Effects of Coloration of Spinel CoAl2O4 Cobalt Blue Pigments: Composition, Structure, and Cation Distribution
Abstract
:1. Introduction
2. Results and Discussion
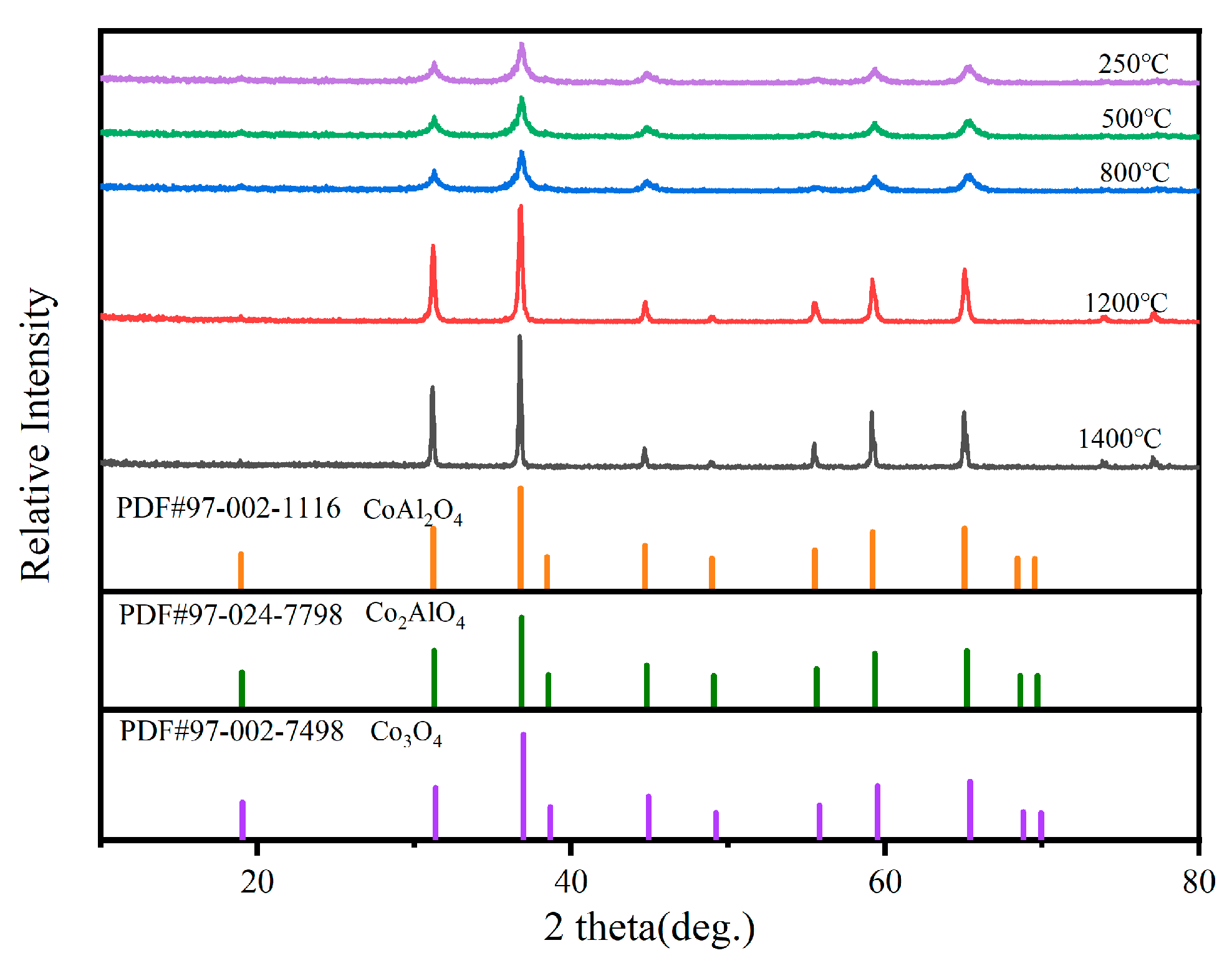
2.1. Crystal Phases after Heat Treatment at Different Temperatures
2.2. Influence Factors of Structural Properties
2.2.1. Crystallite Size
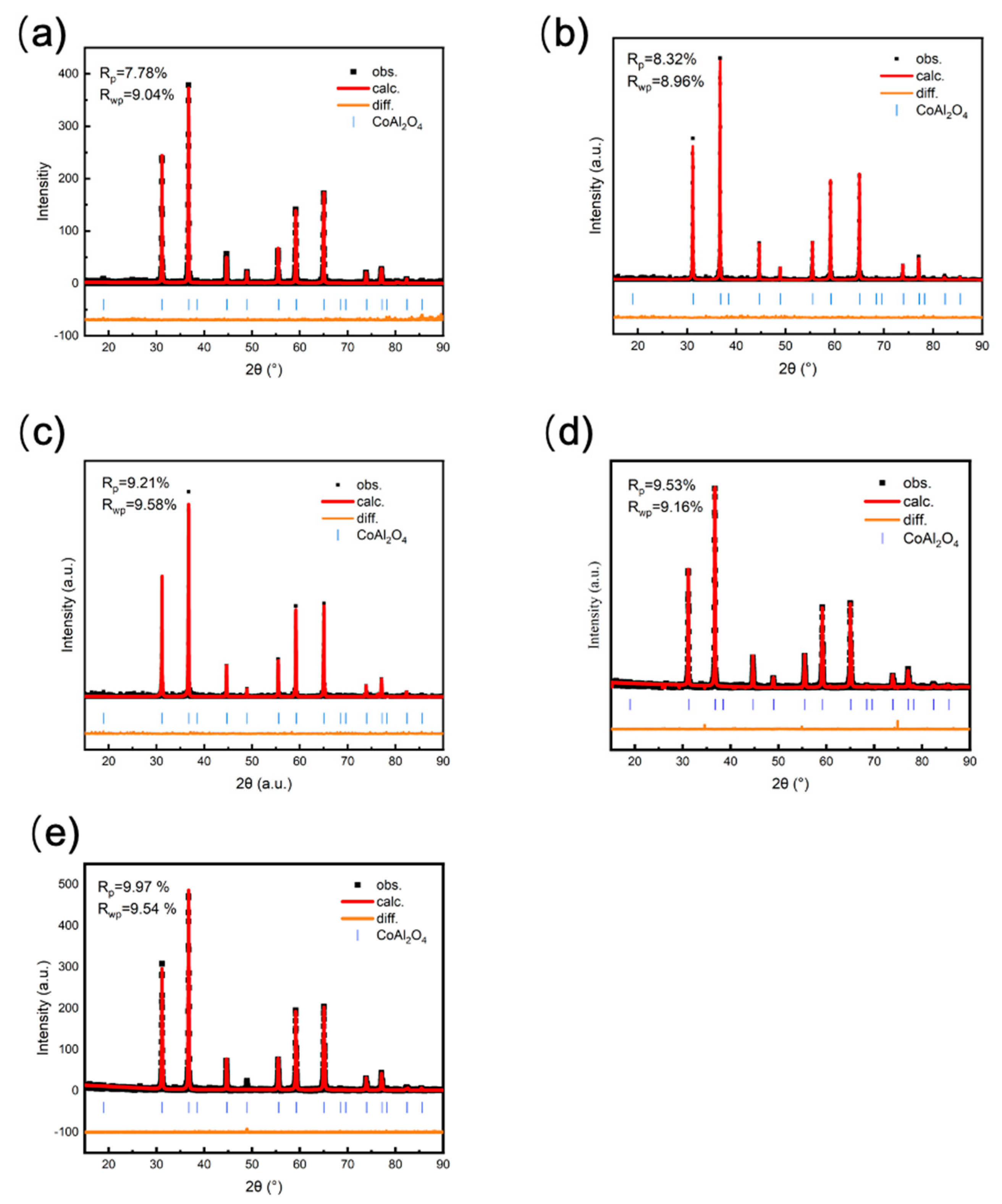
2.2.2. Cation Distribution
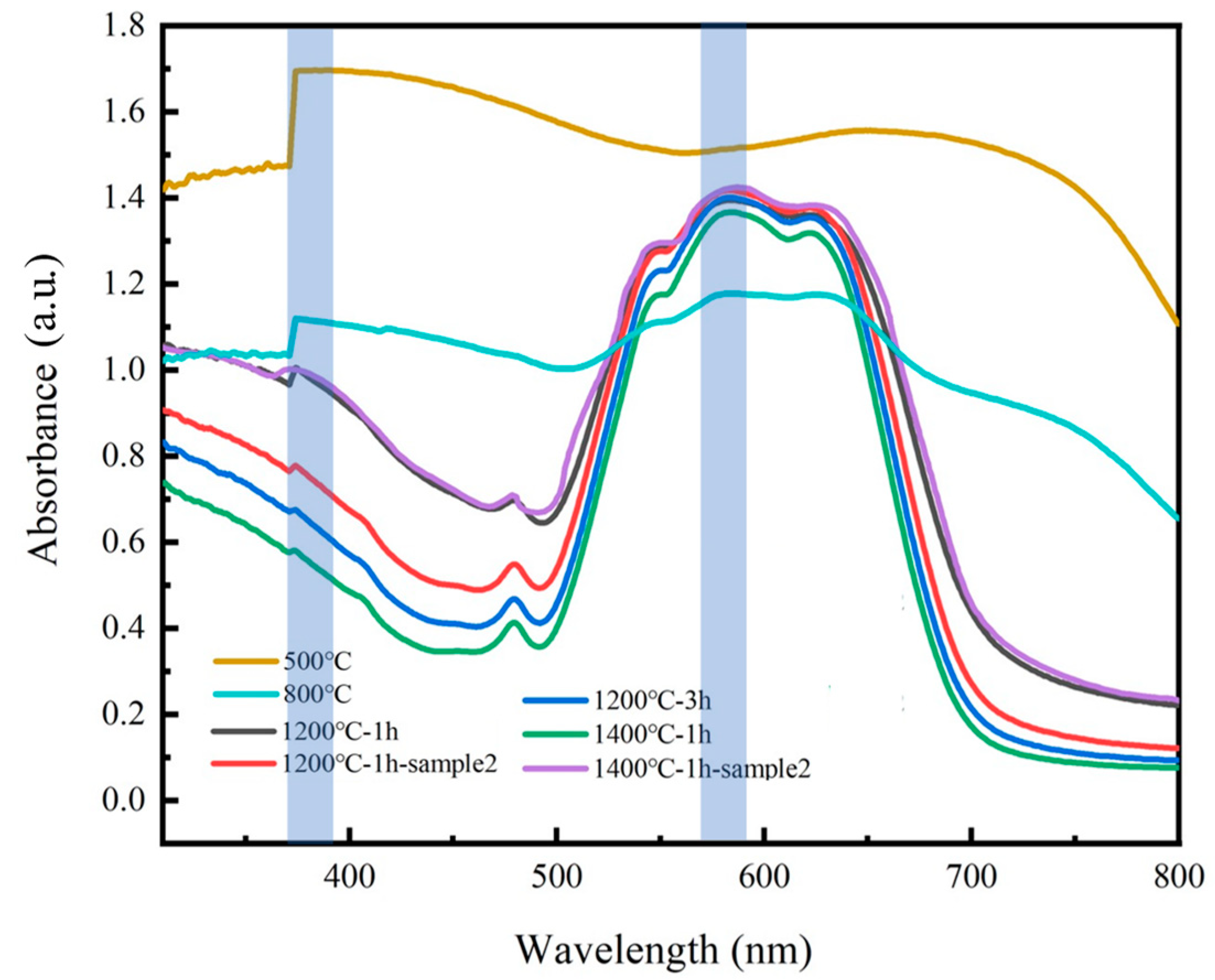
2.3. Color Analysis
3. Experimental
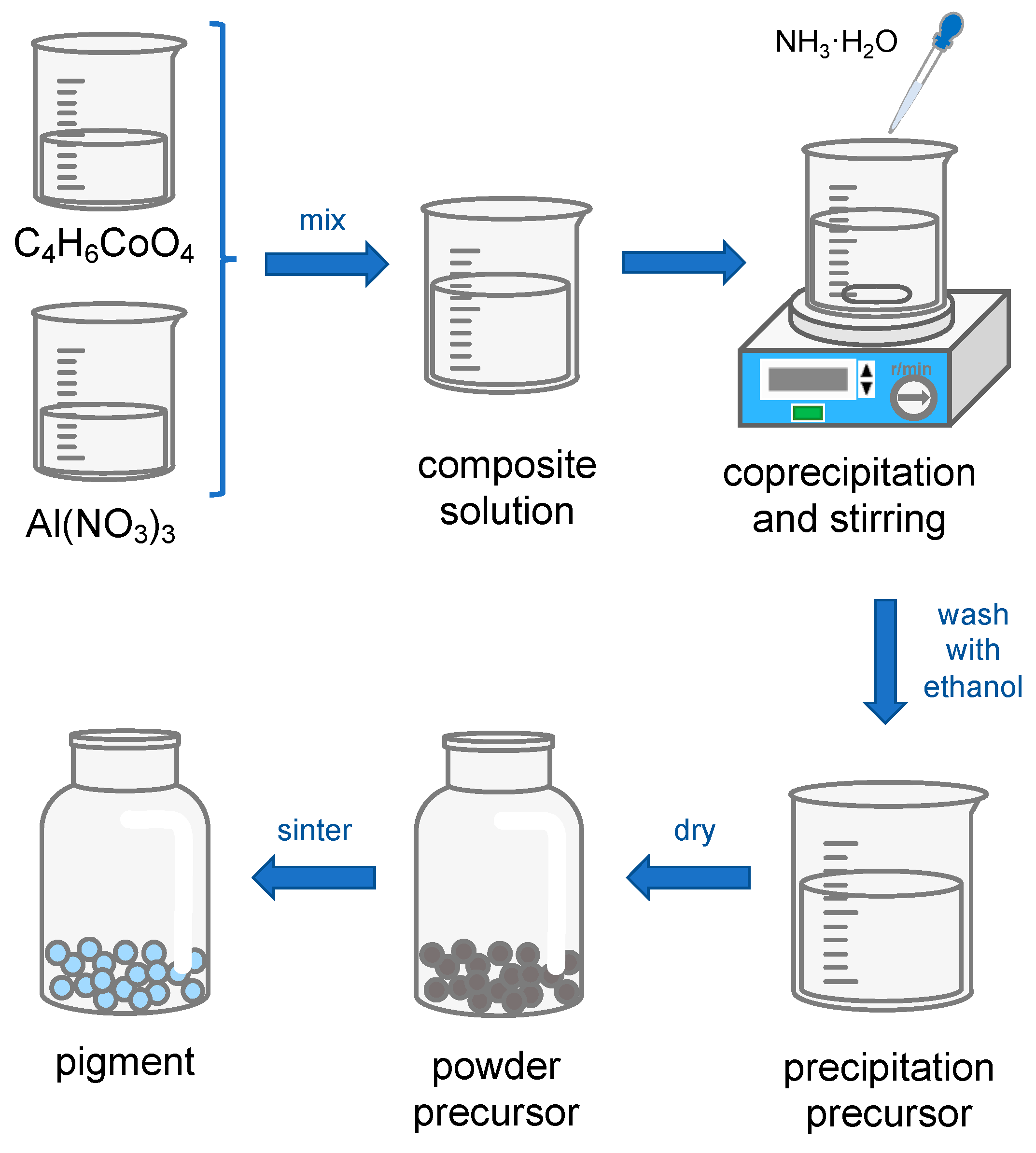
3.1. Synthesis Process
3.2. Characterization
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Bernardi, M.; Feitosa, C.; Paskocimas, C.; Longo, E.; Paiva-Santos, C. Development of metal oxide nanoparticles by soft chemical method. Ceram. Int. 2009, 35, 463–466. [Google Scholar] [CrossRef]
- Akdemir, S.; Ozel, E.; Suvaci, E. Solubility of blue CoAl2O4 ceramic pigments in water and diethylene glycol media. Ceram. Int. 2011, 37, 863–870. [Google Scholar] [CrossRef]
- Merino, M.C.G.; Estrella, A.L.; Rodriguez, M.E.; Acuña, L.; Lassa, M.S.; Lascalea, G.E.; Vázquez, P. Combustion Syntheses of CoAl2O4 Powders Using Different Fuels. Procedia Mater. Sci. 2015, 8, 519–525. [Google Scholar] [CrossRef]
- Wu, T.; Sun, S.; Song, J.; Xi, S.; Du, Y.; Chen, B.; Sasangka, W.A.; Liao, H.; Gan, C.L.; Scherer, G.G.; et al. Iron-facilitated dynamic active-site generation on spinel CoAl2O4 with self-termination of surface reconstruction for water oxidation. Nat. Catal. 2019, 2, 763–772. [Google Scholar] [CrossRef]
