Survey of Main Group Metals and Metalloids in Cancer Treatment
Abstract
:1. Introduction
2. Properties of Metals and Their Significance for Biological Systems
- Tendency to exhibit charge variations: Metal ions exist mainly as positively charged species, although they can form cationic, anionic and neutral species which depends on the coordination environment, which can modify their charges. The positively charged metal ions bind to negatively charged biomolecules in physiological conditions. In the s- and p-block elements of the periodic table, this tendency appears for the IVA (+2, +4), VA (+3, +5) and VIA (+2, +4, +6) groups.
- Chemical bonds and structural geometries: Corresponding to organic biomolecules, metal complex compounds can aggregate to various coordination geometries, giving them unique polyhedral shapes. The bond lengths, bond angles and coordination states can vary; this is contingent on the metal ions and their oxidation states. The variation in the different geometries can also be observed for the s- and p-block elements; see below.
- Metal–bioligand interactions: Different kinds of metal–ligand interactions may exist in the body and these bio-interactions generally initiate complex formations where the properties of obtained complexes are different from those of the free bioligands and metal cations. This characteristic feature is especially pronounced for the metals and metalloids from the s- and p-blocks of the periodic table.
- Features of the acid–base properties: Characterized by high electron affinity and a large set of oxidation states, most metal cations can readily polarize the coordinated functional groups, therefore facilitating the hydrolysis processes. The oxidation states dramatically affect the acid–base properties. These alterations with the variations in the oxidation states are specifically pronounced in p-elements; for instance, selenium and tellurium occur in different chemical forms with varied bioactivity: selenates (SeO42−, HSeO4−, H2SeO4), selenides (H2Se, HSe−), selenites (SeO32−, HSeO3−, H2SeO3), parts of many enzymes and the tellurium oxyanions tellurite (TeO32−) and tellurate (TeO42−).
- Partially filled d-shells: For transition metals, the variable number of electrons in the d- or f-shell (for lanthanides and actinides) impacts the magnetic and electronic properties of the resulting metal complexes. Due to the incompleteness of d- and f-shells and the presence of energetically similar unfilled shells, d- and f-elements are predisposed to complexation reactions in which the complex compounds formed are usually colored and paramagnetic. When moving along a large period, an increase in the ability to chelate in both directions toward the center of the period is clearly observed and maximal complexation capacity is typical for the VIIIB group metals (Fe, Co, Ni, Pt, etc.), elements with unfilled d-subshells.
- Tendency to exhibit oxidation–reduction properties: Many transition metals are strong reducing agents with a strong predisposition to undergoing oxidation–reduction reactions. Their reducing capacity is determined by the electronic configuration structures and the size of the respective cations. Only heavy metals of the VIIIB and IB groups are termed noble because of their inertness. The oxidation states of metal cations are responsible for the modulation and the rational design of bio-coordination compounds. In biochemical redox catalytic reactions, metal cations are activators of the coordinated substrates. Compounds of p-block metals in the maximal oxidation state possess oxidative properties.
3. s-Elements and Their Compounds in Cancer Chemotherapy
4. p-Elements and Their Compounds as Anticancer Chemotherapeutics
4.1. Gallium
4.2. Indium
4.3. Thallium
4.4. Germanium
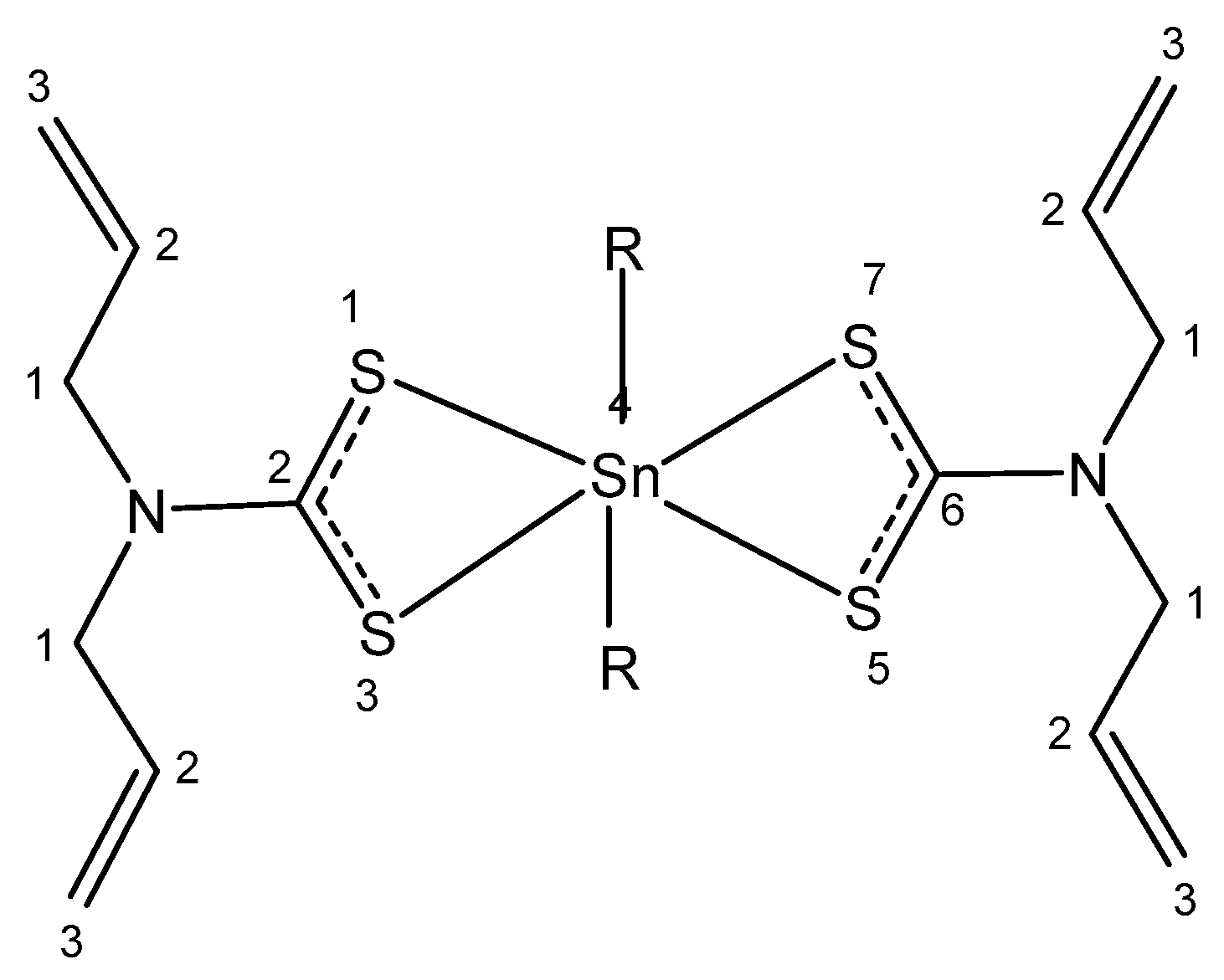
4.5. Tin
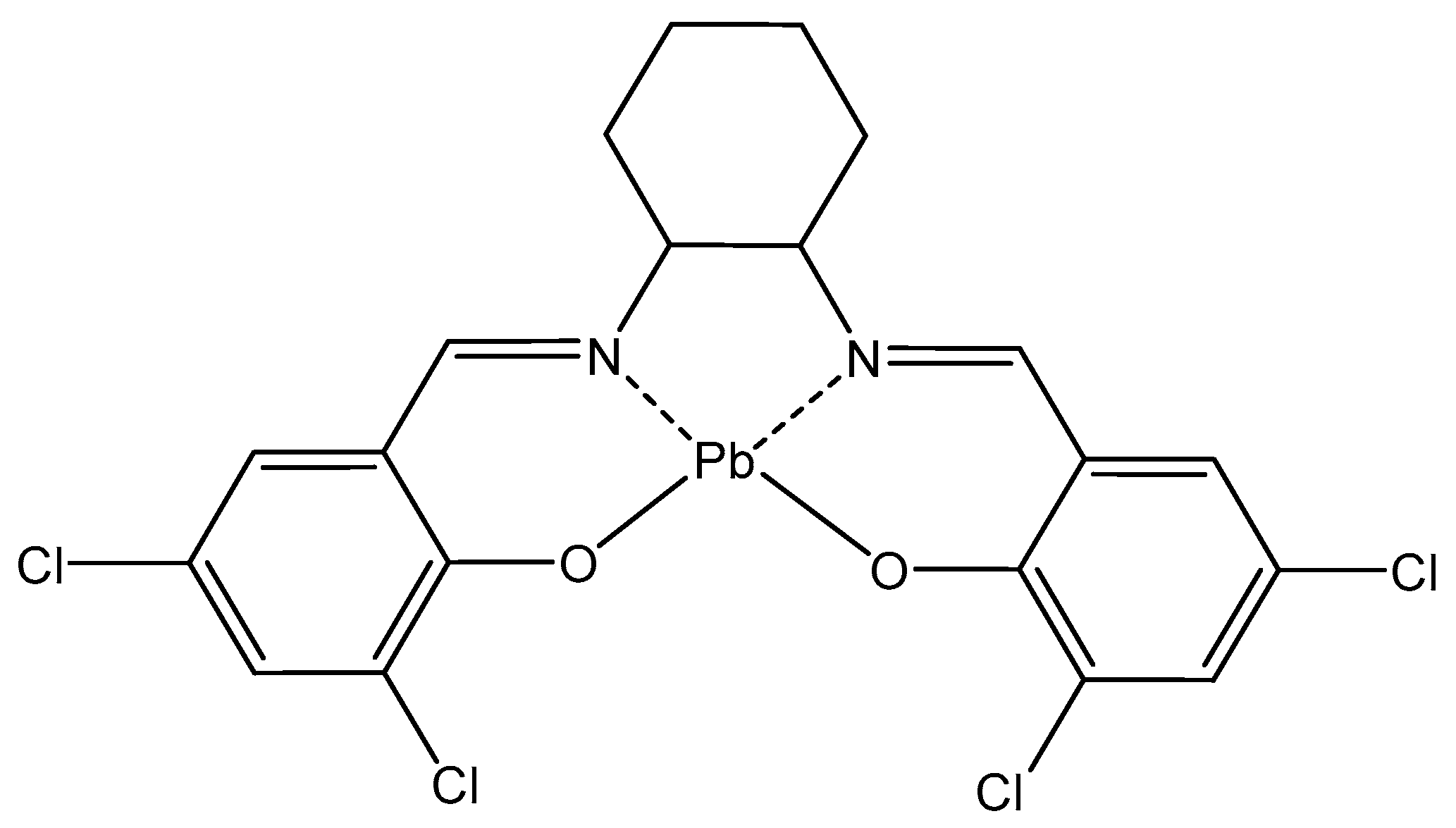
4.6. Lead
4.7. Arsenic
4.8. Antimony
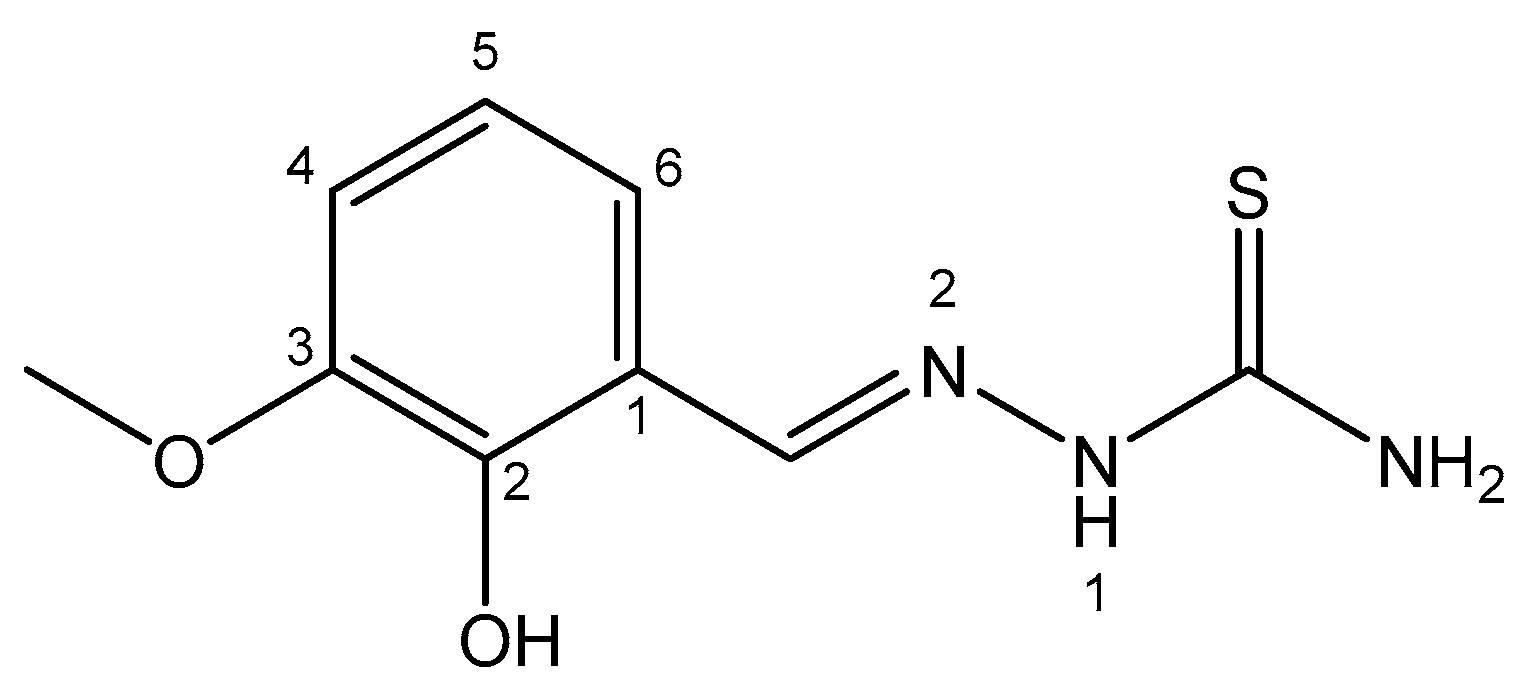
4.9. Bismuth
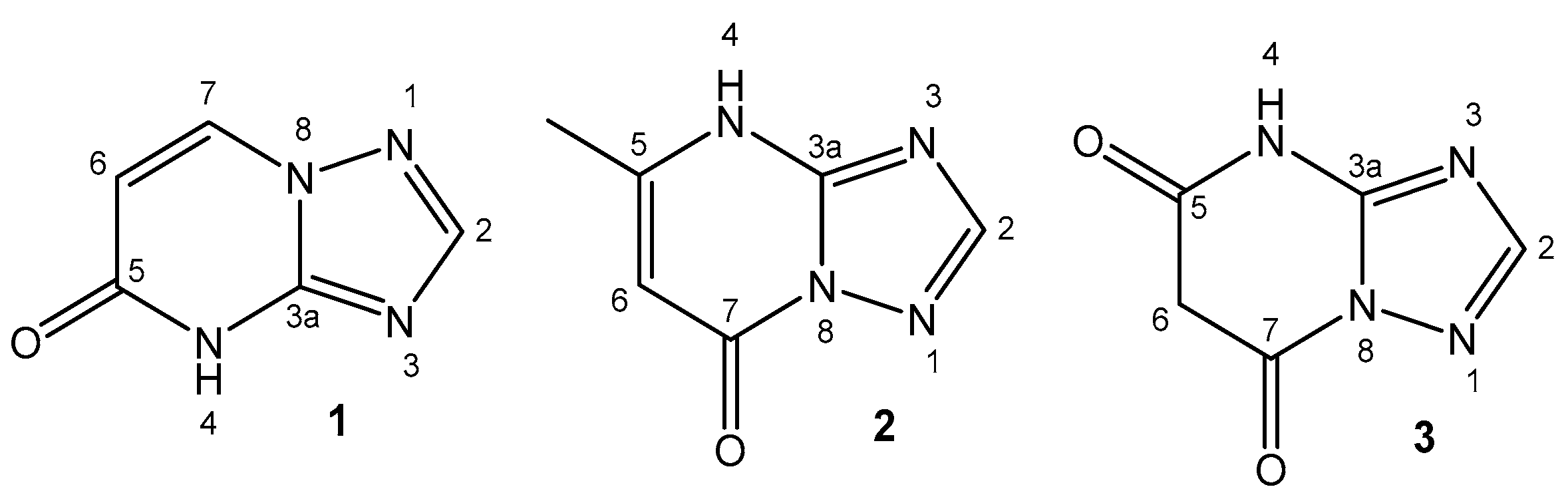
4.10. Selenium
4.11. Tellurium
4.12. Astatine
5. Conclusions
Funding
Acknowledgments
Conflicts of Interest
References
- Kenny, R.G.; Marmion, C.J. Toward multi-targeted platinum and ruthenium drugs—A new paradigm in cancer drug treatment regimens? Chem. Rev. 2019, 119, 1058–1137. [Google Scholar] [CrossRef] [PubMed]
- Coverdale, J.P.; Laroiya-McCarron, T.; Romero-Canelón, I. Designing ruthenium anticancer drugs: What have we learnt from the key drug candidates? Inorganics 2019, 7, 31. [Google Scholar] [CrossRef]
- Sohrabi, M.; Saeedi, M.; Larijani, B.; Mahdavi, M. Recent advances in biological activities of rhodium complexes: Their applications in drug discovery research. Eur. J. Med. Chem. 2021, 216, 113308. [Google Scholar] [CrossRef] [PubMed]
- Scattolin, T.; Voloshkin, V.A.; Visentin, F.; Nolan, S.P. A critical review of palladium organometallic anticancer agents. Cell Rep. Phys. Sci. 2021, 2, 100446. [Google Scholar] [CrossRef]
- Ma, D.L.; Wu, C.; Wu, K.J.; Leung, C.H. Iridium (III) complexes targeting apoptotic cell death in cancer cells. Molecules 2019, 24, 2739. [Google Scholar] [CrossRef]
- Nardon, C.; Boscutti, G.; Fregona, D. Beyond platinums: Gold complexes as anticancer agents. Anticancer Res. 2014, 34, 487–492. [Google Scholar] [PubMed]
- Soumya, R.S.; Hela, P.G. Nano silver based targeted drug delivery for treatment of cancer. Pharm. Lett. 2013, 5, 189–197. [Google Scholar]
