Biosynthesis and Characterization of Zinc Oxide Nanoparticles (ZnO-NPs) Utilizing Banana Peel Extract
Abstract
:1. Introduction
2. Results and Discussion
2.1. Banana Species Identification
2.2. Characterization and Analysis Technique for Banana Peel Extract
2.2.1. Manual Screening Tests
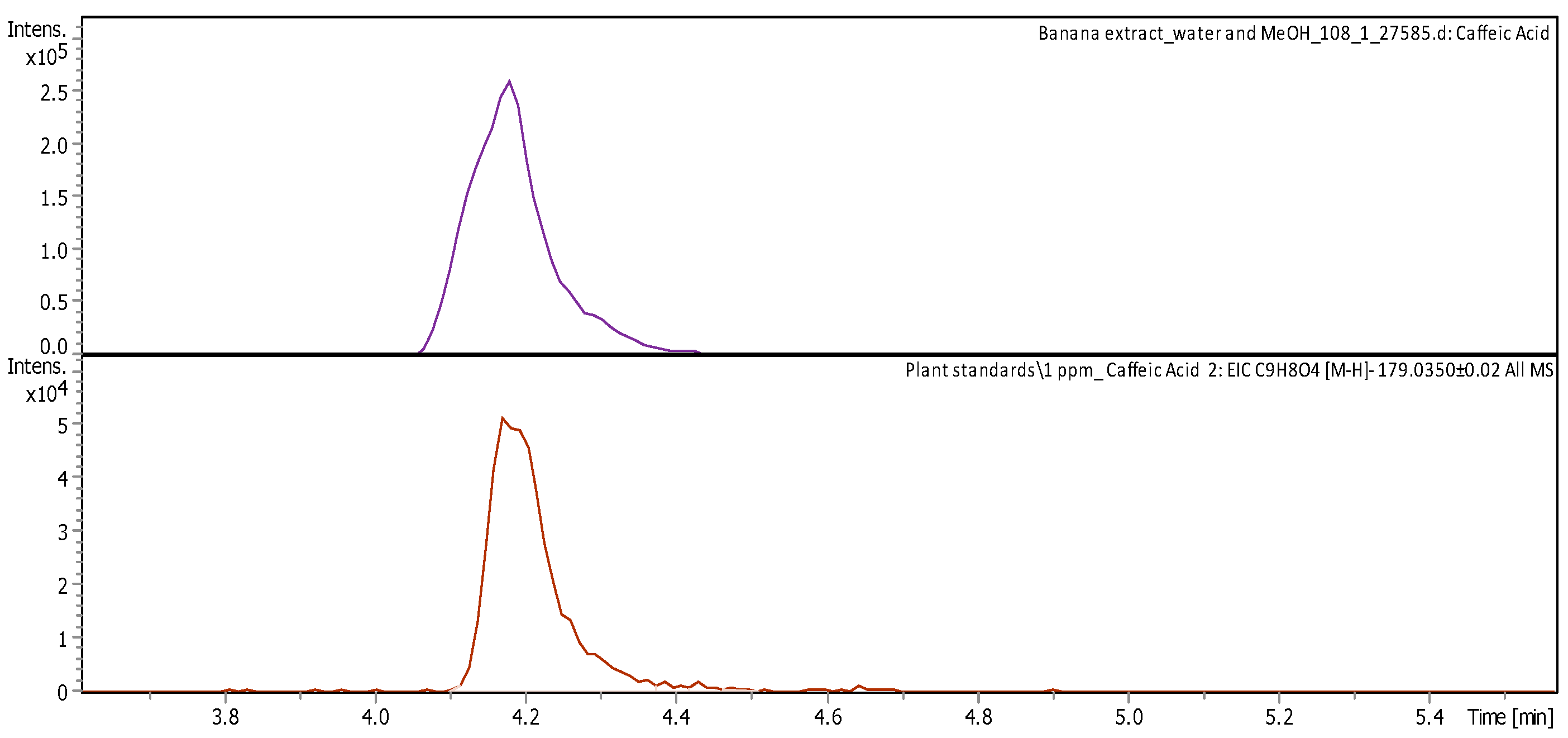
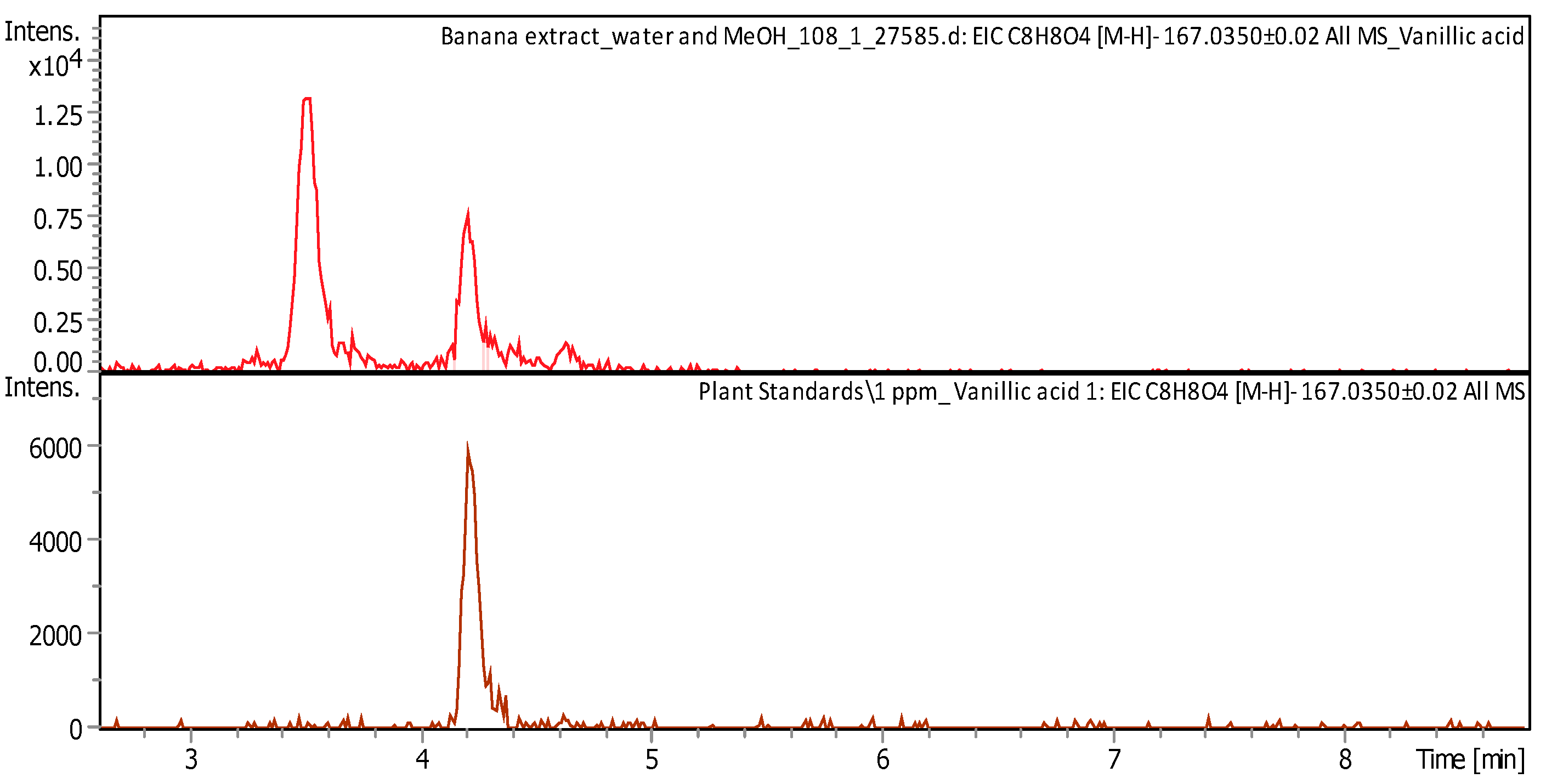
2.2.2. Liquid Chromatography/Mass Spectroscopy (LC/MS) Analysis
2.3. Synthesis of ZnO NPs and Yield
2.4. Characterization of ZnO NPs
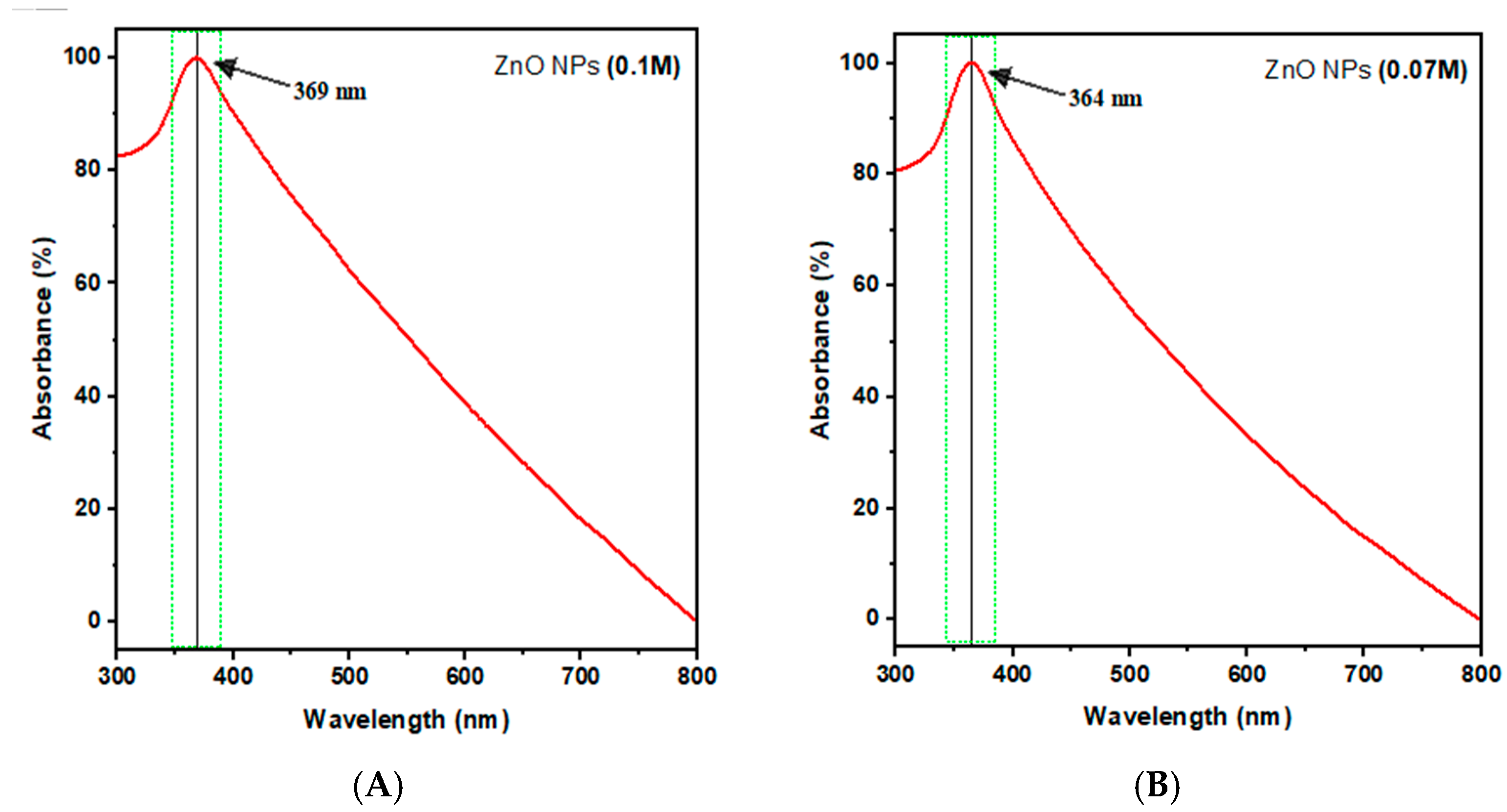
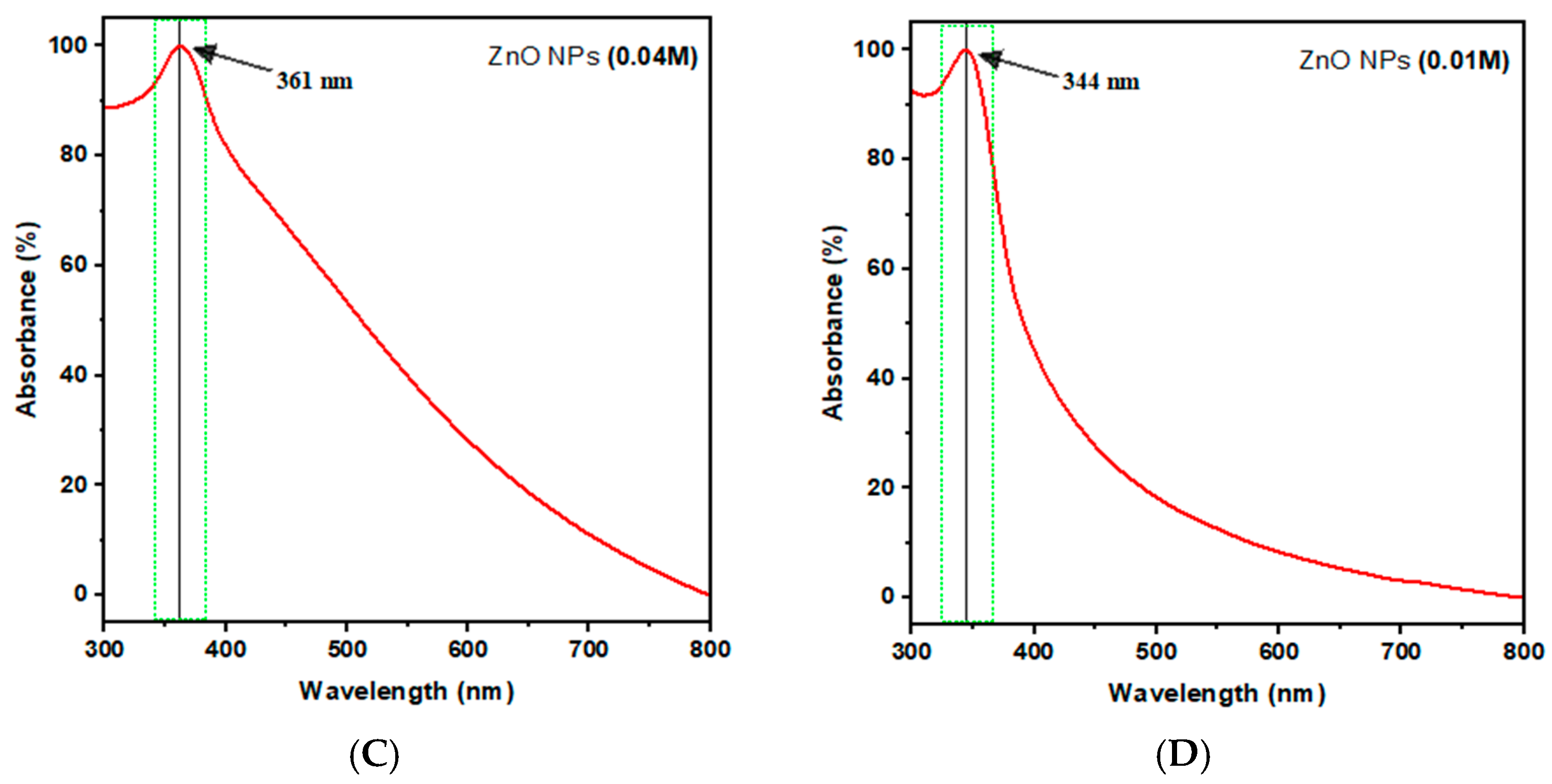
2.4.1. UV–Visible Spectroscopy
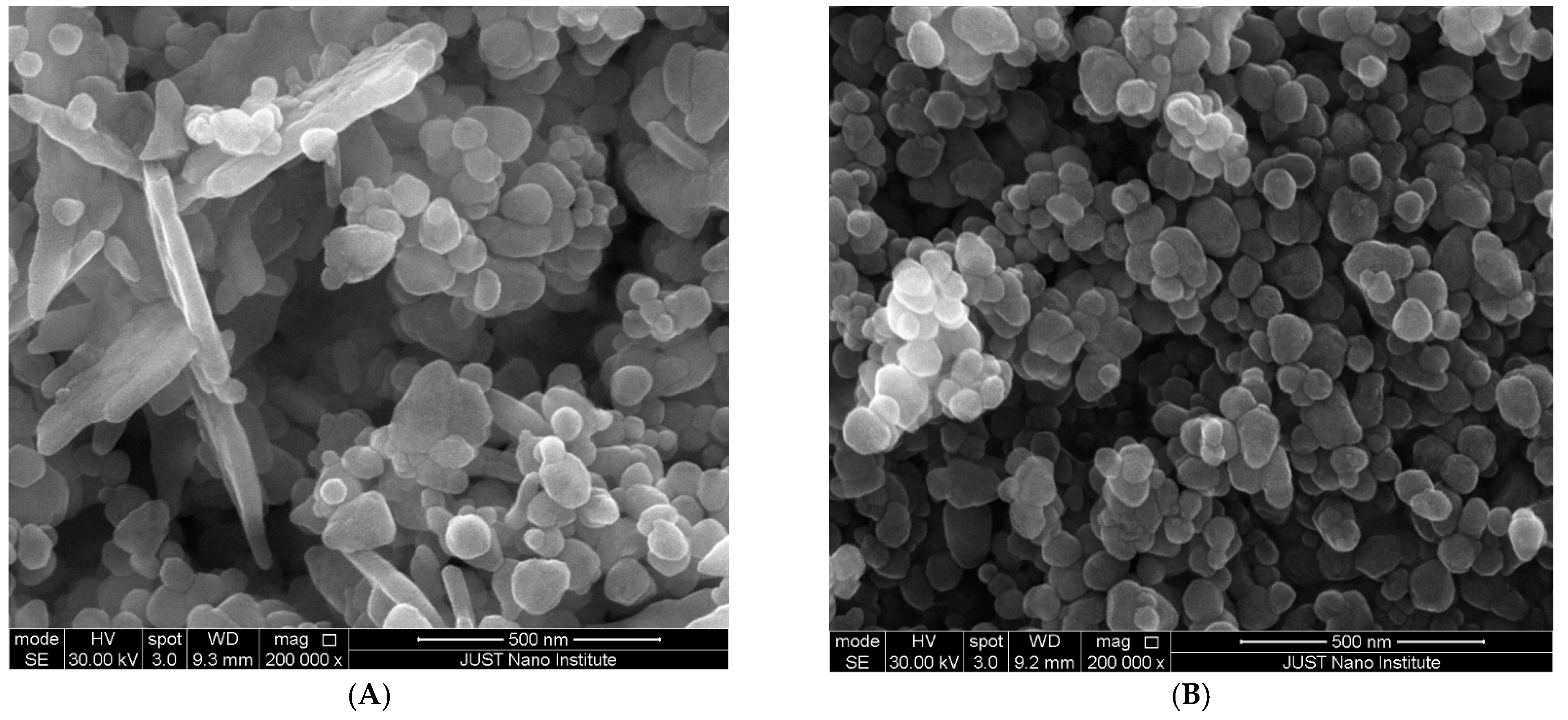
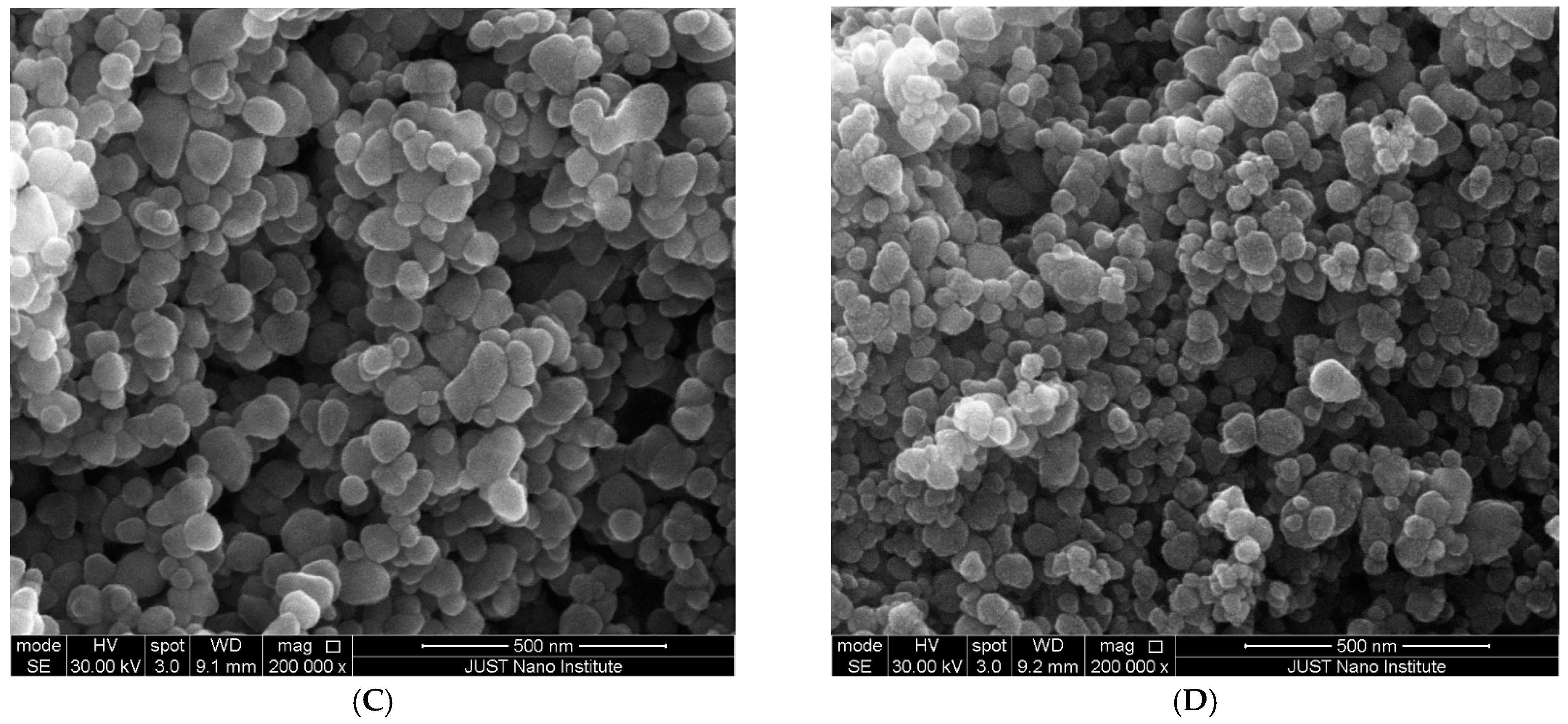
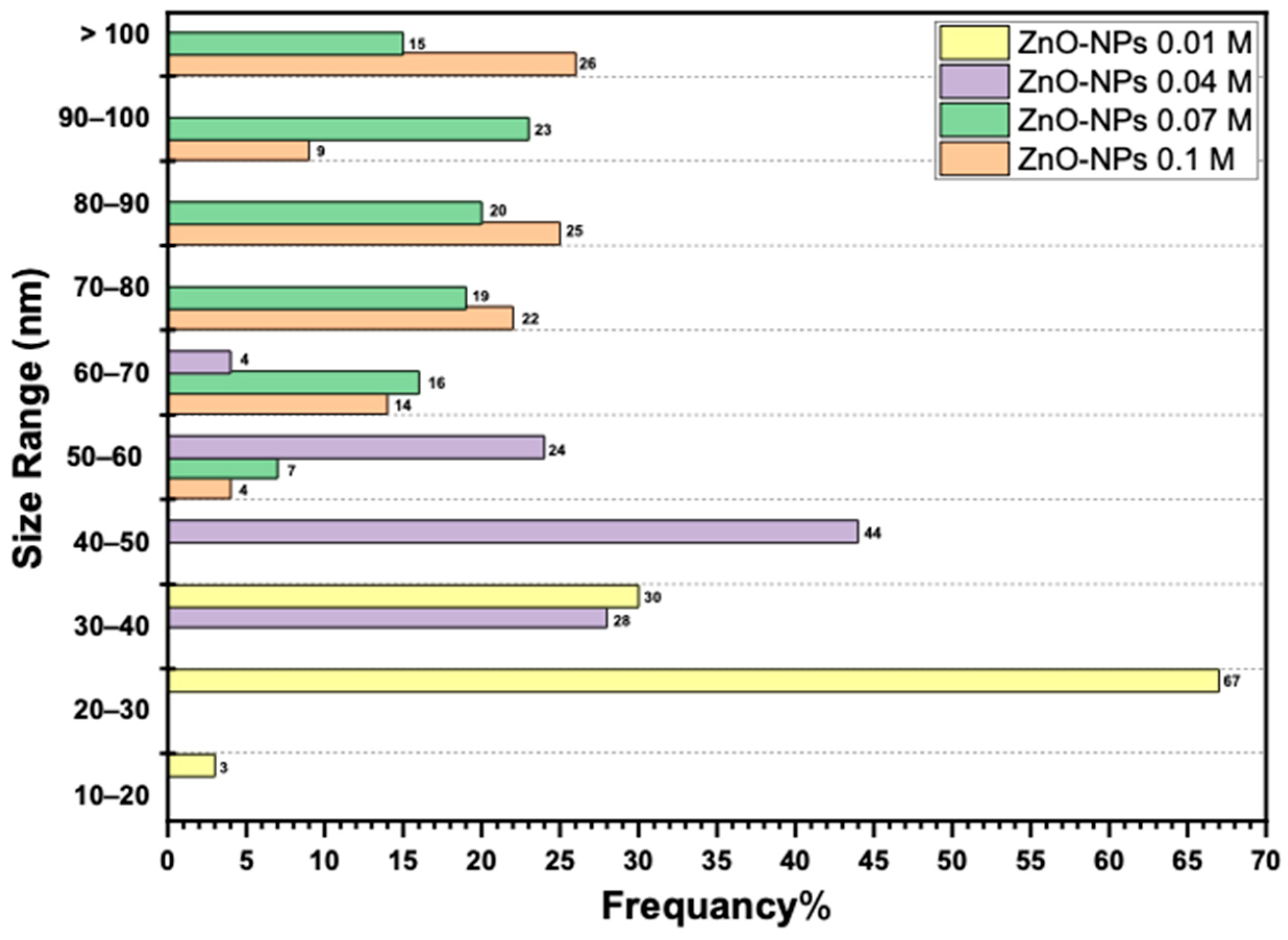
2.4.2. Scanning Electron Microscopy (SEM)
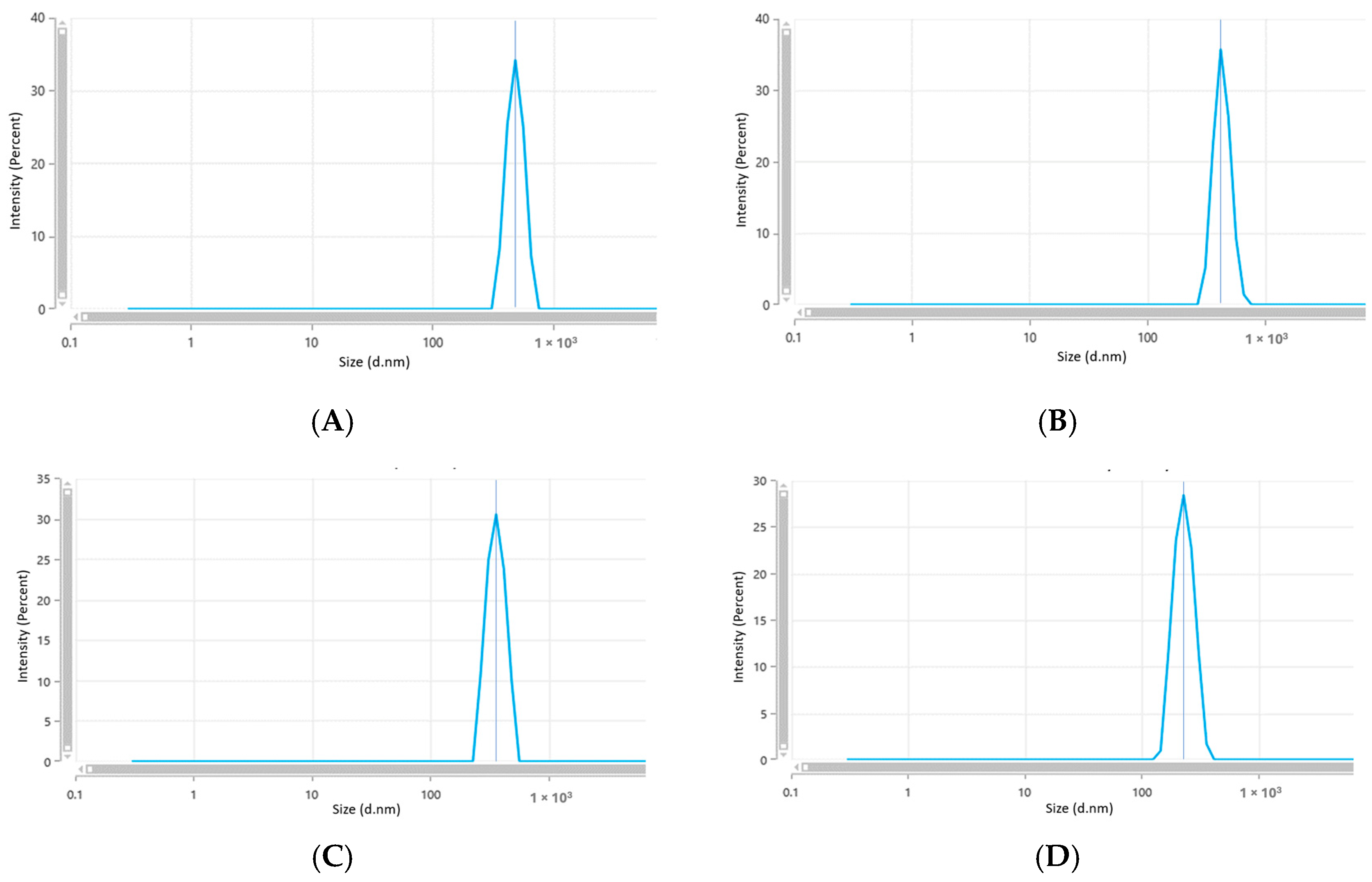
2.4.3. Dynamic Light Scattering (DLS) Analysis
2.4.4. Attenuated Total Reflectance–Fourier-Transform Infrared (ATR-FTIR)
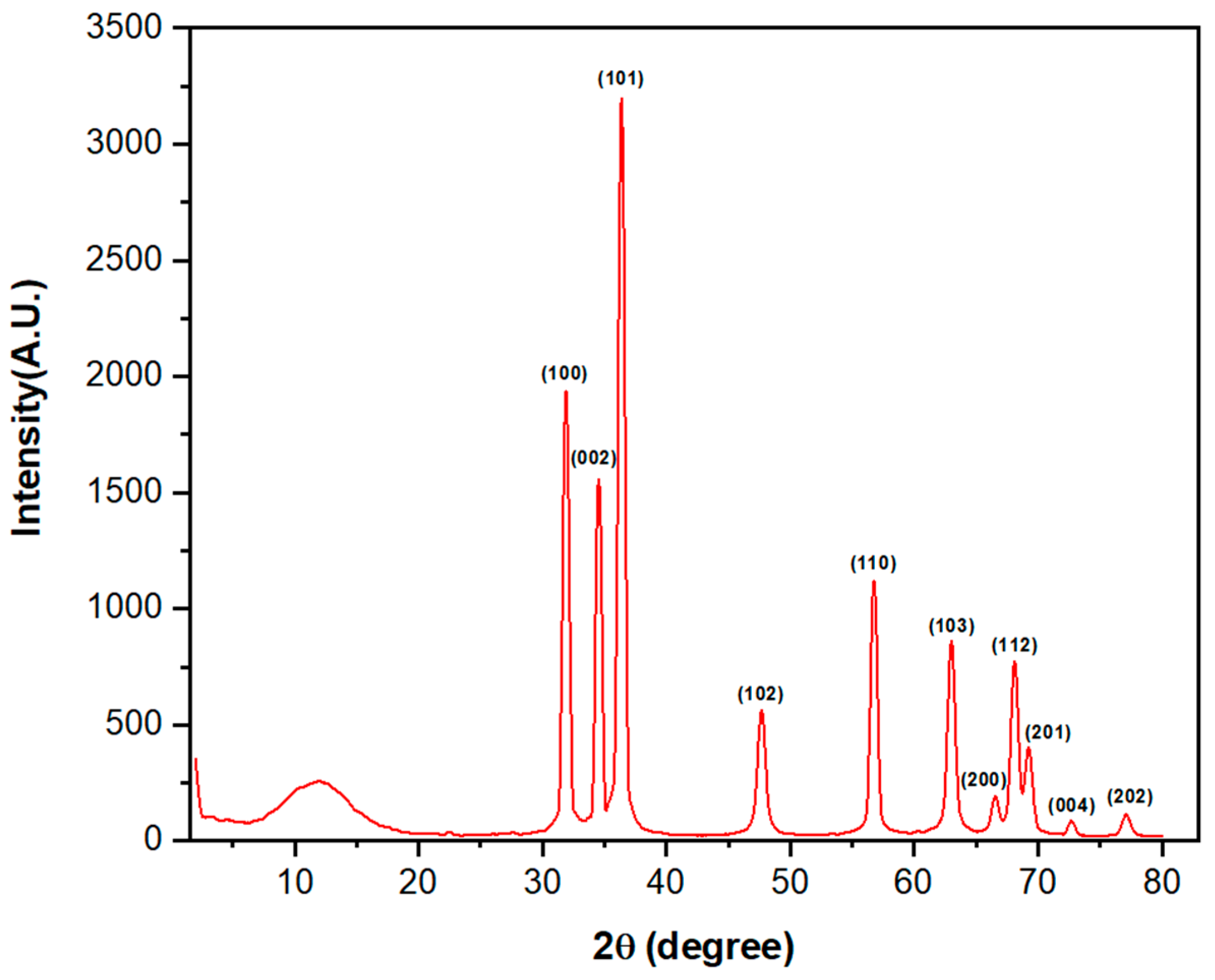
2.4.5. X-ray Powder Diffraction (XRD)
2.5. Antibacterial Activity of ZnO NPs
3. Materials and Methods
3.1. Materials and Reagents
3.2. Banana Species Identification and Banana Peel Extract Preparation
3.3. Characterization and Analysis Technique for Banana Peel Extract
3.3.1. Manual Screening Tests
3.3.2. Liquid Chromatography/Mass Spectroscopy (LC/MS) Analysis
3.4. Synthesis of ZnO Nanoparticles
3.5. Characterization of Zinc Oxide NPs
3.5.1. UV–Visible Spectroscopy
3.5.2. Scanning Electron Microscopy (SEM)
3.5.3. Dynamic Light Scattering (DLS) Analysis
3.5.4. Attenuated Total Reflectance–Fourier-Transform Infrared (ATR-FTIR)
3.5.5. X-ray Powder Diffraction (XRD)
3.6. Antibacterial Activity of ZnO NPs
3.7. Statical Data Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
References
- Mohd Yusof, H.; Mohamad, R.; Zaidan, U.H.; Abdul Rahman, N.A. Microbial Synthesis of Zinc Oxide Nanoparticles and Their Potential Application as an Antimicrobial Agent and a Feed Supplement in Animal Industry: A Review. J. Anim. Sci. Biotechnol. 2019, 10, 57. [Google Scholar] [CrossRef]
- Khan, S.; Mansoor, S.; Rafi, Z.; Kumari, B.; Shoaib, A.; Saeed, M.; Alshehri, S.; Ghoneim, M.M.; Rahamathulla, M.; Hani, U.; et al. A Review on Nanotechnology: Properties, Applications, and Mechanistic Insights of Cellular Uptake Mechanisms. J. Mol. Liq. 2022, 348, 118008. [Google Scholar] [CrossRef]