- Angeletti, C.; Pepe, F.; Porta, P. Structure and catalytic activity of CoχMg1–χAl2O4 spinel solid solutions. Part 1.—Cation distribution of Co2+ ions. J. Chem. Soc. Faraday Trans. 1 Phys. Chem. Condens. Phases 1977, 73, 1972–1982. [Google Scholar] [CrossRef]
- Llusar, M.; Forés, A.; Badenes, J.; Calbo, J.; Tena, M.; Monrós, G. Colour analysis of some cobalt-based blue pigments. J. Eur. Ceram. Soc. 2001, 21, 1121–1130. [Google Scholar] [CrossRef]
- Monari, G.; Manfredini, T. Coloring Effects of Synthetic Inorganic Cobalt Pigments in Fast-Fired Porcelainized Tiles. In A Collection of Papers Presented at the 97th Annual Meeting and the 1995 Fall Meetings of the Materials & Equipment/Whitewares: Ceramic Engineering and Science Proceedings; Wachtman, J.B., Jr., Ed.; The American Ceramic Society: Columbus, OH, USA, 1996; Volume 17, pp. 167–172. [Google Scholar] [CrossRef]
- Tatarchuk, T.; Bououdina, M.; Paliychuk, N.; Yaremiy, I.; Moklyak, V. Structural characterization and antistructure modeling of cobalt-substituted zinc ferrites. J. Alloys Compd. 2017, 694, 777–791. [Google Scholar] [CrossRef]
- Agilandeswari, K.; Kumar, A.R. Synthesis, characterisation, optical and luminescence properties of CoAl2O4. AIP Conf. Proc. 2015, 1665, 120022. [Google Scholar] [CrossRef]
- Hudson, K.; Winbow, H.; Cowley, J. Colors for Ceramic Bodies. In A Collection of Papers Presented at the 97th Annual Meeting and the 1995 Fall Meetings of the Materials & Equipment/Whitewares: Ceramic Engineering and Science Proceedings; Wachtman, J.B., Jr., Ed.; The American Ceramic Society: Columbus, OH, USA, 1996; Volume 17, pp. 102–110. [Google Scholar] [CrossRef]
