Luminescent Cadmium(II)-Based 12-MC-4 Metallacrown Complex with 2-Methylmercaptobenzohydroxamic Acid Ligand
Abstract
:1. Introduction
2. Results and Discussion
2.1. Synthesis
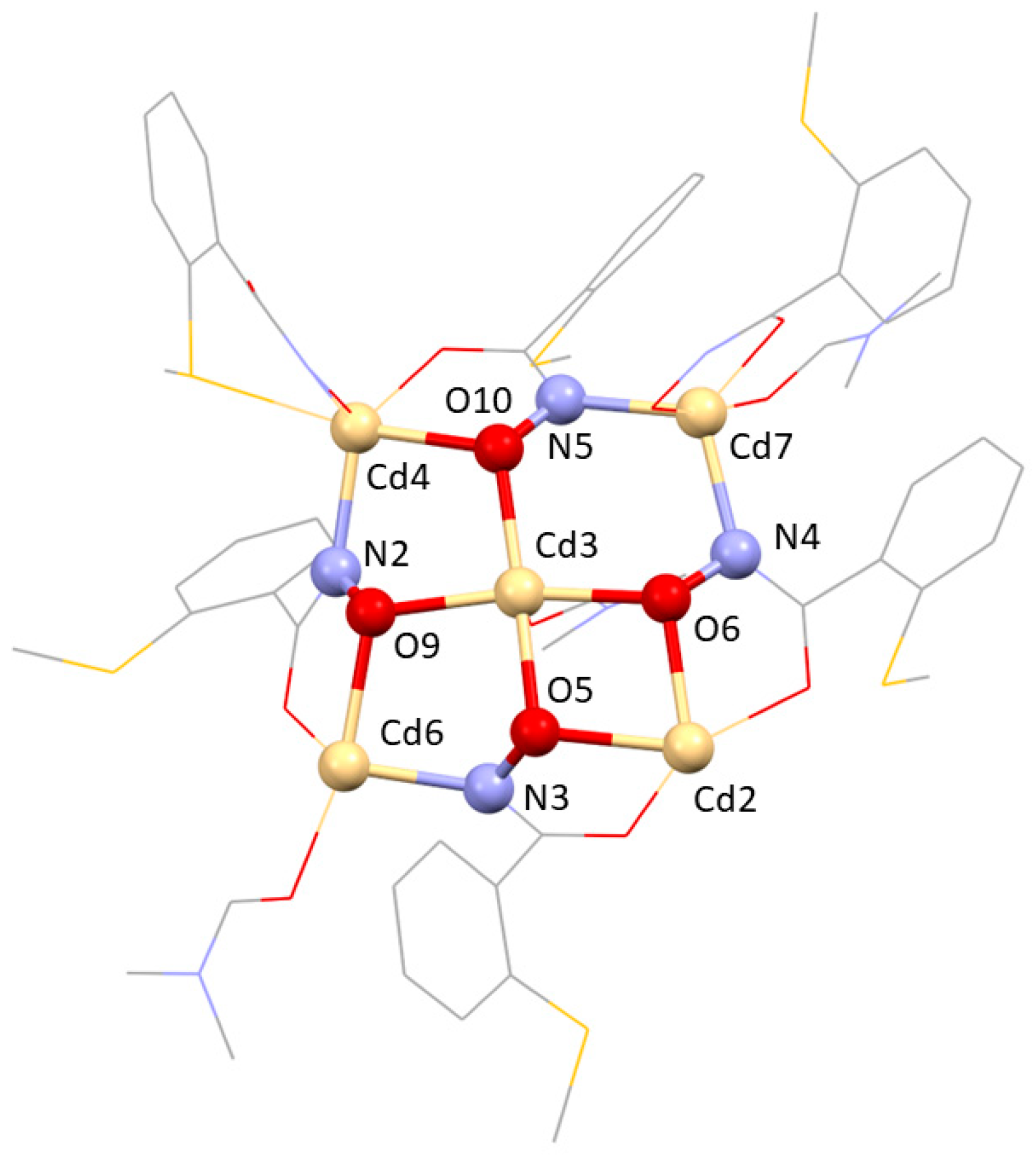
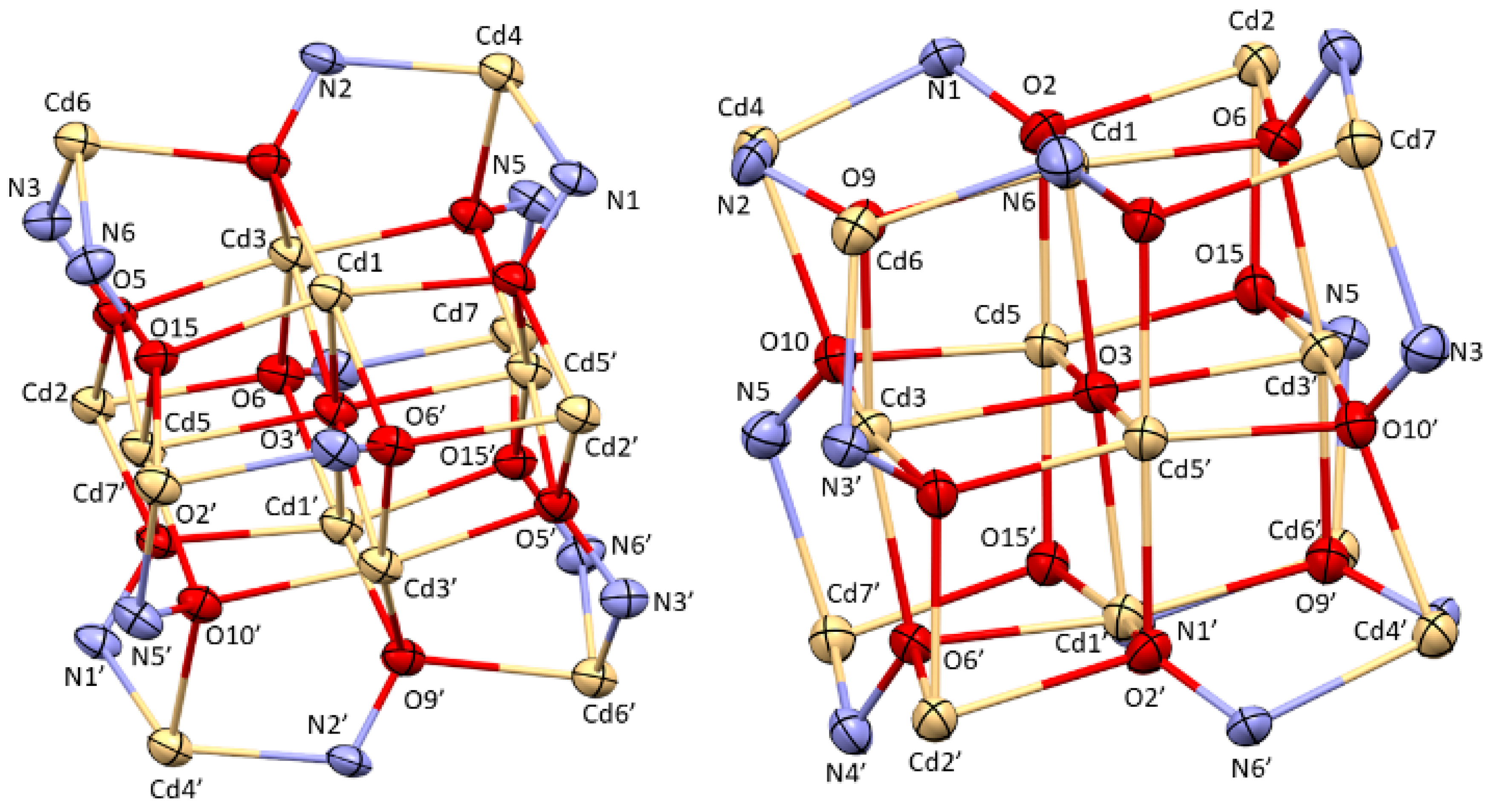
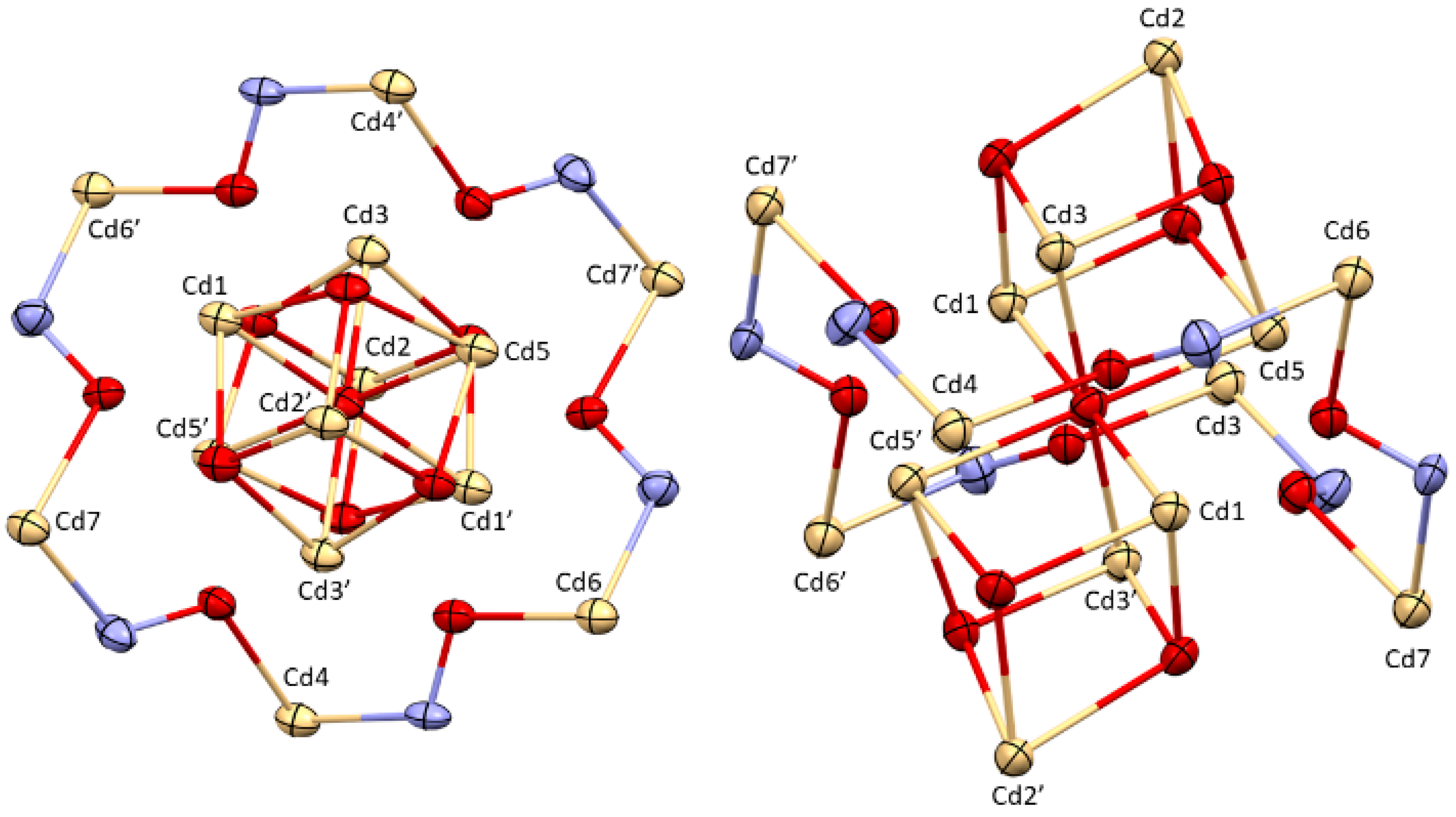
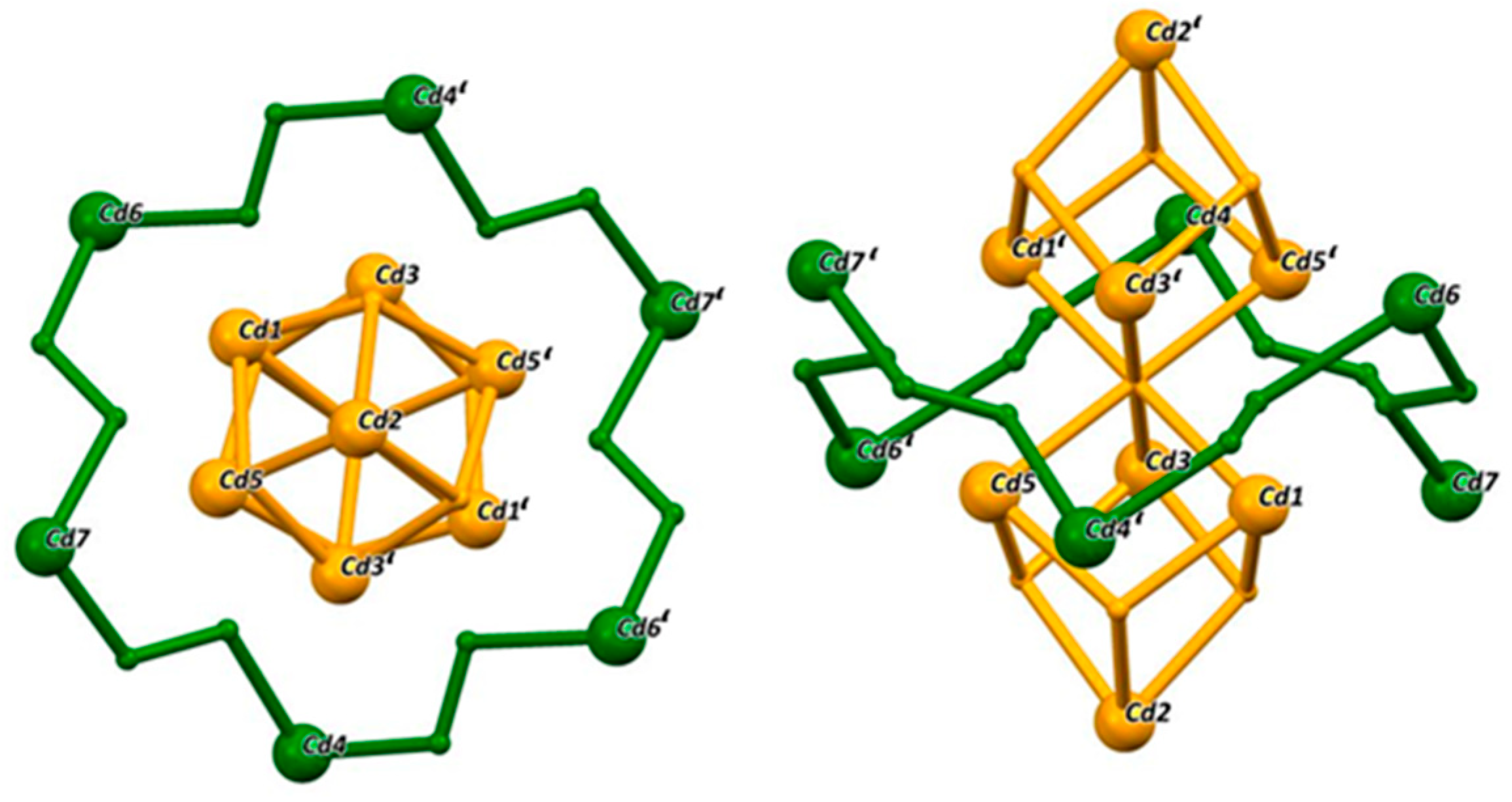
2.2. Crystallographic Discussion
2.3. Infrared Spectroscopy
2.4. Luminescence
3. Materials and Methods
Complex Synthesis
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hardy, J.G. Metallosupramolecular Grid Complexes: Towards Nanostructured Materials with High-Tech Applications. Chem. Soc. Rev. 2013, 42, 7881. [Google Scholar] [CrossRef]
- Leininger, S.; Olenyuk, B.; Stang, P.J. Self-Assembly of Discrete Cyclic Nanostructures Mediated by Transition Metals. Chem. Rev. 2000, 100, 853–908. [Google Scholar] [CrossRef]
- Lehn, J.-M. Supramolecular Chemistry—Scope and Perspectives Molecules, Supermolecules, and Molecular Devices (Nobel Lecture). Angew. Chem. Int. Ed. Engl. 1988, 27, 89–112. [Google Scholar] [CrossRef]
- Smulders, M.M.J.; Riddell, I.A.; Nitschke, J.R. Building on Architectural Principles for Three-Dimensional Metallosupramolecular Construction. Chem. Soc. Rev. 2013, 42, 1728. [Google Scholar] [CrossRef] [PubMed]
- Lyu, Q.; Li, M.; Zhang, L.; Zhu, J. Bioinspired Supramolecular Photonic Composites: Construction and Emerging Applications. Macromol. Rapid Commun. 2022, 43, 2100867. [Google Scholar] [CrossRef] [PubMed]