- Skoupilova, H.; Hrstka, R.; Bartosik, M. Titanocenes as anticancer agents: Recent insights. Med. Chem. 2017, 13, 334–344. [Google Scholar] [CrossRef]
- Kowalski, S.; Wyrzykowski, D.; Inkielewicz-Stępniak, I. Molecular and cellular mechanisms of cytotoxic activity of vanadium compounds against cancer cells. Molecules 2020, 25, 1757. [Google Scholar] [CrossRef]
- Chundawat, N.S.; Jadoun, S.; Zarrintaj, P.; Chauhan, N.P.S. Lanthanide complexes as anticancer agents: A review. Polyhedron 2021, 207, 115387. [Google Scholar] [CrossRef]
- Kostova, I. Biological and Medical Significance of Chemical Elements; Bentham Science Publishers: Sharjah, United Arab Emirates, 2023. [Google Scholar]
- Kostova, I. The Role of Complexes of Biogenic Metals in Living Organisms. Inorganics 2023, 11, 56. [Google Scholar] [CrossRef]
- Goswami, A.K.; Kostova, I. Medicinal and Biological Inorganic Chemistry; Walter de Gruyter GmbH & Co KG: Berlin, Germany, 2022. [Google Scholar]
- Palmisciano, P.; Haider, A.S.; Balasubramanian, K.; D’Amico, R.S.; Wernicke, A.G. The role of cesium-131 brachytherapy in brain tumors: A scoping review of the literature and ongoing clinical trials. J. Neuro-Oncol. 2022, 159, 117–133. [Google Scholar] [CrossRef]
- Surdacka, A.; Stopa, J.; Torlinski, L. In situ effect of strontium toothpaste on artificially decalcified human enamel. Biol. Trace Elem. Res. 2007, 116, 147–153. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, S.P. The biological role of strontium. Bone 2004, 35, 583–588. [Google Scholar] [CrossRef]
- Querido, W.; Campos, A.P.; Martins Ferreira, E.H.; San Gil, R.A.; Rossi, A.M.; Farina, M. Strontium ranelate changes the composition and crystal structure of the biological bone-like apatite produced in osteoblast cell cultures. Cell Tissue Res. 2014, 357, 793–801. [Google Scholar] [CrossRef]
- Tomblyn, M. The role of bone-seeking radionuclides in the palliative treatment of patients with painful osteoblastic skeletal metastases. Cancer Control 2012, 19, 137–144. [Google Scholar] [CrossRef] [PubMed]
- Gunawardana, D.H.; Lichtenstein, M.; Better, N.; Rosenthal, M. Results of strontium-89 therapy in patients with prostate cancer resistant to chemotherapy. Clin. Nucl. Med. 2004, 29, 81–85. [Google Scholar] [CrossRef]
- Bruland, O.S.; Nilsson, S.; Fisher, D.R.; Larsen, R.H. High-linear energy transfer irradiation targeted to skeletal metastases by the α-emitter 223Ra: Adjuvant or alternative to conventional modalities? Clin. Cancer Res. 2006, 12, 6250s–6257s. [Google Scholar] [CrossRef]
- Lewington, V.J. Bone-seeking radionuclides for therapy. J. Nucl. Med. 2005, 46, 38S–47S. [Google Scholar]
- Henriksen, G.; Fisher, D.R.; Roeske, J.C.; Bruland, O.S.; Larsen, R.H. Targeting of osseous sites with α-emitting 223Ra: Comparison with the beta-emitter 89Sr in mice. J. Nucl. Med. 2003, 44, 252–259. [Google Scholar]
- Larsen, R.H.; Saxtorph, H.; Skydsgaard, M.; Borrebaek, J.; Jonasdottir, T.J.; Bruland, O.S.; Klastrup, S.; Harling, R.; Ramdahl, T. Radiotoxicity of the alpha-emitting bone-seeker 223Ra injected intravenously into mice: Histology, clinical chemistry and hematology. In Vivo 2006, 20, 325–331. [Google Scholar] [PubMed]
- Nilsson, S.; Strang, P.; Aksnes, A.K.; Franzèn, L.; Olivier, P.; Pecking, A.; Bruland, O.S. A randomized, dose-response, multicenter phase II study of radium-223 chloride for the palliation of painful bone metastases in patients with castration-resistant prostate cancer. Eur. J. Cancer 2012, 48, 678–686. [Google Scholar] [CrossRef]
- Peng, X.X.; Gao, S.; Zhang, J.L. Gallium(III) complexes in cancer chemotherapy. Eur. J. Inorg. Chem. 2022, 2022, e202100953. [Google Scholar] [CrossRef]
- Verron, E.; Bouler, J.M.; Scimeca, J.C. Gallium as a potential candidate for treatment of osteoporosis. Drug Discov. Today 2012, 17, 1127–1132. [Google Scholar] [CrossRef] [PubMed]
- Chitambar, C.R. Medical applications and toxicities of gallium compounds. Int. J. Environ. Res. Public Health 2010, 7, 2337–2361. [Google Scholar] [CrossRef]
- Leyland-Jones, B. Treating cancer-related hypercalcemia with gallium nitrate. J. Support. Oncol. 2004, 2, 509–516. [Google Scholar]
- Todorov, L.; Kostova, I.; Traykova, M. Lanthanum, Gallium and their Impact on Oxidative Stress. Curr. Med. Chem. 2019, 26, 4280–4295. [Google Scholar] [CrossRef]
- Wilke, N.L.; Abodo, L.O.; Frias, C.; Frias, J.; Baas, J.; Jakupec, M.A.; Keppler, B.K.; Prokop, A. The gallium complex KP46 sensitizes resistant leukemia cells and overcomes Bcl-2-induced multidrug resistance in lymphoma cells via upregulation of Harakiri and downregulation of XIAP in vitro. Biomed. Pharmacother. 2022, 156, 113974. [Google Scholar] [CrossRef]
- Hreusova, M.; Novohradsky, V.; Markova, L.; Kostrhunova, H.; Potočňák, I.; Brabec, V.; Kasparkova, J. Gallium (III) Complex with Cloxyquin Ligands Induces Ferroptosis in Cancer Cells and Is a Potent Agent against Both Differentiated and Tumorigenic Cancer Stem Rhabdomyosarcoma Cells. Bioinorg. Chem. Appl. 2022, 2022, 3095749. [Google Scholar] [CrossRef]
- Chitambar, C.R.; Antholine, W.E. Iron-targeting antitumor activity of gallium compounds and novel insights into triapine®-metal complexes. Antiox. Redox Signal. 2013, 18, 956–972. [Google Scholar] [CrossRef]
- Lessa, J.A.; Parrilha, G.L.; Beraldo, H. Gallium complexes as new promising metallodrug candidates. Inorg. Chim. Acta 2012, 393, 53–63. [Google Scholar] [CrossRef]