- Li, W.; Li, J.; Guo, J. Synthesis and characterization of nanocrystalline CoAl2O4 spinel powder by low temperature combustion. J. Eur. Ceram. Soc. 2003, 23, 2289–2295. [Google Scholar] [CrossRef]
- Cho, W.-S.; Kakihana, M. Crystallization of ceramic pigment CoAl2O4 nanocrystals from Co–Al metal organic precursor. J. Alloys Compd. 1999, 287, 87–90. [Google Scholar] [CrossRef]
- Pozas, R.; Orera, V.; Ocaña, M. Hydrothermal synthesis of Co-doped willemite powders with controlled particle size and shape. J. Eur. Ceram. Soc. 2005, 25, 3165–3172. [Google Scholar] [CrossRef]
- Hu, Z.; Xue, M.; Zhang, Q.; Sheng, Q.; Liu, Y. Nanocolorants: A novel class of colorants, the preparation and performance characterization. Dye. Pigment. 2008, 76, 173–178. [Google Scholar] [CrossRef]
- Zhang, W.; Li, J.; Zhong, F.; Wu, G.; Jiang, H.; Zhang, W.; Liu, Q. Preparation and characterization of supercritical fluid–fried (CoAl2O4) cobalt blue nano-pigment. J. Asian Ceram. Soc. 2021, 10, 33–39. [Google Scholar] [CrossRef]
- Yuan, S.; Yuanlin, Z.; Yufei, T.; Yangfeng, J. Fabrication and Coloration Mechanism of CoAl2O4 Ceramic Pigment by Co-precipitation Method. J. Synth. Cryst. 2017, 46, 79–84. [Google Scholar]
- Zhang, A.; Mu, B.; Luo, Z.; Wang, A. Bright blue halloysite/CoAl2O4 hybrid pigments: Preparation, characterization and application in water-based painting. Dye. Pigment. 2017, 139, 473–481. [Google Scholar] [CrossRef]
- Ahmed, I.; Shama, S.; Moustafa, M.; Dessouki, H.; Ali, A. Synthesis and spectral characterization of CoxMg1−xAl2O4 as new nano-coloring agent of ceramic pigment. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2009, 74, 665–672. [Google Scholar] [CrossRef] [PubMed]
