Preparation of Environmentally Friendly BiVO4@SiO2 Encapsulated Yellow Pigment with Remarkable Thermal and Chemical Stability
Abstract
:1. Introduction
2. Results and Discussion
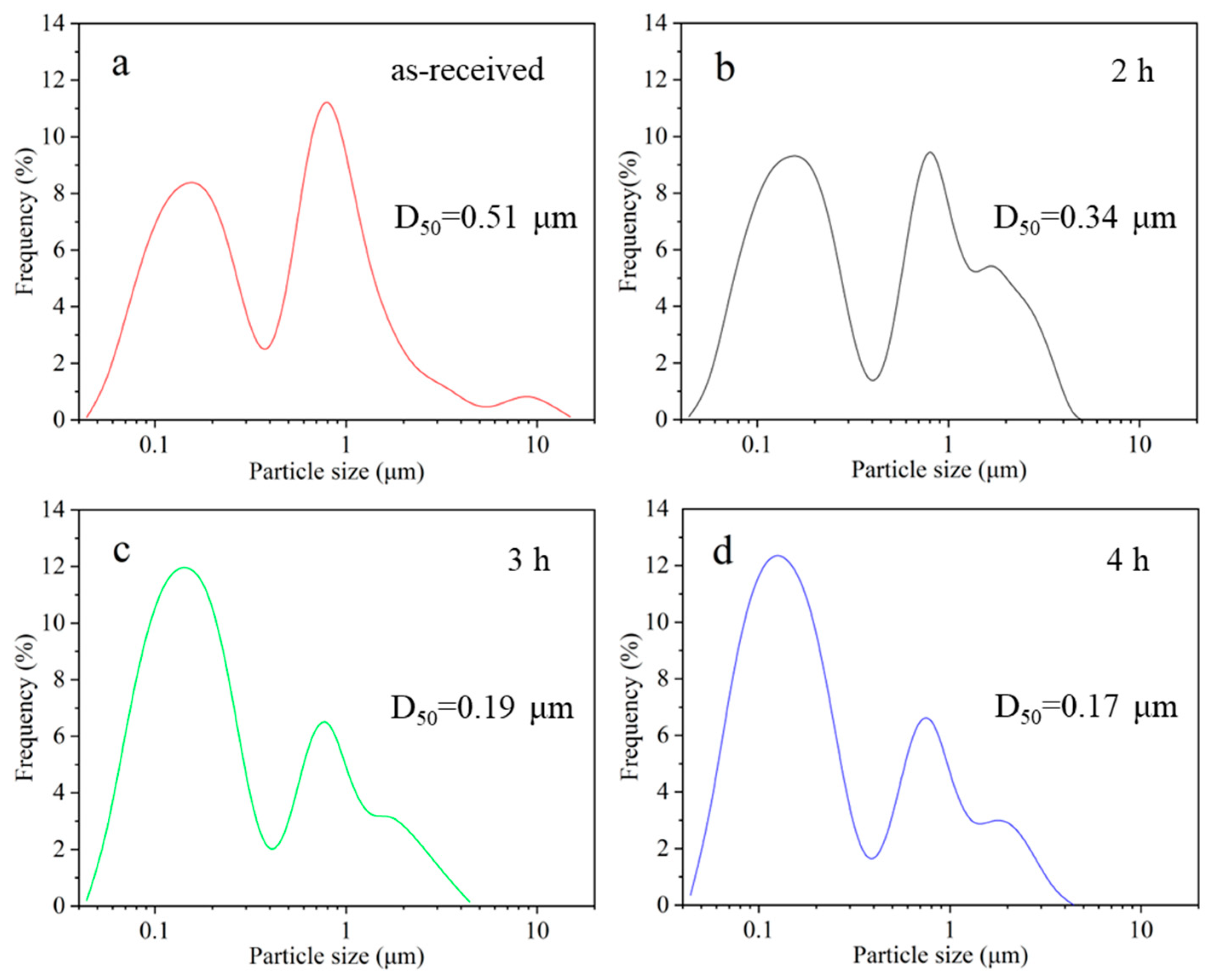
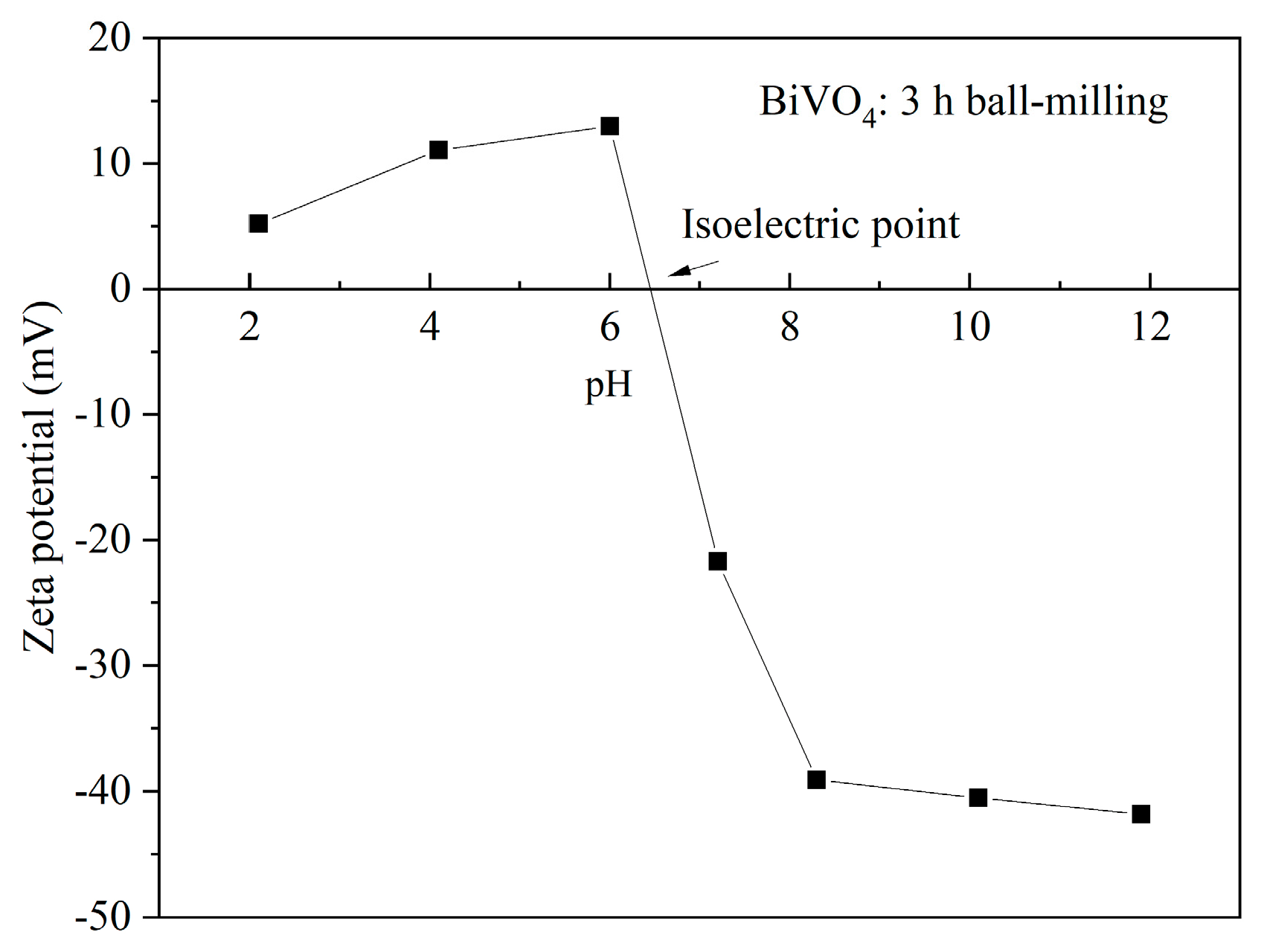
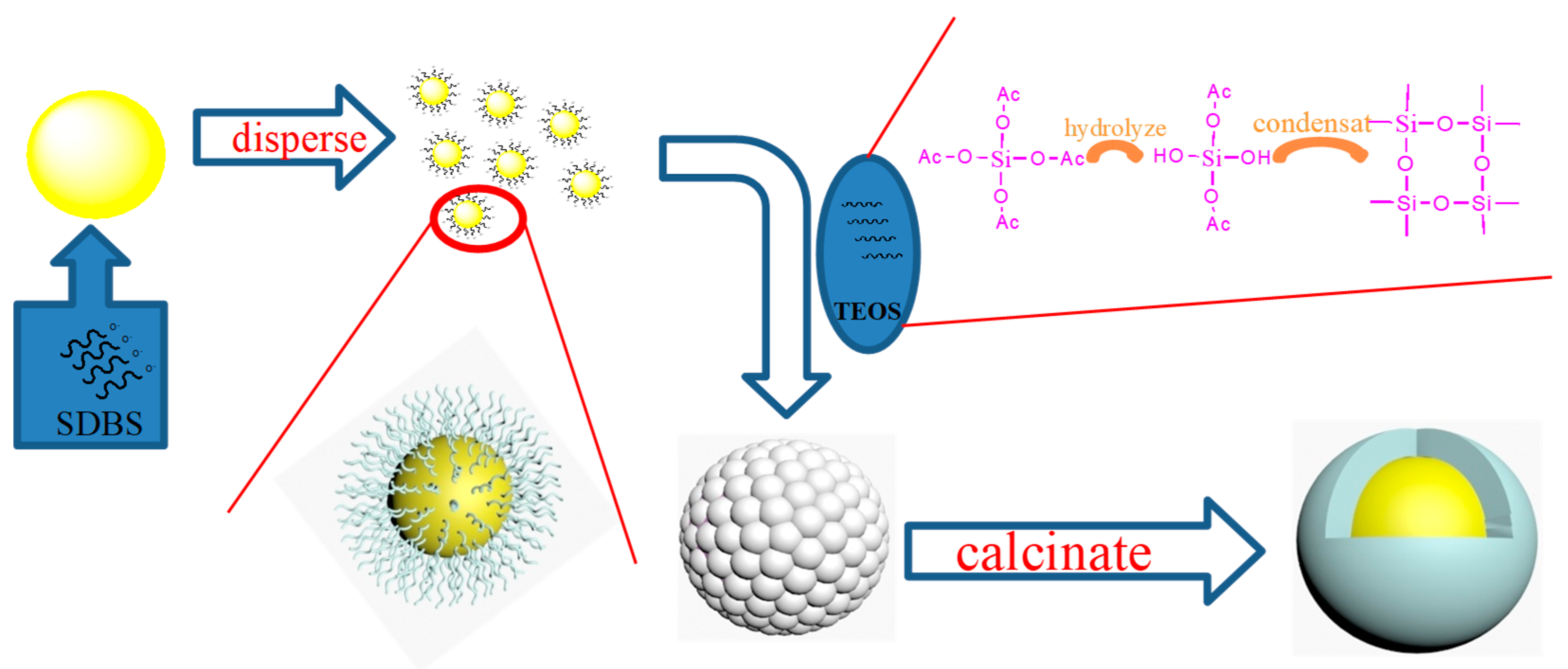
2.1. Pretreatment and Dispersion of BiVO4 Pigment
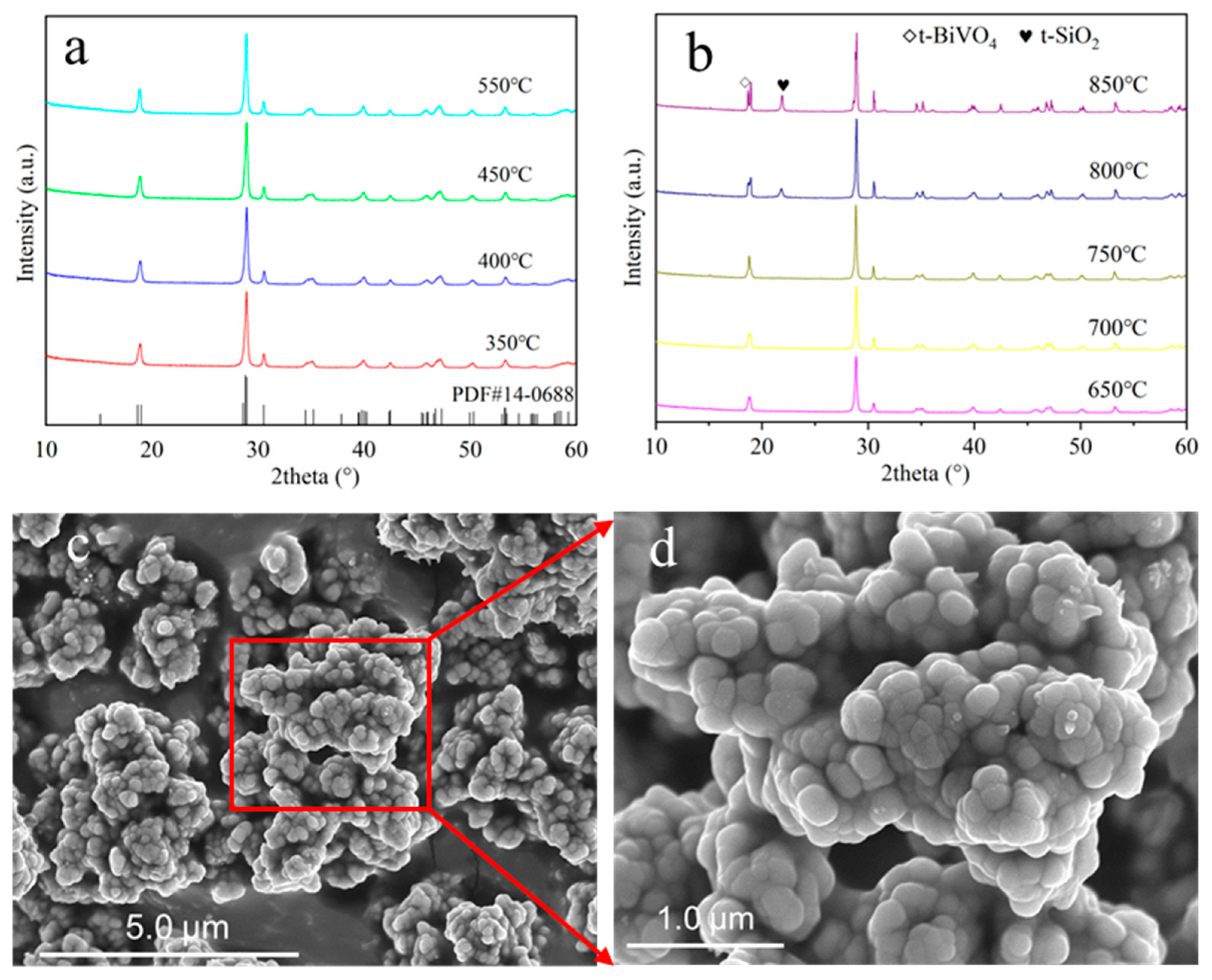
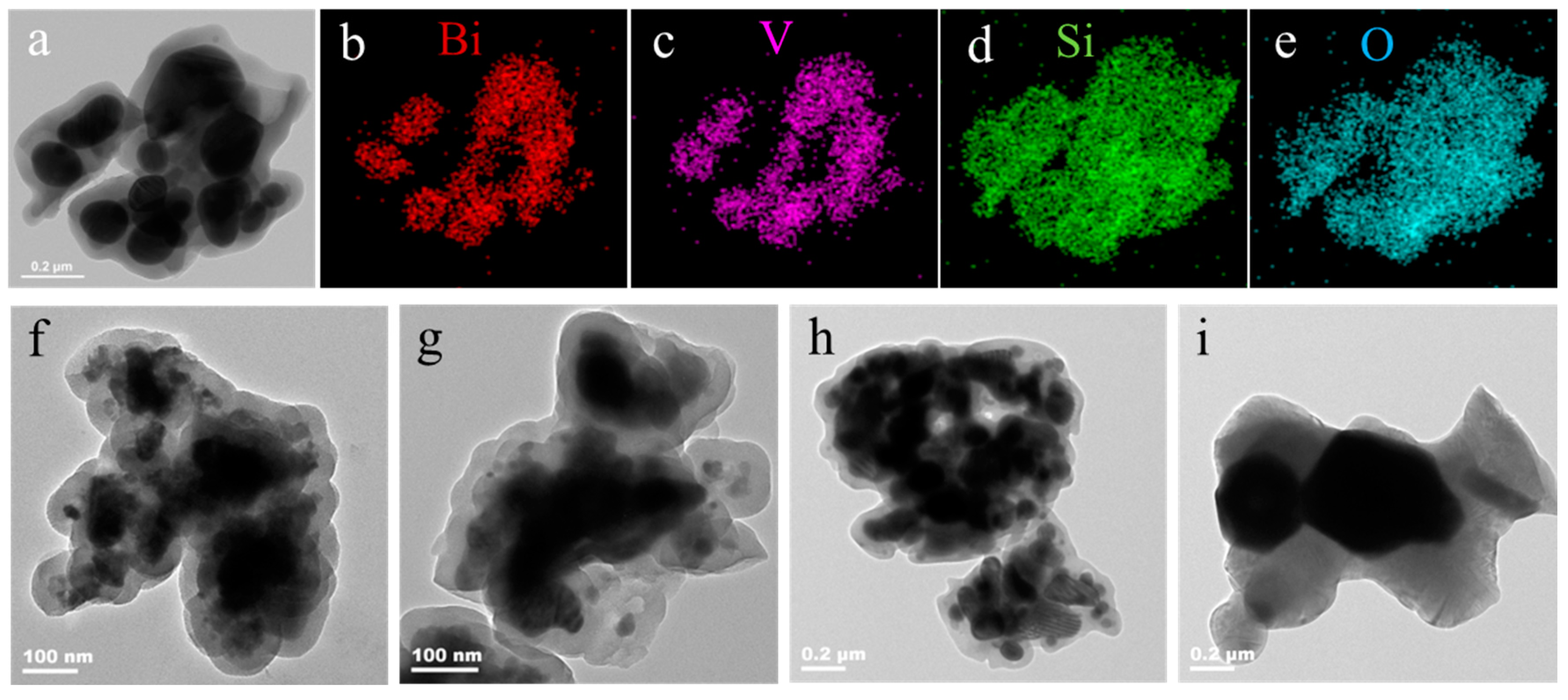
2.2. Phase Composition and Morphology of BiVO4@SiO2 Pigment
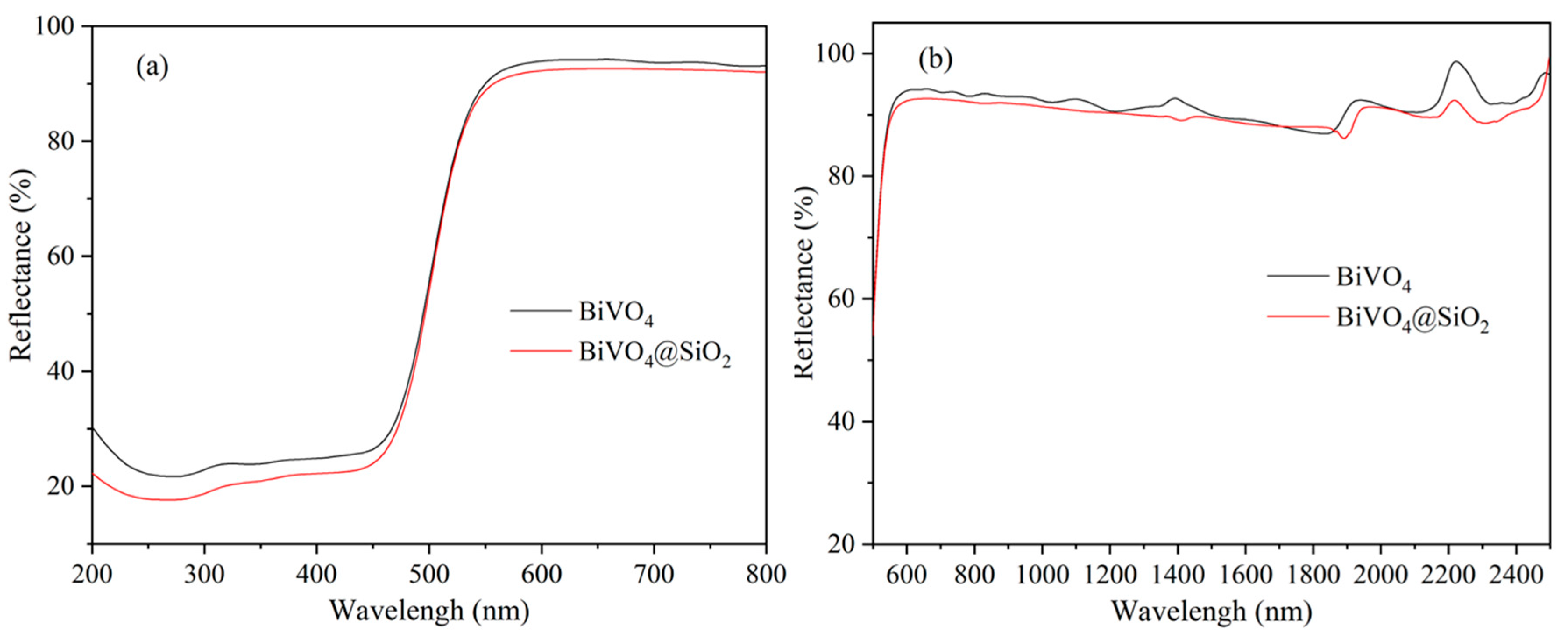
2.3. Chromatic and Spectroscopic Properties of BiVO4@SiO2 Pigments
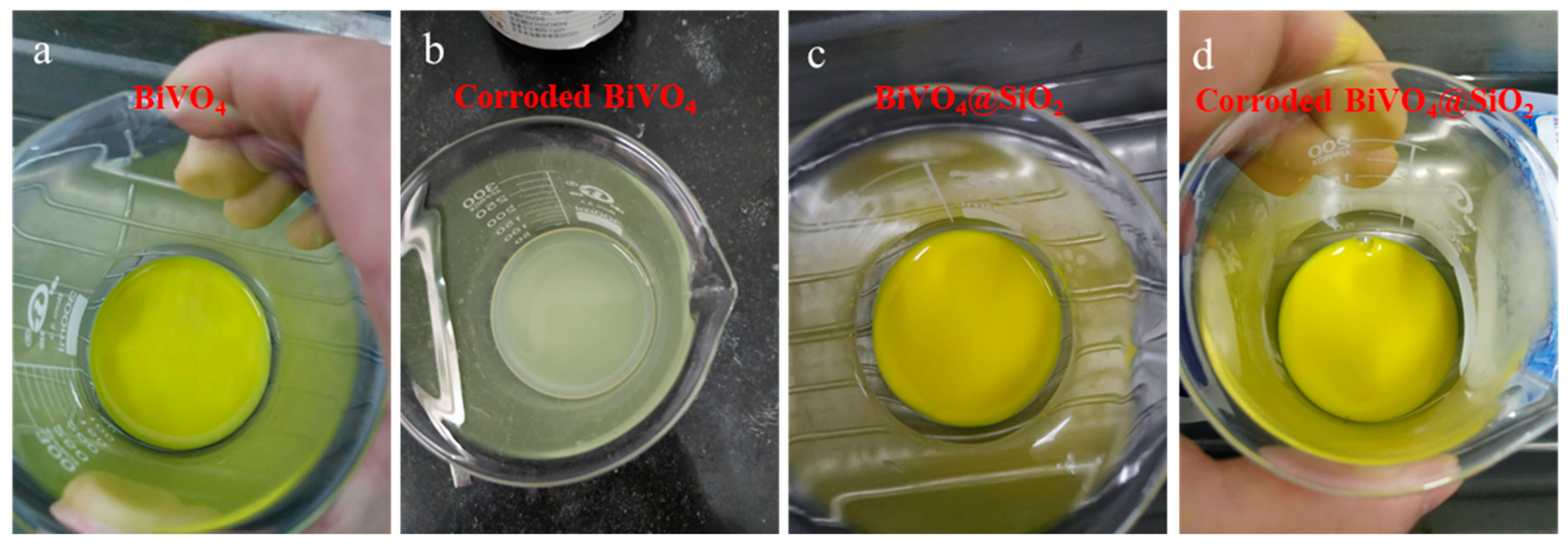
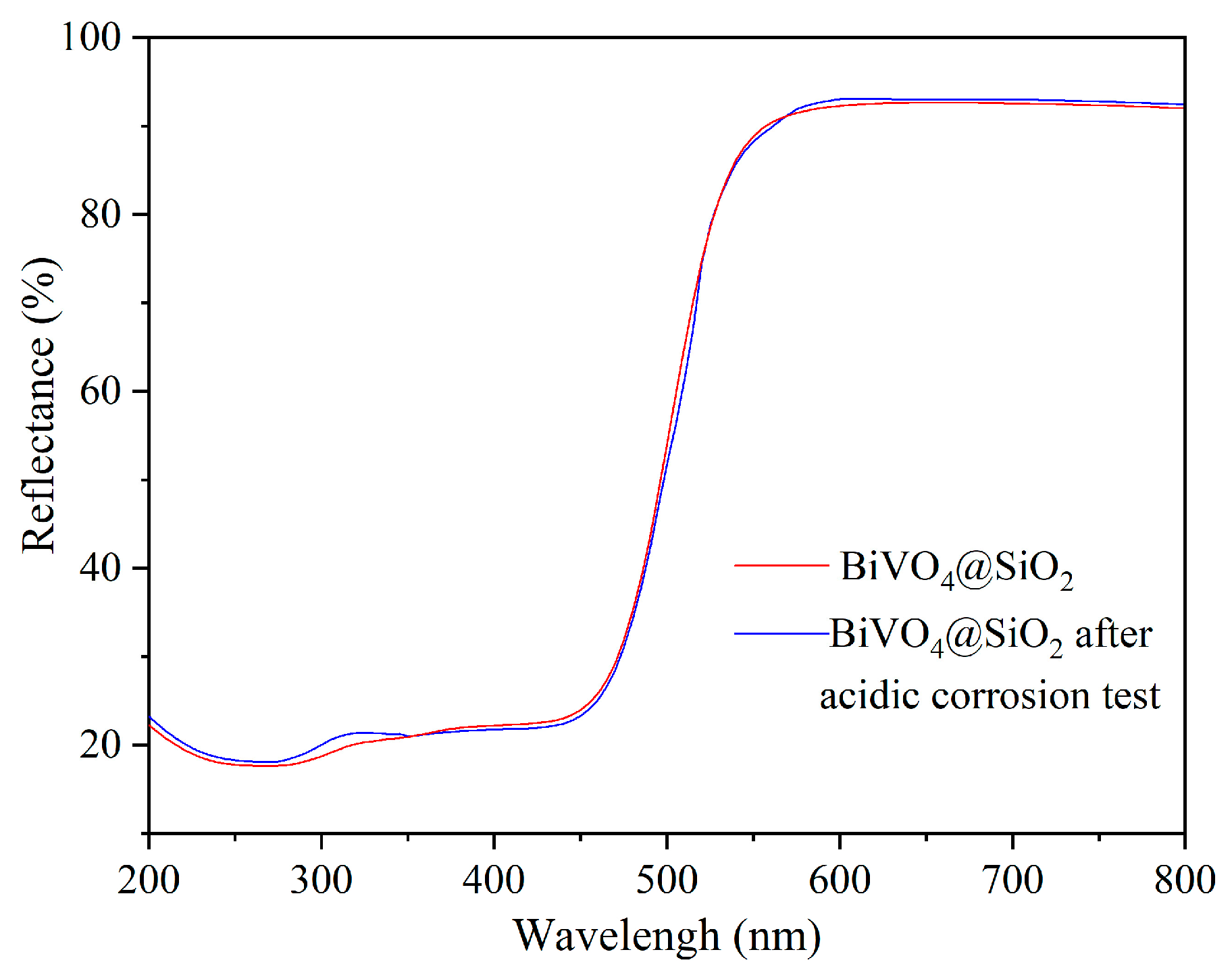
2.4. Evaluation of Thermal and Chemical Stability of BiVO4@SiO2 Pigment
3. Experimental Section
3.1. Preparation of Encapsulated Pigments
3.2. Materials’ Characterization
3.3. Colorimetric Measurements
3.4. Stability Tests
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Masui, T.; Honda, T.; Wendusu; Imanaka, N. Novel and environmentally friendly (Bi,Ca,Zn)VO4 yellow pigments. Dye. Pigment. 2013, 99, 636–641. [Google Scholar] [CrossRef]
- Pailhé, N.; Gaudon, M.; Demourgues, A. (Ca2+, V5+) co-doped Y2Ti2O7 yellow pigment. Mater. Res. Bull. 2009, 44, 1771–1777. [Google Scholar] [CrossRef]