- Ahmed, S.; Annu; Chaudhry, S.A.; Ikram, S. A Review on Biogenic Synthesis of ZnO Nanoparticles Using Plant Extracts and Microbes: A Prospect towards Green Chemistry. J. Photochem. Photobiol. B Biol. 2017, 166, 272–284. [Google Scholar] [CrossRef] [PubMed]
- Carofiglio, M.; Barui, S.; Cauda, V.; Laurenti, M. Doped Zinc Oxide Nanoparticles: Synthesis, Characterization and Potential Use in Nanomedicine. Appl. Sci. 2020, 10, 5194. [Google Scholar] [CrossRef]
- Anjum, S.; Hashim, M.; Malik, S.A.; Khan, M.; Lorenzo, J.M.; Abbasi, B.H.; Hano, C. Recent Advances in Zinc Oxide Nanoparticles (ZnO NPs) for Cancer Diagnosis, Target Drug Delivery, and Treatment. Cancers 2021, 13, 4570. [Google Scholar] [CrossRef]
- Saravanan, K.; Natarajan, B.; Nallamuthu, N.; Sadiq, M.; Ramanujam, G.M. Evaluation of Nanomedicine Applications of Silver and Zinc Oxide Nanoparticles Using Water Extract of Fresh Turmeric. ECS J. Solid State Sci. Technol. 2023, 12, 021003. [Google Scholar] [CrossRef]
- Sivakumar, P.; Lee, M.; Kim, Y.-S.; Shim, M.S. Photo-Triggered Antibacterial and Anticancer Activities of Zinc Oxide Nanoparticles. J. Mater. Chem. B 2018, 6, 4852–4871. [Google Scholar] [CrossRef]
- Bedi, P.; Kaur, A. An Overview on Uses of Zinc Oxide Nanoparticles. World J. Pharm. Pharm. Sci. 2015, 4, 1177–1196. [Google Scholar]
- Kulkarni, S.S.; Shirsat, M.D. Optical and Structural Properties of Zinc Oxide Nanoparticles. Int. J. Adv. Res. Phys. Sci. 2015, 2, 14–18. [Google Scholar]
- Manjunatha, R.L.; Usharani, K.V.; Naik, D. Synthesis and Characterization of ZnO Nanoparticles: A Review. J. Pharmacogn. Phytochem. 2019, 8, 1095–1101. [Google Scholar]
- Islam, F.; Shohag, S.; Uddin, M.J.; Islam, M.R.; Nafady, M.H.; Akter, A.; Mitra, S.; Roy, A.; Emran, T.B.; Cavalu, S. Exploring the Journey of Zinc Oxide Nanoparticles (ZnO-NPs) toward Biomedical Applications. Materials 2022, 15, 2160. [Google Scholar] [CrossRef] [PubMed]
- Sierra, M.J.; Herrera, A.P.; Ojeda, K.A. Synthesis of Zinc Oxide Nanoparticles from Mango and Soursop Leaf Extracts. Contemp. Eng. Sci. 2018, 11, 395–403. [Google Scholar] [CrossRef]
- Jamdagni, P.; Khatri, P.; Rana, J.-S. Green Synthesis of Zinc Oxide Nanoparticles Using Flower Extract of Nyctanthes Arbor-Tristis and Their Antifungal Activity. J. King Saud Univ. -Sci. 2018, 30, 168–175. [Google Scholar] [CrossRef]
- Barani, D.; Benhaoua, B.; Laouini, S.E.; Bentemam, H.; Allag, N.; Berra, D.; Guerram, A. Green Synthesis of Zno Nanoparticles Using Phoenix Dactylifera. L Leaf Extract: Effect of Zinc Acetate Concentration on the Type of Product. Dig. J. Nanomater. Biostructures (DJNB) 2019, 14, 581–591. Available online: https://www.chalcogen.ro/581_BaraniD.pdf (accessed on 14 October 2021).
