath, A.B.; Harpham, M.R.; Huang, J.; Zhang, X.; Møller, K.B.; Chakraborty, A.; et al. Butterfly Deformation Modes in a Photoexcited Pyrazolate-Bridged Pt Complex Measured by Time-Resolved X-ray Scattering in Solution. J. Phys. Chem. A 2016, 120, 7475–7483. [Google Scholar] [CrossRef] [PubMed]
- Rose, T.S.; Rosker, M.J.; Zewail, A.H. Femtosecond real-time probing of reactions. IV. The reactions of alkali halides. J. Chem. Phys. 1989, 91, 7415–7436. [Google Scholar] [CrossRef]
- Shen, Y.-C.; Cina, J.A. What can short-pulse pump-probe spectroscopy tell us about Franck-Condon dynamics? J. Chem. Phys. 1999, 110, 9793–9806. [Google Scholar] [CrossRef]
- Vos, M.H.; Rappaport, F.; Lambry, J.-C.; Breton, J.; Martin, J.-L. Visualization of coherent nuclear motion in a membrane protein by femtosecond spectroscopy. Nature 1993, 363, 320–325. [Google Scholar] [CrossRef]
- Jonas, D.M.; Bradforth, S.E.; Passino, S.A.; Fleming, G.R. Femtosecond Wavepacket Spectroscopy: Influence of Temperature, Wavelength, and Pulse Duration. J. Phys. Chem. 1995, 99, 2594–2608. [Google Scholar] [CrossRef]
- Dantus, M.; Lozovoy, V.V. Experimental Coherent Laser Control of Physicochemical Processes. Chem. Rev. 2004, 104, 1813–1860. [Google Scholar] [CrossRef]
- Letokhov, V.S.; Tyakht, V.V. Coherent Vibrational Wave Packet Dynamics in Femtosecond Laser Excitation of Diatomic Molecules. Isr. J. Chem. 1990, 30, 189–195. [Google Scholar] [CrossRef]
- Song, Y.; Clafton, S.N.; Pensack, R.D.; Kee, T.W.; Scholes, G.D. Vibrational coherence probes the mechanism of ultrafast electron transfer in polymer–fullerene blends. Nat. Commun. 2014, 5, 4933. [Google Scholar] [CrossRef]
- Cina, J.A.; Fleming, G.R. Vibrational Coherence Transfer and Trapping as Sources for Long-Lived Quantum Beats in Polarized Emission from Energy Transfer Complexes. J. Phys. Chem. A 2004, 108, 11196–11208. [Google Scholar] [CrossRef]
- Duan, H.G.; Jha, A.; Li, X.; Tiwari, V.; Ye, H.; Nayak, P.K.; Zhu, X.L.; Li, Z.; Martinez, T.J.; Thorwart, M.; et al. Intermolecular vibrations mediate ultrafast singlet fission. Sci. Adv. 2020, 6, eabb0052. [Google Scholar] [CrossRef] [PubMed]
- Turner, D.B.; Wilk, K.E.; Curmi, P.M.G.; Scholes, G.D. Comparison of Electronic and Vibrational Coherence Measured by Two-Dimensional Electronic Spectroscopy. J. Phys. Chem. Lett. 2011, 2, 1904–1911. [Google Scholar] [CrossRef]
- Lanzani, G.; Cerullo, G.; De Silvestri, S. Coherent Vibrational Dynamics; CRC Press: Boca Raton, FL, USA, 2007. [Google Scholar]
- Schmidbaur, H.; Raubenheimer, H.G. Excimer and exciplex formation in gold (I) complexes preconditioned by aurophilic interactions. Angew. Chem. Int. Ed. 2020, 59, 14748–14771. [Google Scholar] [CrossRef] [PubMed]
- Haldrup, K.; Levi, G.; Biasin, E.; Vester, P.; Laursen, M.G.; Beyer, F.; Kjær, K.S.; Brandt van Driel, T.; Harlang, T.; Dohn, A.O.; et al. Ultrafast X-Ray Scattering Measurements of Coherent Structural Dynamics on the Ground-State Potential Energy Surface of a Diplatinum Molecule. Phys. Rev. Lett. 2019, 122, 063001. [Google Scholar] [CrossRef]
