Endometallofullerenes in the Gas Phase: Progress and Prospect
Abstract
:1. Introduction
2. Experimental Method
3. Novel EMFs in the Gas Phase
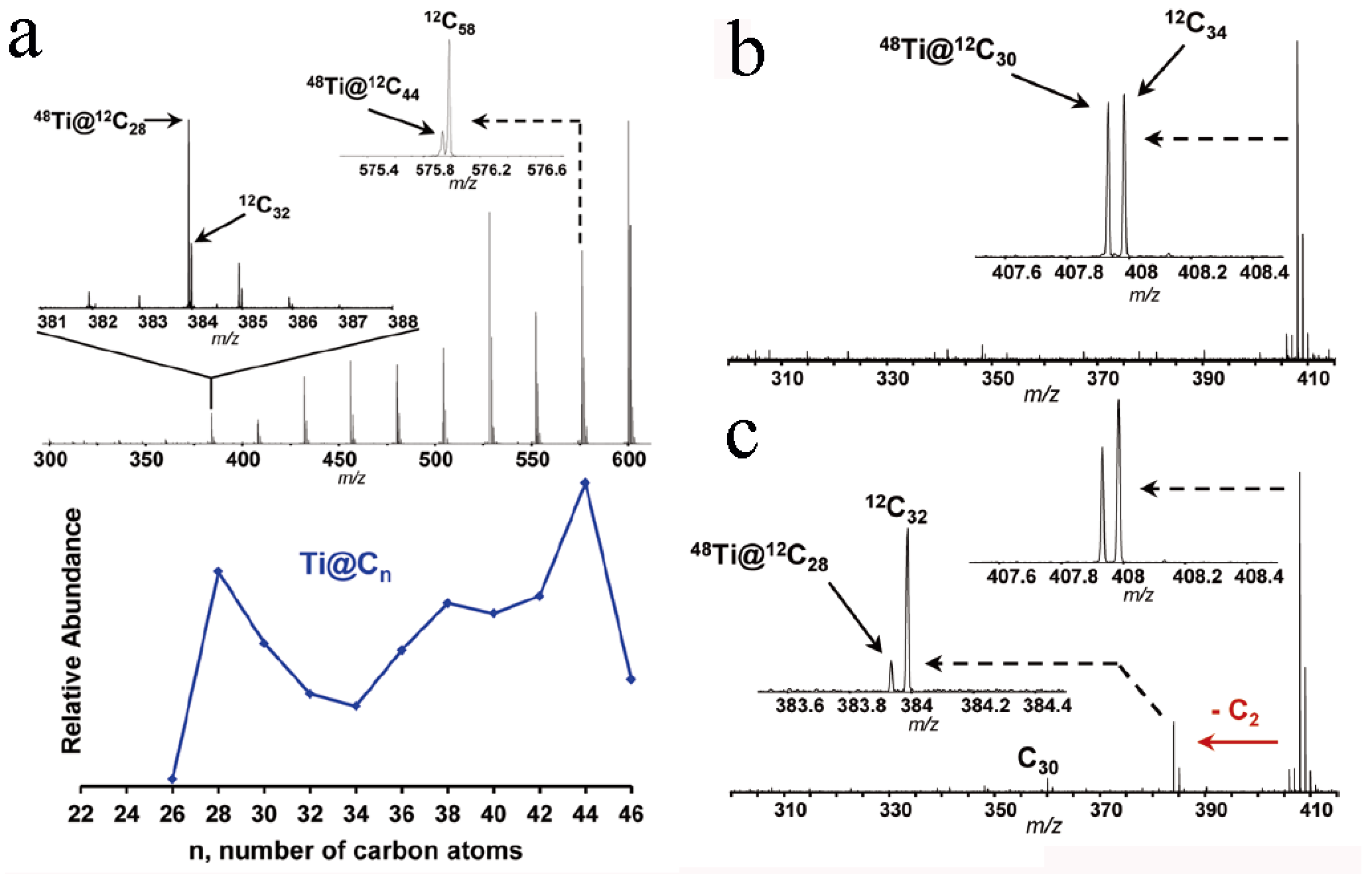
3.1. EMFs with Small-Size Carbon Cages of C2n (n < 60)
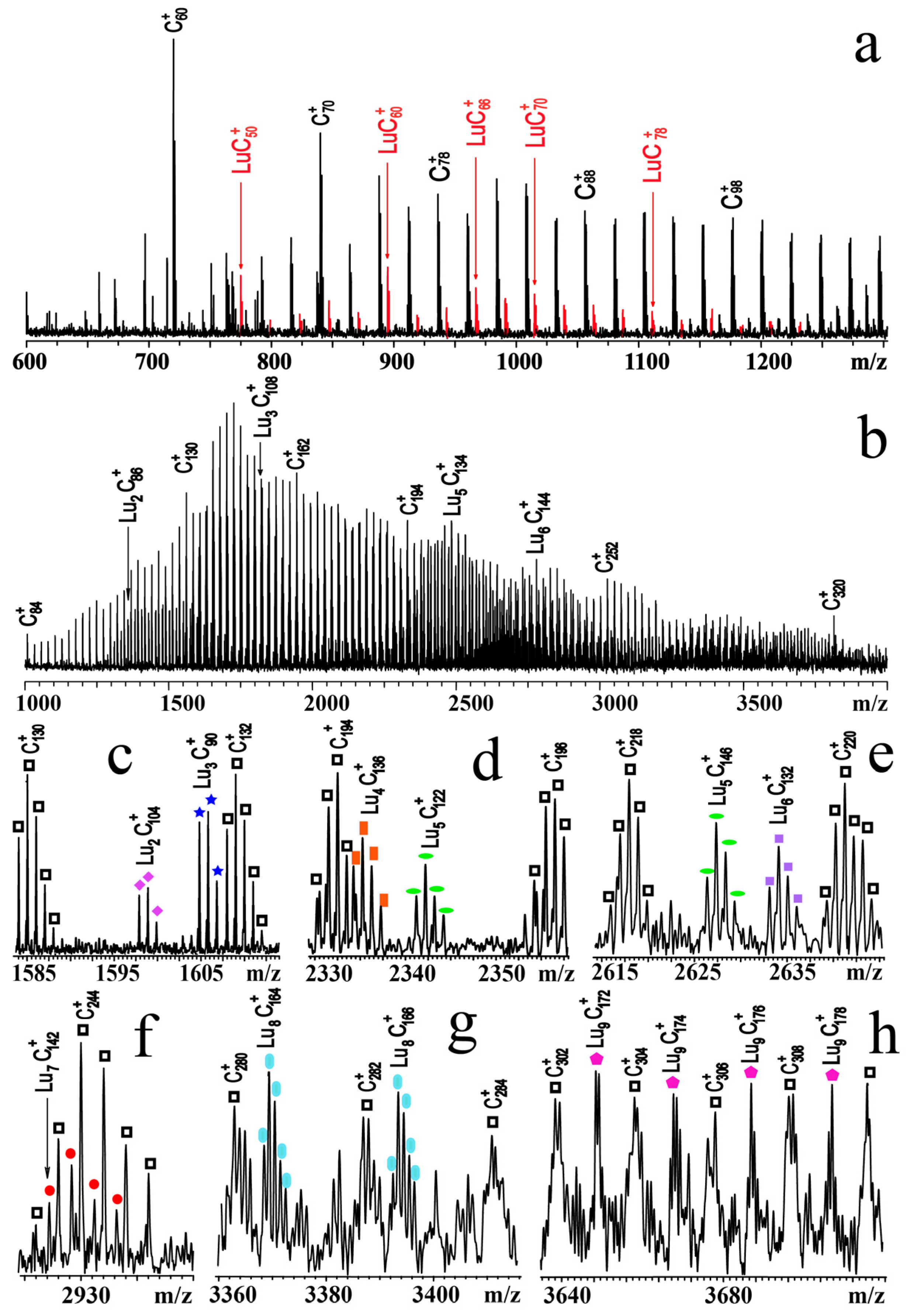
3.2. EMFs including Multiple Metal Atoms (Mx@C2n, x ≥ 3)
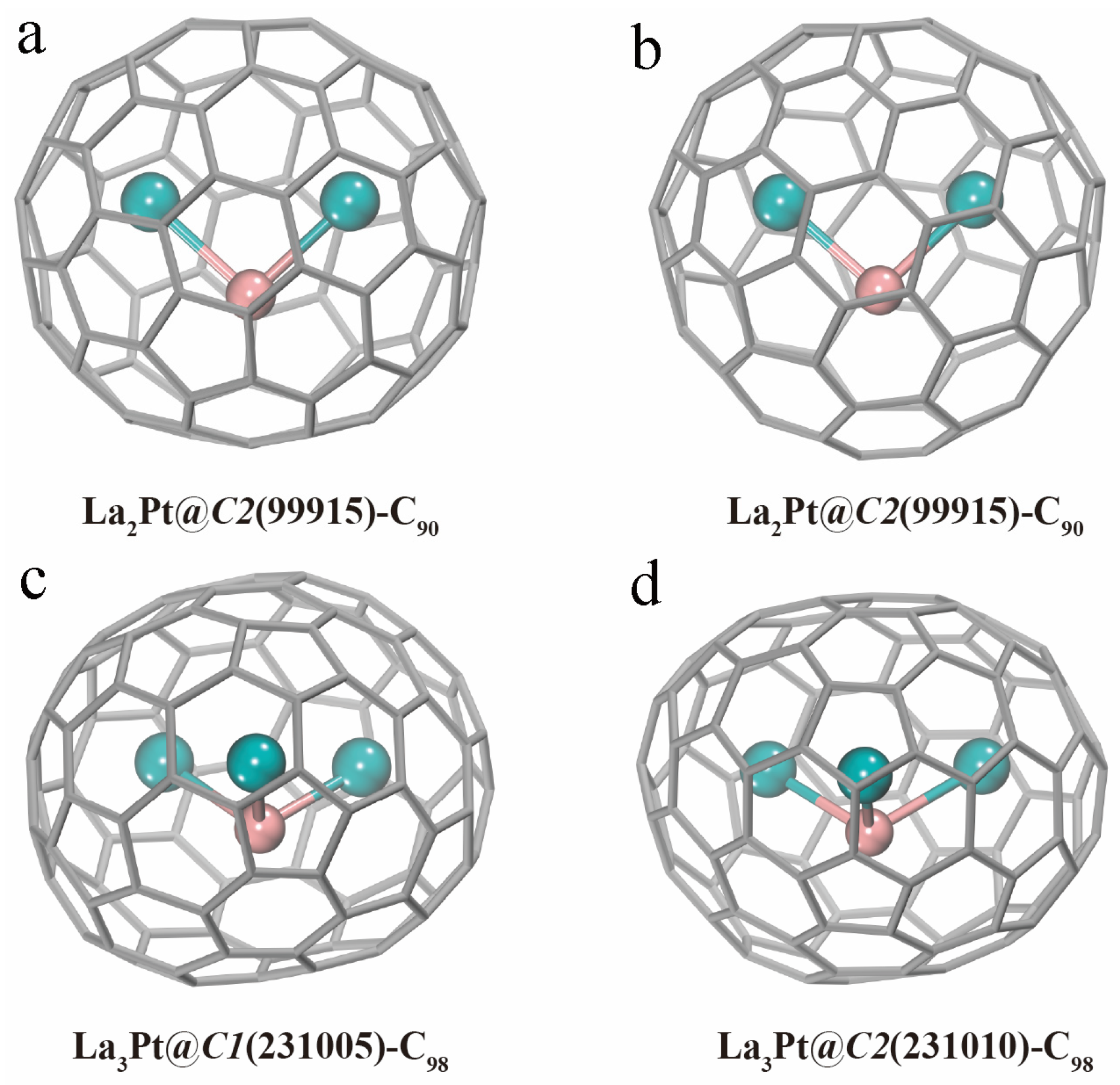
3.3. EMFs including Late Transition Metals
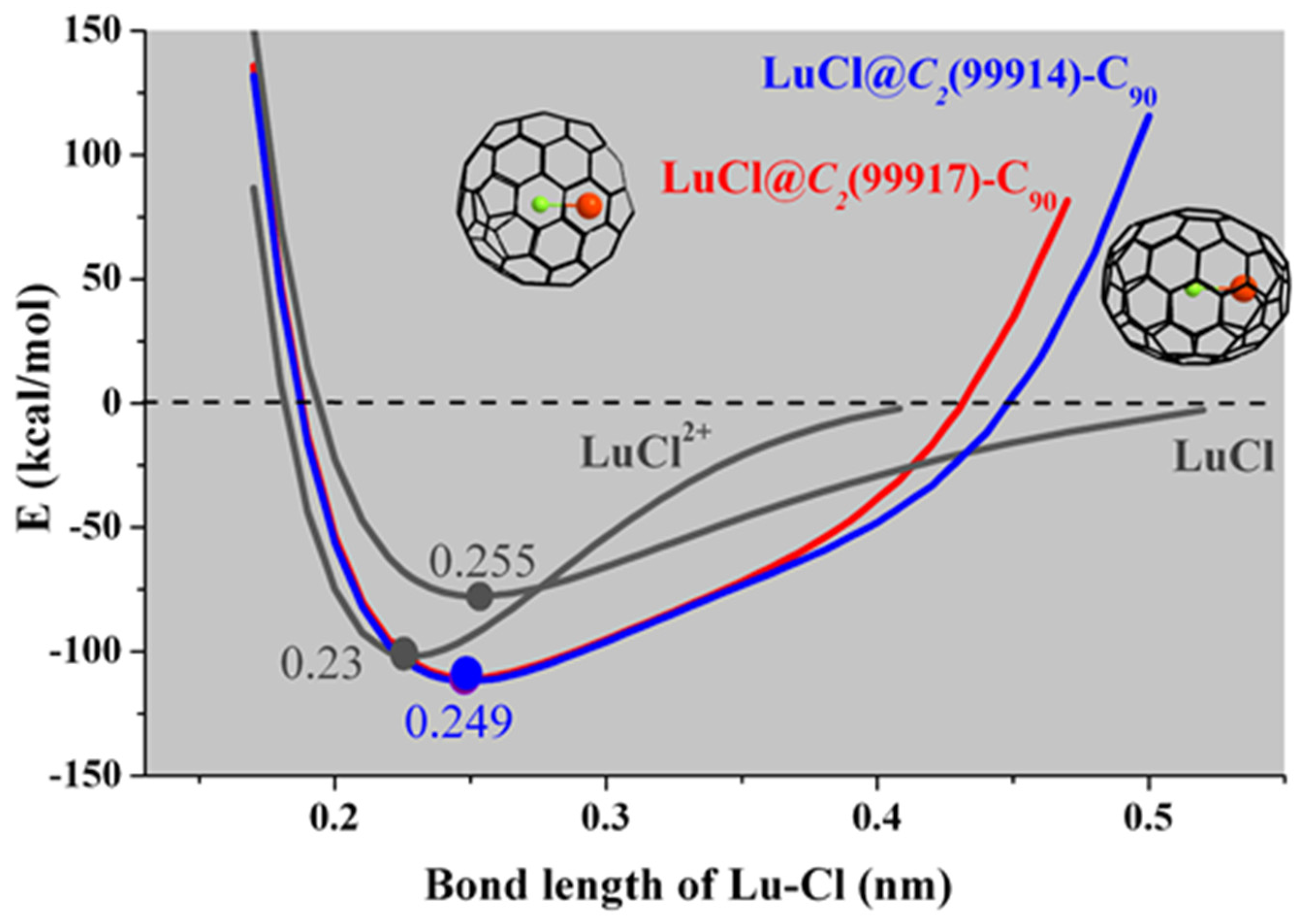
3.4. EMFs including Ionic Bonds
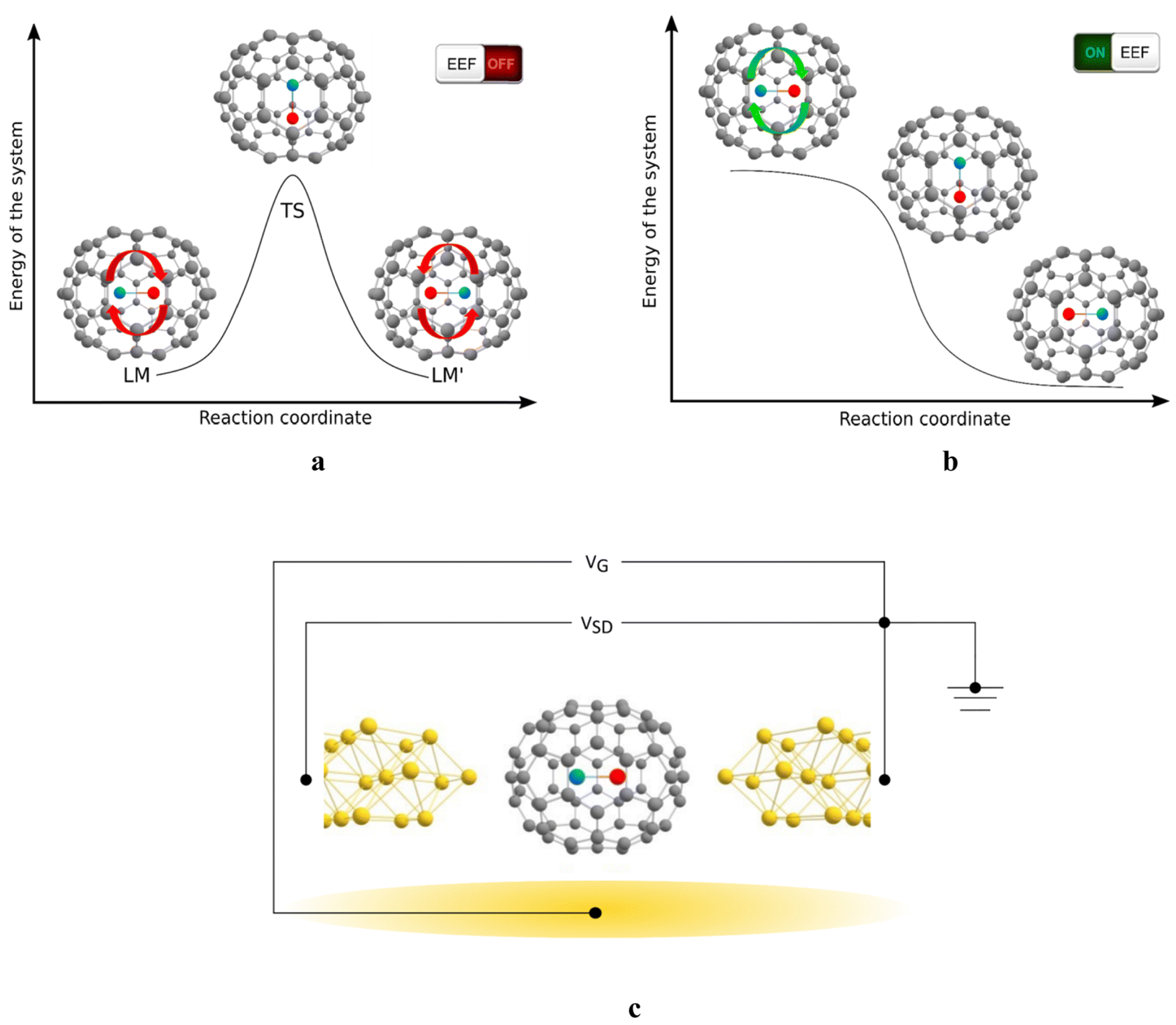
4. Mechanism Study
5. Prospect
Author Contributions
Funding
Conflicts of Interest
References
- Kroto, H.W.; Heath, J.R.; Obrien, S.C.; Curl, R.F.; Smalley, R.E. C60: Buckminsterfullerene. Nature 1985, 318, 162–163. [Google Scholar] [CrossRef]
- Heath, J.R.; O’Brien, S.C.; Zhang, Q.; Liu, Y.; Curl, R.F.; Kroto, H.W.; Tittel, F.K.; Smalley, R.E. Lanthanum complexes of spheroidal carbon shells. J. Am. Chem. Soc. 1985, 107, 7779. [Google Scholar] [CrossRef]
- Weiss, F.D.; O’Brien, S.C.; Elkind, J.L.; Curl, R.F.; Smalley, R.E. Photophysics of metal complexes of spheroidal carbon shells. J. Am. Chem. Soc. 1988, 110, 4464. [Google Scholar] [CrossRef]
- Chai, Y.; Guo, T.; Jin, C.; Haufler, R.E.; Chibante, L.P.F.; Fure, J.; Wang, L.; Alford, J.M.; Smalley, R.E. Fullerenes with metal inside. J. Phys. Chem. 1991, 95, 7564–7568. [Google Scholar] [CrossRef]
- Krätschmer, W.; Lamb, L.D.; Fostiropoulos, K.; Huffman, D.R. Solid C60: A new form of carbon. Nature 1990, 347, 354. [Google Scholar] [CrossRef]
- Alvarez, M.M.; Gillan, E.G.; Holczer, K.; Kaner, R.B.; Min, K.S.; Whetten, R.L. Lanthanum carbide (La2C80): A soluble dimetallofullerene. J. Phys. Chem. 1991, 95, 10561–10563. [Google Scholar] [CrossRef]
- Lu, X.; Feng, L.; Akasaka, T.; Nagase, S. Current status and future developments of endohedral metallofullerenes. Chem. Soc. Rev. 2012, 41, 7723–7760. [Google Scholar] [CrossRef] [PubMed]
- Popov, A.A.; Yang, S.F.; Dunsch, L. Endohedral fullerenes. Chem. Rev. 2013, 113, 5989–6113. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Fortea, A.; Balch, A.L.; Poblet, J.M. Endohedral metallofullerenes: A unique host–guest association. Chem. Soc. Rev. 2011, 40, 3551–3563. [Google Scholar] [CrossRef] [PubMed]
- Shen, W.; Hu, S.; Lu, X. Endohedral metallofullerenes: New structures and unseen phenomena. Chem. Eur. J. 2020, 26, 5748–5757. [Google Scholar] [CrossRef] [PubMed]
- Shen, W.; Bao, L.; Lu, X. Endohedral metallofullerenes: An ideal platform of sub-nano chemistry. Chin. J. Chem. 2022, 40, 275–284. [Google Scholar] [CrossRef]