- Chan, M.H.Y.; Yam, V.W.W. Toward the Design and Construction of Supramolecular Functional Molecular Materials Based on Metal-Metal Interactions. J. Am. Chem. Soc. 2022, 144, 22805–22825. [Google Scholar] [CrossRef] [PubMed]
- Schnitzer, T.; Preuss, M.D.; van Basten, J.; Schoenmakers, S.M.C.; Spiering, A.J.H.; Vantomme, G.; Meijer, E.W. How Subtle Changes Can Make a Difference: Reproducibility in Complex Supramolecular Systems. Angew. Chem. 2022, 134, e202206738. [Google Scholar] [CrossRef]
- Constable, E.C. Stereogenic Metal Centres-from Werner to Supramolecular Chemistry. Chem. Soc. Rev. 2013, 42, 1637. [Google Scholar] [CrossRef]
- Constable, E.C. Expanded Ligands-An Assembly Principle for Supramolecular Chemistry. Coord. Chem. Rev. 2008, 252, 842–855. [Google Scholar] [CrossRef]
- Lehn, J.M. Perspectives in Chemistry—Steps towards Complex Matter. Angew. Chem.—Int. Ed. 2013, 52, 2836–2850. [Google Scholar] [CrossRef]
- Lehn, J.M. Perspectives in Chemistry—Aspects of Adaptive Chemistry and Materials. Angew. Chem.—Int. Ed. 2015, 54, 3276–3289. [Google Scholar] [CrossRef]
- Mezei, G.; Zaleski, C.M.; Pecoraro, V.L. Structural and Functional Evolution of Metallacrowns. Chem. Rev. 2007, 107, 4933–5003. [Google Scholar] [CrossRef] [PubMed]
- Bodwin, J.J.; Cutland, A.D.; Malkani, R.G.; Pecoraro, V.L. The Development of Chiral Metallacrowns into Anion Recognition Agents and Porous Materials. Coord. Chem. Rev. 2001, 216–217, 489–512. [Google Scholar] [CrossRef]