- Chitambar, C.R. Gallium-containing anticancer compounds. Fut. Med. Chem. 2012, 4, 1257–1272. [Google Scholar] [CrossRef]
- Qi, J.; Liu, T.; Zhao, W.; Zheng, X.; Wang, Y. Synthesis, crystal structure and antiproliferative mechanisms of gallium (III) complexes with benzoylpyridine thiosemicarbazones. RSC Adv. 2020, 10, 18553–18559. [Google Scholar] [CrossRef]
- Firmino, G.d.S.S.; Andre, S.C.; Hastenreiter, Z.; Campos, V.K.; Abdel-Salam, M.A.; de Souza-Fagundes, E.M.; Lessa, J.A. In vitro assessment of the cytotoxicity of Gallium (III) complexes with Isoniazid-Derived Hydrazones: Effects on clonogenic survival of HCT-116 cells. Inorg. Chim. Acta 2019, 497, 119079. [Google Scholar] [CrossRef]
- Robin, P.; Singh, K.; Suntharalingam, K. Gallium(III)-polypyridyl complexes as anti-osteosarcoma stem cell agents. Chem. Commun. 2020, 56, 1509–1512. [Google Scholar] [CrossRef] [PubMed]
- Litecká, M.; Hreusova, M.; Kašpárková, J.; Gyepes, R.; Smolková, R.; Obuch, J.; David, T.; Potočňák, I. Low-dimensional compounds containing bioactive ligands. Part XIV: High selective antiproliferative activity of tris (5-chloro-8-quinolinolato) gallium(III) complex against human cancer cell lines. Bioorg. Med. Chem. Lett. 2020, 30, 127206. [Google Scholar] [CrossRef]
- Banerjee, S.R.; Pomper, M.G. Clinical applications of gallium-68. Appl. Radiat. Isot. 2013, 76, 2–13. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.U.; Khan, S.; El-Refaie, S.; Win, Z.; Rubello, D.; Al-Nahhas, A. Clinical indications for gallium-68 positron emission tomography imaging. Eur. J. Surg. Oncol. 2009, 35, 561–567. [Google Scholar] [CrossRef]
- Nishiyama, Y.; Yamamoto, Y.; Toyama, Y.; Satoh, K.; Nagai, M.; Ohkawa, M. Usefulness of 67Ga scintigraphy in extranodal malignant lymphoma patients. Ann. Nucl. Med. 2003, 17, 657–662. [Google Scholar] [CrossRef]
- Beraldo, H. Pharmacological applications of non-radioactive indium(III) complexes: A field yet to be explored. Coord. Chem. Rev. 2020, 419, 213375. [Google Scholar] [CrossRef]
- Xu, X.; Liu, J.H.; Yuan, C.X.; Xing, Y.M.; Wang, M.; Zhang, Y.H.; Xu, F.R. Masses of ground and isomeric states of In 101 and configuration-dependent shell evolution in odd-A indium isotopes. Phys. Rev. C 2019, 100, 051303. [Google Scholar] [CrossRef]
- Goldsmith, S.J. Targeted radionuclide therapy: A historical and personal review. In Seminars in Nuclear Medicine; WB Saunders: Philadelphia, PA, USA, 2020; Volume 50, pp. 87–97. [Google Scholar]
- Kurdziel, K.A.; Mena, E.; McKinney, Y.; Wong, K.; Adler, S.; Sissung, T.; Lee, J.; Lipkowitz, S.; Lindenberg, L.; Turkbey, B.; et al. First-in-human phase 0 study of 111In-CHX-A"-DTPA trastuzumab for HER2 tumor imaging. J. Transl. Sci. 2019, 5, 10. [Google Scholar]
- Banerjee, S.R.; Kumar, V.; Lisok, A.; Plyku, D.; Nováková, Z.; Brummet, M.; Pomper, M.G. Evaluation of 111In-DOTA-5D3, a surrogate SPECT imaging agent for radioimmunotherapy of prostate-specific membrane antigen. J. Nucl. Med. 2019, 60, 400–406. [Google Scholar] [CrossRef]
- Eghtesadi, R.; Safavi, S.; Shahmirzayi, F.; Banafshe, H.R.; Omidi, S.; Ghaderi, A. A narrative review of thallium toxicity; preventive measures. Int. J. Pharm. Res. 2019, 11, 322–330. [Google Scholar]
- Campanella, B.; Colombaioni, L.; Benedetti, E.; Di Ciaula, A.; Ghezzi, L.; Onor, M.; Bramanti, E. Toxicity of thallium at low doses: A review. Int. J. Environ. Res. Public Health 2019, 16, 4732. [Google Scholar] [CrossRef]
- Genchi, G.; Carocci, A.; Lauria, G.; Sinicropi, M.S.; Catalano, A. Thallium use, toxicity, and detoxification therapy: An overview. Appl. Sci. 2021, 11, 8322. [Google Scholar] [CrossRef]
- Al Badarin, F.J.; Malhotra, S. Diagnosis and prognosis of coronary artery disease with SPECT and PET. Curr. Card. Rep. 2019, 21, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Azam, F.; Volcani, B.E. Germanium-silicon interactions in biological systems. In Silicon and Siliceous Structures in Biological Systems; Springer: New York, NY, USA, 1981; pp. 43–67. [Google Scholar]
- Tao, S.H.; Bolger, P.M. Hazard assessment of germanium supplements. Regul. Toxicol. Pharmacol. 1997, 25, 211–219. [Google Scholar] [CrossRef]
- Sabbioni, E.; Fortaner, S.; Bosisio, S.; Farina, M.; Del Torchio, R.; Edel, J.; Fischbach, M. Metabolic fate of ultratrace levels of GeCl4 in the rat and in vitro studies on its basal cytotoxicity and carcinogenic potential in Balb/3T3 and HaCaT cell lines. J. Appl. Toxicol. 2010, 30, 34–41. [Google Scholar] [CrossRef]
- Schauss, A.G. Nephrotoxicity in humans by the ultratrace element germanium. Ren. Fail. 1991, 13, 1–4. [Google Scholar] [CrossRef]
- Gerber, G.B.; Léonard, A. Mutagenicity, carcinogenicity and teratogenicity of germanium compounds. Mutat. Res. 1997, 387, 141–146. [Google Scholar] [CrossRef]
- Gerik, S.; Maypole, J. Overview of biologically based therapies in rehabilitation. In Complementary Therapies for Physical Therapy; Deutsch, J.E., Anderson, E.Z., Eds.; W.B. Saunders: Saint Louis, MO, USA, 2008; pp. 156–175. [Google Scholar]
- Menchikov, L.G.; Ignatenko, M.A. Biological activity of organogermanium compounds (a review). Pharm. Chem. J. 2013, 46, 635–638. [Google Scholar] [CrossRef]
- Aldridge, W.N. The toxicology and biological properties of organotin compounds. In Tin as a Vital Nutrient; CRC Press: Boca Raton, FL, USA, 2019; pp. 245–262. [Google Scholar]
- Kucklick, J.R.; Ellisor, M.D. A review of organotin contamination in arctic and subarctic regions. Emerg. Contamin. 2019, 5, 150–156. [Google Scholar] [CrossRef]