- Gan, L.B. Molecular containers derived from [60] fullerene through peroxide chemistry. Acc. Chem. Res. 2019, 52, 1793–1801. [Google Scholar] [CrossRef]
- Yang, S.F.; Ioffe, I.N.; Troyanov, S.I. Chlorination-promoted skeletal transformations of fullerenes. Acc. Chem. Res. 2019, 52, 1783–1792. [Google Scholar] [CrossRef]
- Li, M.; Zhao, R.; Dang, J.; Zhao, X. Theoretical study on the stabilities, electronic structures, and reaction and formation mechanisms of fullerenes and endohedral metallofullerenes. Coord. Chem. Rev. 2022, 471, 214762. [Google Scholar] [CrossRef]
- Boltalina, O.V. Ions of Endometallofullerenes in the Gas Phase. In Endohedral Fullerenes: Electron Transfer and Spin; Springer: Berlin/Heidelberg, Germany, 2017; pp. 81–102. [Google Scholar]
- Marshall, A.G.; Hendrickson, C.L.; Jackson, G.S. Fourier transform ion cyclotron resonance mass spectrometry: A primer. Mass Spectrom. Rev. 1998, 17, 1–35. [Google Scholar] [CrossRef]
- Duncan, M.A. Invited review article: Laser vaporization cluster sources. Rev. Sci. Instrum. 2012, 83, 041101. [Google Scholar] [CrossRef] [PubMed]
- Dunk, P.W.; Kaiser, N.K.; Hendrickson, C.L.; Quinn, J.P.; Ewels, C.P.; Nakanishi, Y.; Sasaki, Y.; Shinohara, H.; Marshall, A.G.; Kroto, H.W. Closed network growth of fullerenes. Nat. Commun. 2012, 3, 855. [Google Scholar] [CrossRef]
- Dunk, P.W.; Adjizian, J.J.; Kaiser, N.K.; Quinn, J.P.; Blakney, G.T.; Ewels, C.P.; Marshall, A.G.; Kroto, H.W. Metallofullerene and fullerene formation from condensing carbon gas under conditions of stellar outflows and implication to stardust. Proc. Natl. Acad. Sci. USA 2013, 110, 18081–18086. [Google Scholar] [CrossRef] [PubMed]
- Dunk, P.W.; Mulet-Gas, M.; Nakanishi, Y.; Kaiser, N.K.; Rodríguez-Fortea, A.; Shinohara, H.; Poblet, J.M.; Marshall, A.G.; Kroto, H.W. Bottom-up formation of endohedral mono-metallofullerenes is directed by charge transfer. Nat. Commun. 2014, 5, 5844. [Google Scholar] [CrossRef]
- Kong, X.L.; Li, S.Q.; Zhang, S.; Huang, Y.; Cheng, Y.S. Large carbon cluster anions generated by laser ablation of graphene. J. Am. Soc. Mass Spectrom. 2011, 22, 2033–2041. [Google Scholar] [CrossRef]
- Kong, X.L.; Huang, Y.; Chen, Y.S. Difference in Formation of Carbon Cluster Cations by Laser Ablation of Graphene and Graphene Oxide. J. Mass. Spectrom. 2012, 47, 523–528. [Google Scholar] [CrossRef]
- Kong, X.L.; Bao, X.D. Generation of M@C2n+ (M = Ca, Sr, Ba, 2n = 50–230) by Laser Ablation of Graphene. Int. J. Mass. Spectrom. 2014, 372, 1–7. [Google Scholar] [CrossRef]
