A Study on Surface Modification Characteristics and Charge–Discharge Mechanism of Natural Serpentinite Ore Secondary Battery
Abstract
:1. Introduction
2. Experimental Procedure
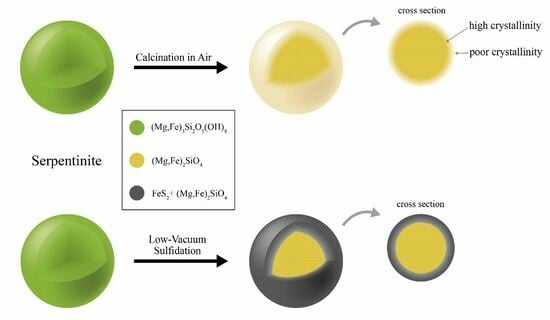
2.1. Preparation of MFS-H and MFS-S Powders
2.2. Structural and Morphological Characterization
2.3. Electrochemical Measurements
3. Results and Discussion
3.1. Morphology and Structure
3.2. Electrochemical Properties
4. Limitation
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Watanabe, A.; Yamamoto, K.; Orikasa, Y.; Masese, T.; Mori, T.; Uchiyama, T.; Matsunaga, T.; Uchimoto, Y. Reaction mechanism of electrochemical insertion/extraction of magnesium ions in olivine-type FePO4. Solid State Ionics 2020, 349, 115311. [Google Scholar] [CrossRef]
- Truong, Q.D.; Kobayashi, H.; Nayuki, K.; Sasaki, Y.; Honma, I. Atomic-scale observation of phase transition of MgMn2O4 cubic spinel upon the charging in Mg-ion battery. Solid State Ionics 2020, 344, 115136. [Google Scholar] [CrossRef]
- Callegari, D.; Colombi, S.; Nitti, A.; Simari, C.; Nicotera, I.; Ferrara, C.; Mustarelli, P.; Pasini, D.; Quartarone, E. Quartarone, Autonomous self-healing strategy for stable sodium-ion battery: A case study of black phosphorus anodes. ACS Appl. Mater. Interfaces 2021, 13, 13170–13182. [Google Scholar] [CrossRef] [PubMed]
- Huang, Z.-D.; Masese, T.; Orikasa, Y.; Mori, T.; Yamamoto, K. Vanadium phosphate as a promising high-voltage magnesium ion (de)-intercalation cathode host. RSC Adv. 2015, 5, 8598–8603. [Google Scholar] [CrossRef]
- Tarascon, J.-M.; Armand, M. Issues and challenges facing rechargeable lithium batteries. Nature 2001, 414, 359–367. [Google Scholar] [CrossRef] [PubMed]
- Zhan, Y.; Zhang, W.; Lei, B.; Liu, H.; Li, W. Recent development of Mg ion solid electrolyte. Front. Chem. 2020, 8, 125. [Google Scholar] [CrossRef] [PubMed]
- Aurbach, D.; Weissman, I.; Gofer, Y.; Levi, E. Nonaqueous magnesium electrochemistry and its application in secondary batteries. Chem. Rec. 2003, 3, 61–73. [Google Scholar] [CrossRef]
- Dompablo, M.A.-D.; Armand, M.; Tarascon, J.; Amador, U. On-demand design of polyoxianionic cathode materials based on electronegativity correlations: An exploration of the Li2MSiO4 system (M = Fe, Mn, Co, Ni). Electrochem. Commun. 2006, 8, 1292–1298. [Google Scholar] [CrossRef]
- Zhou, F.; Cococcioni, M.; Kang, K.; Ceder, G. The Li intercalation potential of LiMPO4 and LiMSiO4 olivines with M = Fe, Mn, Co, Ni. Electrochem. Commun. 2004, 6, 1144–1148. [Google Scholar] [CrossRef]
- Li, Y.; Nuli, Y.; Yang, J.; Yilinuer, T.; Wang, J. MgFeSiO4 prepared via a molten salt method as a new cathode material for rechargeable magnesium batteries. Chin. Sci. Bull. 2011, 56, 386–390. [Google Scholar] [CrossRef]