- Wang, Q.; Seto, T.; Du, Y.; Liu, W.; Wang, Y. Design and preparation of high color rendering red inorganic pigment via red light emission phosphor. Opt. Mater. 2023, 140, 113886. [Google Scholar] [CrossRef]
- Blasco-Zarzoso, S.; Beltrán-Mir, H.; Cordoncillo, E. Su stainable inorganic pigments with high near-infra-red reflectance based on Fe3+ doped YAlO3 for high temperature applications. J. Alloys Compd. 2023, 960, 170695. [Google Scholar] [CrossRef]
- Zou, J.; Chen, Y.; Zhang, P. Influence of crystallite size on color properties and NIR reflectance of TiO2@NiTiO3 inorganic pigments. Ceram. Int. 2021, 47, 12661–12666. [Google Scholar] [CrossRef]
- Liu, H.; Wang, Q.; Chang, Q.; Wang, C.; Wang, Y.; Wang, Y.; Zhang, X. Relationship between the colour and particle size of the ultrafine V-ZrSiO4 and Pr-ZrSiO4 pigments and their mixture. Mater. Res. Express 2019, 6, 075214. [Google Scholar] [CrossRef]
- Zhou, X.; Zhang, X.; Zou, C.; Chen, R.; Cheng, L.; Han, B.; Liu, H. Insight into the Effect of Counterions on the Chromatic Properties of Cr-Doped Rutile TiO2-Based Pigments. Materials 2022, 15, 2049. [Google Scholar] [CrossRef]
- Kumar, B.; Sharma, R.; Bhoi, H.; Punia, K.; Kumar, S.; Barbar, S.K. Synthesis, structural, morphological and optical properties of environment friendly yellow inorganic pigment Bi4Zr3O12. Opt. Mater. 2023, 142, 114040. [Google Scholar] [CrossRef]