- Karas, M.; Hillenkamp, F. Laser desorption ionization of proteins with molecular masses exceeding 10,000 daltons. Anal. Chem. 1988, 60, 2299–2301. [Google Scholar] [CrossRef] [PubMed]
- Guan, S.; Marshall, A.G. Stored waveform inverse Fourier transform (SWIFT) ion excitation in trapped-ion mass spectrometry: Theory and applications. Int. J. Mass Spectrom. Ion Proc. 1996, 158, 5–37. [Google Scholar] [CrossRef]
- Laskin, J.; Futrell, J.H. Collisional activation of peptide ions in FT-ICR mass spectrometry. Mass Spectrom. Rev. 2003, 22, 158–181. [Google Scholar] [CrossRef] [PubMed]
- Wahl, F.; Wörth, J.; Prinzbach, H. The pagodane route to dodecahedranes: An improved approach to the C20H20 parent framework; partial and total functionalizations—Does C20-fullerene exist? Angew. Chem. Int. Ed. 1993, 32, 1722–1726. [Google Scholar] [CrossRef]
- Prinzbach, H.; Weiler, A.; Landenberger, P.; Wahl, F.; Wörth, J.; Scott, L.T.; Gelmont, M.; Olevano, D.; Issendorff, B.V. Gas-phase production and photoelectron spectroscopy of the smallest fullerene, C20. Nature 2000, 407, 60–63. [Google Scholar] [CrossRef] [PubMed]
- An, Y.P.; Yang, C.L.; Wang, M.S.; Ma, X.G.; Wang, D.H. Geometrical and electronic properties of the clusters of C20 Cage doped with alkali metal atoms. J. Clust. Sci. 2011, 22, 31–39. [Google Scholar] [CrossRef]
- Baei, M.T.; Soltani, A.; Torabi, P.; Hosseini, F. Formation and electronic structure of C20 fullerene transition metal clusters. Monatshefte für Chemie-Chem. Mon. 2014, 145, 1401–1405. [Google Scholar] [CrossRef]
- Rad, A.S.; Ayub, K. Nonlinear optical and electronic properties of Cr-, Ni-, and Ti- substituted C20 fullerenes: A quantum-chemical study. Mater. Res. Bull. 2018, 97, 399–404. [Google Scholar] [CrossRef]
- Rad, A.S.; Ayub, K. Substitutional doping of zirconium-, molybdenum-, ruthenium-, and palladium: An effective method to improve nonlinear optical and electronic property of C20 fullerene. Comput. Theor. Chem. 2017, 1121, 68–75. [Google Scholar]
- Zhou, Z.H.; Liu, Z.F.; Li, Z.; Wang, Z.Y. Structures, stability, electronic, magnetic properties of TM@Cn (n = 20, 24, 28) and polymerization of cyclocarbon-24: A DFT study. Mater. Chem. Phys. 2021, 260, 124154. [Google Scholar] [CrossRef]
- Yang, Y.F.; Gromov, E.V.; Cederbaum, L.S. Charge separated states of endohedral fullerene Li@C20. J. Chem. Phys. 2019, 151, 114306. [Google Scholar] [CrossRef] [PubMed]
- Li, J.R.; Wang, R.; Huang, W.R.; Zhu, Y.; Teo, B.K.; Wang, Z.G. Smallest endohedral metallofullerenes [Mg@C20]n (n = 4, 2, 0, −2, and −4): Endo-ionic interaction in superatoms. J. Phys. Chem. Lett. 2023, 14, 2862–2868. [Google Scholar] [CrossRef] [PubMed]
