Circular Dichroism Spectroscopic Studies on Solution Chemistry of M(II)-Monensinates in Their Competition Reactions
Abstract
:1. Introduction
2. Results and Discussion
2.1. Binary Systems of Monensinate with Ni(II) or Co(II)
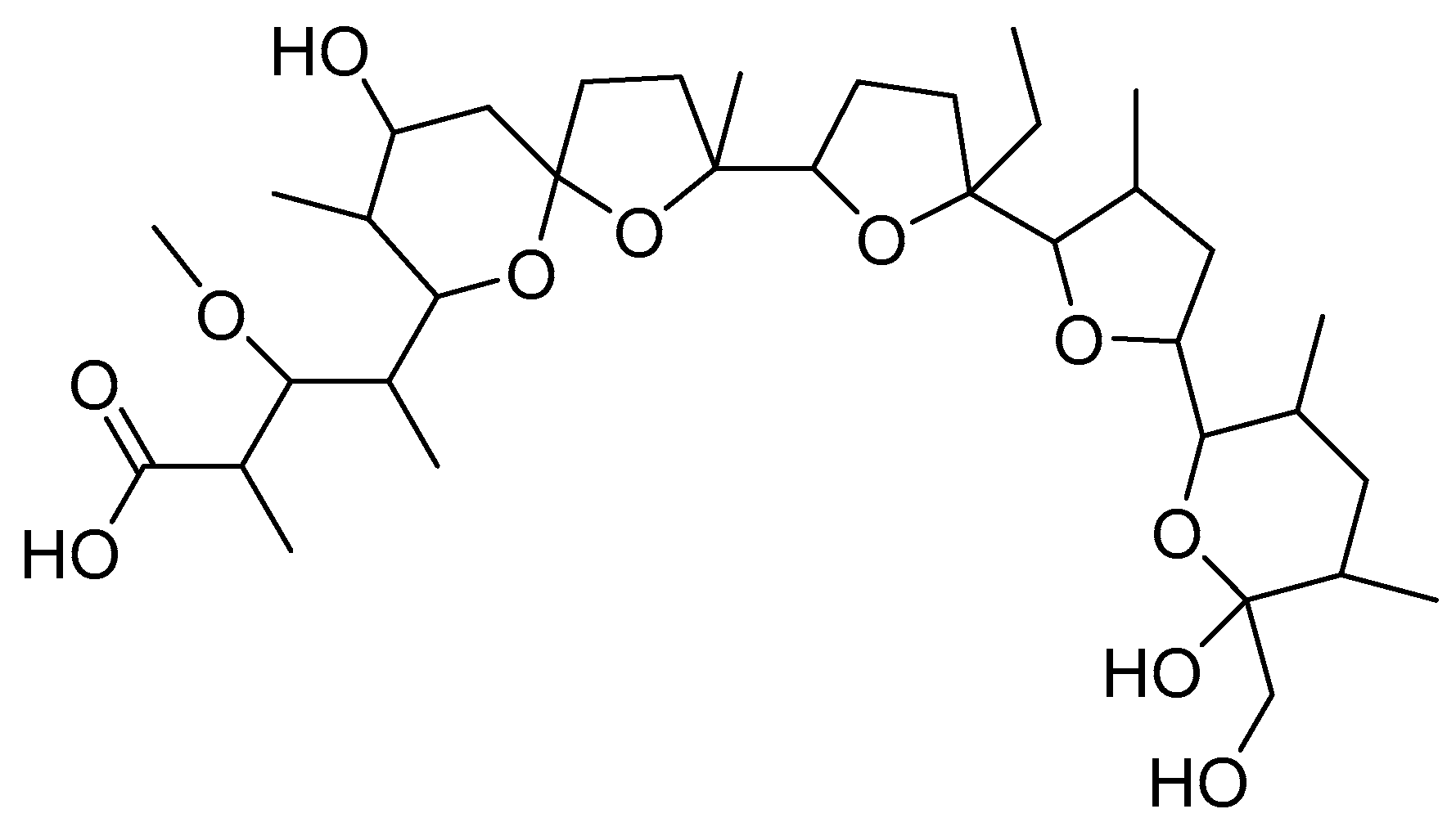
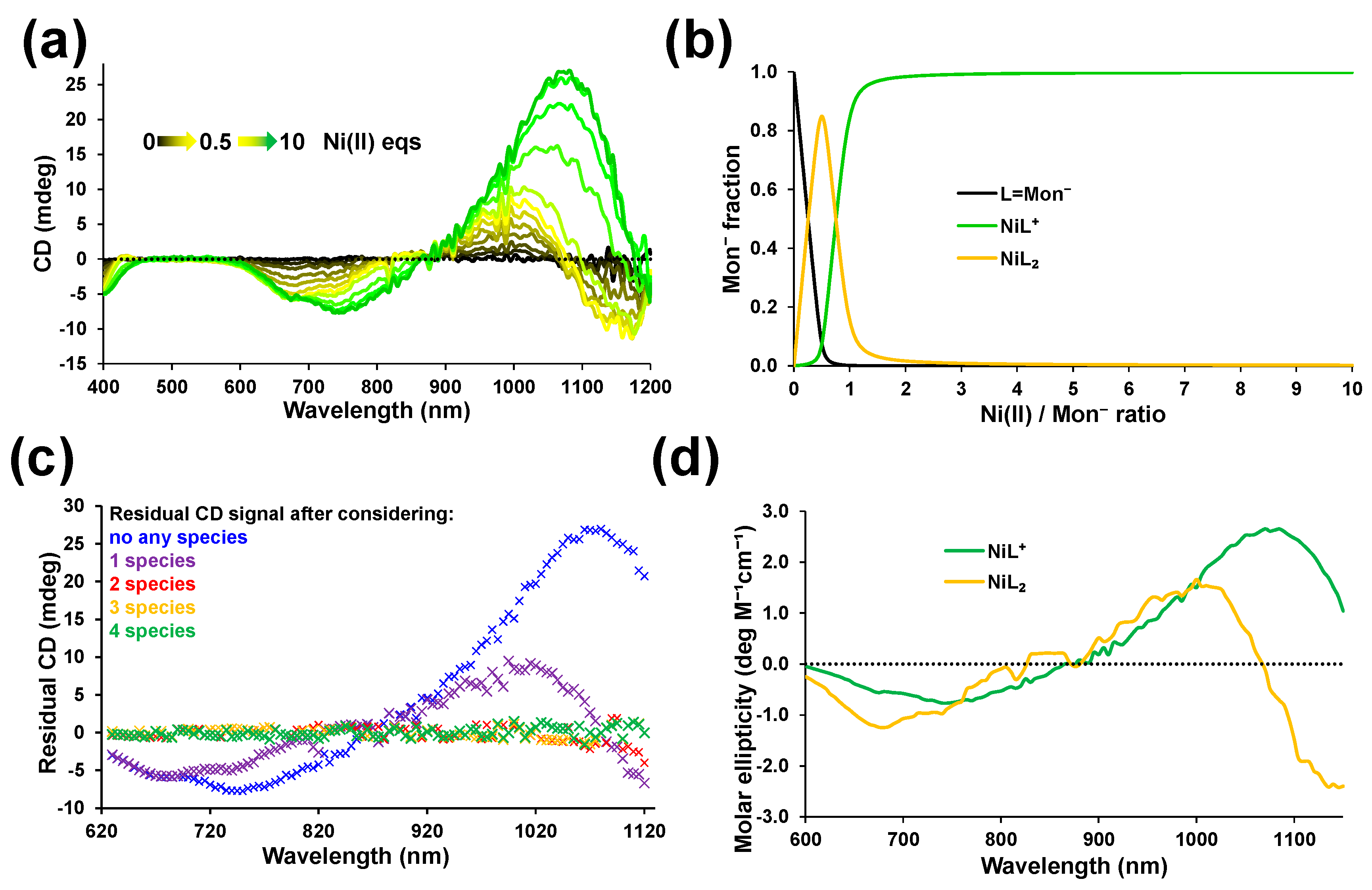
2.1.1. Interactions with Ni(II)
2.1.2. Interactions with Co(II)
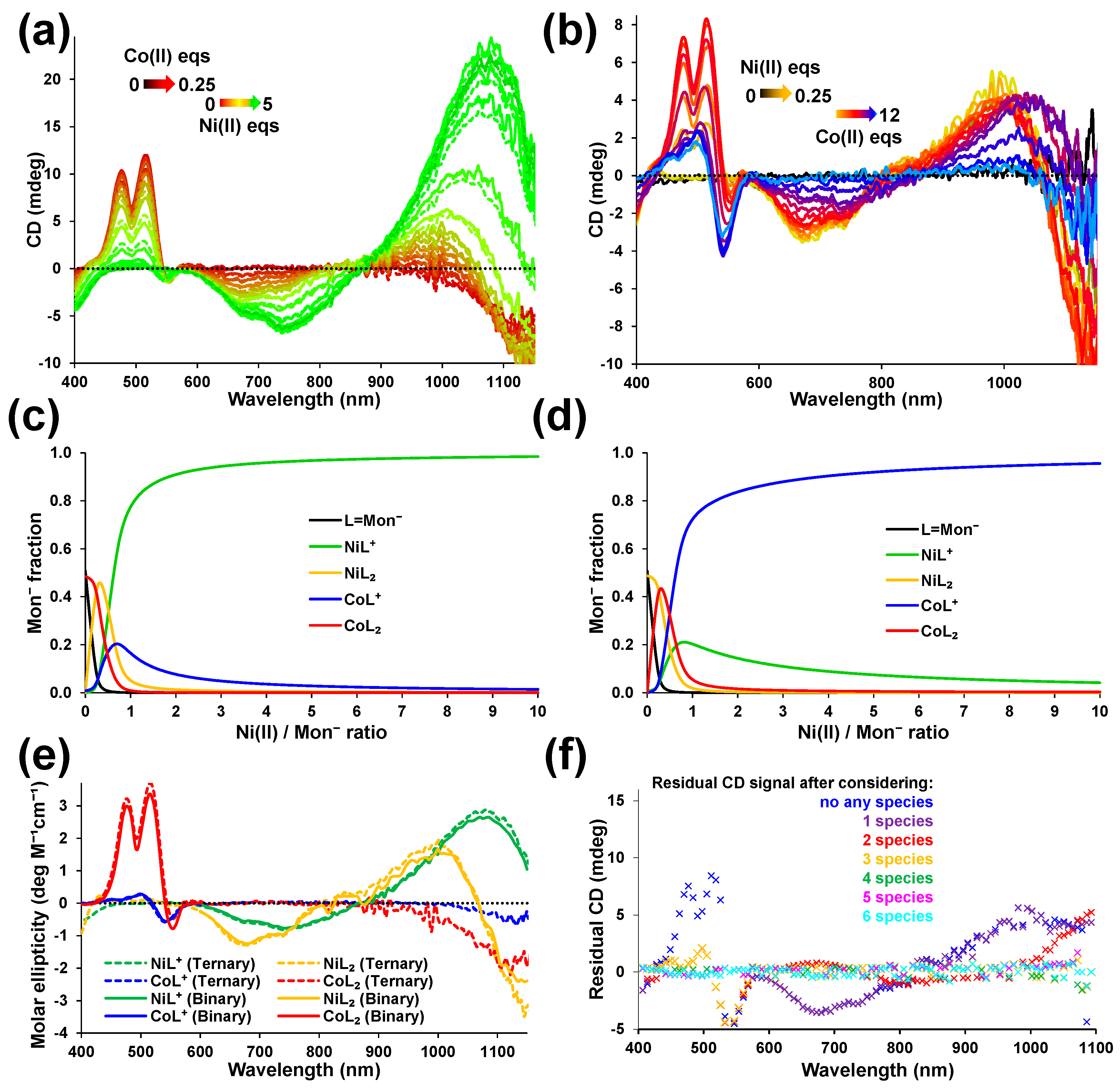
2.2. Competition between Ni(II) and Co(II) for Monensinate
2.3. Competition between Ni(II) or Co(II) and Colorless Divalent Metal Ions for Monensinate
3. Materials and Methods
3.1. Materials
3.2. CD Spectroscopy
3.3. Mathematical Treatment of the Experimental Data
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Agtarap, A.; Chamberlin, J.W.; Pinkerton, M.; Steinrauf, L. The structure of monensic acid, a new biologically active compound. J. Am. Chem. Soc. 1967, 89, 5737–5739. [Google Scholar] [CrossRef]
- Chapman, H.D.; Jeffers, T.K.; Williams, R.B. Forty years of monensin for the control of coccidiosis in poultry. Poult. Sci. 2010, 89, 1788–1801. [Google Scholar] [CrossRef]
- Wischer, G.; Boguhn, J.; Steingaß, H.; Schollenberger, M.; Hartung, K.; Rodehutscord, M. Effect of monensin on in vitro fermentation of silages and microbial protein synthesis. Arch. Anim. Nutr. 2013, 67, 219–234. [Google Scholar] [CrossRef]
- Odongo, N.E.; Bagg, R.; Vessie, G.; Dick, P.; Or-Rashid, M.M.; Hook, S.E.; Gray, J.T.; Kebreab, E.; France, J.; McBride, B.W. Long-term effects of feeding monensin on methane production in lactating dairy cows. J. Dairy Sci. 2007, 90, 1781–1788. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- de Jesus, E.F.; Del Valle, T.A.; Calomeni, G.D.; Silva, T.H.; Takiya, C.S.; Vendramini, T.H.A.; Paiva, P.G.; Silva, G.G.; Netto, A.S.; Rennó, F.P. Influence of a blend of functional oils or monensin on nutrient intake and digestibility, ruminal fermentation and milk production of dairy cows. Anim. Feed Sci. Technol. 2016, 219, 59–67. [Google Scholar] [CrossRef] [Green Version]