- Happ, P.; Plenk, C.; Rentschler, E. 12-MC-4 Metallacrowns as Versatile Tools for SMM Research. Coord. Chem. Rev. 2015, 289–290, 238–260. [Google Scholar] [CrossRef]
- Lah, M.S.; Pecoraro, V.L. Isolation and Characterization of {mnii[Mniii(Salicylhydroximate)]4(Acetate)2(Dmf)6}. 2dmf: An Inorganic Analogue of M2+(12-Crown-4). J. Am. Chem. Soc. 1989, 111, 7258–7259. [Google Scholar] [CrossRef]
- Lüpke, A.; Carrella, L.M.; Rentschler, E. Filling the Gap in the Metallacrown Family: The 9-MC-3 Chromium Metallacrown. Chem.—Eur. J. 2021, 27, 4283–4286. [Google Scholar] [CrossRef]
- Athanasopoulou, A.A.; Baldoví, J.J.; Carrella, L.M.; Rentschler, E. Field-Induced Slow Magnetic Relaxation in the First Dy(III)-Centered 12-Metallacrown-4 Double-Decker. Dalton Trans. 2019, 48, 15381–15385. [Google Scholar] [CrossRef]
- Xue, J.; Yang, F.; Jin, J.; Li, Y.; Wu, D.; Yang, G.-P.; Wang, Y.-Y. Design and Synthesis of Four Newly Water-Stable Pb-Based Heterometallic Organic Frameworks: How Do the Second Metals (Zn, Cd, Co, and Mn) Optimize Their Fluorescent and Catalytic Properties? Cryst. Growth Des. 2022, 14, 33. [Google Scholar] [CrossRef]
- Lee, Y.H.; Kubota, E.; Fuyuhiro, A.; Kawata, S.; Harrowfield, J.M.; Kim, Y.; Hayami, S. Synthesis, structure and luminescence properties of Cu(II), Zn(II) and Cd(II) complexes with 4′-tetraphenylterpyridine. Dalton Trans. 2012, 41, 10825–10831. [Google Scholar] [CrossRef]