- Tománek, D.; Schluter, M.A. Growth regimes of carbon clusters. Phys. Rev. Lett. 1991, 67, 2331. [Google Scholar] [CrossRef] [PubMed]
- Kent, P.R.C.; Towler, M.D.; Needs, R.J.; Rajagopal, G. Carbon clusters near the crossover to fullerene stability. Phys. Rev. B 2000, 62, 15394. [Google Scholar] [CrossRef]
- Guo, T.; Smalley, R.E.; Scuseria, G.E. Ab initio theoretical predictions of C28, C28H4, C28F4, (Ti@C28) H4, and M@C28 (M = Mg, Al, Si, S, Ca, Sc, Ti, Ge, Zr, and Sn. J. Chem. Phys. 1993, 99, 352–359. [Google Scholar] [CrossRef]
- Garg, I.; Sharma, H.; Kapila, N.; Dharamvir, K.; Jindal, V.K. Transition metal induced magnetism in smaller fullerenes (Cn for n ≤ 36). Nanoscale 2011, 3, 217–224. [Google Scholar] [CrossRef] [PubMed]
- Koley, S.; Sen, S.; Chakrabarti, S. Role of molecule-electrode coupling strength in inducing inelastic transmission spectra of Hf@C28. Chem. Phys. 2020, 539, 110930. [Google Scholar] [CrossRef]
- Dai, X.; Gao, Y.; Jiang, W.R.; Lei, Y.Y.; Wang, Z.G. U@C28: The electronic structure induced by the 32-electron principle. Phys. Chem. Chem. Phys. 2015, 17, 23308. [Google Scholar] [CrossRef]
- Zhao, Y.X.; Yuan, K.; Li, M.Y.; Zhao, X. Oxidation states of gallium (infrequent i and common iii) tunable via medium-sized C60 and small-sized C28 fullerenes. Inorg. Chem. Front. 2020, 7, 4113. [Google Scholar] [CrossRef]
- Guo, T.; Diener, M.D.; Chai, Y.; Alford, M.J.; Haufler, R.E.; McClure, S.M.; Ohno, T.; Weaver, J.H.; Scuseria, G.E.; Smalley, R.E. Uranium stabilization of C28: A tetravalent fullerene. Science 1992, 257, 1661–1664. [Google Scholar] [CrossRef]
- Dunk, P.W.; Kaiser, N.K.; Mulet-Gas, M.; Rodríguez-Fortea, A.; Poblet, J.M.; Shinohara, H.; Hendrickson, C.L.; Marshall, A.G.; Kroto, H.W. The smallest stable fullerene, M@C28 (m = Ti, Zr, U): Stabilization and growth from carbon vapor. J. Am. Chem. Soc. 2012, 134, 9380–9389. [Google Scholar] [CrossRef]
- Gómez-Torres, A.; Esper, R.; Dunk, P.W.; Morales-Martínez, R.; Rodríguez-Fortea, A.; Echegoyen, L.; Poblet, J.M. Small cage uranofullerenes: 27 years after their first observation. Helv. Chim. Acta 2019, 102, e1900046. [Google Scholar] [CrossRef]
- Moreno-Vicente, A.; Alías-Rodríguez, M.; Dunk, P.W.; de Graaf, C.; Poblet, J.M.; Rodríguez-Fortea, A. Highly oxidized U(vi) within the smallest fullerene: Gas-phase synthesis and computational study of boron-doped U@C27B. Inorg. Chem. Front. 2023, 10, 908. [Google Scholar] [CrossRef]