- Ghorbani, B.; Ghoorchi, T.; Amanlou, H.; Zerehdaran, S. Effects of using monensin and different levels of crude protein on milk production, blood metabolites and digestion of dairy cows. Asian-Australas. J. Anim. Sci. 2011, 24, 65–72. [Google Scholar] [CrossRef]
- Rezaei Ahvanooei, M.R.; Norouzian, M.A.; Piray, A.H.; Vahmani, P.; Ghaffari, M.H. Effects of monensin supplementation on lactation performance of dairy cows: A systematic review and dose-response meta-analysis. Sci. Rep. 2023, 13, 568. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, K.; Tohda, K.; Aruga, H.; Matsuzoe, M.; Inoue, H.; Shirai, T. Ion-selective electrodes based on natural carboxylic polyether antibiotics. Anal. Chem. 1988, 60, 1714–1721. [Google Scholar] [CrossRef]
- Zahran, E.M.; Gavalas, V.; Valiente, M.; Bachas, L.G. Can temperature be used to tune the selectivity of membrane ion-selective electrodes? Anal. Chem. 2010, 82, 3622–3628. [Google Scholar] [CrossRef]
- Lowicki, D.; Huczynski, A. Structure and antimicrobial properties of monensin A and its derivatives: Summary of the achievements. Biomed. Res. Int. 2013, 2013, 742149. [Google Scholar] [CrossRef] [Green Version]
- Greenstein, R.J.; Su, L.; Whitlock, R.H.; Brown, S.T. Monensin causes dose dependent inhibition of Mycobacterium avium subspecies paratuberculosis in radiometric culture. Gut. Pathog. 2009, 1, 4. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mimouni, M.; Khardli, F.Z.; Warad, I.; Ahmad, M.; Mubarak, M.S.; Sultana, S.; Hadda, T.B. Antimicrobial activity of naturally occurring antibiotics monensin, lasalocid and their metal complexes. J. Mater. Environ. Sci. 2014, 5, 207–214. [Google Scholar]
- Hendrick, S.H.; Kelton, D.F.; Leslie, K.E.; Lissemore, K.D.; Archambault, M.; Bagg, R.; Dick, P.; Duffield, T.F. Efficacy of monensin sodium for the reduction of fecal shedding of Mycobacterium avium subsp. paratuberculosis in infected dairy cattle. Prev. Vet. Med. 2006, 75, 206–220. [Google Scholar] [CrossRef]
- Rajendran, V.; Rohra, S.; Raza, M.; Hasan, G.M.; Dutt, S.; Ghosh, P.C. Stearylamine liposomal delivery of monensin in combination with free artemisinin eliminates blood stages of Plasmodium falciparum in culture and P. berghei infection in murine malaria. Antimicrob. Agents Chemother. 2015, 60, 1304–1318. [Google Scholar] [CrossRef] [Green Version]