- Fakhari, S.; Jamzad, M.; Kabiri Fard, H. Green Synthesis of Zinc Oxide Nanoparticles: A Comparison. Green Chem. Lett. Rev. 2019, 12, 19–24. [Google Scholar] [CrossRef]
- Król, A.; Railean-Plugaru, V.; Pomastowski, P.; Buszewski, B. Phytochemical Investigation of Medicago sativa L. Extract and Its Potential as a Safe Source for the Synthesis of ZnO Nanoparticles: The Proposed Mechanism of Formation and Antimicrobial Activity. Phytochem. Lett. 2019, 31, 170–180. [Google Scholar] [CrossRef]
- Prasad, A.R.; Williams, L.; Garvasis, J.; Shamsheera, K.O.; Basheer, S.M.; Kuruvilla, M.; Joseph, A. Applications of Phytogenic ZnO Nanoparticles: A Review on Recent Advancements. J. Mol. Liq. 2021, 331, 115805. [Google Scholar] [CrossRef]
- Devi, D.; Julkapli, N.M.; Sagadevan, S.; Johan, M.R. Eco-Friendly Green Synthesis Approach and Evaluation of Environmental and Biological Applications of Iron Oxide Nanoparticles. Inorg. Chem. Commun. 2023, 152, 110700. [Google Scholar]
- Colon, G.; Ward, B.C.; Webster, T.J. Increased Osteoblast and Decreased Staphylococcus epidermidis Functions on Nanophase ZnO and TiO2. J. Biomed. Mater. Res. 2006, 78A, 595–604. [Google Scholar] [CrossRef]
- Padmavathy, N.; Vijayaraghavan, R. Enhanced Bioactivity of ZnO Nanoparticles—An Antimicrobial Study. Sci. Technol. Adv. Mater. 2008, 9, 035004. [Google Scholar] [CrossRef]
- Aswathi, V.P.; Meera, S.; Maria, C.G.A.; Nidhin, M. Green Synthesis of Nanoparticles from Biodegradable Waste Extracts and Their Applications: A Critical Review. Nanotechnol. Environ. Eng. 2023, 8, 377–397. [Google Scholar] [CrossRef]
- Šimoníková, D.; Čížková, J.; Zoulová, V.; Christelová, P.; Hřibová, E. Advances in the Molecular Cytogenetics of Bananas, Family Musaceae. Plants 2022, 11, 482. [Google Scholar] [CrossRef] [PubMed]
- Chugh, R.; Kaur, G. A Mini Review on Green Synthesis of Nanoparticles by Utilization of Musa- Balbisiana Waste Peel Extract. Mater. Today Proc. 2022; in press. [Google Scholar] [CrossRef]
- Dmochowska, A.; Czajkowska, J.; Jędrzejewski, R.; Stawiński, W.; Migdał, P.; Fiedot-Toboła, M. Pectin Based Banana Peel Extract as a Stabilizing Agent in Zinc Oxide Nanoparticles Synthesis. Int. J. Biol. Macromol. 2020, 165, 1581–1592. [Google Scholar] [CrossRef]