- Mulet-Gas, M.; Abella, L.; Dunk, P.W.; Rodríguez-Fortea, A.; Kroto, H.W.; Poblet, J.M. Small endohedral metallofullerenes: Exploration of the structure and growth mechanism in the Ti@C2n (2n = 26–50) family. Chem. Sci. 2015, 6, 675–686. [Google Scholar] [CrossRef] [PubMed]
- Meng, F.C.; Zhou, Z.W.; Zhang, P.L.; Jiang, M.; Xu, X.L.; Wang, Y.; Gou, J.H.; Huie, D.; Die, D. Encapsulation of an f-block metal atom/ion to enhance the stability of C20 with the Ih symmetry. Phys. Chem. Chem. Phys. 2015, 17, 4328. [Google Scholar] [CrossRef] [PubMed]
- Muñoz-Castro, A. U@C36. Is there enough room for a second uranium? RSC Adv. 2016, 6, 78176. [Google Scholar] [CrossRef]
- Hou, Y.M.; Mu, L.; Zhou, S.Z.; Xu, Y.; Kong, X. Structure and bonding properties of the platinum-mediated tetrametallic endohedral fullerene La3Pt@C98. Dalton Trans. 2023, 52, 7021–7030. [Google Scholar] [CrossRef] [PubMed]
- Kong, X.L.; Huang, Y. Applications of graphene in mass spectrometry. J. Nanosci. Nanotechnol. 2014, 14, 4719–4732. [Google Scholar] [CrossRef] [PubMed]
- Bao, X.D.; Kong, X.L. Generation of M@C2n+ (M = Cs, Rb, K, 2n = 70–220) by Laser Ablation of Graphene. Rapid Comm. Mass. Spectrom. 2015, 29, 1774–1778. [Google Scholar] [CrossRef] [PubMed]
- Kong, X.; Bao, X. Formation of Endohedral Metallofullerene (EMF) ions of MnC2m+ (M = La, Y, n ≤ 6, 50 ≤ 2m ≤ 194) in the Laser Ablation Process with Graphene as Precursor. Rapid Comm. Mass. Spectrom. 2017, 31, 865–872. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.Y.; Ren, J.; Mu, L.; Kong, X.L. Metallofullerene ions of LunC2m+ (1 ≤ n ≤ 9, 50 ≤ 2m ≤ 198) generated by laser ablation of graphene/ LuCl3. Int. J. Mass. Spectrom. 2017, 422, 105–110. [Google Scholar] [CrossRef]
- Wang, Y.; Ma, L.; Ren, J.; Mu, L.; Kong, X.L. A systematic study on the generation of multimetallic lanthanide fullerene ions by laser ablation mass spectrometry. Rapid Commun. Mass Spectrom. 2018, 32, 1396–1402. [Google Scholar] [CrossRef] [PubMed]
- Mu, L.; Yang, S.; Feng, R.X.; Kong, X.L. Encapsulation of Platinum in Fullerenes: Is that Possible? Inorg. Chem. 2017, 56, 6035–6038. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.Y.; Shi, Y.Y.; Fan, X.T.; Ren, J.; Kong, X.L. Encapsulation of an Ionic Bond in Fullerenes: What is the Difference? Inorg. Chem. 2019, 58, 3601–3605. [Google Scholar] [CrossRef] [PubMed]
- Fan, X.T.; Wang, Y.Y.; Kong, X.L. Generation of sodium halide endohedral metallofullerenes in the gas phase. Rapid Comm. Mass. Spectrom. 2020, 34, e8826. [Google Scholar] [CrossRef] [PubMed]