- Lavine, M.D.; Arrizabalaga, G. Analysis of monensin sensitivity in Toxoplasma gondii reveals autophagy as a mechanism for drug induced death. PLoS ONE 2012, 7, e42107. [Google Scholar] [CrossRef] [Green Version]
- Lad, V.J.; Gupta, A.K. Effect of brefelidin A and monensin on Japanese encephalitis virus maturation and virus release from cells. Microbiol. Res. 2011, 2, e9. [Google Scholar] [CrossRef] [Green Version]
- Feneant, L.; Potel, J.; Francois, C.; Sane, F.; Douam, F.; Belouzard, S.; Calland, N.; Vausselin, T.; Rouille, Y.; Descamps, V.; et al. New insights into the understanding of Hepatitis C virus entry and cell-to-cell transmission by using the ionophore Monensin A. J. Virol. 2015, 89, 8346–8364. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Iacoangeli, A.; Melucci-Vigo, G.; Risuleo, G. The ionophore monensin inhibits mouse polyomavirus DNA replication and destabilizes viral early mRNAs. Biochimie 2000, 82, 35–39. [Google Scholar] [CrossRef]
- Rajendran, V.; Ilamathi, H.S.; Dutt, S.; Lakshminarayana, T.S.; Ghosh, P.C. Chemotherapeutic potential of monensin as an anti-microbial agent. Curr. Top. Med. Chem. 2018, 18, 1976–1986. [Google Scholar] [CrossRef]
- Markowska, A.; Kaysiewicz, J.; Markowska, J.; Huczyński, A. Doxycycline, salinomycin, monensin and ivermectin repositioned as cancer drugs. Bioorg. Med. Chem. Lett. 2019, 29, 1549–1554. [Google Scholar] [CrossRef]
- Choi, H.S.; Jeong, E.H.; Lee, T.G.; Kim, S.Y.; Kim, H.R.; Kim, C.H. Autophagy inhibition with monensin enhances cell cycle arrest and apoptosis induced by mTOR or epidermal growth factor receptor inhibitors in lung cancer cells. Tuberc. Respir. Dis. 2013, 75, 9–17. [Google Scholar] [CrossRef] [Green Version]
- Yao, S.; Wang, W.; Zhou, B.; Cui, X.; Yang, H.; Zhang, S. Monensin suppresses cell proliferation and invasion in ovarian cancer by enhancing MEK1 SUMOylation. Exp. Therap. Med. 2021, 22, 1390. [Google Scholar] [CrossRef] [PubMed]