- Hussien, N.A.; Al Malki, J.S.; Al Harthy, F.A.; Mazi, A.W.; Al Shadadi, J.A. Sustainable Eco-Friendly Synthesis of Zinc Oxide Nanoparticles Using Banana Peel and Date Seed Extracts, Characterization, and Cytotoxicity Evaluation. Sustainability 2023, 15, 9864. [Google Scholar] [CrossRef]
- Ruangtong, J.; T-Thienprasert, J.; T-Thienprasert, N.P. Green Synthesized ZnO Nanosheets from Banana Peel Extract Possess Anti-Bacterial Activity and Anti-Cancer Activity. Mater. Today Commun. 2020, 24, 101224. [Google Scholar] [CrossRef]
- Bag, S.S.; Bora, A.; Golder, A.K. Biomimetic Synthesis of Silver Nanoparticles Using Bhimkol (Musa Balbisiana) Peel Extract as Biological Waste: Its Antibacterial Activity and Role of Ripen Stage of the Peel. Curr. Nanomater. 2020, 5, 47–65. [Google Scholar] [CrossRef]
- Abdol Aziz, R.A.; Abd Karim, S.F.; Ibrahim, U.K.; Sanuddin, N. Precursor Concentration Effect on Physicochemical Properties of Zinc Oxide Nanoparticle Synthesized with Banana Peel Extract. Key Eng. Mater. 2019, 797, 262–270. [Google Scholar] [CrossRef]
- Vishnukumar, P.; Vivekanandhan, S.; Misra, M.; Mohanty, A.K. Recent Advances and Emerging Opportunities in Phytochemical Synthesis of ZnO Nanostructures. Mater. Sci. Semicond. Process. 2018, 80, 143–161. [Google Scholar] [CrossRef]
- Wang, Q.; Mei, S.; Manivel, P.; Ma, H.; Chen, X. Zinc Oxide Nanoparticles Synthesized Using Coffee Leaf Extract Assisted with Ultrasound as Nanocarriers for Mangiferin. Curr. Res. Food Sci. 2022, 5, 868–877. [Google Scholar] [CrossRef]
- Mohammadi, F.M.; Ghasemi, N. Influence of Temperature and Concentration on Biosynthesis and Characterization of Zinc Oxide Nanoparticles Using Cherry Extract. J. Nanostructure Chem. 2018, 8, 93–102. [Google Scholar] [CrossRef]
- Hooshmand, S.; Mohammadian, M.; Es’haghi, Z. Green and Chemical Synthesis of Zinc Oxide Nanoparticles and Size Evaluation by UV–Vis Spectroscopy. J. Nanomed. Res. 2018, 7, 00175. [Google Scholar] [CrossRef]
- Chaudhuri, S.K.; Malodia, L. Biosynthesis of Zinc Oxide Nanoparticles Using Leaf Extract of Calotropis Gigantea: Characterization and Its Evaluation on Tree Seedling Growth in Nursery Stage. Appl. Nanosci. 2017, 7, 501–512. [Google Scholar] [CrossRef]
- Rajan, A.; Cherian, E.; Gurunathan, D.B. Biosynthesis of Zinc Oxide Nanoparticles Using Aspergillus Fumigatus JCF and Its Antibacterial Activity. Int. J. Mod. Sci. Technol. 2016, 1, 52–57. [Google Scholar]
- Ahmad, N.; Ang, B.C.; Amalina, M.A.; Bong, C.W. Influence of Precursor Concentration and Temperature on the Formation of Nanosilver in Chemical Reduction Method. Sains Malays. 2018, 47, 157–168. [Google Scholar]