- Xin, L.; Jiake, L.; Kai, C. Preparation of Spinel Type ZnxCo1-xAl2O4 Ultrafine Ceramic Pigment by Solution Combustion Method. J. Synth. Cryst. 2014, 43, 2086–2090. [Google Scholar]
- Gilabert, J.; Palacios; Sanz, V.; Mestre, S. Characteristics reproducibility of (Fe, Co)(Cr, Al)2O4 pigments obtained by solution combustion synthesis. Ceram. Int. 2016, 42, 12880–12887. [Google Scholar] [CrossRef]
- Chemlal, S.; Larbot, A.; Persin, M.; Sarrazin, J.; Sghyar, M.; Rafiq, M. Cobalt spinel CoAl2O4 via sol-gel process: Elaboration and surface properties. Mater. Res. Bull. 2000, 35, 2515–2523. [Google Scholar] [CrossRef]
- Valenzuela, M.; Bosch, P.; Aguilar-Rios, G.; Montoya, A.; Schifter, I. Comparison Between Sol-Gel, Coprecipitation and Wet Mixing Synthesis of ZnAl2O. J. Sol-Gel Sci. Technol. 1997, 8, 107–110. [Google Scholar] [CrossRef]
- Gaudon, M.; Robertson, L.; Lataste, E.; Duttine, M.; Ménétrier, M.; Demourgues, A. Cobalt and nickel aluminate spinels: Blue and cyan pigments. Ceram. Int. 2014, 40, 5201–5207. [Google Scholar] [CrossRef]
- Fernández-Osorio, A.; Pineda-Villanueva, E.; Chávez-Fernández, J. Synthesis of nanosized (Zn1−Co )Al2O4 spinels: New pink ceramic pigments. Mater. Res. Bull. 2012, 47, 445–452. [Google Scholar] [CrossRef]
- Tsurumi, T.; Hirayama, H.; Vacha, M.; Taniyama, T. Nanoscale Physics for Materials Science; CRC Press: Boca Raton, FL, USA, 2009. [Google Scholar] [CrossRef]
- Zawadzki, M.; Wrzyszcz, J. Hydrothermal synthesis of nanoporous zinc aluminate with high surface area. Mater. Res. Bull. 2000, 35, 109–114. [Google Scholar] [CrossRef]