- Tumova, L.; Pombinho, A.R.; Vojtechova, M.; Stancikova, J.; Gradl, D.; Krausova, M.; Sloncova, E.; Horazna, M.; Kriz, V.; Machonova, O.; et al. Monensin inhibits canonical Wnt signaling in human col-orectal cancer cells and suppresses tumor growth in multiple intestinal neoplasia mice. Mol. Cancer Ther. 2014, 13, 812–822. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, Y.; Sun, Q.; Chen, S.; Yu, X.; Jing, H. Monensin inhibits anaplastic thyroid cancer via disrupting mitochondrial respiration and AMPK/mTOR signaling. Anti-Cancer Agents Med. Chem. 2022, 22, 2539–2547. [Google Scholar] [CrossRef]
- Zhou, Y.; Deng, Y.; Wang, J.; Yan, Z.; Wei, Q.; Ye, J.; Zhang, J.; He, T.C.; Qiao, M. Effect of antibiotic monensin on cell prolifera-tion and IGF1R signaling pathway in human colorectal cancer cells. Annals Med. 2023, 55, 954–964. [Google Scholar] [CrossRef]
- Ketola, K.; Vainio, P.; Fey, V.; Kallioniemi, O.; Iljin, K. Monensin is a potent inducer of oxidative stress and inhibitor of androgen signaling leading to apoptosis in prostate cancer cells. Mol. Cancer Ther. 2010, 9, 3175–3185. [Google Scholar] [CrossRef] [Green Version]
- Kim, S.H.; Kim, K.Y.; Yu, S.N.; Park, S.G.; Yu, H.S.; Seo, Y.K.; Ahn, S.C. Monensin induces PC-3 prostate cancer cell apoptosis via ROS production and Ca2+ homeostasis disruption. Anticancer Res. 2016, 36, 5835–5843. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Agtarap, A.; Chamberlin, J.W. Monensin, a new biologically active compound. IV. Chem. Antimicrob. Agents Chemother. 1967, 7, 359–362. [Google Scholar]
- Lutz, W.K.; Winkler, F.K.; Dunitz, J.D. Crystal structure of the antibiotic monensin. Similarities and differences between free acid and metal complex. Helv. Chim. Acta 1971, 54, 1103–1108. [Google Scholar] [CrossRef]
- Walba, D.M.; Hermsmeier, M.; Haltiwanger, R.C.; Noordik, J.H. Crystal structures of monensin B lithium and silver salts. J. Org. Chem. 1986, 51, 245–247. [Google Scholar] [CrossRef]
- Duax, W.L.; Smith, G.D.; Strong, P.D. Complexation of metal ions by monensin. Crystal and molecular structure of hydrated and anhydrous crystal forms of sodium monensin. J. Am. Chem. Soc. 1980, 102, 6725–6729. [Google Scholar] [CrossRef]