- Lockard, J.V.; Rachford, A.A.; Smolentsev, G.; Stickrath, A.B.; Wang, X.; Zhang, X.; Atenkoffer, K.; Jennings, G.; Soldatov, A.; Rheingold, A.L.; et al. Triplet Excited State Distortions in a Pyrazolate Bridged Platinum Dimer Measured by X-ray Transient Absorption Spectroscopy. J. Phys. Chem. A 2010, 114, 12780–12787. [Google Scholar] [CrossRef]
- van der Veen, R.M.; Milne, C.J.; El Nahhas, A.; Lima, F.A.; Pham, V.-T.; Best, J.; Weinstein, J.A.; Borca, C.N.; Abela, R.; Bressler, C.; et al. Structural determination of a photochemically active diplatinum molecule by time-resolved EXAFS spectroscopy. Angew. Chem. Int. Ed. 2009, 48, 2711–2714. [Google Scholar] [CrossRef]
- Novozhilova, I.V.; Volkov, A.V.; Coppens, P. Theoretical analysis of the triplet excited state of the [Pt2(H2P2O5)4]4- ion and comparison with time-resolved X-ray and spectroscopic results. J. Am. Chem. Soc. 2003, 125, 1079–1087. [Google Scholar] [CrossRef]
- Mewes, L.; Ingle, R.A.; Megow, S.; Böhnke, H.; Baranoff, E.; Temps, F.; Chergui, M. Ultrafast Intersystem Crossing and Structural Dynamics of [Pt(ppy)(μ-tBu2pz)]2. lnorg. Chem. 2020, 59, 14643–14653. [Google Scholar] [CrossRef]

| Technique | Advantages | Disadvantages |
|---|---|---|
| Visible transient absorption (TA) | Information about excited state dynamics such as internal conversion, ISC and so on. Simple setup. | Blurred signals due to broadening. Mandatory requirement of optically allowed electronic transition. |
| Time-resolved vibrational spectroscopy (IR) | Information about molecular vibrations at excited state. | Mandatory requirement of symmetry allowed vibrational modes. Challenging to set up. Costly lasers and detectors. |
| Time-resolved X-ray diffraction | Information about molecular structural changes at excited state. High temporal and spatial resolution. | Requirement of specially prepared samples. Potentially sample damage by radiation. Complexity and synchrotron radiation facilities are usually required. |
| 2D spectroscopy | Enhanced resolution for signals mixed due to inhomogeneous broadening, compared to TA technique. Direct inspection of correlated state. | Long data acquisition time, stable samples required. Challenging to set up. |
| Transient Raman | Information about molecular vibrations and conformational changes. | Weak signal. Interfered by fluorescence signal. |
| Time-resolved photoluminescence | Wide range of detection time delay. Simple setup. | Mandatory requirement of detectable photoluminescence of the sample. Inferior time resolution. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yan, T.; Wan, Q. Vibrational Coherence in the Metal–Metal-Bonded Excited State of Pt(II) Complexes. Inorganics 2023, 11, 441. https://doi.org/10.3390/inorganics11110441
Yan T, Wan Q. Vibrational Coherence in the Metal–Metal-Bonded Excited State of Pt(II) Complexes. Inorganics. 2023; 11(11):441. https://doi.org/10.3390/inorganics11110441
Chicago/Turabian StyleYan, Tengfei, and Qingyun Wan. 2023. "Vibrational Coherence in the Metal–Metal-Bonded Excited State of Pt(II) Complexes" Inorganics 11, no. 11: 441. https://doi.org/10.3390/inorganics11110441