- Wang, Y.; Jiang, P.; Subramanian, M.A.; Cao, W. Synthesis, properties and applications of novel inorganic yellow pigments based on Ni-doped Al2TiO5. Solid State Sci. 2023, 135, 107088. [Google Scholar] [CrossRef]
- Thejusa, P.K.; Nishantha, K.G. Rational approach to synthesis low-cost BiVO4–ZnO complex inorganic pigment for energy efficient buildings. Sol. Energy Mater. Sol. Cells 2019, 200, 109999. [Google Scholar] [CrossRef]
- Sameera, S.; Rao, P.P.; James, V.; Divya, S.; Raj, A.K.V. Influence of (LiLa)1/2MoO4 substitution on the pigmentary properties of BiVO4. Dye. Pigment. 2014, 104, 41–47. [Google Scholar] [CrossRef]
- Sameera, S.F.; Rao, P.P.; Kumari, L.S.; James, V.; Divya, S. Potential NIR Reflecting Yellow Pigments in (BiV)1−x(YNb)xO4 Solid Solutions. Chem. Lett. 2013, 42, 521523. [Google Scholar] [CrossRef]
- Zhao, G.; Liu, W.; Dong, M.; Li, W.; Chang, L. Synthesis of monoclinic sheet-like BiVO4 with preferentially exposed (040) facets as a new yellow-green pigment. Dye. Pigment. 2016, 134, 91–98. [Google Scholar] [CrossRef]
- Kumari, L.S.; Rao, P.P.; Radhakrishnan, A.N.P.; James, V.; Sameera, S.; Koshy, P. Brilliant yellow color and enhanced NIR reflectance of monoclinic BiVO4 through distortion in VO43− tetrahedra. Sol. Energy Mater. Sol. Cells 2013, 112, 134–143. [Google Scholar] [CrossRef]