- Sidhu, A.K.; Verma, N.; Kaushal, P. Role of Biogenic Capping Agents in the Synthesis of Metallic Nanoparticles and Evaluation of Their Therapeutic Potential. Front. Nanotechnol. 2022, 3, 801620. [Google Scholar] [CrossRef]
- Shrestha, S.; Wang, B.; Dutta, P. Nanoparticle Processing: Understanding and Controlling Aggregation. Adv. Colloid Interface Sci. 2020, 279, 102162. [Google Scholar] [CrossRef] [PubMed]
- Vimala, K.; Sundarraj, S.; Paulpandi, M.; Vengatesan, S.; Kannan, S. Green Synthesized Doxorubicin Loaded Zinc Oxide Nanoparticles Regulates the Bax and Bcl-2 Expression in Breast and Colon Carcinoma. Process Biochem. 2014, 49, 160–172. [Google Scholar] [CrossRef]
- Geremew, A.; Carson, L.; Woldesenbet, S.; Wang, H.; Reeves, S.; Brooks, N.; Saganti, P.; Weerasooriya, A.; Peace, E. Effect of Zinc Oxide Nanoparticles Synthesized from Carya Illinoinensis Leaf Extract on Growth and Antioxidant Properties of Mustard (Brassica Juncea). Front. Plant Sci. 2023, 14, 1108186. [Google Scholar] [CrossRef]
- Figueiredo, M. Sizing Nanoparticles in Liquids: An Overview of Methods. In Drug Delivery Systems: Advanced Technologies Potentially Applicable in Personalised Treatment; Coelho, J., Ed.; Advances in Predictive, Preventive and Personalised Medicine; Springer: Dordrecht, The Netherlands, 2013; Volume 4, pp. 87–107. [Google Scholar] [CrossRef]
- Reddy, N.K.; Pérez-Juste, J.; Pastoriza-Santos, I.; Lang, P.R.; Dhont, J.K.G.; Liz-Marzán, L.M.; Vermant, J. Flow Dichroism as a Reliable Method to Measure the Hydrodynamic Aspect Ratio of Gold Nanoparticles. ACS Nano 2011, 5, 4935–4944. [Google Scholar] [CrossRef]
- Kibria, A.A.; Kamrunnessa; Rahman, M.M.; Kar, A. Extraction and Evaluation of Phytochemicals from Banana Peels (Musa sapientum) and Banana Plants (Musa paradisiaca). Malays. J. Halal Res. 2019, 2, 22–26. [Google Scholar] [CrossRef]
- Hess, W.; Frisch, H.L.; Klein, R. On the Hydrodynamic Behavior of Colloidal Aggregates. Z. Für Phys. B Condens. Matter 1986, 64, 65–67. [Google Scholar] [CrossRef]
- Maguire, C.M.; Rösslein, M.; Wick, P.; Prina-Mello, A. Characterisation of Particles in Solution—A Perspective on Light Scattering and Comparative Technologies. Sci. Technol. Adv. Mater. 2018, 19, 732–745. [Google Scholar] [CrossRef]
- Alamdari, S.; Sasani Ghamsari, M.; Lee, C.; Han, W.; Park, H.-H.; Tafreshi, M.J.; Afarideh, H.; Ara, M.H.M. Preparation and Characterization of Zinc Oxide Nanoparticles Using Leaf Extract of Sambucus Ebulus. Appl. Sci. 2020, 10, 3620. [Google Scholar] [CrossRef]