- Almeida Paz, F.A.; Gates, P.J.; Fowler, S.; Gallimore, A.; Harvey, B.; Lopes, N.P.; Stark, C.B.W.; Staunton, J.; Klinowski, J.; Spencer, J.B. Sodium monensin dehydrate. Acta Cryst. 2003, E59, m1050–m1052. [Google Scholar] [CrossRef]
- Pangborn, W.; Duax, W.; Langs, D. The hydrated potassium complex of the ionophore monensin A. J. Am. Chem. Soc. 1987, 109, 2163–2165. [Google Scholar] [CrossRef]
- Yildirim, S.Ö.; McKee, V.; Khardli, F.Z.; Mimouni, M.; Hadda, T.B. Rubidium(I) monensinate dihydrate. Acta Cryst. 2007, E64, m154–m155. [Google Scholar] [CrossRef]
- Huczynski, A.; Ratajczak-Sitarz, M.; Katrusiak, A.; Brzezinski, B. Molecular structure of rubidium six-coordinated dihydrate complex with monensin A. J. Mol. Struct. 2008, 888, 224–229. [Google Scholar] [CrossRef]
- Turner, D.L. The conformation of the monensin-A-sodium complex in solution determined from self-consistent NOE distance constraints. J. Magn. Reson. B. 1995, 108, 137–142. [Google Scholar] [CrossRef]
- Briggs, R.W.; Hinton, J.F. Thallium-205 and proton nuclear magnetic resonance investigation of the complexation of thallium by the ionophores monensin and nigericin. Biochemistry 1978, 17, 55765582. [Google Scholar] [CrossRef]
- Cox, B.G.; van Truong, N.; Rzeszotarska, J.; Schneider, H. Rates and equilibria of alkali metal and silver ion complex formation with monensin in ethanol. J. Am. Chem. Soc. 1984, 106, 5965–5969. [Google Scholar] [CrossRef]
- Hoogerheide, J.G.; Popov, A.I. Study of monensin complexes with monovalent metal ions in anhydrous methanol solutions. J. Sol. Chem. 1978, 7, 357–372. [Google Scholar] [CrossRef]
- Hoogerheide, J.G.; Popov, A.I. A study of metal complexes of a naturally occurring macrocyclic ionophore-monensin. J. Sol. Chem. 1979, 8, 83–95. [Google Scholar] [CrossRef]
- Huczyński, A.; Janczak, J.; Łowicki, D.; Brzezinski, B. Monensin A acid complexes as a model of electrogenic transport of so-dium cation. Biochim. Biophys. Acta (BBA) Biomembr. 2012, 1818, 2108–2119. [Google Scholar] [CrossRef] [Green Version]
- Chow, J.M.; Russell, J.B. Effect of ionophores and pH on growth of Streptococcus bovis in batch and continuous culture. Appl. Environ. Microbiol. 1990, 56, 1588–1593. [Google Scholar] [CrossRef]