- Zhang, A.; Mu, B.; Hui, A.; Wang, A. A facile approach to fabricate bright blue heat-resisting paint with self-cleaning ability based on CoAl2O4/kaolin hybrid pigment. Appl. Clay Sci. 2018, 160, 153–161. [Google Scholar] [CrossRef]
- Angeletti, C.; Pepe, F.; Porta, P. Structure and catalytic activity of CoxMg1–xAl2O4 spinel solid solutions. Part 2.—Decomposition of N2O. J. Chem. Soc. Faraday Trans. 1 Phys. Chem. Condens. Phases 1978, 74, 1595–1603. [Google Scholar] [CrossRef]
- Zayat, M.; Levy, D. Blue CoAl2O4 Particles Prepared by the Sol−Gel and Citrate−Gel Methods. Chem. Mater. 2000, 12, 2763–2769. [Google Scholar] [CrossRef]
- Holzwarth, U.; Gibson, N. The Scherrer equation versus the ‘Debye-Scherrer equation’. Nat. Nanotechnol. 2011, 6, 534. [Google Scholar] [CrossRef] [PubMed]
- Mindru, I.; Marinescu, G.; Gingasu, D.; Patron, L.; Ghica, C.; Giurginca, M. Blue CoAl2O4 spinel via complexation method. Mater. Chem. Phys. 2010, 122, 491–497. [Google Scholar] [CrossRef]
- Zhang, A.; Wang, N.; Mu, B.; Li, H.; Wang, A. Amino-acid-assisted preparation of CoAl2O4/kaolin hybrid pigments. Appl. Clay Sci. 2020, 191, 105611. [Google Scholar] [CrossRef]
- Peng, X.; Cheng, J.; Yuan, J.; Jin, N.; Kang, J.; Hou, Y.; Zhang, Q. Environmental blue CoAl2O4 pigment co-doped by Zn2+ and Mg2+: Synthesis, structure and optical properties. Adv. Appl. Ceram. 2017, 117, 303–311. [Google Scholar] [CrossRef]
- Lilova, K.I.; Navrotsky, A.; Melot, B.C.; Seshadri, R. Thermodynamics of CoAl2O4–CoGa2O4 solid solutions. J. Solid State Chem. 2010, 183, 1266–1271. [Google Scholar] [CrossRef]