- Zhang, S.; Pan, Z.; Wang, Y. Synthesis and characterization of (Ni, Sb)-co-doped rutile ceramic pigment via mechanical activation-assisted solid-state reaction. Particuology 2018, 41, 20–29. [Google Scholar] [CrossRef]
- Sameera, S.; Rao, P.P.; Divya, S.; Raj, A.K.V. Brilliant IR reflecting yellow colorants in rare earth double molybdate substituted BiVO4 solid solutions for energy saving applications. ACS Sustain. Chem. Eng. 2015, 3, 1227–1233. [Google Scholar] [CrossRef]
- Tao, R.; Jiang, Y.; Liu, H.; Zhang, X.; Chen, R.; Chang, Q.; Wang, Y. Effects of metal ions doping on the chromatic performance of BiVO4 yellow pigments. J. Ceram. 2019, 40, 371–376. [Google Scholar]
- Tao, R.; Zhang, X.; Liu, H.; Jiang, Y.; Pan, T.; Chang, Q.; Wang, Y.; Zhou, J. Effect of Zn doping on the properties of nano BiVO4 yellow pigments. J. Synth. Cryst. 2019, 6, 1144–1149. [Google Scholar]
- Sameera, S.F.; Rao, P.P.; James, V.; Raj, A.K.V.; Chitradevi, G.R.; Leela, S. Probing structural variation and multifunctionality in niobium doped bismuth vanadate materials. Dalton Trans. 2014, 43, 15851–15860. [Google Scholar]
- Wendusu; Ken-ichi, I.; Toshiyuki, M.; Nobuhito, I. Novel Environment-friendly Yellow Pigments Based on (Bi, La)VO4. Chem. Lett. 2011, 40, 792–794. [Google Scholar] [CrossRef]