- Werlin, R.; Priester, J.H.; Mielke, R.E.; Krämer, S.; Jackson, S.; Stoimenov, P.K.; Stucky, G.D.; Cherr, G.N.; Orias, E.; Holden, P.A. Biomagnification of Cadmium Selenide Quantum Dots in a Simple Experimental Microbial Food Chain. Nat. Nanotechnol. 2011, 6, 65–71. [Google Scholar] [CrossRef]
- Oh, E.; Liu, R.; Nel, A.; Gemill, K.B.; Bilal, M.; Cohen, Y.; Medintz, I.L. Meta-Analysis of Cellular Toxicity for Cadmium-Containing Quantum Dots. Nat. Nanotechnol. 2016, 11, 479–486. [Google Scholar] [CrossRef] [PubMed]
- Santra, P.K.; Kamat, P.V. Tandem-Layered Quantum Dot Solar Cells: Tuning the Photovoltaic Response with Luminescent Ternary Cadmium Chalcogenides The Influence of the Se:S Ratio in Tuning. J. Am. Chem. Soc. 2013, 135, 32. [Google Scholar] [CrossRef]
- Chu, T.L.; Chu, S.S. Recent Progress in Thin-Film Cadmium Telluride Solar Cells. Prog. Photovolt. Res. Appl. 1993, 1, 31–42. [Google Scholar] [CrossRef]
- Bati, A.S.R.; Lin Zhong, Y.; Burn, P.L.; Khaja Nazeeruddin, M.; Shaw, P.E.; Batmunkh, M. Next-Generation Applications for Integrated Perovskite Solar Cells. Commun. Mater. 2023, 4, 2. [Google Scholar] [CrossRef]
- Gamer, C.; Sundaresan, S.; Carrella, L.M.; Rentschler, E. Single- vs Double-Decker Copper 12-MC-4 Metallacrown Using the Coordination Flexibility of a Soft Donor Ligand. Eur. J. Inorg. Chem. 2022, 2022, e202200261. [Google Scholar] [CrossRef]
- Li, Q.-W.; Wan, R.-C.; Chen, Y.-C.; Liu, J.-L.; Wang, L.-F.; Jia, J.-H.; Chilton, N.F.; Tong, M.-L. Unprecedented Hexagonal Bipyramidal Single-Ion Magnets Based on Metallacrowns. Chem. Commun. 2016, 52, 13365–13368. [Google Scholar] [CrossRef] [PubMed]