- Wong-Ng, W.; McMurdie, H.; Hubbard, C.; Mighell, A. JCPDS-ICDD Research Associateship (cooperative program with NBS/NIST). J. Res. Natl. Inst. Stand. Technol. 2001, 106, 1013–1028. [Google Scholar] [CrossRef]
- Visinescu, D.; Paraschiv, C.; Ianculescu, A.; Jurca, B.; Vasile, B.; Carp, O. The environmentally benign synthesis of nanosized CoxZn1−xAl2O4 blue pigments. Dye. Pigment. 2010, 87, 125–131. [Google Scholar] [CrossRef]
- Chen, Z.-Z.; Shi, E.-W.; Li, W.-J.; Zheng, Y.-Q.; Zhuang, J.-Y.; Xiao, B.; Tang, L.-A. Preparation of nanosized cobalt aluminate powders by a hydrothermal method. Mater. Sci. Eng. B 2004, 107, 217–223. [Google Scholar] [CrossRef]
- Yu, F.; Yang, J.; Ma, J.; Du, J.; Zhou, Y. Preparation of nanosized CoAl2O4 powders by sol–gel and sol–gel-hydrothermal methods. J. Alloys Compd. 2009, 468, 443–446. [Google Scholar] [CrossRef]
- Štangar, U.L.; Orel, B.; Krajnc, M. Preparation and Spectroscopic Characterization of Blue CoAl2O4 Coatings. J. Sol-Gel Sci. Technol. 2003, 26, 771–775. [Google Scholar] [CrossRef]
- Luo, M.R.; Cui, G.; Rigg, B. The development of the CIE 2000 colour-difference formula: CIEDE2000. Color Res. Appl. 2001, 26, 340–350. [Google Scholar] [CrossRef]
- Gaudon, M.; Apheceixborde, A.; Ménétrier, M.; Le Nestour, A.; Demourgues, A. Synthesis Temperature Effect on the Structural Features and Optical Absorption of Zn1−xCoxAl2O4 Oxides. Inorg. Chem. 2009, 48, 9085–9091. [Google Scholar] [CrossRef]
- Vandewater, L.; Bezemer, G.L.; Bergwerff, J.A.; Versluijshelder, M.; Weckhuysen, B.M.; Dejong, K. Spatially resolved UV–vis microspectroscopy on the preparation of alumina-supported Co Fischer–Tropsch catalysts: Linking activity to Co distribution and speciation. J. Catal. 2006, 242, 287–298. [Google Scholar] [CrossRef]