- NuLi, Y.; Zheng, Y.; Wang, F.; Yang, J.; Minett, A.I.; Wang, J.; Chen, J. MWNT/C/Mg1.03Mn0.97SiO4 hierarchical nanostructure for superior reversible magnesium ion storage. Electrochem. Commun. 2011, 13, 1143–1146. [Google Scholar] [CrossRef]
- Zheng, Y.; NuLi, Y.; Chen, Q.; Wang, Y.; Yang, J.; Wang, J. Magnesium cobalt silicate materials for reversible magnesium ion storage. Electrochimica Acta 2012, 66, 75–81. [Google Scholar] [CrossRef]
- Zhao, J.-R.; Wang, I.-H.; Hung, F.-Y. A New Secondary Battery Technology: Electrode Structure and Charge–Discharge Mechanism of All-Solid-State Zinc-Graphite Batteries. Mater. Sci. Eng. B 2024, 299, 116975. [Google Scholar] [CrossRef]
- Kiyoura, R.; Ito, Y. Study on Thermal Transformation in the Synthetic and Natural Serpentines, especialy Exothermic Reaction of Serpentines. J. Ceram. Assoc. Jpn. 1953, 61, 525. [Google Scholar] [CrossRef]
- Lin, X.; Wang, P.; Li, P.; Yu, H.; Qian, S.; Shui, M.; Wang, D.; Long, N.; Shu, J. Improved the lithium storage capability of BaLi2Ti6O14 by electroless silver coating. Electrochimica Acta 2015, 186, 24–33. [Google Scholar] [CrossRef]
- Shih, Y.-T.; Wu, C.-H.; Hung, F.-Y.; Lui, T.-S.; Chen, L.-H. A study at room temperature and 55 °C on the charge–discharge characteristics of Si(100−x)Alx thin film anode for Li-ion batteries. Surf. Coat. Technol. 2013, 215, 79–84. [Google Scholar] [CrossRef]
- Maxwell, J.C. Ophiolites—Ancient oceanic lithosphere?: R.G. Coleman. Springer, Berlin, 1977, 229 pp., DM 68.00 or US $ 30.00. Mar. Geol. 1979, 30, 322–324. [Google Scholar] [CrossRef]
- Jafari, A.; Shayesteh, S.F.; Salouti, M.; Boustani, K. Effect of annealing temperature on magnetic phase transition in Fe3O4 nanoparticles. J. Magn. Magn. Mater. 2015, 379, 305–312. [Google Scholar] [CrossRef]
- Jia, L.; Ashtiani, S.; Liang, F.; He, G.; Jiang, H. Hydrogen permeation through dual-phase ceramic membrane derived from automatic phase-separation of SrCe0.50Fe0.50O3-δ precursor. Int. J. Hydrogen Energy 2020, 45, 4625–4634. [Google Scholar] [CrossRef]
- Palayangoda, S.S.; Nguyen, Q.P. Thermal behavior of raw oil shale and its components. Oil Shale 2015, 32, 160. [Google Scholar] [CrossRef]
- Deng, J.; Wen, S.; Chen, X.; Xian, Y.; Wu, D. Dynamic Simulation of the Thermal Decomposition of Pyrite Under Vacuum. Met. Mater. Trans. A 2014, 45, 2445–2452. [Google Scholar] [CrossRef]
- Sweeney, R.E.; Kaplan, I.R. Pyrite Framboid Formation; Laboratory Synthesis and Marine Sediments. Econ. Geol. 1973, 68, 618–634. [Google Scholar] [CrossRef]
- Luther, G.W. Pyrite synthesis via polysulfide compounds. Geochim. Cosmochim. Acta 1991, 55, 2839–2849. [Google Scholar] [CrossRef]
