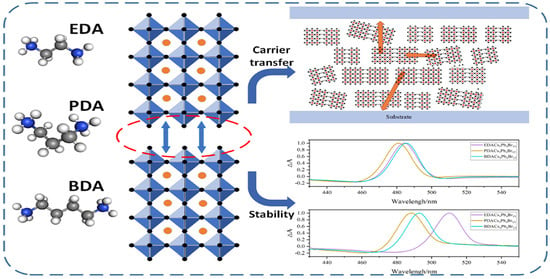
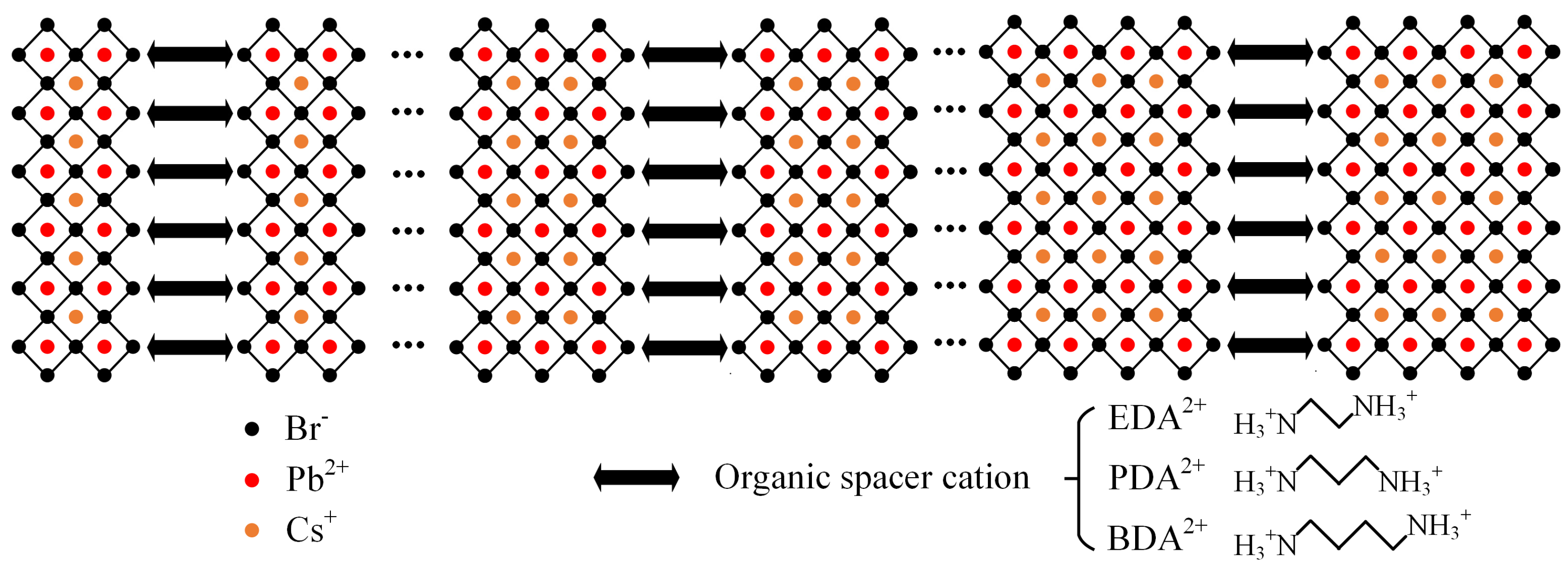
The Effect of Organic Spacer Cations with Different Chain Lengths on Quasi-Two-Dimensional Perovskite Properties
Abstract
:1. Introduction
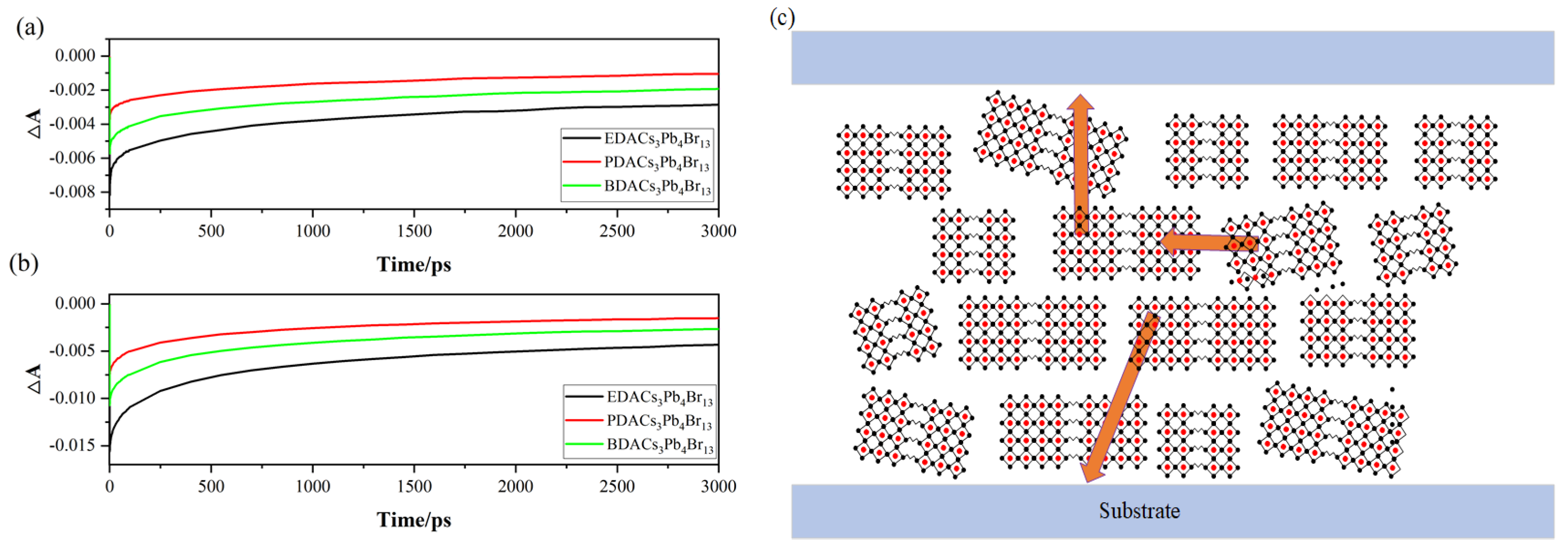
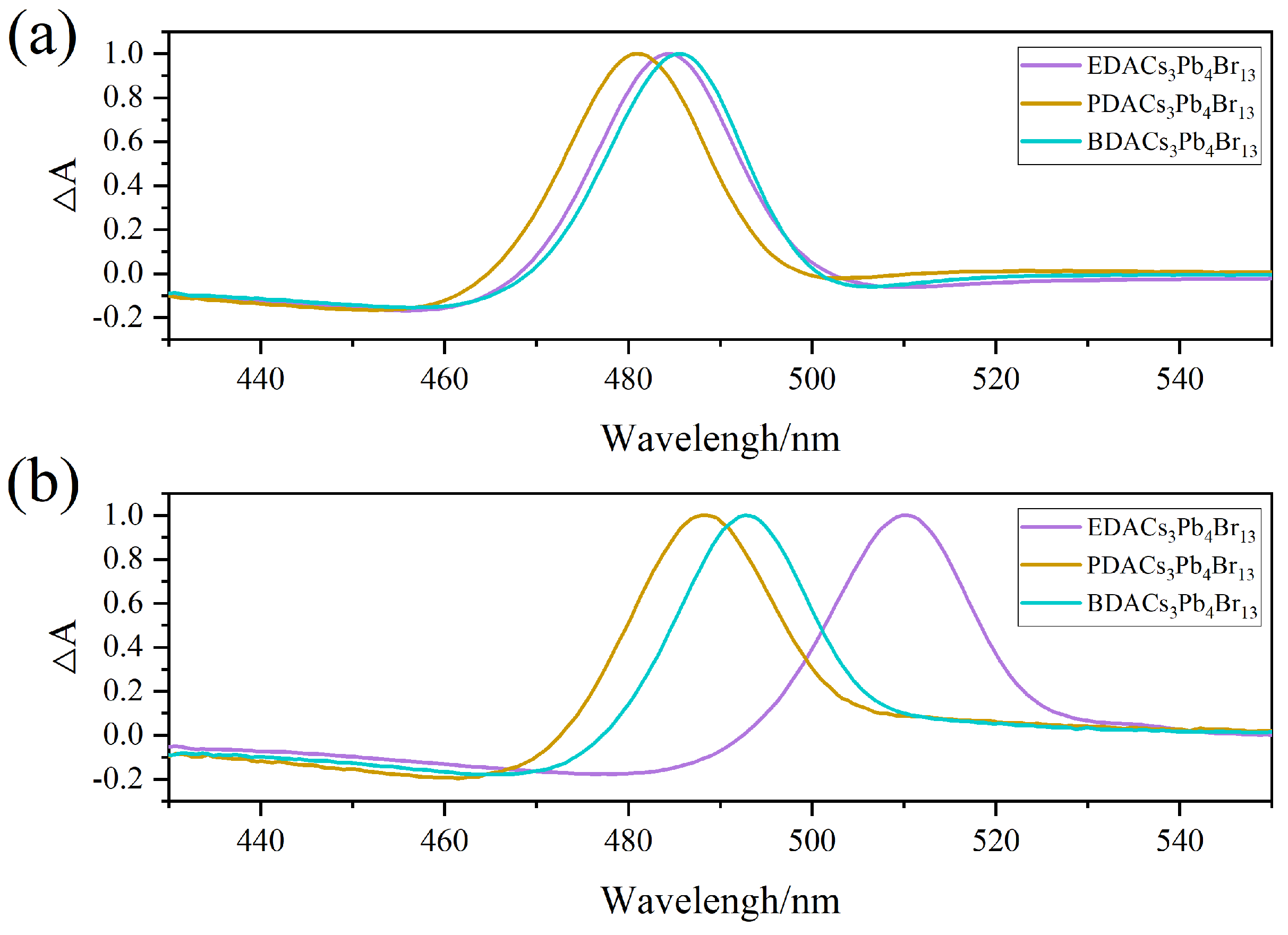
2. Results
3. Discussion
4. Materials and Methods
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Stranks, S.D.; Snaith, H.J. Metal-halide perovskites for photovoltaic and light-emitting devices. Nat. Nanotechnol. 2015, 10, 391–402. [Google Scholar] [CrossRef] [PubMed]
- Huang, P.; Kazim, S.; Wang, M.; Ahmad, S. Toward Phase Stability: Dion–Jacobson Layered Perovskite for Solar Cells. ACS Energy Lett. 2019, 4, 2960–2974. [Google Scholar] [CrossRef]
- Zhao, W.; Dong, Q.; Zhang, J.; Wang, S.; Chen, M.; Zhao, C.; Hu, M.; Jin, S.; Padture, N.P.; Shi, Y. Asymmetric alkyl diamine based Dion–Jacobson low-dimensional perovskite solar cells with efficiency exceeding 15%. J. Mater. Chem. A 2020, 8, 9919–9926. [Google Scholar] [CrossRef]
- He, T.; Li, S.; Jiang, Y.; Qin, C.; Cui, M.; Qiao, L.; Xu, H.; Yang, J.; Long, R.; Wang, H.; et al. Reduced-dimensional perovskite photovoltaics with homogeneous energy landscape. Nature 2020, 11, 1672. [Google Scholar] [CrossRef] [PubMed]
- Fu, S.; Li, X.; Wan, L.; Zhang, W.; Song, W.; Fang, J. Effective Surface Treatment for High-Performance Inverted CsPbI2Br Perovskite Solar Cells with Efficiency of 15.92. Nanomicro Lett. 2020, 12, 170. [Google Scholar] [CrossRef] [PubMed]
- Fu, P.; Liu, Y.; Yu, S.; Yin, H.; Yang, B.; Ahmad, S.; Guo, X.; Li, C. Dion-Jacobson and Ruddlesden-Popper double-phase 2D perovskites for solar cells. Nano Energy 2021, 88, 106249. [Google Scholar] [CrossRef]
- Yang, J.; Yang, T.; Liu, D.; Zhang, Y.; Luo, T.; Lu, J.; Fang, J.; Wen, J.; Deng, Z.; Liu, S.; et al. Stable 2D Alternating Cation Perovskite Solar Cells with Power Conversion Efficiency >19% via Solvent Engineering. Sol. RRL 2021, 5, 2100286. [Google Scholar] [CrossRef]