- Oliveira, R.N.; Mancini, M.C.; de Oliveira, F.C.S.; Passos, T.M.; Quilty, B.; Thiré, R.M.d.S.M.; McGuinness, G.B. FTIR Analysis and Quantification of Phenols and Flavonoids of Five Commercially Available Plants Extracts Used in Wound Healing. Matéria 2016, 21, 767–779. [Google Scholar]
- Rojas Flores, S.; Cruz-Noriega, M.; Nazario-Naveda, R.; Benites, S.; Delfin Narciso, D.; Angelats-Silva, L.; Murga-Torres, E. Use of Banana Waste as a Source for Bioelectricity Generation. Processes 2022, 10, 942. [Google Scholar] [CrossRef]
- Sangeetha, M.; Rajendran, S.; Sathiyabama, J.; Prabhakar, P. Eco Friendly Extract of Banana Peel as Corrosion Inhibitor for Carbon Steel in Sea Water. J. Nat. Prod. Plant Resour. 2012, 2, 601–610. [Google Scholar]
- Das, J.; Velusamy, P. Antibacterial Effects of Biosynthesized Silver Nanoparticles Using Aqueous Leaf Extract of Rosmarinus officinalis L. Mater. Res. Bull. 2013, 48, 4531–4537. [Google Scholar] [CrossRef]
- Alaa El-Din, G.; Amer, A.A.; Malsh, G.; Hussein, M. Study on the Use of Banana Peels for Oil Spill Removal. Alex. Eng. J. 2018, 57, 2061–2068. [Google Scholar] [CrossRef]
- Dudek, M.; Zajac, G.; Szafraniec, E.; Wiercigroch, E.; Tott, S.; Malek, K.; Kaczor, A.; Baranska, M. Raman Optical Activity and Raman Spectroscopy of Carbohydrates in Solution. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2019, 206, 597–612. [Google Scholar] [CrossRef]
- Gnanasangeetha, D.; Thambavani, D.S. Biogenic Production of Zinc Oxide Nanoparticles Using Acalypha Indica. J. Chem. Biol. Phys. Sci. (JCBPS) 2013, 4, 238. [Google Scholar]
- Ramesh, M.; Anbuvannan, M.; Viruthagiri, G. Green Synthesis of ZnO Nanoparticles Using Solanum Nigrum Leaf Extract and Their Antibacterial Activity. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2015, 136, 864–870. [Google Scholar] [CrossRef] [PubMed]
- Stan, M.; Popa, A.; Toloman, D.; Silipas, T.-D.; Vodnar, D.C. Antibacterial and Antioxidant Activities of ZnO Nanoparticles Synthesized Using Extracts of Allium Sativum, Rosmarinus officinalis and Ocimum Basilicum. Acta Metall. Sin. (Engl. Lett.) 2016, 29, 228–236. [Google Scholar] [CrossRef]
- Ghamsari, M.S.; Alamdari, S.; Razzaghi, D.; Pirlar, M.A. ZnO Nanocrystals with Narrow-Band Blue Emission. J. Lumin. 2019, 205, 508–518. [Google Scholar] [CrossRef]
- Ibrahem, E.J.; Yasin, Y.S.; Jasim, O.K. Antibacterial Activity of Zinc Oxide Nanoparticles Against Staphylococcus Aureus and Pseudomonas Aeruginosa Isolated from Burn Wound Infections. Cihan Univ.-Erbil Sci. J. 2017, 2017, 265–277. [Google Scholar] [CrossRef]