- Sundaresan, S.; Becker, J.-G.; Eppelsheimer, J.; Sedykh, A.E.; Carrella, L.; Müller-Buschbaum, K.; Rentschler, E. Synergetic Spin Singlet-Quintet Switching and Luminescence in Mononuclear Fe(II) 1,3,4-Oxadiazole Tetradentate Chelates with NCBH3 Co-Ligand. Dalton Trans. 2023; accepted manuscript. [Google Scholar] [CrossRef]
- Sundaresan, S.; Kiehl, J.; Carrella, L.M.; Rentschler, E. Effect of Methoxy Ligand-Substituent and NCE Coligands in Tuning T 1/2 of Coordinatively Elastic Fe(II) Spin Crossover Complexes. Cryst. Growth Des. 2023, 23, 1648–1655. [Google Scholar] [CrossRef]
- Zhong, X.; Hu, Z.-B.; Luo, T.-K.; Peng, Y.; Liu, S.-J.; Anson, C.E.; Powell, A.K.; Wen, H.-R. Nine-Coordinate Ln III-Based One-Dimensional Complexes: Synthesis and Magnetic Properties. Cryst. Growth Des. 2023, 11, 43. [Google Scholar] [CrossRef]
- Sundaresan, S.; Diehl, M.; Carrella, L.M.; Rentschler, E. Triggering of Valence Tautomeric Transitions in Dioxolene-Based Cobalt Complexes Influenced by Ligand Substituents, Co-Ligands, and Anions. Magnetochemistry 2022, 8, 109. [Google Scholar] [CrossRef]
- Harris, M.M.; Kühne, I.A.; Kelly, C.T.; Jakobsen, V.B.; Jordan, R.; O’Brien, L.; Müller-Bunz, H.; Felton, S.; Morgan, G.G. Compressed and Expanded Lattices—Barriers to Spin-State Switching in Mn3+ Complexes. Cryst. Growth Des. 2023, 23, 3996–4012. [Google Scholar] [CrossRef]