- Dodokhova, M.A.; Safronenko, A.V.; Kotieva, I.M.; Alkhuseyn-Kulyaginova, M.S.; Shpakovsky, D.B.; Milaeva, E.R. Impact of organotin compounds on the growth of epidermoid Lewis carcinoma. Res. Res. Pharmacol. 2021, 7, 81–88. [Google Scholar] [CrossRef]
- Alama, A.; Tasso, B.; Novelli, F.; Sparatore, F. Organometallic compounds in oncology: Implications of novel organotins as antitumor agents. Drug Discov. Today 2009, 14, 500–508. [Google Scholar] [CrossRef]
- Nath, M. Toxicity and the cardiovascular activity of organotin compounds: A review. Appl. Organomet. Chem. 2008, 22, 598–612. [Google Scholar] [CrossRef]
- Graisa, A.M.; Zainulabdeen, K.; Salman, I.; Al-Ani, A.; Mohammed, R.; Hairunisa, N.; Yousif, E. Toxicity and anti-tumour activity of organotin (IV) compounds. Baghdad J. Biochem. Appl. Biol. Sci. 2022, 3, 99–108. [Google Scholar] [CrossRef]
- Iornumbe, E.N.; Yiase, S.G.; Sha’Ato, R.; Tor-Anyiin, T.A. Synthesis, characterization and antifungal activity of some organotin(IV) derivatives of octanedioic acid. Int. J. Sci. Res. 2015, 4, 2095–2101. [Google Scholar]
- Allison, R.R.; Sibata, C.H. Oncologic photodynamic therapy photosensitizers: A clinical review. Photodiagn. Photodyn. Ther. 2010, 7, 61–75. [Google Scholar] [CrossRef]
- Flora, G.; Gupta, D.; Tiwari, A. Toxicity of lead: A review with recent updates. Interdiscip. Toxicol. 2012, 5, 47–58. [Google Scholar] [CrossRef]
- Kumar, A.; Kumar, A.; Cabral Pinto, M.M.S.; Chaturvedi, A.K.; Shabnam, A.A.; Subrahmanyam, G.; Yadav, K.K. Lead toxicity: Health hazards, influence on food chain, and sustainable remediation approaches. Int. J. Environ. Res. Public Health 2020, 17, 2179. [Google Scholar] [CrossRef] [PubMed]
- Matović, V.; Buha, A.; Dukić-Ćosić, D.; Bulat, Z. Insight into the oxidative stress induced by lead and/or cadmium in blood, liver and kidneys. Food Chem. Toxicol. 2015, 78, 130–140. [Google Scholar] [CrossRef] [PubMed]
- Kumar, V.; Dwivedi, S.K.; Oh, S. A critical review on lead removal from industrial wastewater: Recent advances and future outlook. J. Water Proc. Engin. 2022, 45, 102518. [Google Scholar] [CrossRef]
- Bjørklund, G.; Crisponi, G.; Nurchi, V.M.; Cappai, R.; Buha Djordjevic, A.; Aaseth, J. A review on coordination properties of thiol-containing chelating agents towards mercury, cadmium, and lead. Molecules 2019, 24, 3247. [Google Scholar] [CrossRef]
- Patrick, L. Lead toxicity, a review of the literature. Part 1: Exposure, evaluation, and treatment. Altern. Med. Rev. 2006, 11, 2–22. [Google Scholar] [PubMed]
- Tan, Z.; Chen, P.; Schneider, N.; Glover, S.; Cui, L.; Torgue, J.; Rixe, O.; Spitz, H.B.; Dong, Z. Significant systemic therapeutic effects of high-LET immunoradiation by 212Pb-trastuzumab against prostatic tumors of androgen-independent human prostate cancer in mice. Int. J. Oncol. 2012, 40, 1881–1888. [Google Scholar]
- Meredith, R.F.; Torgue, J.; Azure, M.T.; Shen, S.; Saddekni, S.; Banaga, E.; Alvarez, R. Pharmacokinetics and imaging of 212Pb-TCMC-trastuzumab after intraperitoneal administration in ovarian cancer patients. Cancer Biother. Radiopharm. 2014, 29, 12–17. [Google Scholar] [PubMed]
- Milenic, D.E.; Garmestani, K.; Brady, E.D.; Baidoo, K.E.; Albert, P.S.; Wong, K.J.; Flynn, J.; Brechbiel, M.W. Multimodality therapy: Potentiation of high linear energy transfer radiation with paclitaxel for the treatment of disseminated peritoneal disease. Clin. Cancer Res. 2008, 14, 5108–5115. [Google Scholar] [CrossRef]
- Prakash, S.; Verma, A.K. Arsenic: It’s Toxicity and Impact on Human health. Int. J. Biol. Innov. 2021, 3, 38–47. [Google Scholar] [CrossRef]
- Flora, S.J.S. Preventive and therapeutic strategies for acute and chronic human arsenic exposure. In Arsenic in Drinking Water and Food; Springer: Dordrecht, The Netherlands, 2020. [Google Scholar]
- Rajavardhan, R.; Mamadapur, A.; Shyamala, N. Acute Arsenic Suicidal Poisoning—A Rare Case. Int. J. Med. Dent. Sci. 2021, 10, 1961–1965. [Google Scholar] [CrossRef]
- Naujokas, M.F.; Anderson, B.; Ahsan, H.; Aposhian, H.V.; Graziano, J.H.; Thompson, C.; Suk, W.A. The broad scope of health effects from chronic arsenic exposure: Update on a worldwide public health problem. Environ. Health Perspect. 2013, 121, 295–302. [Google Scholar] [CrossRef] [PubMed]
- Fang, J.; Chen, S.-J.; Tong, J.-H.; Wang, Z.-G.; Chen, G.-Q.; Chen, Z. Treatment of acute promyelocytic leukemia with ATRA and As2O3. Cancer Biol. Ther. 2002, 1, 614–620. [Google Scholar] [CrossRef] [PubMed]
- Blower, P.J. 30 Inorganic pharmaceuticals. Annu. Rep. Prog. Chem. Sect. A 2004, 100, 633–658. [Google Scholar] [CrossRef]
- Bisser, S.; N’Siesi, F.-X.; Lejon, V.; Preux, P.-M.; Van Nieuwenhove, S.; Miaka Mia Bilenge, C.; Büscher, P. Equivalence trial of melarsoprol and nifurtimox monotherapy and combination therapy for the treatment of second-stage Trypanosoma brucei gambiense sleeping sickness. J. Infect. Dis. 2007, 195, 322–329. [Google Scholar] [CrossRef]
- Garnier, N.; Redstone, G.G.; Dahabieh, M.S.; Nichol, J.N.; del Rincon, S.V.; Gu, Y.; Miller, W.H. The novel arsenical darinaparsin is transported by cystine importing systems. Mol. Pharmacol. 2014, 85, 576–585. [Google Scholar] [CrossRef]
- Lloyd, N.C.; Morgan, H.W.; Nicholson, B.K.; Ronimus, R.S. The composition of Ehrlich’s salvarsan: Resolution of a century-old debate. Angew. Chem. Int. Ed. Engl. 2005, 44, 941–944. [Google Scholar] [CrossRef]