- Zhang, Y.; Wen, J.; Xu, Z.; Liu, D.; Yang, T.; Niu, T.; Luo, T.; Lu, J.; Fang, J.; Chang, X.; et al. Effective Phase-Alignment for 2D Halide Perovskites Incorporating Symmetric Diammonium Ion for Photovoltaics. Adv. Sci. 2021, 8, 2001433. [Google Scholar] [CrossRef]
- Ren, G.; Yan, C.; Xiao, L.; Wu, X.; Peng, S.; Lin, W.; Tan, W.; Liu, Y.; Min, Y. Additive-Induced Film Morphology Evolution for Inverted Dion–Jacobson Quasi-Two-Dimensional Perovskite Solar Cells with Enhanced Performance. ACS Appl. Energy Mater. 2022, 5, 9837–9845. [Google Scholar] [CrossRef]
- Yang, Z.; Liu, Z.; Ahmadi, V.; Chen, W.; Qi, Y. Recent Progress on Metal Halide Perovskite Solar Minimodules. Sol. RRL 2022, 6, 2100458. [Google Scholar] [CrossRef]
- Li, M.; Gao, Q.; Liu, P.; Liao, Q.; Zhang, H.; Yao, J.; Hu, W.; Wu, Y.; Fu, H. Amplified Spontaneous Emission Based on 2D Ruddlesden-Popper Perovskites. Adv. Funct. Mater. 2018, 28, 1707006. [Google Scholar] [CrossRef]
- Yu, M.; Yi, C.; Wang, N.; Zhang, L.; Zou, R.; Tong, Y.; Chen, H.; Cao, Y.; He, Y.; Wang, Y.; et al. Control of Barrier Width in Perovskite Multiple Quantum Wells for High Performance Green Light–Emitting Diodes. Adv. Opt. Mater. 2018, 7, 1801575. [Google Scholar] [CrossRef]
- Chen, P.; Meng, Y.; Ahmadi, M.; Peng, Q.; Gao, C.; Xu, L.; Shao, M.; Xiong, Z.; Hu, B. Charge-transfer versus energy-transfer in quasi-2D perovskite light-emitting diodes. Nano Energy 2018, 50, 615–622. [Google Scholar] [CrossRef]
- Das, S.; Gholipour, S.; Saliba, M. Perovskites for Laser and Detector Applications. Energy Environ. Mater. 2019, 2, 146–153. [Google Scholar] [CrossRef]
- Li, Z.; Chen, Z.; Yang, Y.; Xue, Q.; Yip, H.L.; Cao, Y. Modulation of recombination zone position for quasi-two-dimensional blue perovskite light-emitting diodes with efficiency exceeding 5. Nat. Commun. 2019, 10, 1027. [Google Scholar] [CrossRef]
- Zhu, L.; Liu, D.; Wang, J.; Wang, N. Large Organic Cations in Quasi-2D Perovskites for High-Performance Light-Emitting Diodes. J. Phys. Chem. Lett. 2020, 11, 8502–8510. [Google Scholar] [CrossRef]