- Chirase, N.K.; Greene, L.W.; Schelling, G.T.; Byers, F.M. Effect of magnesium and potassium on microbial fermentation in a continuous culture fermentation system with different levels of monensin or lasalocid. J. Anim. Sci. 1987, 65, 1633–1638. [Google Scholar] [CrossRef] [Green Version]
- Dorkov, P.; Pantcheva, I.N.; Sheldrick, W.S.; Mayer-Figge, H.; Petrova, R.; Mitewa, M. Synthesis, structure and antimicrobial activity of manganese(II) and cobalt(II) complexes of the polyether ionophore antibiotic sodium monensin A. J. Inorg. BioChem. 2008, 102, 26–32. [Google Scholar] [CrossRef]
- Pantcheva, I.N.; Dorkov, P.; Atanasov, V.N.; Mitewa, M.; Shivachev, B.L.; Nikolova, R.P.; Mayer-Figge, H.; Sheldrick, W.S. Crystal structure and properties of the copper(II) complex of sodium monensin A. J. Inorg. Biochem. 2009, 103, 1419–1424. [Google Scholar] [CrossRef] [PubMed]
- Ivanova, J.; Pantcheva, I.N.; Mitewa, M.; Simova, S.; Mayer-Figge, H.; Sheldrick, W.S. Crystal structures and spectral properties of new Cd(II) and Hg(II) complexes of monensic acid with different coordination modes of the ligand. Centr. Eur. J. Chem. 2010, 8, 852–860. [Google Scholar] [CrossRef]
- Pantcheva, I.N.; Mitewa, M.I.; Sheldrick, W.S.; Oppel, I.M.; Zhorova, R.; Dorkov, P. First divalent metal complexes of the pol-yether ionophore monensin A: X-ray structures of [Co(Mon)2(H2O)2] and [Mn(Mon)2(H2O)2] and their properties. Curr. Drug Discov. Technol. 2008, 5, 154–161. [Google Scholar] [CrossRef]
- Pantcheva, I.N.; Zhorova, R.; Mitewa, M.; Simova, S.; Mayer-Figge, H.; Sheldrick, W.S. First solid state alkaline-earth complex-es of monensic A acid: X-ray crystal structure of [M(Mon)2(H2O)2] (M = Mg, Ca), spectral properties and cytotoxicity against Gram-positive bacteria. BioMetals 2010, 23, 59–70. [Google Scholar] [CrossRef] [PubMed]
- Pantcheva, I.N.; Ivanova, J.; Zhorova, R.; Mitewa, M.; Simova, S.; Mayer-Figge, H.; Sheldrick, W.S. Nickel(II) and zinc(II) dim-onensinates: Crystal structure, spectral properties and bactericidal activity. Inorg. Chim. Acta 2010, 363, 1879–1886. [Google Scholar] [CrossRef]