- Sanders, V.A.; Cutler, C.S. Radioarsenic: A promising theragnostic candidate for nuclear medicine. Nucl. Med. Biol. 2021, 92, 184–201. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Shen, C.; Zhang, X.; Yu, H.; Wang, F.; Wang, Y.; Zhang, L.W. Exposure and nephrotoxicity concern of bismuth with the occurrence of autophagy. Toxicol. Ind. Health 2018, 34, 188–199. [Google Scholar] [CrossRef]
- Li, H.; Sun, H. Recent advances in bioinorganic chemistry of bismuth. Curr. Opin. Chem. Biol. 2012, 16, 74–83. [Google Scholar] [CrossRef]
- Alkim, H.; Koksal, A.R.; Boga, S.; Sen, I.; Alkim, C. Role of bismuth in the eradication of Helicobacter pylori. Am. J. Therap. 2017, 24, e751–e757. [Google Scholar] [CrossRef]
- Thomas, F.; Bialek, B.; Hensel, R. Medical use of bismuth: The two sides of the coin. J. Clin. Toxicol. 2011, 3, 004. [Google Scholar]
- Shiotani, A.; Roy, P.; Lu, H.; Graham, D.Y. Helicobacter pylori diagnosis and therapy in the era of antimicrobial stewardship. Therap. Adv. Gastroenterol. 2021, 14, 17562848211064080. [Google Scholar] [CrossRef]
- Wang, R.; Li, H.; Ip, T.K.Y.; Sun, H. Bismuth drugs as antimicrobial agents. In Advances in Inorganic Chemistry; Academic Press: Cambridge, MA, USA, 2020; Volume 75, pp. 183–205. [Google Scholar]
- Halani, S.; Wu, P.E. Salicylate toxicity from chronic bismuth subsalicylate use. BMJ Case Rep. 2020, 13, e236929. [Google Scholar] [CrossRef]
- Sadler, P.J.; Li, H.; Sun, H. Coordination chemistry of metals in medicine: Target sites for bismuth. Coord. Chem. Rev. 1999, 185–186, 689–709. [Google Scholar] [CrossRef]
- Xie, Y.; Pan, X.; Li, Y.; Wang, H.; Du, Y.; Xu, J.; Wang, J.; Zeng, Z.; Chen, Y.; Zhang, G.; et al. New single capsule of bismuth, metronidazole and tetracycline given with omeprazole versus quadruple therapy consisting of bismuth, omeprazole, amoxicillin and clarithromycin for eradication of Helicobacter pylori in duodenal ulcer patients: A Chinese prospective, randomized, multicentre trial. J. Antimicrob. Chemother. 2018, 73, 1681–1687. [Google Scholar] [PubMed]
- Shetu, S.A.; Sanchez-Palestino, L.M.; Rivera, G.; Bandyopadhyay, D. Medicinal bismuth: Bismuth-organic frameworks as pharmaceutically privileged compounds. Tetrahedron 2022, 129, 133117. [Google Scholar] [CrossRef]
- Kowalik, M.; Masternak, J.; Barszcz, B. Recent research trends on bismuth compounds in cancer chemo-and radiotherapy. Curr. Med. Chem. 2019, 26, 729–759. [Google Scholar] [CrossRef]
- Yang, N.; Sun, H. Biocoordination chemistry of bismuth: Recent advances. Coord. Chem. Rev. 2007, 251, 2354–2366. [Google Scholar] [CrossRef]
- Bartoli, M.; Jagdale, P.; Tagliaferro, A. A short review on biomedical applications of nanostructured bismuth oxide and related Nanomaterials. Materials 2020, 13, 5234. [Google Scholar] [CrossRef]
- Peng, Z.; Tang, J. Intestinal Infection of Candida albicans: Preventing the Formation of Biofilm by C. albicans and Protecting the Intestinal Epithelial Barrier. Front. Microbiol. 2022, 12, 4413. [Google Scholar] [CrossRef]
- Prakash, M.; Kavitha, H.P.; Abinaya, S.; Vennila, J.P.; Lohita, D. Green synthesis of bismuth-based nanoparticles and its applications—A review. Sust. Chem. Pharm. 2022, 25, 100547. [Google Scholar] [CrossRef]
- Rosenblat, T.L.; McDevitt, M.R.; Mulford, D.A.; Pandit-Taskar, N.; Divgi, C.R.; Panageas, K.S.; Jurcic, J.G. Sequential cytarabine and alpha-particle immunotherapy with bismuth-213-lintuzumab (HuM195) for acute myeloid leukemia. Clin. Cancer Res. 2010, 16, 5303–5311. [Google Scholar] [CrossRef] [PubMed]
- Jurcic, J. Alpha-Particle Therapy for Acute Myeloid Leukemia. J. Med. Imag. Rad. Sci. 2019, 50, S86–S87. [Google Scholar]
- Zare, O.; Afzalipour, R.; Yazdi, M.S.G. Review of Alloy Containing Bismuth Oxide Nanoparticles on X-Ray Absorption in Radiology Shields. Dis. Diagn. 2022, 11, 31–35. [Google Scholar] [CrossRef]
- Hu, W.; Zhao, C.; Hu, H.; Yin, S. Food sources of selenium and its relationship with chronic diseases. Nutrients 2021, 13, 1739. [Google Scholar] [CrossRef] [PubMed]
- Wesolowski, L.T.; Semanchik, P.L.; White-Springer, S.H. Beyond antioxidants: Selenium and skeletal muscle mitochondria. Front. Veter. Sci. 2022, 9, 1868. [Google Scholar] [CrossRef] [PubMed]
- Parish, L.; Parish, J.; Routh, H. Selenium sulfide in the 21st century. J. Am. Acad. Dermatol. 2008, 58, AB72. [Google Scholar]
- Radomska, D.; Czarnomysy, R.; Radomski, D.; Bielawski, K. Selenium compounds as novel potential anticancer agents. Int. J. Mol. Sci. 2021, 22, 1009. [Google Scholar] [CrossRef]
- Medina-Cruz, D.; Tien-Street, W.; Vernet-Crua, A.; Zhang, B.; Huang, X.; Murali, A.; Webster, T. Tellurium, the forgotten element: A review of the properties, processes, and biomedical applications of the bulk and nanoscale metalloid. In Racing for the Surface; Li, B., Moriarty, T., Webster, T., Xing, M., Eds.; Springer: Cham, Switzerland, 2020. [Google Scholar]
- Chiaverini, L.; Marzo, T.; La Mendola, D. AS101: An Overview on a Leading Tellurium-Based Prodrug. Inorg. Chim. Acta 2022, 540, 121048. [Google Scholar] [CrossRef]
- Halpert, G.; Eitan, T.; Voronov, E.; Apte, R.N.; Rath-Wolfson, L.; Albeck, M.; Kalechman, Y.; Sredni, B. Multifunctional activity of a small tellurium redox immunomodulator compound, AS101, on dextran sodium sulfate-induced murine colitis. J. Biol. Chem. 2014, 289, 17215–17227. [Google Scholar] [CrossRef]