- Navarre, W.W.; Schneewind, O. Surface Proteins of Gram-Positive Bacteria and Mechanisms of Their Targeting to the Cell Wall Envelope. Microbiol. Mol. Biol. Rev. 1999, 63, 174–229. [Google Scholar] [CrossRef]
- Zare, E.; Pourseyedi, S.; Khatami, M.; Darezereshki, E. Simple Biosynthesis of Zinc Oxide Nanoparticles Using Nature’s Source, and It’s in Vitro Bio-Activity. J. Mol. Struct. 2017, 1146, 96–103. [Google Scholar] [CrossRef]
- Li, Y.; Liao, C.; Tjong, S.C. Recent Advances in Zinc Oxide Nanostructures with Antimicrobial Activities. Int. J. Mol. Sci. 2020, 21, 8836. [Google Scholar] [CrossRef]
- Sakthivel, R.; Geetha, A. Investigating the Effect of Post Annealing on Antibacterial Activity of Zno Thin Films Prepared by Modified Silar Technique. Prog. Chem. Sci. Res. 2023, 7, 18–29. [Google Scholar]
- Yang, J.; Liu, X.; Yang, L.; Wang, Y.; Zhang, Y.; Lang, J.; Gao, M.; Feng, B. Effect of Annealing Temperature on the Structure and Optical Properties of ZnO Nanoparticles. J. Alloys Compd. 2009, 477, 632–635. [Google Scholar] [CrossRef]
- Nazir, A.; Akbar, A.; Baghdadi, H.B.; ur Rehman, S.; Al-Abbad, E.; Fatima, M.; Iqbal, M.; Tamam, N.; Alwadai, N.; Abbas, M. Zinc Oxide Nanoparticles Fabrication Using Eriobotrya Japonica Leaves Extract: Photocatalytic Performance and Antibacterial Activity Evaluation. Arab. J. Chem. 2021, 14, 103251. [Google Scholar] [CrossRef]
- Miri, A.; Khatami, M.; Ebrahimy, O.; Sarani, M. Cytotoxic and Antifungal Studies of Biosynthesized Zinc Oxide Nanoparticles Using Extract of Prosopis Farcta Fruit. Green Chem. Lett. Rev. 2020, 13, 27–33. [Google Scholar] [CrossRef]
- Hussien, N.A. Antimicrobial Potential of Biosynthesized Zinc Oxide Nanoparticles Using Banana Peel and Date Seeds Extracts. Sustainability 2023, 15, 9048. [Google Scholar] [CrossRef]
- Shanavas, S.; Duraimurugan, J.; Kumar, G.S.; Ramesh, R.; Acevedo, R.; Anbarasan, P.M.; Maadeswaran, P. Ecofriendly Green Synthesis of ZnO Nanostructures Using Artabotrys Hexapetalu and Bambusa Vulgaris Plant Extract and Investigation on Their Photocatalytic and Antibacterial Activity. Mater. Res. Express 2019, 6, 105098. [Google Scholar] [CrossRef]
- Yuvakkumar, R.; Suresh, J.; Hong, S.I. Green Synthesis of Zinc Oxide Nanoparticles. Adv. Mater. Res. 2014, 952, 137–140. [Google Scholar] [CrossRef]