- Sameera, S.F.; Rao, P.P.; Kumari, L.S.; Koshy, P. New Scheelite-based Environmentally Friendly Yellow Pigments: (BiV)x(CaW)1−xO4. Chem. Lett. 2009, 38, 1088–1089. [Google Scholar] [CrossRef]
- Chang, Q.; Wang, X.; Wang, Y.; Bao, Q.; Zhou, J.; Zhu, Q. Encapsulated carbon black prepared by sol-gel-spraying: A new black ceramic pigment. J. Eur. Ceram. Soc. 2014, 34, 3151–3157. [Google Scholar] [CrossRef]
- Chen, T.; Jiang, W.; Zhang, X.; Liu, J.; Jiang, W.; Xie, Z. Ionic liquid-assisted synthesis of C@ZrSiO4 ceramic inclusion pigment. Mater. Sci. Forum 2016, 848, 256–261. [Google Scholar] [CrossRef]
- Tang, H.; Hu, Q.; Jiang, F.; Jiang, W.; Liu, J.; Chen, T.; Feng, G.; Wang, T.; Luo, W. Size control of C@ZrSiO4 pigments via soft mechano-chemistry assisted non-aqueous sol-gel method and their application in ceramic glaze. Ceram. Int. 2019, 45, 10756–10764. [Google Scholar] [CrossRef]
- Liu, S.; Li, Y.; Wang, Z.; Shen, Z.; Xie, Z. Enhanced high temperature oxidization resistance of silica coated γ-Ce2S3 red pigments. Appl. Surf. Sci. 2016, 387, 1147–1153. [Google Scholar] [CrossRef]
- Zhang, W.; Wang, Q.; Chen, X.; Li, X.; Long, Q.; Wang, C.; Liu, K.; Wang, Y.; Chang, Q. Synthesis of high color performance V-ZrSiO4 blue pigment with low doping amount via inorganic sol–gel route. Adv. Powder Technol. 2021, 32, 3355–3363. [Google Scholar] [CrossRef]