- De Giorgi, M.L.; Creti, A.; La-Placa, M.G.; Boix, P.P.; Bolink, H.J.; Lomascolo, M.; Anni, M. Amplified spontaneous emission in thin films of quasi-2D BA3MA3Pb5Br16 lead halide perovskites. Nanoscale 2021, 13, 8893–8900. [Google Scholar] [CrossRef]
- Kar, S.; Jamaludin, N.F.; Yantara, N.; Mhaisalkar, S.G.; Leong, W.L. Recent advancements and perspectives on light management and high performance in perovskite light-emitting diodes. Nanophotonics 2021, 10, 2103–2143. [Google Scholar] [CrossRef]
- Lian, Y.; Yang, Y.; He, L.; Yang, X.; Gao, J.; Qin, C.; Niu, L.; Yang, X. Enhancing the Luminance Efficiency of Formamidinium-Based Dion-Jacobson Perovskite Light-Emitting Diodes via Compositional Engineering. ACS Appl. Mater. Interfaces 2021, 14, 1659–1669. [Google Scholar] [CrossRef]
- Qin, X.; Liu, F.; Leung, T.L.; Sun, W.; Chan, C.C.S.; Wong, K.S.; Kanižaj, L.; Popović, J.; Djurišić, A.B. Compositional optimization of mixed cation Dion–Jacobson perovskites for efficient green light emission. J. Mater. Chem. C 2022, 10, 108–114. [Google Scholar] [CrossRef]
- Lian, Y.; Yang, Y.; Gao, J.; Qin, C. Efficient Dion-Jacobson perovskite light-emitting diodes via mixed cation engineering. Opt. Lett. 2022, 47, 657–660. [Google Scholar] [CrossRef] [PubMed]
- Zhong, Y.; Liu, G.; Su, Y.; Sheng, W.; Gong, L.; Zhang, J.; Tan, L.; Chen, Y. Diammonium molecular configuration-induced regulation of crystal orientation and carrier dynamics for highly efficient and stable 2D/3D perovskite solar cells. Angew. Chem. Int. Ed. 2022, 61, e202114588. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.; Xie, Y.; Zhang, J.; Duan, J.; Chen, X.; Liang, Y.; Wang, K.; Xu, L. Thermal and humidity stability of mixed spacer cations 2D perovskite solar cells. Adv. Sci. 2021, 8, 2004510. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Chen, J.; Chen, S.; Gao, H.; Li, Y.; Jiang, Z.; Zhang, Y.; Wang, X.; Zhu, X.; Xu, B. Organic spacer engineering of ruddlesden-popper perovskite materials toward efficient and stable solar cells. Chem. Eng. J. 2023, 453, 139790. [Google Scholar] [CrossRef]