- Song, Y.; Liu, J.C.; Liu, Y.J.; Zhu, D.R.; Zhuang, J.Z.; You, X.Z. Preparation, Crystal Structures and Magnetic Properties of 12-Metallacrown-4 Complexes with the Donors on the Organic Periphery of Molecule. Inorganica Chim. Acta 2000, 305, 135–142. [Google Scholar] [CrossRef]
- Sheldrick, G.M. Foundations and Advances SHELXT-Integrated Space-Group and Crystal-Structure Determination. Acta Cryst. 2015, 71, 3–8. [Google Scholar] [CrossRef]
- Sheldrick, G.M. Crystal Structure Refinement with SHELXL. Acta Cryst. 2015, 71, 3–8. [Google Scholar] [CrossRef]
- Sheldrick, G.M.; Schneider, T.R. [16] SHELXL: High-Resolution Refinement. Methods Enzymol. 1997, 277, 319–343. [Google Scholar] [CrossRef]
- Dolomanov, O.V.; Bourhis, L.J.; Gildea, R.J.; Howard, J.A.K.; Puschmann, H. OLEX2: A Complete Structure Solution, Refinement and Analysis Program. J. Appl. Cryst. 2009, 42, 339–341. [Google Scholar] [CrossRef]


| Compound | [CdII14(LmmbHA)12(µ6−O)(DMF)10](ClO4)2·3H2O |
|---|---|
| Empirical formula | C144H196Cd14Cl2N28O49S12 |
| Formula weight/g mol−1 | 5132.50 |
| Crystal size/mm | 0.14 × 0.097 × 0.02 |
| Crystal system | monoclinic |
| Space group | P21/c |
| CCDC No. | 2239578 |
| Unit cell dimensions | |
| a/Å | 18.1706(5) |
| b/Å | 17.2534(3) |
| c/Å | 30.5506(7) |
| α/ | 90 |
| β/ | 105.688(2) |
| γ/ | 90 |
| Volume/Å3 | 9221.0(4) |
| Z | 2 |
| ρcalc./g·cm−1 | 1.849 |
| µ/mm−1 | 1.826 |
| F(000) | 5092 |
| Temperature/K | 193(2) |
| Diffractometer | STOE IPDS 2T |
| Radiation | MoK α |
| θ-range for data collection/ | 1.658 < θ < 25.999 |
| Index ranges | |
| −22 < h < 21 | |