- Hu, Z.; Wang, Q.; Liu, J.; Liu, K.; Zhang, W.; Yang, Y.; Wang, Y.; Chang, Q. Synthesis and chromatic properties of V-doped and V/Y-codoped ZrO2 yellow pigments. J. Alloys Compd. 2021, 856, 157397. [Google Scholar] [CrossRef]
- Li, Y.; Liu, S.; Song, F.; Wang, Z.; Shen, Z.; Xie, Z. Preparation and thermal stability of silica layer multicoated γ-Ce2S3 red pigment microparticles. Surf. Coat. Technol. 2018, 345, 70–75. [Google Scholar] [CrossRef]
- Sultan, S.; Kareem, K.; He, L. Synthesis, characterization and resistant performance of α-Fe2O3@SiO2 composite as pigment protective coatings. Surf. Coat. Technol. 2016, 300, 42–49. [Google Scholar] [CrossRef]
- Huang, J.; Feng, L.; Cao, L. Sol-Gel Technology and Applications; Higher Education Press: Beijing, China, 2021; pp. 79–91. (In Chinese) [Google Scholar]
- Kweon, K.E.; Hwang, G.S. Structural phase-dependent hole localization and transport in bismuth vanadate. Phys. Rev. B 2013, 87, 205202. [Google Scholar] [CrossRef]
- Qin, W.; Wang, K.; Zhang, Y. Preparation of submicron CdSxSe1−x@ZrSiO4 inclusion pigment and its application in ink-jet printing. J. Eur. Ceram. Soc. 2021, 41, 7878–7885. [Google Scholar] [CrossRef]