- Makvandi, M.; Dupis, E.; Engle, J.W.; Nortier, F.M.; Fassbender, M.E.; Simon, S.; Norenberg, J.P. Alpha-emitters and targeted alpha therapy in oncology: From basic science to clinical investigations. Targ. Oncol. 2018, 13, 189–203. [Google Scholar] [CrossRef] [PubMed]
- Guerra Liberal, F.D.; O’Sullivan, J.M.; McMahon, S.J.; Prise, K.M. Targeted alpha therapy: Current clinical applications. Cancer Biother. Radiopharm. 2020, 35, 404–417. [Google Scholar] [CrossRef] [PubMed]
- Lindegren, S.; Albertsson, P.; Bäck, T.; Jensen, H.; Palm, S.; Aneheim, E. Realizing clinical trials with astatine-211: The chemistry infrastructure. Cancer Biother. Radiopharm. 2020, 35, 425–436. [Google Scholar] [CrossRef] [PubMed]
- Zalutsky, M.R.; Pruszynski, M. Astatine-211: Production and availability. Curr. Radiopharm. 2011, 4, 177–185. [Google Scholar] [CrossRef] [PubMed]
- Vaidyanathan, G.; Zalutsky, M.R. Astatine radiopharmaceuticals: Prospects and problems. Curr. Radiopharm. 2008, 1, 177–196. [Google Scholar] [CrossRef] [PubMed]
- Reilly, S.W.; Makvandi, M.; Xu, K.; Mach, R.H. Rapid Cu-Catalyzed [211At]Astatination and [125I]Iodination of Boronic Esters at Room Temperature. Org. Lett. 2018, 20, 1752–1755. [Google Scholar] [CrossRef]
- Mohammadi, K.; Thompson, K.H.; Patrick, B.O.; Storr, T.; Martins, C.; Polishchuk, E.; Yuen, V.G.; McNeill, J.H.; Orvig, C. Synthesis and Characterization of Dual Function Vanadyl, Gallium and Indium Curcumin Complexes for Medicinal Applications. J. Inorg. Biochem. 2005, 99, 2217–2225. [Google Scholar] [CrossRef] [PubMed]
- Meza-Morales, W.; Alvarez-Ricardo, Y.; Pérez-González, L.L.; Tavera-Hernández, R.; Ramírez-Apan, M.T.; Toscano, R.A.; Sánchez-Obregón, R.; Obregón-Mendoza, M.A.; Enríquez, R.G. First Gallium and Indium Crystal Structures of Curcuminoid Homoleptic Complexes: All-Different Ligand Stereochemistry and Cytotoxic Potential. Int. J. Mol. Sci. 2023, 24, 16324. [Google Scholar] [CrossRef]
- Shorkaei, M.R.; Asadi, Z.; Asadi, M. Synthesis, characterization, molecular docking and DNA binding studies of Al (III), Ga (III) and In (III) water-soluble complexes. J. Mol. Struct. 2016, 1109, 22–30. [Google Scholar] [CrossRef]
- Yao, S.; Jiang, J.; Yang, P.; Cai, J.-Y. Synthesis, characterization and antioxidant activity of a novel organogermanium sesquioxide with resveratrol. Bull. Korean Chem. Soc. 2012, 33, 1121–1122. [Google Scholar] [CrossRef]
- Menchikov, L.G.; Popov, A.V. Physiological Activity of Trace Element Germanium Including Anticancer Properties. Biomedicines 2023, 11, 1535. [Google Scholar] [CrossRef] [PubMed]
- Oh, C.; Li, M.; Kim, E.H.; Park, J.S.; Lee, J.C.; Ham, S.W. Antioxidant and Radical Scavenging Activities of Ascorbic Acid Derivatives Conjugated with Organogermanium. Bull. Korean Chem. Soc. 2010, 31, 3513–3514. [Google Scholar] [CrossRef]
- Jiang, J.; Yao, S.; Cai, H.-H.; Yang, P.-H.; Cai, J. Synthesis and synergetic effects of chrysin–organogermanium (IV) complex as potential anti-oxidant. Bioorg. Med. Chem. Lett. 2013, 23, 5727–5732. [Google Scholar] [CrossRef] [PubMed]
- Lu, P.; Yao, S.; Cai, J.; Yang, P.-H. Synthesis and synergetic anti-tumor activity evaluation of dihydroartemisininorganogermanium(IV) compound. Bioorg. Med. Chem. Lett. 2014, 24, 5294–5297. [Google Scholar] [CrossRef]
- Pi, J.; Zeng, J.; Luo, J.-J.; Yang, P.-H.; Cai, J.-Y. Synthesis and biological evaluation of Germanium(IV)–polyphenol complexes as potential anti-cancer agents. Bioorg. Med. Chem. Lett. 2013, 23, 2902–2908. [Google Scholar] [CrossRef]
- Syed Annuar, S.N.; Kamaludin, N.F.; Awang, N.; Chan, K.M. Cellular Basis of Organotin(IV) Derivatives as Anticancer Metallodrugs: A Review. Front. Chem. 2021, 9, 657599. [Google Scholar] [CrossRef] [PubMed]
- Devi, J.; Yadav, J.; Kumar, D.; Jindal, D.K.; Basu, B. Synthesis, Spectral Analysis and In Vitro Cytotoxicity of Diorganotin (IV) Complexes Derived from Indole-3-Butyric Hydrazide. Appl. Organomet. Chem. 2020, 34, e5815. [Google Scholar] [CrossRef]
- Liu, K.; Yan, H.; Chang, G.; Li, Z.; Niu, M.; Hong, M. Organotin(IV) Complexes Derived from Hydrazone Schiff Base: Synthesis, crystal Structure, In Vitro Cytotoxicity and DNA/BSA Interactions. Inorg. Chim. Acta 2017, 464, 137–146. [Google Scholar] [CrossRef]
- Adeyemi, J.O.; Onwudiwe, D.C.; Ekennia, A.C.; Anokwuru, C.P.; Nundkumar, N.; Singh, M. Synthesis, Characterization and Biological Activities of Organotin(IV) Diallyldithiocarbamate Complexes. Inorg. Chim. Acta 2019, 485, 64–72. [Google Scholar] [CrossRef]
- Attanzio, A.; D’Agostino, S.; Busà, R.; Frazzitta, A.; Rubino, S.; Girasolo, M.A. Cytotoxic Activity of Organotin(IV) Derivatives with Triazolopyrimidine Containing Exocyclic Oxygen Atoms. Molecules 2020, 25, 859. [Google Scholar] [CrossRef]
- Khandani, M.; Sedaghat, T.; Erfani, N.; Haghshenas, M.R.; Khavasi, H.R. Synthesis, Spectroscopic Characterization, Structural Studies and Antibacterial and Antitumor Activities of Diorganotin Complexes with 3- methoxysalicylaldehyde Thiosemicarbazone. J. Mol. Struct. 2013, 1037, 136–143. [Google Scholar] [CrossRef]
- Priya, J.; Madheswari, D. Biomolecular docking interactions, cytotoxicity and antioxidant property evaluations with novel Mn(II), Ni(II), Cd(II) and Pb(II) Schiff base ligand complexes: Synthesis and characterization. J. Biosci. 2022, 47, 29. [Google Scholar] [CrossRef]
- Yang, K.N.; Yuan, K.D.; Jiang, L.L.; Zhang, Y. A new Pb (II)-based coordination polymer constructed from a semirigid tricarboxylate ligand: Crystal structure and anti-lung cancer activity. Main Group Met. Chem. 2019, 42, 8–12. [Google Scholar] [CrossRef]