- Ouertani, B.; Ouerfelli, J.; Saadoun, M.; Bessaïs, B.; Ezzaouia, H.; Bernède, J. Characterization of FeS2-pyrite thin films synthesized by sulphuration of amorphous iron oxide films pre-deposited by spray pyrolysis. Mater. Charact. 2005, 54, 431–437. [Google Scholar] [CrossRef]
- Ouertani, B.; Ouerfelli, J.; Saadoun, M.; Bessaïs, B.; Hajji, M.; Kanzari, M.; Ezzaouia, H.; Hamdadou, N.; Bernède, J. Transformation of amorphous iron oxide films pre-deposited by spray pyrolysis into FeS2-pyrite films. Mater. Lett. 2005, 59, 734–739. [Google Scholar] [CrossRef]
- Brindley, G.W.; Hayami, R. Kinetics and Mechanisms of Dehydration and Recrystallization of Serpentine—I. Clays Clay Miner. 1963, 12, 35–47. [Google Scholar] [CrossRef]
- Tran, D.T.; Dong, H.; Walck, S.D.; Zhang, S.S. Pyrite FeS2–C composite as a high capacity cathode material of rechargeable lithium batteries. RSC Adv. 2015, 5, 87847–87854. [Google Scholar] [CrossRef]
- Bodenes, L.; Naturel, R.; Martinez, H.; Dedryvère, R.; Menetrier, M.; Croguennec, L.; Pérès, J.-P.; Tessier, C.; Fischer, F. Lithium secondary batteries working at very high temperature: Capacity fade and understanding of aging mechanisms. J. Power Source 2013, 236, 265–275. [Google Scholar] [CrossRef]
- Shao-Horn, Y.; Osmialowski, S.; Horn, Q.C. Reinvestigation of Lithium Reaction Mechanisms in FeS2 Pyrite at Ambient Temperature. J. Electrochem. Soc. 2002, 149, A1547–A1555. [Google Scholar] [CrossRef]

















| Element (at.%) | Mg | Si | Fe | O | S |
|---|---|---|---|---|---|
| MFS-S-700 | 19.89 | 14.82 | 2.77 | 62.18 | 0.34 |
| MFS-S-800 | 21.22 | 15.95 | 3.31 | 59.09 | 0.43 |
| MFS-S-900 | 20.55 | 15.49 | 2.18 | 61.47 | 0.31 |
| Categories | MFS-H-900 | MFS-S-900 |
|---|---|---|
| [Mg2+] (ppb) | N.D. | 611 |
| Categories | Rs (Ω) | Rct (Ω) | σ (ΩHz1/2) |
|---|---|---|---|
| MFS-H-900 | 4.62 | 586.49 | 198.12 |
| MFS-S-900 | 7.40 | 47.95 | 20.30 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, J.-R.; Chen, K.-J.; Hung, F.-Y.; Tsai, Y.-Y.; Wu, P.-T. A Study on Surface Modification Characteristics and Charge–Discharge Mechanism of Natural Serpentinite Ore Secondary Battery. Inorganics 2024, 12, 13. https://doi.org/10.3390/inorganics12010013
Zhao J-R, Chen K-J, Hung F-Y, Tsai Y-Y, Wu P-T. A Study on Surface Modification Characteristics and Charge–Discharge Mechanism of Natural Serpentinite Ore Secondary Battery. Inorganics. 2024; 12(1):13. https://doi.org/10.3390/inorganics12010013
Chicago/Turabian StyleZhao, Jun-Ren, Kuan-Jen Chen, Fei-Yi Hung, Yung-Yi Tsai, and Po-Ting Wu. 2024. "A Study on Surface Modification Characteristics and Charge–Discharge Mechanism of Natural Serpentinite Ore Secondary Battery" Inorganics 12, no. 1: 13. https://doi.org/10.3390/inorganics12010013