- He, X.; Wang, F.; Liu, H.; Wang, X.; Gao, J. Synthesis and coloration of highly dispersive SiO2/BiVO4 hybrid pigments with low cost and high NIR reflectance. Nanotechnology 2019, 30, 295701. [Google Scholar] [CrossRef] [PubMed]
- Guan, L.; Fan, J.; Zhang, Y.; Guo, Y.; Duan, H.; Chen, Y.; Li, H.; Liu, H. Facile preparation of highly cost-effective BaSO4@BiVO4 core-shell structured brilliant yellow pigment. Dye. Pigment. 2016, 128, 49–53. [Google Scholar] [CrossRef]
- Kalarikkal, T.P.; Veedu, K.K.; Gopalan, N.K. Inorganic ferrite brown; transformation towards a versatile pigment for energy efficient constructions. J. Ind. Eng. Chem. 2023, 122, 349–358. [Google Scholar] [CrossRef]
- He, X.; Wang, F.; Liu, H.; Li, J.; Niu, L. Fabrication of highly dispersed NiTiO3@TiO2 yellow pigments with enhanced NIR reflectance. Mater. Lett. 2017, 208, 82–85. [Google Scholar] [CrossRef]


| Sample | Lattice Parameter | |||||
|---|---|---|---|---|---|---|
| a/Å | b/Å | c/Å | α/° | β/° | γ/° | |
| BiVO4 | 5.186 | 11.698 | 5.101 | 90.000 | 90.287 | 90.000 |
| BiVO4@SiO2 | 5.192 | 11.717 | 5.116 | 90.000 | 90.184 | 90.000 |
| Calcination Temperature | Color Parameter | |||||
|---|---|---|---|---|---|---|
| L* | a* | b* | C* | h° (°) | ΔE* 1 | |
| BiVO4 | 88.94 | −3.93 | 80.09 | 80.19 | 87.19 | / |
| 500 °C | 90.29 | −4.24 | 80.38 | 80.49 | 86.98 | 2.00 |
| 600 °C | 88.69 | −4.42 | 83.83 | 83.95 | 86.99 | 3.78 |