- Lv, L.L.; Xia, W.M.; Cheng, Y.Z.; Zhang, L.P.; Wang, X.D. Synthesis, structure, DNA binding and anticancer activity of a new tetranuclear Pb (II) complex constructed by 8-hydroxyquinolinate and 4-nitrobenzoate ligands. Main Group Met. Chem. 2019, 42, 60–66. [Google Scholar] [CrossRef]
- Müller, S.; Miller, W.H., Jr.; Dejean, A. Trivalent Antimonials Induce Degradation of the PML-RARα Oncoprotein and Reorganization of the Promyelocytic Leukemia Nuclear Bodies in Acute Promyelocytic Leukemia NB4 Cells. Blood J. Am. Soc. Hematol. 1998, 92, 4308–4316. [Google Scholar]
- Islam, A.; Rodrigues, B.L.; Marzano, I.M.; Perreira-Maia, E.C.; Dittz, D.; Lopes, M.T.P.; Demicheli, C. Cytotoxicity and apoptotic activity of novel organobismuth (V) and organoantimony (V) complexes in different cancer cell lines. Eur. J. Med. Chem. 2016, 109, 254–267. [Google Scholar] [CrossRef]
- Ouyang, R.; Yang, Y.; Tong, X.; Feng, K.; Yang, Y.; Tao, H.; Zhou, S. Potent anticancer activity of a new bismuth (III) complex against human lung cancer cells. J. Inorg. Biochem. 2017, 168, 18–26. [Google Scholar] [CrossRef]
- Khan, M.H.; Cai, M.; Li, S.; Zhang, Z.; Zhang, J.; Wen, X.; Yang, F. Developing a binuclear multi-target Bi(III) complex by optimizing 2-acetyl-3-ethylpyrazine thiosemicarbazides. Eur. J. Med. Chem. 2019, 182, 111616. [Google Scholar] [CrossRef]
- Li, M.; Lu, Y.; Yang, M.; Li, Y.; Zhang, L.; Xie, S. One dodecahedral bismuth(III) complex derived from 2- acetylpyridine N(4)-pyridylthiosemicarbazone: Synthesis, crystal structure and biological evaluation. Dalton Trans. 2012, 41, 12882–12887. [Google Scholar] [CrossRef]
- Zhang, L.-Z.; An, G.-Y.; Yang, M.; Li, M.-X.; Zhu, X.-F. Synthesis, characterization, crystal structure and biological activities of the unusual main group 8-coordinate bismuth(III) complex derived from 2-acetylpyrazine N4- pyridylthiosemicarbazone. Inorg. Chem. Commun. 2012, 20, 37–40. [Google Scholar] [CrossRef]
- Li, Y.-K.; Yang, M.; Li, M.-X.; Yu, H.; Wu, H.-C.; Xie, S.-Q. Synthesis, crystal structure and biological evaluation of a main group seven-coordinated bismuth(III) complex with 2- acetylpyridine N4-phenylthiosemicarbazone. Bioorg. Med. Chem. Lett. 2013, 23, 2288–2292. [Google Scholar] [CrossRef] [PubMed]
- Parrilha, G.L.; Ferraz, K.S.O.; Lessa, J.A.; de Oliveira, K.N.; Rodrigues, B.L.; Ramos, J.P.; Souza-Fagundes, E.M.; Ott, I.; Beraldo, H. Metal complexes with 2-acetylpyridineN(4)-orthochlorophenylthiosemicarbazone: Cytotoxicity and effect on the enzymatic activity of thioredoxin reductase and glutathione reductase. Eur. J. Med. Chem. 2014, 84, 537–544. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, I.P.; Piló, E.D.L.; Recio-Despaigne, A.A.; Da Silva, J.G.; Ramos, J.P.; Marques, L.B.; Prazeres, P.H.D.M.; Takahashi, J.A.; Souza-Fagundes, E.M.; Rocha, W.; et al. Bismuth(III) complexes with 2- acetylpyridine- and 2-benzoylpyridine-derived hydrazones: Antimicrobial and cytotoxic activities and effects on the clonogenic survival of human solid tumor cells. Bioorg. Med. Chem. 2016, 24, 2988–2998. [Google Scholar] [CrossRef] [PubMed]
- Ferraz, K.S.O.; Silva, N.F.; da Silva, J.G.; de Miranda, L.F.; Romeiro, C.F.D.; Souza-Fagundes, E.M.; Mendes, I.C.; Beraldo, H. Investigation on the pharmacological profile of 2,6-diacetylpyridine bis(benzoylhydrazone) derivatives and their antimony(III) and bismuth(III) complexes. Eur. J. Med. Chem. 2012, 53, 98–106. [Google Scholar] [CrossRef]
- Zhang, N.; Tai, Y.; Li, M.; Ma, P.; Zhao, J.; Niu, J. Main group bismuth(III), gallium(III) and diorganotin(IV) complexes derived from bis(2- acetylpyrazine)thiocarbonohydrazone: Synthesis, crystal structures and biological evaluation. Dalton Trans. 2014, 43, 5182–5189. [Google Scholar] [CrossRef]
- Ozturk, I.I.; Banti, C.N.; Kourkoumelis, N.; Manos, M.J.; Tasiopoulos, A.J.; Owczarzak, A.M.; Kubicki, M.; Hadjikakou, S.K. Synthesis, characterization and biological activity of antimony(III) or bismuth(III) chloride complexes with dithiocarbamate ligands derived from thiuram degradation. Polyhedron 2014, 67, 89–103. [Google Scholar] [CrossRef]
- Tiekink, E.R.T. Antimony and bismuth compounds in oncology. Crit. Rev. Oncol. Hematol. 2002, 42, 217–224. [Google Scholar] [CrossRef]
- Zhang, X.W.; Xia, J.; Yan, H.W.; Luo, S.L.; Yin, S.F.; Au, C.T.; Wong, W.Y. Synthesis, structure, and in vitro antiproliferative activity of cyclic hypervalent organobismuth(III) chlorides and their triphenylgermylpropionate derivatives. J. Organomet. Chem. 2009, 694, 3019–3026. [Google Scholar] [CrossRef]
- Hariharan, S.; Dharmaraj, S. Selenium and selenoproteins: It’s role in regulation of inflammation. Inflammopharmacology 2020, 28, 667–695. [Google Scholar] [CrossRef]
- Garbo, S.; Di Giacomo, S.; Łazewska, D.; Honkisz-Orzechowska, E.; Di Sotto, A.; Fioravanti, R.; Zwergel, C.; Battistelli, C. Selenium-Containing Agents Acting on Cancer—A New Hope? Pharmaceutics 2023, 15, 104. [Google Scholar] [CrossRef]
- Ali, W.; Benedetti, R.; Handzlik, J.; Zwergel, C.; Battistelli, C. The innovative potential of selenium-containing agents for fighting cancer and viral infections. Drug Discov. Today 2021, 26, 256–263. [Google Scholar] [CrossRef] [PubMed]
- Domínguez-Álvarez, E.; Rácz, B.; Marć, M.A.; Nasim, M.J.; Szemerédi, N.; Viktorová, J.; Spengler, G. Selenium and tellurium in the development of novel small molecules and nanoparticles as cancer multidrug resistance reversal agents. Drug Resist. Updates 2022, 63, 100844. [Google Scholar] [CrossRef] [PubMed]