- Pantcheva, I.; Stamboliyska, R.; Nedzhib, A.; Dorkov, P. Spectral properties of copper(II) bis-monensinate. C. R. Acad. Bulg. Sci. 2022, 75, 519–526. [Google Scholar] [CrossRef]
- Hamidinia, S.A.; Shimelis, O.I.; Tan, B.; Erdahl, W.L.; Chapman, C.J.; Renkes, G.D.; Taylor, R.W.; Pfeiffer, D.R. Monensin mediates a rapid and selective transport of Pb(2+). Possible application of monensin for the treatment of Pb(2+) intoxication. J. Biol. Chem. 2002, 277, 38111–38120. [Google Scholar] [CrossRef] [Green Version]
- Hamidinia, S.A.; Tan, B.; Erdahl, W.L.; Chapman, C.J.; Taylor, R.W.; Pfeiffer, D.R. The ionophore nigericin transports Pb2+ with high activity and selectivity: A comparison to monensin and ionomycin. Biochemistry 2004, 43, 15956–15965. [Google Scholar] [CrossRef] [PubMed]
- Cox, B.G.; van Truong, N.; Rzeszotarska, J.; Schneider, H. Stability constants of complexes of monensin and lasalocid with alkali-metal and alkaline-earth-metal ions in protic and polar aprotic solvents. J. Chem. Soc. Faraday Trans. 1984, 80, 3275–3284. [Google Scholar] [CrossRef]
- Newbold, C.J.; Wallace, R.J.; Walker-Bax, N.D. Potentiation by metal ions of the efficacy of the ionophores, monensin and tetronasin, towards four species of ruminal bacteria. FEMS Microbiol. Lett. 2013, 338, 161–167. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Elsasser, T.H. Potential interactions of ionophore drugs with divalent cations and their function in the animal body. J. Anim. Sci. 1984, 59, 845–853. [Google Scholar] [CrossRef] [PubMed]
- Dubreuil, P.; Sauvageau, R. Intoxication chronique au cuivre chez des agneaux lourds par l’eau d’abreuvement. Can. Vet. J. 1993, 34, 428430. [Google Scholar]
- Nedzhib, A.; Kessler, J.; Bour, P.; Gyurcsik, B.; Pantcheva, I. Circular dichroism is sensitive to monovalent cation binding in monensin complexes. Chirality 2016, 28, 420–428. [Google Scholar] [CrossRef] [Green Version]
- Pantcheva, I.; Nedzhib, A.; Antonov, L.; Gyurcsik, B.; Dorkov, P. New insights into coordination chemistry of monensin to-wards divalent metal ions. Inorg. Chim. Acta 2020, 505, 119481. [Google Scholar] [CrossRef]