| 700 °C | 88.26 | −2.57 | 81.52 | 81.56 | 88.20 | 2.06 |
| 800 °C | 87.60 | 4.63 | 79.03 | 79.17 | 86.65 | 8.73 |
| Temperature (°C) | Sample | Chromatic Parameter | ||||
|---|---|---|---|---|---|---|
| L* | a* | b* | C* | h° (°) | ||
| 500 | BiVO4 | 88.21 | 0.21 | 79.91 | 79.91 | 89.85 |
| BiVO4@SiO2 | 89.71 | −4.28 | 82.43 | 82.54 | 87.03 | |
| 700 | BiVO4 | 78.48 | 11.28 | 62.31 | 63.32 | 79.74 |
| BiVO4@SiO2 | 87.72 | 2.73 | 80.89 | 80.93 | 88.07 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, R.; Zhang, X.; Tao, R.; Jiang, Y.; Liu, H.; Cheng, L. Preparation of Environmentally Friendly BiVO4@SiO2 Encapsulated Yellow Pigment with Remarkable Thermal and Chemical Stability. Inorganics 2024, 12, 17. https://doi.org/10.3390/inorganics12010017
Chen R, Zhang X, Tao R, Jiang Y, Liu H, Cheng L. Preparation of Environmentally Friendly BiVO4@SiO2 Encapsulated Yellow Pigment with Remarkable Thermal and Chemical Stability. Inorganics. 2024; 12(1):17. https://doi.org/10.3390/inorganics12010017
Chicago/Turabian StyleChen, Renhua, Xiaozhen Zhang, Rui Tao, Yuhua Jiang, Huafeng Liu, and Lanlan Cheng. 2024. "Preparation of Environmentally Friendly BiVO4@SiO2 Encapsulated Yellow Pigment with Remarkable Thermal and Chemical Stability" Inorganics 12, no. 1: 17. https://doi.org/10.3390/inorganics12010017