- Yang, T.; Ma, C.; Cai, W.; Wang, S.; Wu, Y.; Feng, J.; Wu, N.; Li, H.; Huang, W.; Ding, Z. Amidino-based Dion-Jacobson 2D perovskite for efficient and stable 2D/3D heterostructure perovskite solar cells. Joule 2023, 7, 574–586. [Google Scholar] [CrossRef]
- Ngai, K.H.; Wei, Q.; Chen, Z.; Guo, X.; Qin, M.; Xie, F.; Chan, C.C.S.; Xing, G.; Lu, X.; Chen, J. Enhanced electrochemical stability by alkyldiammonium in Dion–Jacobson perovskite toward ultrastable light-emitting diodes. Adv. Opt. Mater. 2021, 9, 2100243. [Google Scholar] [CrossRef]
- Lei, Y.; Li, Z.; Wang, H.; Wang, Q.; Peng, G.; Xu, Y.; Zhang, H.; Wang, G.; Ding, L.; Jin, Z. Manipulate energy transport via fluorinated spacers towards record-efficiency 2D Dion-Jacobson CsPbI3 solar cells. Sci. Bull. 2022, 67, 1352–1361. [Google Scholar] [CrossRef] [PubMed]
- Ke, W.; Mao, L.; Stoumpos, C.C.; Hoffman, J.; Spanopoulos, I.; Mohite, A.D.; Kanatzidis, M.G. Compositional and solvent engineering in Dion–Jacobson 2D perovskites boosts solar cell efficiency and stability. Adv. Energy Mater. 2019, 9, 1803384. [Google Scholar] [CrossRef]
- Wu, H.; Lian, X.; Tian, S.; Zhang, Y.; Qin, M.; Zhang, Y.; Wang, F.; Lu, X.; Wu, G.; Chen, H. Additive-Assisted Hot-Casting Free Fabrication of Dion–Jacobson 2D Perovskite Solar Cell with Efficiency Beyond 16%. Sol. RRL 2020, 4, 2000087. [Google Scholar] [CrossRef]
- Mitzi, D.B. Templating and structural engineering in organic–inorganic perovskites. J. Chem. Soc. Dalton Trans. 2001, 1, 1–12. [Google Scholar] [CrossRef]
- Hu, T.; Smith, M.D.; Dohner, E.R.; Sher, M.-J.; Wu, X.; Trinh, M.T.; Fisher, A.; Corbett, J.; Zhu, X.Y.; Karunadasa, H.I. Mechanism for broadband white-light emission from two-dimensional (110) hybrid perovskites. J. Phys. Chem. Lett. 2016, 7, 2258–2263. [Google Scholar] [CrossRef] [PubMed]
- Tan, M.; Wang, S.; Rao, F.; Yang, S.; Wang, F. Pressures tuning the band gap of organic–inorganic trihalide perovskites (MAPbBr 3): A first-principles study. J. Electron. Mater. 2018, 47, 7204–7211. [Google Scholar] [CrossRef]
- Mao, L.; Guo, P.; Kepenekian, M.; Hadar, I.; Katan, C.; Even, J.; Schaller, R.D.; Stoumpos, C.C.; Kanatzidis, M.G. Structural diversity in white-light-emitting hybrid lead bromide perovskites. J. Am. Chem. Soc. 2018, 140, 13078–13088. [Google Scholar] [CrossRef] [PubMed]