| −21 < k < 21 | |
| −37 < l < 37 | |
| Collected reflections | 38914 |
| Independent reflections | 18092 |
| Completeness | 0.998 |
| Max. and min. transmission | 0.9472 and 0.7877 |
| Rint | 0.0328 |
| Rsigma | 0.0455 |
| Data/restraints/parameters | 18092/116/1230 |
| Goodness-of-fit on F2 | 1.115 |
| Final R1 [I ≥ 2σ(I)] | 0.051 |
| Final wR2 [I ≥ 2σ(I)] | 0.0901 |
| Final R1 [alldata] | 0.0844 |
| Final wR2 [alldata] | 0.1044 |
| Ion | Cd1 | Cd2 | Cd3 | Cd4 | Cd5 | Cd6 | Cd7 |
|---|---|---|---|---|---|---|---|
| Structure | OC-6 | TPR-6 | OC-6 | TBPY-5 | OC-6 | TBPY-5 | TBPY-5 |
| CShM | 0.73 | 3.54 | 0.63 | 2.66 | 0.67 | 1.91 | 1.43 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sundaresan, S.; Gamer, C.; Förster, M.J.; Carrella, L.M.; Rentschler, E. Luminescent Cadmium(II)-Based 12-MC-4 Metallacrown Complex with 2-Methylmercaptobenzohydroxamic Acid Ligand. Inorganics 2023, 11, 362. https://doi.org/10.3390/inorganics11090362
Sundaresan S, Gamer C, Förster MJ, Carrella LM, Rentschler E. Luminescent Cadmium(II)-Based 12-MC-4 Metallacrown Complex with 2-Methylmercaptobenzohydroxamic Acid Ligand. Inorganics. 2023; 11(9):362. https://doi.org/10.3390/inorganics11090362
Chicago/Turabian StyleSundaresan, Sriram, Christoph Gamer, Mortiz J. Förster, Luca M. Carrella, and Eva Rentschler. 2023. "Luminescent Cadmium(II)-Based 12-MC-4 Metallacrown Complex with 2-Methylmercaptobenzohydroxamic Acid Ligand" Inorganics 11, no. 9: 362. https://doi.org/10.3390/inorganics11090362