- Liotta, L.; Pantaleo, G.; Macaluso, A.; Di Carlo, G.; Deganello, G. CoOx catalysts supported on alumina and alumina-baria: Influence of the support on the cobalt species and their activity in NO reduction by C3H6 in lean conditions. Appl. Catal. A Gen. 2003, 245, 167–177. [Google Scholar] [CrossRef]
- Dhas, C.R.; Venkatesh, R.; Jothivenkatachalam, K.; Nithya, A.; Benjamin, B.S.; Raj, A.M.E.; Jeyadheepan, K.; Sanjeeviraja, C. Visible light driven photocatalytic degradation of Rhodamine B and Direct Red using cobalt oxide nanoparticles. Ceram. Int. 2015, 41, 9301–9313. [Google Scholar] [CrossRef]


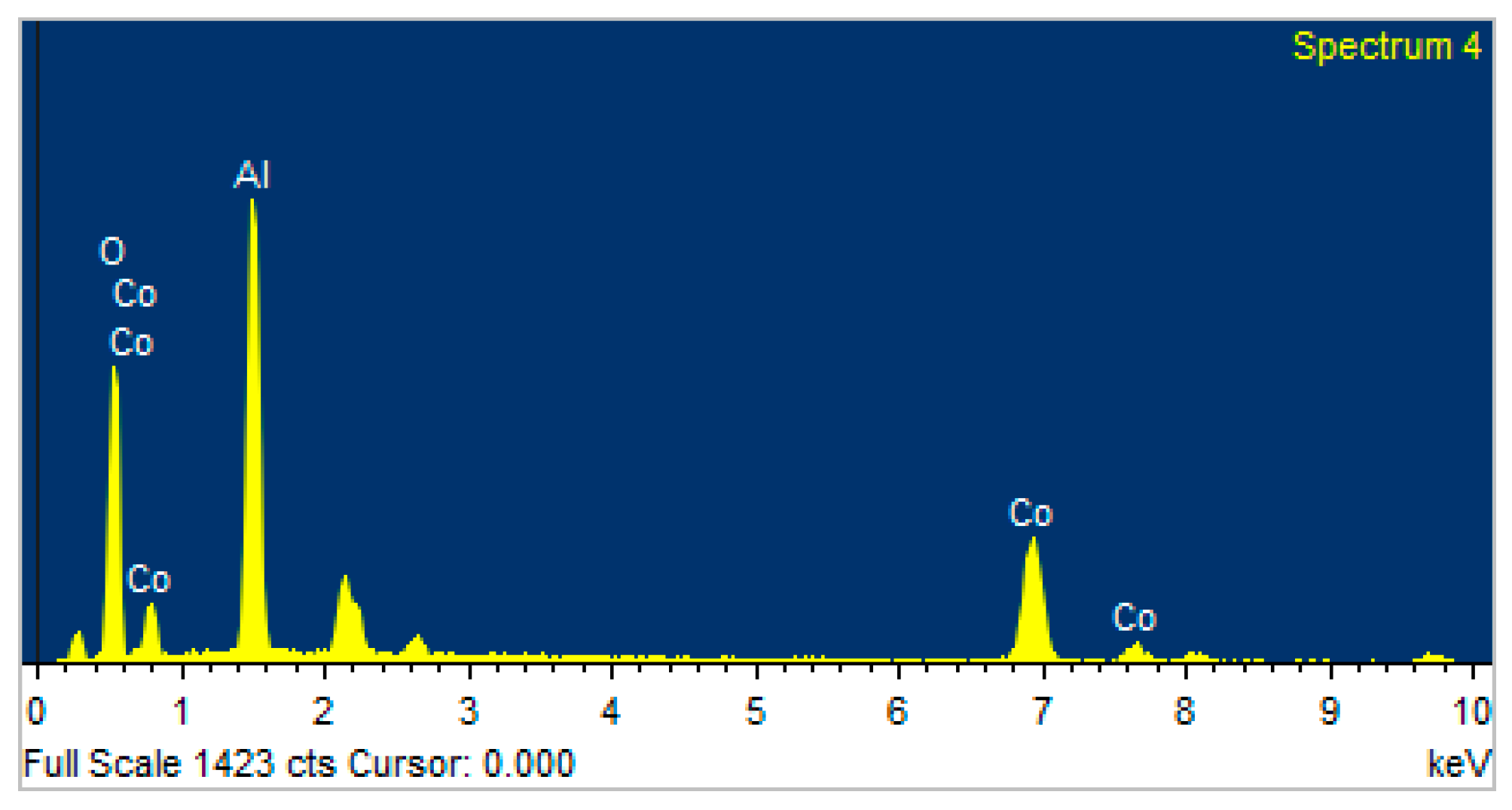

| Element | Weight% | Atomic% |
|---|---|---|
| O K | 37.46 | 58.05 |
| Al K | 31.39 | 28.85 |
| Co K | 31.15 | 13.10 |
| Totals | 100.00 |
| Sample | Temperature (°C) | Time (h) | Xs (Å) |
|---|---|---|---|
| 1 | 1200 | 1 | 553 |
| 2 | 1200 | 1 | 596 |
| 1 | 1200 | 3 | 580 |
| 1 | 1400 | 1 | 624 |
| 2 | 1400 | 1 | 721 |
| Sample | Temperature (°C) | Time (h) | Chemical Formula | Occupation of Cations | Lattice Parameter | |
|---|---|---|---|---|---|---|
| Tetrahedral Site | Octahedral Site | |||||
| 1 | 1200 | 1 | Co1.91Al1.09O4 | 8.096 | ||
| 2 | 1200 | 1 | Co1.46Al1.54O4 | 8.104 | ||
| 1 | 1200 | 3 | Co1.39Al1.61O4 | 8.110 | ||
| 1 | 1400 | 1 | Co1.39Al1.61O4 | 8.111 | ||
| 2 | 1400 | 1 | CoAl2O4 | 8.107 | ||
| Sample | Temperature (°C) | Time (h) | Space Group | a (Å) | α (°) | V (Å3) | Z | Density |