- Saidaminov, M.I.; Mohammed, O.F.; Bakr, O.M. Low-dimensional-networked metal halide perovskites: The next big thing. ACS Energy Lett. 2017, 2, 889–896. [Google Scholar] [CrossRef]
- Ahmad, S.; Hanmandlu, C.; Kanaujia, P.K.; Prakash, G.V. Direct deposition strategy for highly ordered inorganic organic perovskite thin films and their optoelectronic applications. Opt. Mater. Express 2014, 4, 1313–1323. [Google Scholar] [CrossRef]
- Hong, X.; Ishihara, T.; Nurmikko, A.V. Dielectric confinement effect on excitons in PbI 4-based layered semiconductors. Phys. Rev. B 1992, 45, 6961. [Google Scholar] [CrossRef] [PubMed]
- Muljarov, E.A.; Tikhodeev, S.G.; Gippius, N.A.; Ishihara, T. Excitons in self-organized semiconductor/insulator superlattices: PbI-based perovskite compounds. Phys. Rev. B 1995, 51, 14370. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Sun, Y.; Peng, J.; Zhang, W.; Su, X.; Zheng, K.; Pullerits, T.; Liang, Z. Tailoring organic cation of 2D air-stable organometal halide perovskites for highly efficient planar solar cells. Adv. Energy Mater. 2017, 7, 1700162. [Google Scholar] [CrossRef]
- Guo, X.; Gao, Y.; Wei, Q.; Ho Ngai, K.; Qin, M.; Lu, X.; Xing, G.; Shi, T.; Xie, W.; Xu, J. Suppressed Phase Segregation in High-Humidity-Processed Dion–Jacobson Perovskite Solar Cells Toward High Efficiency and Stability. Sol. RRL 2021, 5, 2100555. [Google Scholar] [CrossRef]
- Proppe, A.H.; Quintero-Bermudez, R.; Tan, H.; Voznyy, O.; Kelley, S.O.; Sargent, E.H. Synthetic control over quantum well width distribution and carrier migration in low-dimensional perovskite photovoltaics. J. Am. Chem. Soc. 2018, 140, 2890–2896. [Google Scholar] [CrossRef]
- Li, X.; Hoffman, J.; Ke, W.; Chen, M.; Tsai, H.; Nie, W.; Mohite, A.D.; Kepenekian, M.; Katan, C.; Even, J. Two-dimensional halide perovskites incorporating straight chain symmetric diammonium ions, (NH3CmH2mNH3)(CH3NH3)n−1PbnI3n+1 (m = 4–9; n = 1–4). J. Am. Chem. Soc. 2018, 140, 12226–12238. [Google Scholar] [CrossRef] [PubMed]