- Zékány, L.; Nagypál, I. PSEQUAD, A comprehensive program for the evaluation of potentiometric and/or spectrophotometric equilibrium data using analytical derivatives. In Computational Methods for the Determination of Formation Constants; Leggett, D.J., Ed.; Springer: New York, NY, USA, 1985; pp. 291–353. [Google Scholar] [CrossRef]
- Martinek, T.; Riddell, F.G.; Wilson, C.; Weller, C.T. The conformations of monensin-A metal complexes in solution determined by NMR spectroscopy. J. Chem. Soc. Perkin Trans. 2000, 2, 35–41. [Google Scholar] [CrossRef]
- Peintler, G.; Nagypál, I.; Jancsó, A.; Epstein, I.R.; Kustin, K. Extracting experimental information from large matrixes. 1. A new algorithm for the application of matrix rank analysis. J. Phys. Chem. A 1997, 101, 8013–8020. [Google Scholar] [CrossRef]
- Pinkerton, M.; Steinrauf, L.K. Molecular structure of monovalent metal cation complexes of monensin. J. Mol. Biol. 1970, 49, 533–546. [Google Scholar] [CrossRef] [PubMed]



| logβ’ | [Co(Mon)(H₂O)]⁺ | [Co(Mon)₂(H₂O)₂] | Ref |
|---|---|---|---|
| UV-range | 5.9 ± 0.5 | 10.2 ± 0.8 | [58] |
| VIS-range | 5.74 ± 0.02 | 9.93 ± 0.04 | This work |
| Complex Species | Zn(II) | Mg(II) | Ca(II) |
|---|---|---|---|
| [M(Mon)(H₂O)]⁺ | 6.09 ± 0.02 | 4.81 ± 0.01 | 5.18 ± 0.01 |
| [M(Mon)₂(H₂O)₂] | 10.12 ± 0.02 | 8.36 ± 0.01 | 8.40 ± 0.01 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kis, M.L.; Hajdu, B.; Dorkov, P.; Pantcheva, I.; Gyurcsik, B. Circular Dichroism Spectroscopic Studies on Solution Chemistry of M(II)-Monensinates in Their Competition Reactions. Inorganics 2023, 11, 334. https://doi.org/10.3390/inorganics11080334
Kis ML, Hajdu B, Dorkov P, Pantcheva I, Gyurcsik B. Circular Dichroism Spectroscopic Studies on Solution Chemistry of M(II)-Monensinates in Their Competition Reactions. Inorganics. 2023; 11(8):334. https://doi.org/10.3390/inorganics11080334
Chicago/Turabian StyleKis, Máté Levente, Bálint Hajdu, Petar Dorkov, Ivayla Pantcheva, and Béla Gyurcsik. 2023. "Circular Dichroism Spectroscopic Studies on Solution Chemistry of M(II)-Monensinates in Their Competition Reactions" Inorganics 11, no. 8: 334. https://doi.org/10.3390/inorganics11080334