|---|---|---|---|---|---|---|---|---|
| 1 | 1200 | 1 | Fd-3m | 8.096 | 90 | 533.39 | 8 | 4.4053 |
| 2 | 1200 | 1 | Fd-3m | 8.104 | 90 | 532.37 | 8 | 4.4138 |
| 1 | 1200 | 3 | Fd-3m | 8.11 | 90 | 532.67 | 8 | 4.4113 |
| 1 | 1400 | 1 | Fd-3m | 8.111 | 90 | 533.58 | 8 | 4.4037 |
| 2 | 1400 | 1 | Fd-3m | 8.107 | 90 | 533.18 | 8 | 4.5219 |
| Sample | Temperature (°C) | Time (h) | L* | a* | b* | Color |
|---|---|---|---|---|---|---|
| 1 | 500 | 1 | 22.52 | −0.53 | −11.24 |  |
| 1 | 800 | 1 | 14.54 | −2.82 | −9.44 |  |
| 1 | 1200 | 1 | 27.70 | −4.15 | −27.17 |  |
| 2 | 1200 | 1 | 24.60 | −3.74 | −27.83 |  |
| 1 | 1200 | 3 | 27.87 | −3.68 | −30.80 |  |
| 1 | 1400 | 1 | 27.82 | −1.85 | −31.18 |  |
| 2 | 1400 | 1 | 26.41 | 7.31 | −40.54 |  |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, W.; Li, Z.; Wu, G.; Wu, W.; Zeng, H.; Jiang, H.; Zhang, W.; Wu, R.; Xue, Q. Effects of Coloration of Spinel CoAl2O4 Cobalt Blue Pigments: Composition, Structure, and Cation Distribution. Inorganics 2023, 11, 368. https://doi.org/10.3390/inorganics11090368
Zhang W, Li Z, Wu G, Wu W, Zeng H, Jiang H, Zhang W, Wu R, Xue Q. Effects of Coloration of Spinel CoAl2O4 Cobalt Blue Pigments: Composition, Structure, and Cation Distribution. Inorganics. 2023; 11(9):368. https://doi.org/10.3390/inorganics11090368
Chicago/Turabian StyleZhang, Weiran, Ziyu Li, Guohua Wu, Wei Wu, Hailan Zeng, Haiyun Jiang, Weili Zhang, Ruomei Wu, and Qiong Xue. 2023. "Effects of Coloration of Spinel CoAl2O4 Cobalt Blue Pigments: Composition, Structure, and Cation Distribution" Inorganics 11, no. 9: 368. https://doi.org/10.3390/inorganics11090368