- Ma, C.; Shen, D.; Ng, T.W.; Lo, M.F.; Lee, C.S. 2D perovskites with short interlayer distance for high-performance solar cell application. Adv. Mater. 2018, 30, 1800710. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, S.; Fu, P.; Yu, S.; Yang, Q.; Liu, X.; Wang, X.; Wang, X.; Guo, X.; Li, C. Dion-Jacobson phase 2D layered perovskites for solar cells with ultrahigh stability. Joule 2019, 3, 794–806. [Google Scholar] [CrossRef]
- Zheng, Y.; Niu, T.; Qiu, J.; Chao, L.; Li, B.; Yang, Y.; Li, Q.; Lin, C.; Gao, X.; Zhang, C. Oriented and uniform distribution of Dion–Jacobson phase perovskites controlled by quantum well barrier thickness. Sol. RRL 2019, 3, 1900090. [Google Scholar] [CrossRef]
- Sourisseau, S.; Louvain, N.; Bi, W.; Mercier, N.; Rondeau, D.; Boucher, F.; Buzaré, J.-Y.; Legein, C. Reduced band gap hybrid perovskites resulting from combined hydrogen and halogen bonding at the organic− inorganic interface. Chem. Mater. 2007, 19, 600–607. [Google Scholar] [CrossRef]
- Gan, L.; Li, J.; Fang, Z.; He, H.; Ye, Z. Effects of organic cation length on exciton recombination in two-dimensional layered lead iodide hybrid perovskite crystals. J. Phys. Chem. Lett. 2017, 8, 5177–5183. [Google Scholar] [CrossRef]
- Blancon, J.C.; Tsai, H.; Nie, W.; Stoumpos, C.C.; Pedesseau, L.; Katan, C.; Kepenekian, M.; Soe, C.M.M.; Appavoo, K.; Sfeir, M.Y. Extremely efficient internal exciton dissociation through edge states in layered 2D perovskites. Science 2017, 355, 1288–1292. [Google Scholar] [CrossRef]
- Han, Y.; Li, Y.; Wang, Y.; Cao, G.; Yue, S.; Zhang, L.; Cui, B.B.; Chen, Q. From distortion to disconnection: Linear alkyl diammonium cations tune structure and photoluminescence of lead bromide perovskites. Adv. Opt. Mater. 2020, 8, 1902051. [Google Scholar] [CrossRef]
- Ajayakumar, A.; Muthu, C.; Dev, A.V.; Pious, J.K.; Vijayakumar, C. Two-Dimensional Halide Perovskites: Approaches to Improve Optoelectronic Properties. Chem.—Asian J. 2022, 17, e202101075. [Google Scholar] [CrossRef]