- Krachmalnicoff, A.; Bounds, R.; Mamone, S.; Alom, S.; Concistrè, M.; Meier, B.; Kouril, K.; Light, M.E.; Johnson, M.R.; Rols, S.; et al. The dipolar endofullerene HF@C60. Nat. Chem. 2016, 8, 953–957. [Google Scholar] [CrossRef] [PubMed]
- Gao, R.; Liu, Z.; Liu, Z.; Liang, T.; Su, J.; Gan, L. Open-cage fullerene as a selective molecular trap for LiF/[BeF]+. Angew. Chem. Int. Ed. 2023, 62, e202300151, Erratum in Angew. Chem.2023, 135, e202300151. [Google Scholar] [CrossRef] [PubMed]
- Martin, T.P.; Heinebrodt, M.; Näher, U.; Göhlich, H.; Lange, T.; Schaber, H. Fullerenes doped with metal halides. Int. J. Mod. Phys. B 1992, 6, 3871–3877. [Google Scholar] [CrossRef]
- Li, J.; Hou, S.J.; Yao, Y.R.; Zhang, C.Y.; Wu, Q.Q.; Wang, H.C.; Zhang, H.W.; Liu, X.Y.; Tang, C.; Wei, M.X.; et al. Room-temperature logic-in-memory operations in single-metallofullerene devices. Nat. Mater. 2022, 21, 917–923. [Google Scholar] [CrossRef] [PubMed]
- Kang, D.; Yao, Y.; Su, Z.; Xu, H.L. Probing the structure–property relationships of Na+···Cl–@C50N5H5 under the external electric field. Inorg. Chem. 2022, 61, 17646–17652. [Google Scholar] [CrossRef] [PubMed]
- Jaros, A.; Bonab, E.F.; Straka, M.; Foroutan-Nejad, C. Fullerene-based switching molecular diodes controlled by oriented external electric fields. J. Am. Chem. Soc. 2019, 141, 19644–19654. [Google Scholar] [CrossRef] [PubMed]
- Foroutan-Nejad, C.; Andrushchenko, V.; Straka, M. Dipolar molecules inside C70: An electric field-driven room-temperature single-molecule switch. Phys. Chem. Chem. Phys. 2016, 18, 32673–32677. [Google Scholar] [CrossRef]
- Sabirov, D.S.; Terentyev, A.O.; Shepelevich, I.S.; Bulgakov, R.G. Inverted thermochemistry of “norbornadiene–quadricyclane” molecular system inside fullerene nanocages. Comput. Theor. Chem. 2014, 1045, 86–92. [Google Scholar] [CrossRef]
- Sabirov, D.S.; Terentyev, A.O.; Sokolov, V.I. Activation energies and information entropies of helium penetration through fullerene walls. Insights into the formation of endofullerenes nX@C60/70 (n = 1 and 2) from the information entropy approach. RSC Adv. 2016, 6, 72230–72237. [Google Scholar] [CrossRef]
- Yu, P.; Li, M.; Shen, W.; Hu, S.; Yu, P.Y.; Tian, X.; Zhao, X.; Bao, L.; Lu, X. An unprecedented C80 cage that violates the isolated pentagon rule. Inorg. Chem. Front. 2022, 9, 2264–2270. [Google Scholar] [CrossRef]
- Brotsman, V.A.; Ioffe, I.N.; Troyanov, S.I. Crippling the C70 fullerene: Non-classical C68Cl26(OH)2 and C68Cl25(OH)3 with three heptagons and only fused pentagons via chlorination-promoted skeletal transformations. Chem. Commun. 2022, 58, 6918–6921. [Google Scholar] [CrossRef]