- Wieslander, E.; Engman, L.; Svensjö, E.; Erlansson, M.; Johansson, U.; Linden, M.; Brattsand, R. Antioxidative properties of organotellurium compounds in cell systems. Biochem. Pharmacol. 1998, 55, 573–584. [Google Scholar] [CrossRef] [PubMed]
- Gross, M.; Stanciu, E.; Kenigsbuch-Sredni, D.; Sredni, B.; Pinhasov, A. The immunomodulatory tellurium compound ammonium trichloro (dioxoethylene-O,O’) tellurate reduces anxiety-like behavior and corticosterone levels of submissive mice. Behav. Pharmacol. 2017, 28, 458–465. [Google Scholar] [CrossRef]
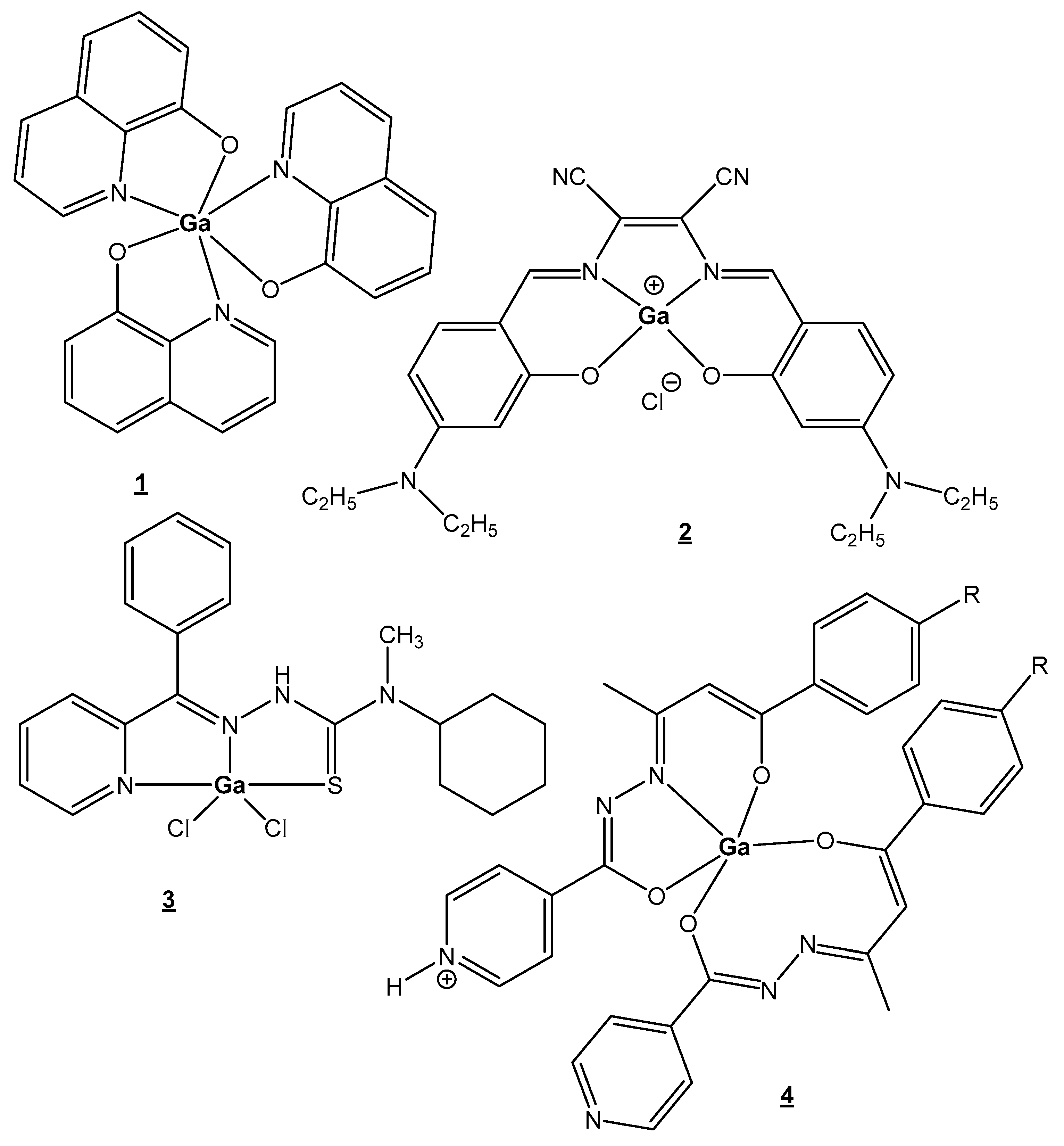
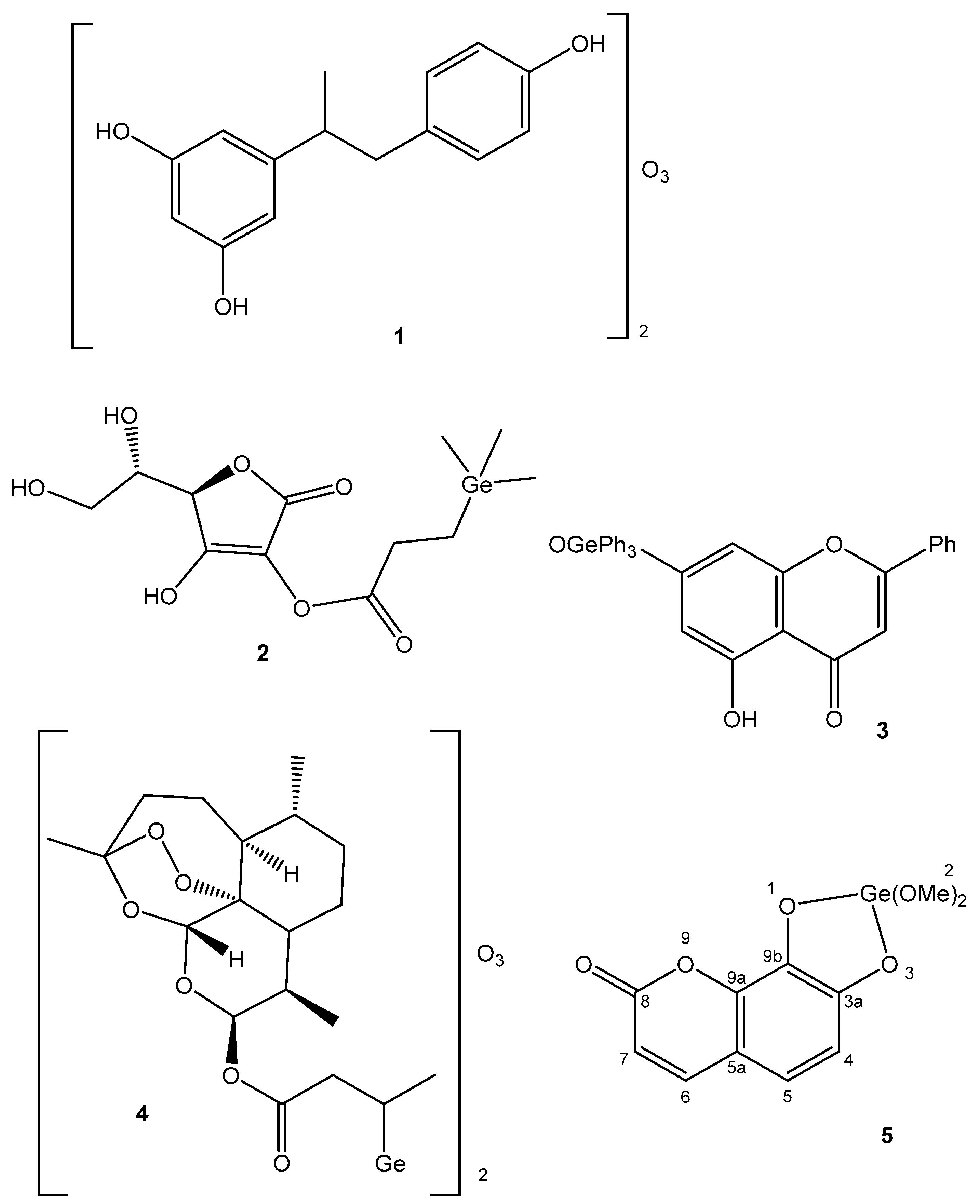
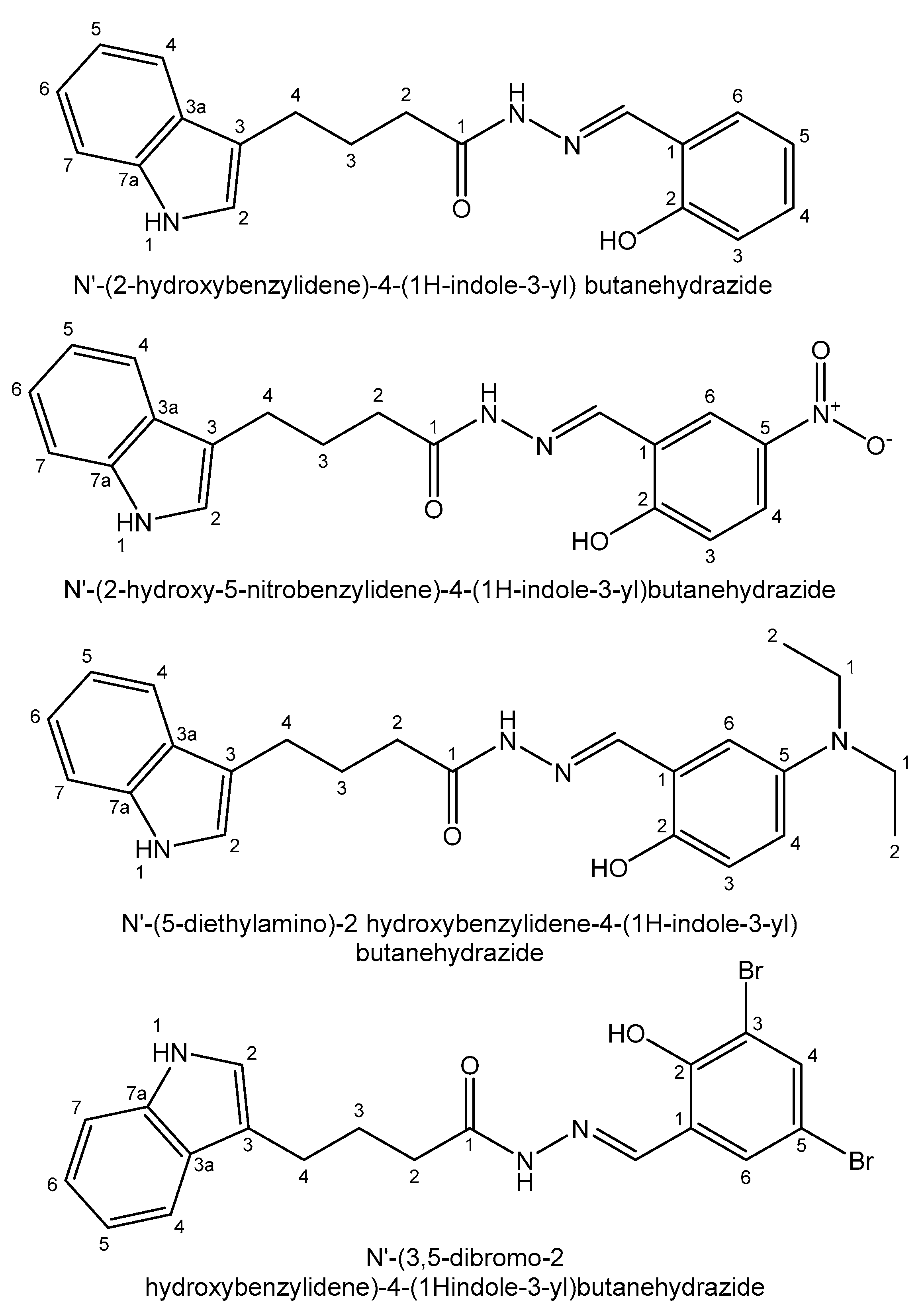
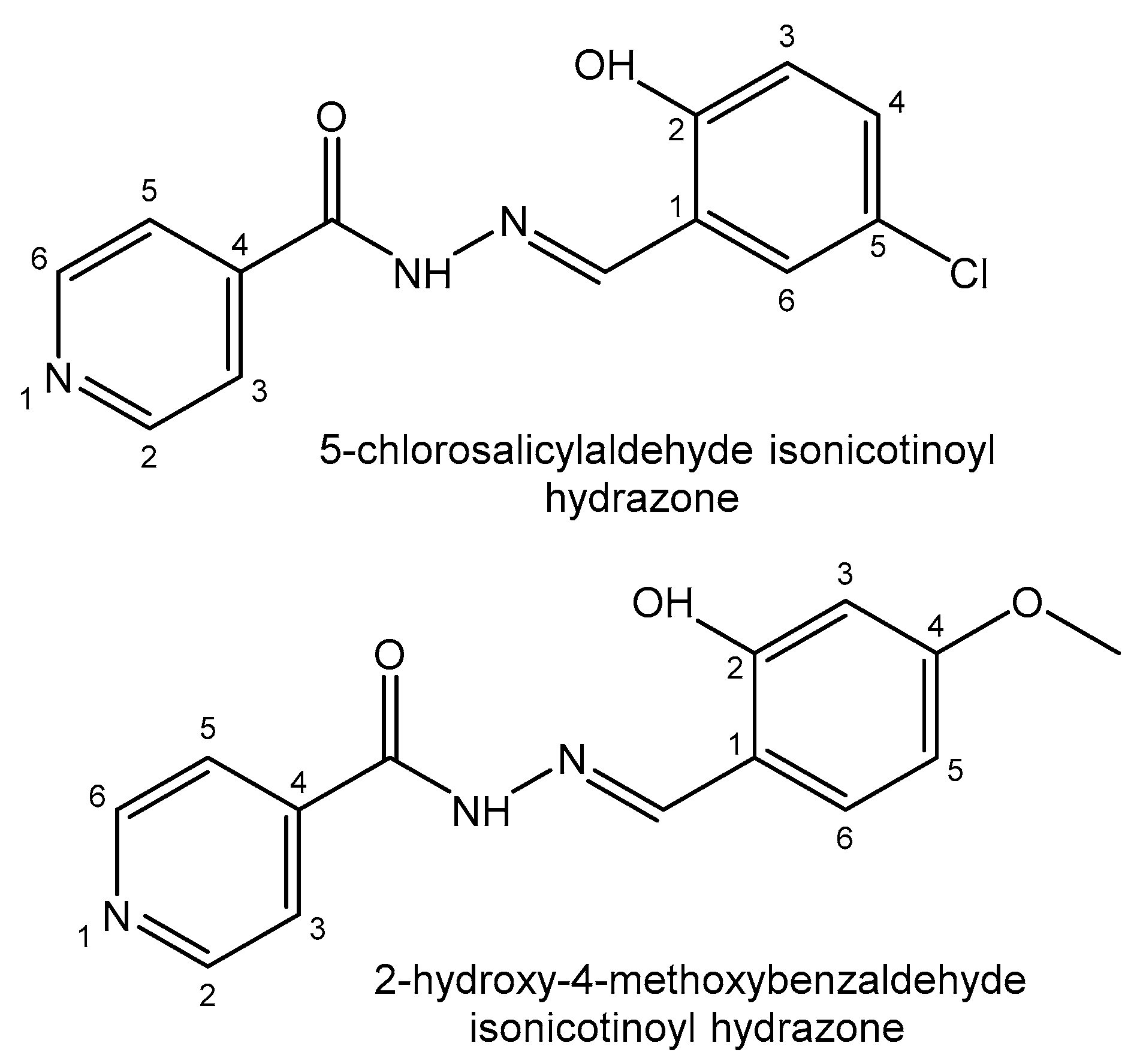
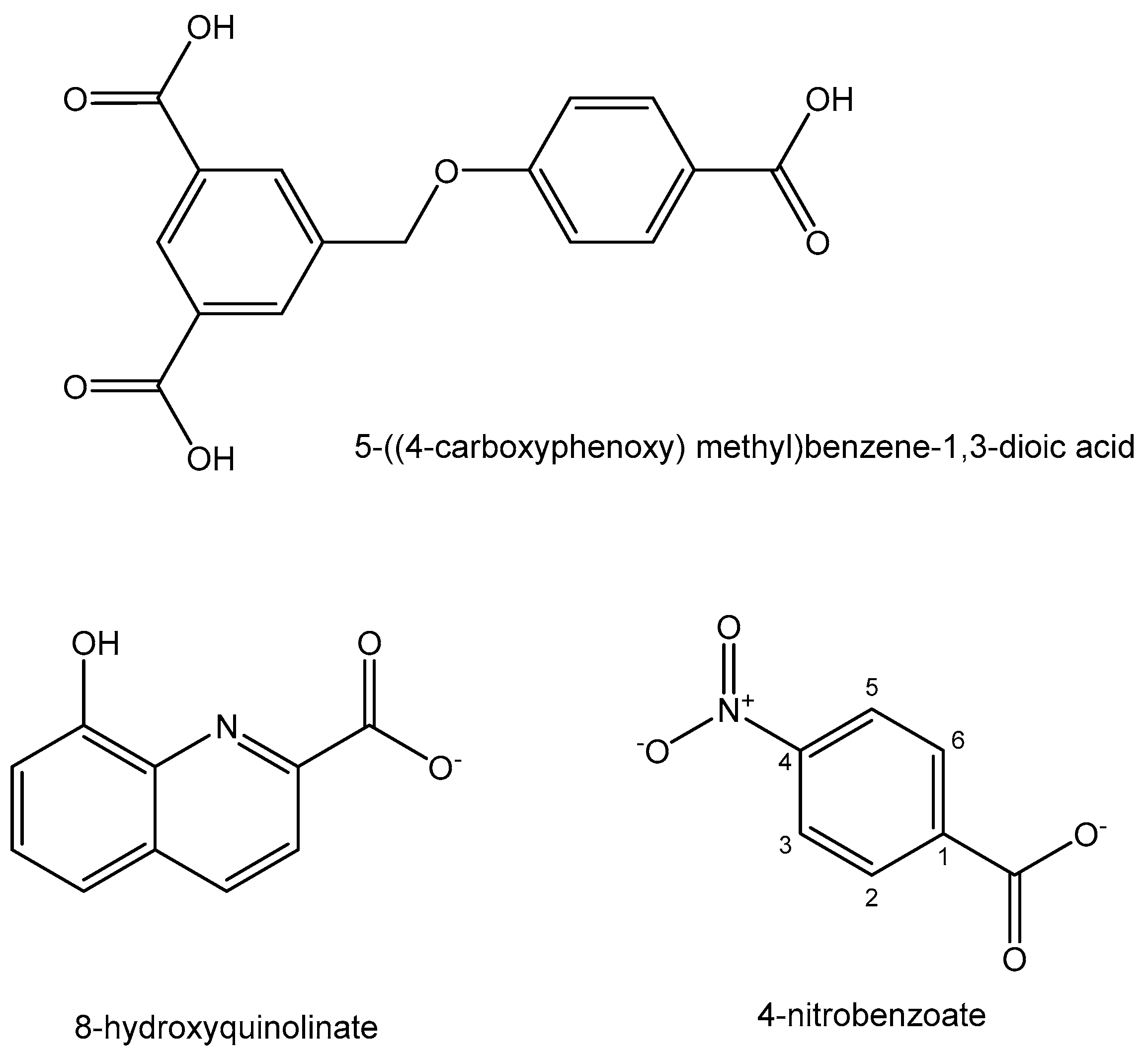
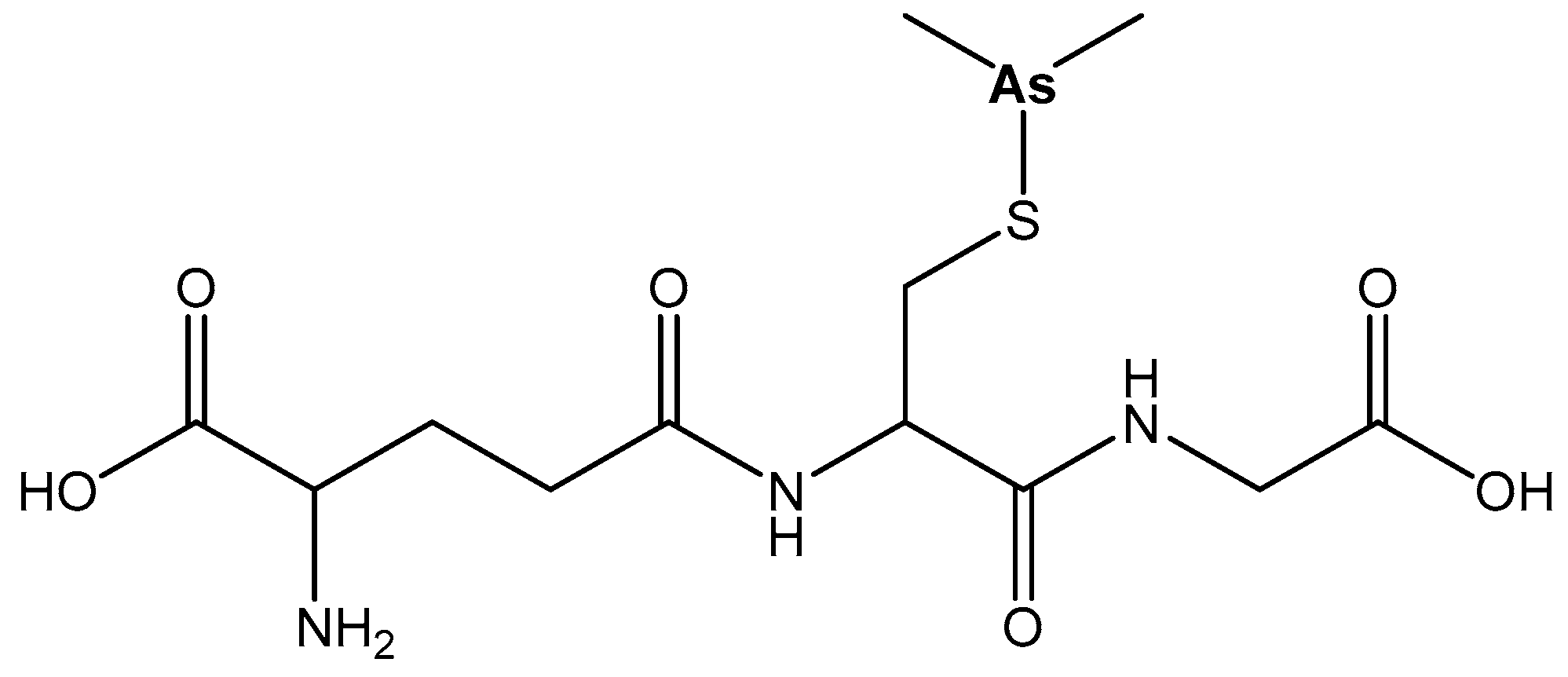

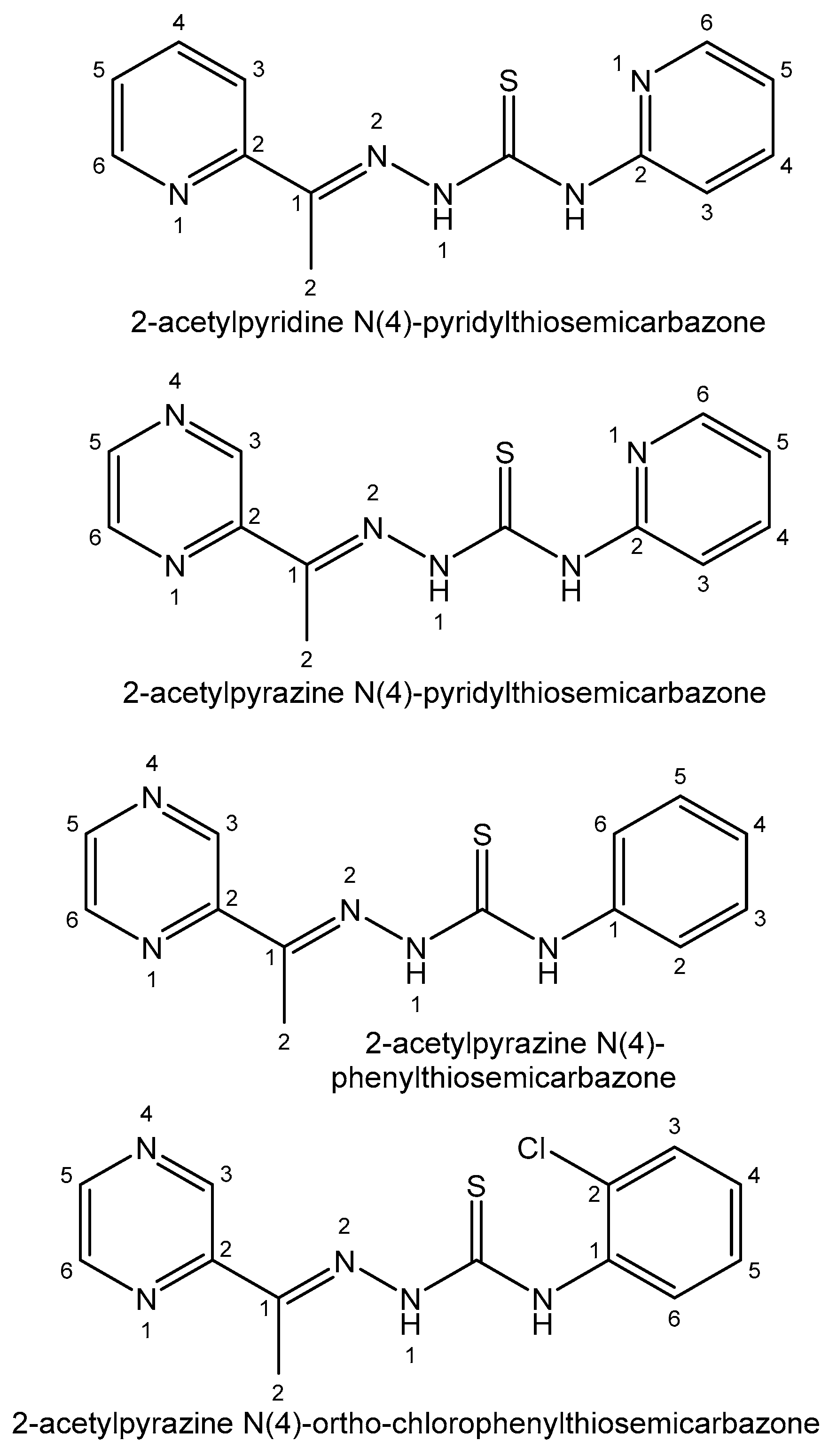
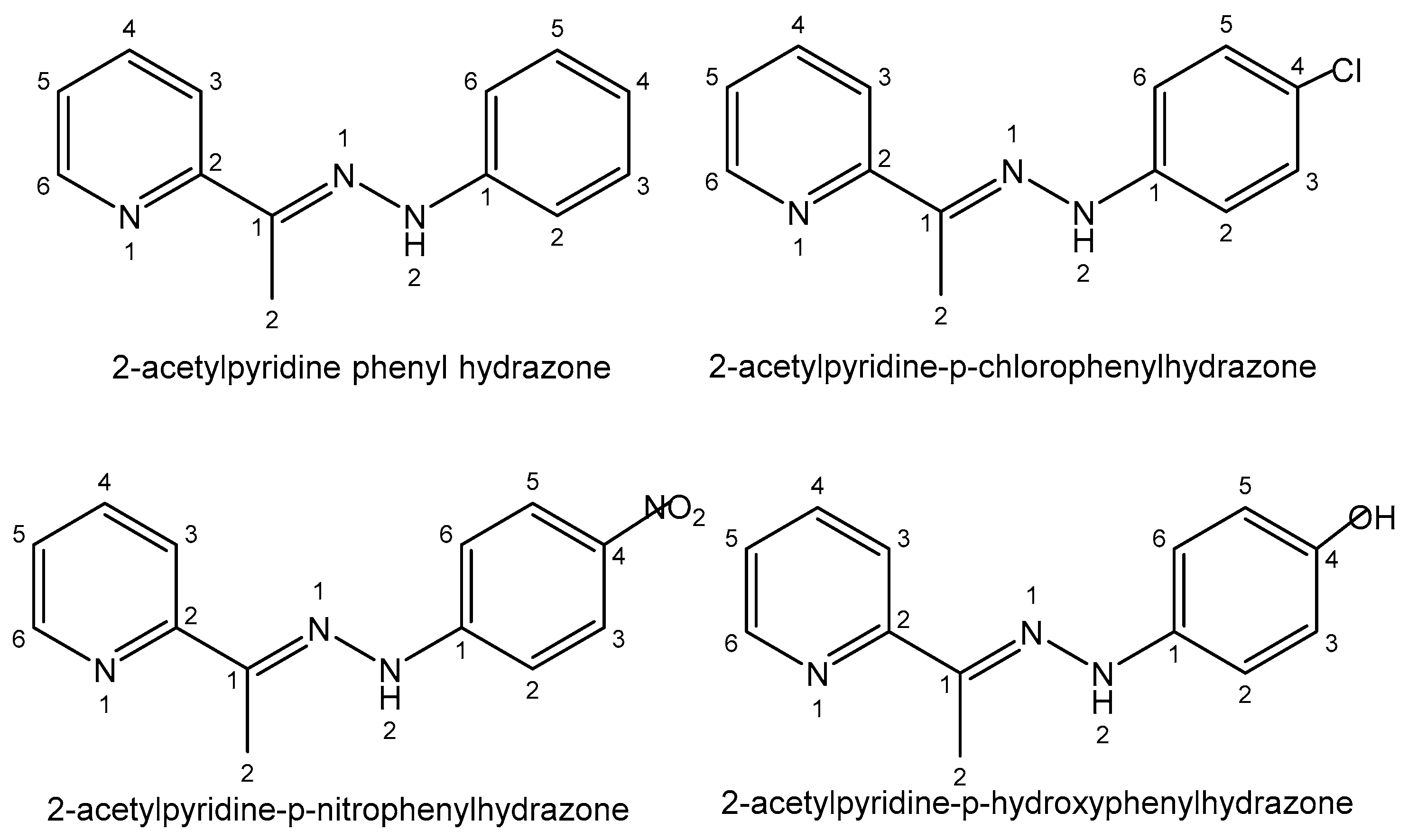
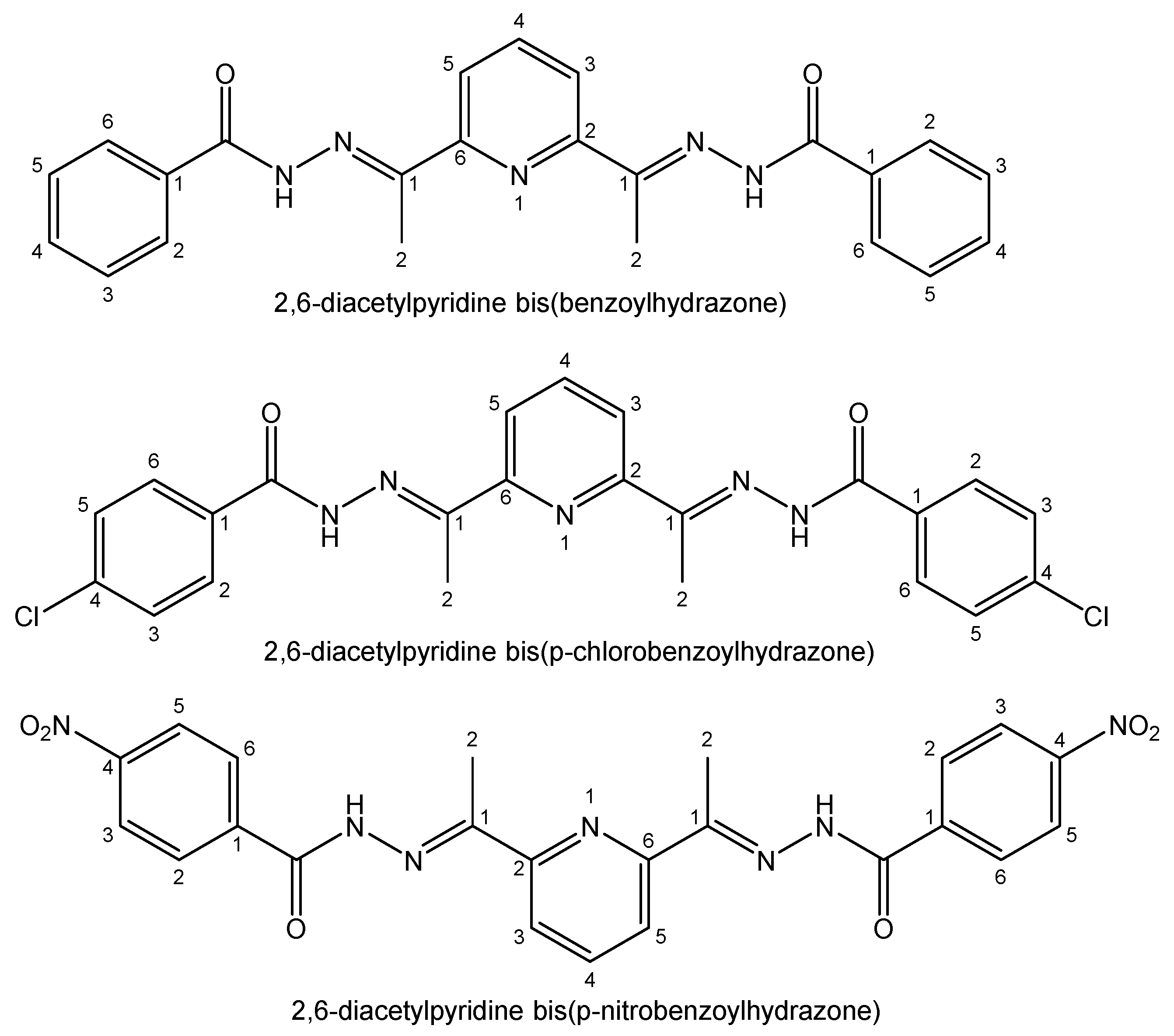
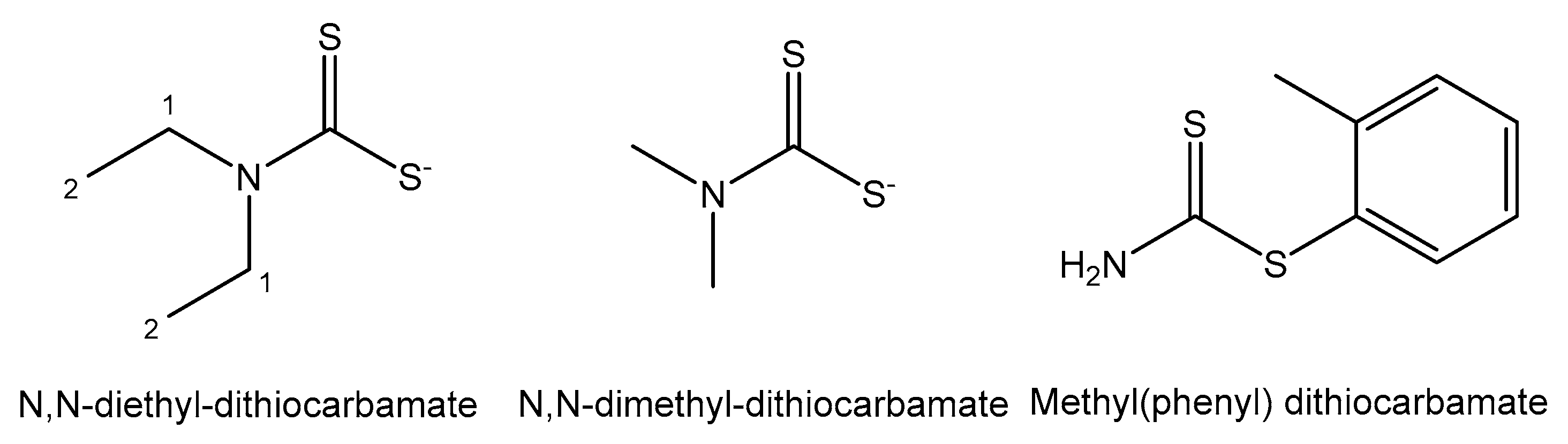

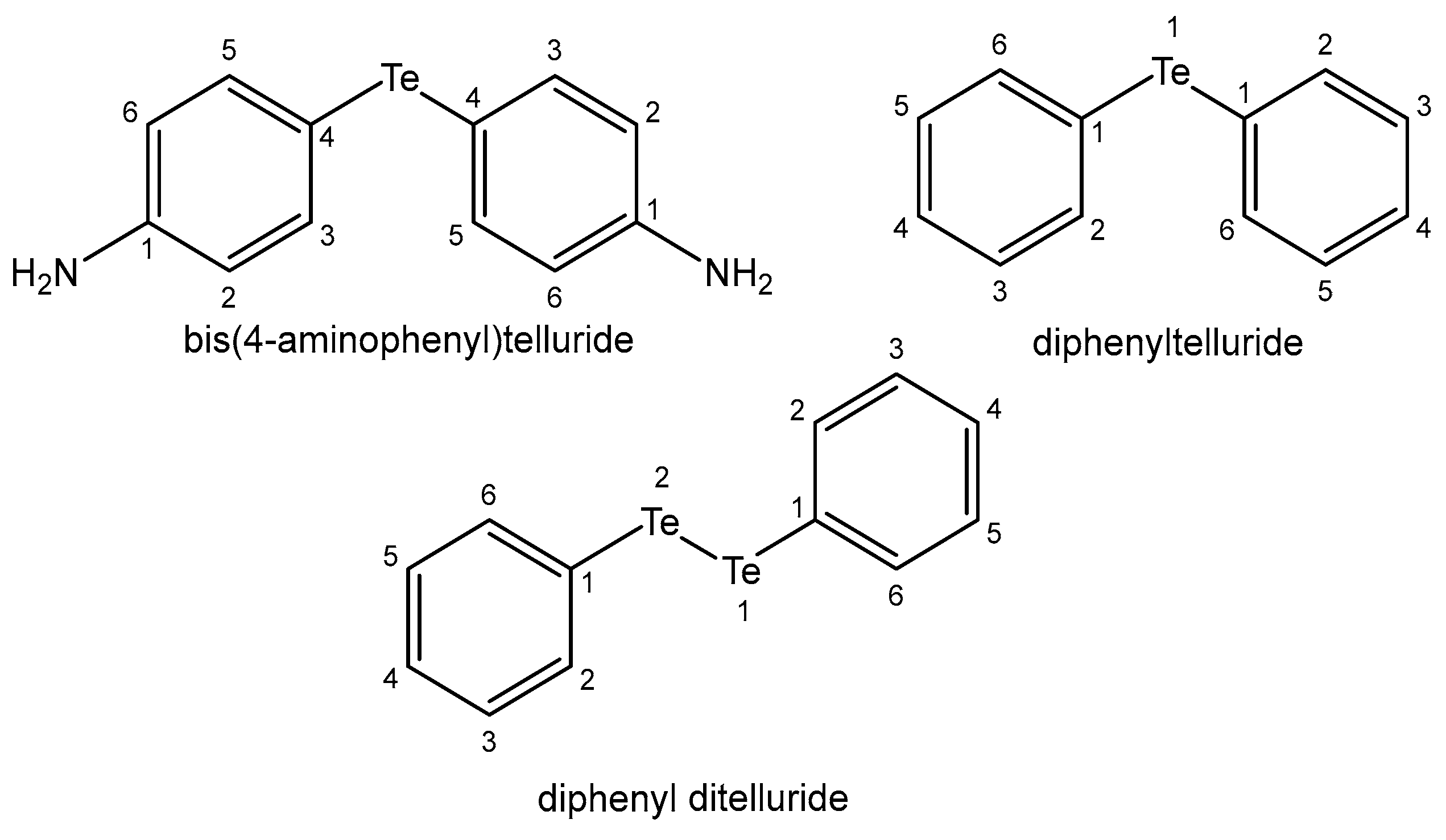
| Element | Location and Biofunctions | Compounds with Anticancer Activity | Toxicity, Antidotes | References |
|---|---|---|---|---|
| Cesium | 134Cs and 137Cs—radioactive pollutants | 131Cs—in brachytherapy and oncology for prostate cancer | Low abundance, insignificant influence | [14] |
| Strontium | In bone tissue affecting bone formation; 90Sr disturbs bone marrow hematopoiesis | Strontium-89—β-emitter in therapy for breast and metastatic prostate cancer | Brittle bones, Sr rickets, extracting Sr from bones is impossible | [15,16,17,18,19] |
| Radium | 223Ra2+ is a Ca2+-mimetic | 223Ra—α-emitter for bone metastases and prostate cancer | Ultratrace amounts in human body | [20,21,22,23,24] |
| Gallium | Ga3+—metabolism stimulator; anticancer, antibacterial, anti-inflammation; Fe3+-mimetic | Ga(NO3)3 in treatment of cancer-related hypercalcemia, 68Ga-, 67Ga-scintigraphy of malignant tumors, incl. Hodgkin’s and non-Hodgkin’s lymphomas | Particularly nontoxic | [25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41] |
| Indium | In3+—metabolism stimulator; 111In—radiotracer for tagging red, white blood cells and proteins | 111In—γ-emitter in radiopharmaceuticals for neuroendocrine and prostate cancer | Damages kidneys; low abundance, insignificant influence | [42,43,44,45,46] |
| Thallium | Tl causes mitochondrial and energy production damages; interferes with K+ pathways | 205Tl—in NMR detection, 201Tl—in SPECT for perfusion tests of myocardium in diagnostics of heart attacks and coronary artery disease | Toxic metal; antidotes—dialysis and Prussian Blue in colloid solutions | [47,48,49,50] |
| Germanium | Improves immunity, removes toxins, controls pain, antitumor | Tumor prevention; Ge-132—in cancer and cardiovascular therapy | Ge compounds with low solubility are not toxic to human cells | [51,52,53,54,55,56,57] |
| Tin | Stimulating growth effect; easily enters the bloodstream | Photodynamic therapy agent Purlytin for cutaneous cancer, Kaposi’s sarcoma, AIDS, breast metastases | Low-abundance nontoxic metal; toxic organotins | [58,59,60,61,62,63,64,65] |
| Lead | In erythrocytes; contributes to other metals’ toxicity; disturbs nervous system | 212Pb (β−emission) in radioimmunotherapy, 212Pb-TCMC-trastuzumab—antitumor agent | Saturnism; Pb2+ binds SH-groups; Antidotes—CaNa2EDTA, dimercaprol | [66,67,68,69,70,71,72,73,74] |
| Arsenic | In the brain and muscle tissues, contributes to hemoglobin synthesis | As2O3—for promyelocytic leukemia; Darinaparsin—anticancer therapeutic | Toxic metal—arsenolysis; antidotes—Na2S2O3, dimercaprol, DMPS, DMSA | [75,76,77,78,79,80,81,82,83,84] |
| Bismuth | In kidneys; inhibits enzymes amino- and carboxy-polypeptidaze | 213Bi (α-emitter); 213Bi-lintuzumab for α-targeted radiotherapy in patients with AML; Bi2O3—shielding γ-rays | Nontoxic with reversible toxicity; antidotes— D-penicillamine | [85,86,87,88,89,90,91,92,93,94,95,96,97,98,99,100,101,102] |
| Selenium | In small amounts is vital for normal growth and metabolism; in selenoproteins | Cancer-preventing; selenomethionine and Se-methyl-seleno-L-cysteine for treatment of prostate cancer | Selenosis; toxic selenites and selenates | [103,104,105,106] |
| Tellurium | Constantly in the human body; its biofunctions are not clear | Te(IV) nontoxic compound AS101—for AML chemotherapy—antiapoptotic | Toxic volatile compounds—in glutathione metabolism | [107,108,109] |
| Astatine | Short-lived At radioisotopes; in spleen, thyroid gland and lungs | 221At, 222At, 223At (α-emitters); 211At in therapy of CNS and malignant brain tumors | Analogous to iodine; thyrotoxicosis | [110,111,112,113,114,115] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kostova, I. Survey of Main Group Metals and Metalloids in Cancer Treatment. Inorganics 2024, 12, 29. https://doi.org/10.3390/inorganics12010029
Kostova I. Survey of Main Group Metals and Metalloids in Cancer Treatment. Inorganics. 2024; 12(1):29. https://doi.org/10.3390/inorganics12010029
Chicago/Turabian StyleKostova, Irena. 2024. "Survey of Main Group Metals and Metalloids in Cancer Treatment" Inorganics 12, no. 1: 29. https://doi.org/10.3390/inorganics12010029
APA StyleKostova, I. (2024). Survey of Main Group Metals and Metalloids in Cancer Treatment. Inorganics, 12(1), 29. https://doi.org/10.3390/inorganics12010029