- Queloz, V.I.E.; Bouduban, M.E.F.; García-Benito, I.; Fedorovskiy, A.; Orlandi, S.; Cavazzini, M.; Pozzi, G.; Trivedi, H.; Lupascu, D.C.; Beljonne, D. Spatial charge separation as the origin of anomalous stark effect in fluorous 2d hybrid perovskites. Adv. Funct. Mater. 2020, 30, 2000228. [Google Scholar] [CrossRef]
- Walters, G.; Wei, M.; Voznyy, O.; Quintero-Bermudez, R.; Kiani, A.; Smilgies, D.M.; Munir, R.; Amassian, A.; Hoogland, S.; Sargent, E. The quantum-confined Stark effect in layered hybrid perovskites mediated by orientational polarizability of confined dipoles. Nat. Commun. 2018, 9, 4214. [Google Scholar] [CrossRef] [PubMed]
- Sharma, D.K.; Hirata, S.; Biju, V.; Vacha, M. Stark effect and environment-induced modulation of emission in single halide perovskite nanocrystals. ACS Nano 2019, 13, 624–632. [Google Scholar] [CrossRef] [PubMed]
- Manser, J.S.; Kamat, P.V. Band filling with free charge carriers in organometal halide perovskites. Nat. Photonics 2014, 8, 737–743. [Google Scholar] [CrossRef]
- Konidakis, I.; Maksudov, T.; Serpetzoglou, E.; Kakavelakis, G.; Kymakis, E.; Stratakis, E. Improved Charge Carrier Dynamics of CH3NH3PbI3 Perovskite Films Synthesized by Means of Laser-Assisted Crystallization. ACS Appl. Energy Mater. 2018, 1, 5101–5111. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, L.; Xia, M.; Zhang, Y.; Song, L.; Guo, X.; Zhang, Y.; Wang, Y.; Xia, Y. The Effect of Organic Spacer Cations with Different Chain Lengths on Quasi-Two-Dimensional Perovskite Properties. Inorganics 2024, 12, 12. https://doi.org/10.3390/inorganics12010012
Zhang L, Xia M, Zhang Y, Song L, Guo X, Zhang Y, Wang Y, Xia Y. The Effect of Organic Spacer Cations with Different Chain Lengths on Quasi-Two-Dimensional Perovskite Properties. Inorganics. 2024; 12(1):12. https://doi.org/10.3390/inorganics12010012
Chicago/Turabian StyleZhang, Lei, Mingze Xia, Yuan Zhang, Li Song, Xiwei Guo, Yong Zhang, Yulei Wang, and Yuanqin Xia. 2024. "The Effect of Organic Spacer Cations with Different Chain Lengths on Quasi-Two-Dimensional Perovskite Properties" Inorganics 12, no. 1: 12. https://doi.org/10.3390/inorganics12010012