- Kirkpatrick, J.; McMorrow, B.; Turban, D.H.P.; Gaunt, A.L.; Spencer, J.S.; Matthews, A.G.D.G.; Obika, A.; Thiry, L.; Fortunato, M.; Pfau, D.; et al. Pushing the frontiers of density functionals by solving the fractional electron problem. Science 2021, 374, 1385–1389. [Google Scholar] [CrossRef]
- Irle, S.; Zheng, G.; Wang, Z.; Morokuma, K. The C60 formation puzzle “solved”: QM/MD simulations reveal the shrinking hot giant road of the dynamic fullerene self-assembly mechanism. J. Phys. Chem. B 2006, 110, 14531–14545. [Google Scholar] [CrossRef]
- Curl, R.F.; Lee, M.K.; Scuseria, G.E. C60 buckminsterfullerene high yields unraveled. J. Phys. Chem. A 2008, 112, 11951–11955. [Google Scholar] [CrossRef] [PubMed]
- Cross, R.J.; Saunders, M. Transmutation of fullerenes. J. Am. Chem. Soc. 2005, 127, 3044–3047. [Google Scholar] [CrossRef] [PubMed]
- Chuvilin, A.; Kaiser, U.; Bichoutskaia, E.; Besley, N.A.; Khlobystov, A.N. Direct transformation of graphene to fullerene. Nat. Chem. 2010, 2, 450–453. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Cheng, Z.; Wang, R.; Li, Q.; Fang, Y. Spontaneous formation of nanostructures in graphene. Nano Lett. 2009, 9, 3599–3602. [Google Scholar] [CrossRef]
- Shenoy, V.B.; Reddy, C.D.; Zhang, Y.W. Spontaneous curling of graphene sheets with reconstructed edges. ACS Nano 2010, 4, 4840–4844. [Google Scholar] [CrossRef] [PubMed]
- Berné, O.; Tielens, A.G.G.M. Formation of buckminsterfullerene (C60) in interstellar space. Proc. Natl. Acad. Sci. USA 2012, 109, 401–406. [Google Scholar] [CrossRef] [PubMed]
- Berné, O.; Montillaud, J.; Joblin, C. Top-down formation of fullerenes in the interstellar medium. Astron. Astrophys. 2015, 588, C1. [Google Scholar] [CrossRef]
- Cai, W.T.; Li, F.F.; Bao, L.P.; Xie, Y.P.; Lu, X. Isolation and crystallographic characterization of La2C2@Cs(574)-C102 and La2C2@C2(816)-C104: Evidence for the top-down formation mechanism of fullerenes. J. Am. Chem. Soc. 2016, 138, 6670–6675. [Google Scholar] [CrossRef]
- Zhang, J.Y.; Bowles, F.L.; Bearden, D.W.; Ray, W.K.; Fuhrer, T.; Ye, Y.Q.; Dixon, C.; Harich, K.; Helm, R.F.; Olmstead, M.M.; et al. A missing link in the transformation from asymmetric to symmetric metallofullerene cages implies a top-down fullerene formation mechanism. Nat. Chem. 2013, 5, 880–885. [Google Scholar] [CrossRef]
- Li, M.Y.; Zhao, Y.X.; Han, Y.B.; Yuan, K.; Nagase, S.; Ehara, M.; Zhao, X. Theoretical investigation of the key roles in fullerene-formation mechanisms: Enantiomer and enthalpy. ACS Appl. Nano Mater. 2020, 3, 547–554. [Google Scholar] [CrossRef]
- Mulet-Gas, M.; Abella, L.; Cerón, M.R.; Castro, E.; Marshall, A.G.; Rodríguez-Fortea, A.; Echegoyen, L.; Poblet, J.M.; Dunk, P.W. Transformation of doped graphite into cluster-encapsulated fullerene cages. Nat. Commun. 2017, 8, 1222. [Google Scholar] [CrossRef] [PubMed]
- Yamaguchi, Y.; Maruyama, S. A molecular dynamics study on the formation of metallofullerene. Eur. Phys. J. D 1999, 9, 385–388. [Google Scholar] [CrossRef]
- Deng, Q.M.; Heine, T.; Irle, S.; Popov, A.A. Self-assembly of endohedral metallofullerenes: A decisive role of cooling gas and metal–carbon bonding. Nanoscale 2016, 8, 3796–3808. [Google Scholar] [CrossRef]
- Gan, L.H.; Lei, D.; Fowler, P.W. Structural interconnections and the role of heptagonal rings in endohedral trimetallic nitride template fullerenes. J. Comput. Chem. 2016, 37, 1907–1913. [Google Scholar] [CrossRef]
- Fan, H.; Liu, Z.; Gan, L.H.; Wang, C.R. The formation mechanism of Sc-based metallofullerenes: A molecular dynamics simulation study. Phys. Chem. Chem. Phys. 2024, 26, 5499–5507. [Google Scholar] [CrossRef] [PubMed]
- Sinitsa, A.S.; Chamberlain, T.W.; Zoberbier, T.; Lebedeva, I.V.; Popov, A.M.; Knizhnik, A.A.; McSweeney, R.L.; Biskupek, J.; Kaiser, U.; Khlobystov, A.N. Formation of Nickel Clusters Wrapped in Carbon Cages: Toward New Endohedral Metallofullerene Synthesis. Nano Lett. 2017, 17, 1082–1089. [Google Scholar] [CrossRef] [PubMed]




| M | Oxide States | Observed Multi-Metallofullerene Ions | Ref. |
|---|---|---|---|
| La | +3 | [53] | |
| Ce | +3 +4 | [55] | |
| Pr | +3 +4 | [55] | |
| Nd | +3 | [55] | |
| Sm | +2 +3 | None | [55] |
| Eu | +2 +3 | None | [55] |
| Gd | +3 | [55] | |
| Tb | +3 +4 | [55] | |
| Dy | +3 | [55] | |
| Ho | +3 | [55] | |
| Er | +3 | [54] | |
| Tm | +2 +3 | [54] | |
| Yb | +2 +3 | [54] | |
| Lu | +3 | [54] | |
| Y | +3 | [53] | |
| Li | +1 | None | [52] |
| Na | +1 | None | [52] |
| Rb | +1 | None | [52] |
| Cs | +1 | None | [52] |
| Mg | +2 | None | [23] |
| Ca | +2 | [23] | |
| Sr | +2 | None | [23] |
| Ba | +2 | [23] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hou, Y.; Kong, X. Endometallofullerenes in the Gas Phase: Progress and Prospect. Inorganics 2024, 12, 68. https://doi.org/10.3390/inorganics12030068
Hou Y, Kong X. Endometallofullerenes in the Gas Phase: Progress and Prospect. Inorganics. 2024; 12(3):68. https://doi.org/10.3390/inorganics12030068
Chicago/Turabian StyleHou, Yameng, and Xianglei Kong. 2024. "Endometallofullerenes in the Gas Phase: Progress and Prospect" Inorganics 12, no. 3: 68. https://doi.org/10.3390/inorganics12030068