- Devi, R.S.; Gayathri, R. Green Synthesis of Zinc Oxide Nanoparticles by Using Hibiscus Rosa-Sinensis. Int. J. Curr. Eng. Technol. 2014, 4, 2444–2446. [Google Scholar]
- Hameed, A.S.H.; Karthikeyan, C.; Ahamed, A.P.; Thajuddin, N.; Alharbi, N.S.; Alharbi, S.A.; Ravi, G. In Vitro Antibacterial Activity of ZnO and Nd Doped ZnO Nanoparticles against ESBL Producing Escherichia Coli and Klebsiella Pneumoniae. Sci. Rep. 2016, 6, 24312. [Google Scholar] [CrossRef]
- Sultan, A.; Khan, H.M.; Malik, A.; Ansari, A.; Azam, A.; Perween, N. Antibacterial Activity of ZnO Nanoparticles against ESBL and Amp-C Producing Gram Negative Isolates from Superficial Wound Infections. Int. J. Curr. Microbiol. App. Sci. 2015, 1, 38–47. [Google Scholar]
- Hoseinzadeh, E.; Alikhani, M.-Y.; Samarghandi, M.-R.; Shirzad-Siboni, M. Antimicrobial Potential of Synthesized Zinc Oxide Nanoparticles against Gram Positive and Gram Negative Bacteria. Desalination Water Treat. 2014, 52, 4969–4976. [Google Scholar] [CrossRef]
- Jones, N.; Ray, B.; Ranjit, K.T.; Manna, A.C. Antibacterial Activity of ZnO Nanoparticle Suspensions on a Broad Spectrum of Microorganisms. FEMS Microbiol. Lett. 2008, 279, 71–76. [Google Scholar] [CrossRef]
- Kumari, S.; Tyagi, M.; Jagadevan, S. Mechanistic Removal of Environmental Contaminants Using Biogenic Nano-Materials. Int. J. Environ. Sci. Technol. 2019, 16, 7591–7606. [Google Scholar] [CrossRef]
- Hussain, I.; Singh, N.B.; Singh, A.; Singh, H.; Singh, S.C. Green Synthesis of Nanoparticles and Its Potential Application. Biotechnol. Lett. 2016, 38, 545–560. [Google Scholar] [CrossRef] [PubMed]
- Ying, S.; Guan, Z.; Ofoegbu, P.C.; Clubb, P.; Rico, C.; He, F.; Hong, J. Green Synthesis of Nanoparticles: Current Developments and Limitations. Environ. Technol. Innov. 2022, 26, 102336. [Google Scholar] [CrossRef]
- Duan, H.; Wang, D.; Li, Y. Green Chemistry for Nanoparticle Synthesis. Chem. Soc. Rev. 2015, 44, 5778–5792. [Google Scholar] [CrossRef]
- Naghdi, M.; Taheran, M.; Brar, S.K.; Verma, M.; Surampalli, R.Y.; Valéro, J.R. Green and Energy-Efficient Methods for the Production of Metallic Nanoparticles. Beilstein J. Nanotechnol. 2015, 6, 2354–2376. [Google Scholar] [CrossRef]
- Virkutyte, J.; Varma, R.S. Green Synthesis of Metal Nanoparticles: Biodegradable Polymers and Enzymes in Stabilization and Surface Functionalization. Chem. Sci. 2011, 2, 837–846. [Google Scholar] [CrossRef]
- Mulvihill, M.J.; Beach, E.S.; Zimmerman, J.B.; Anastas, P.T. Green Chemistry and Green Engineering: A Framework for Sustainable Technology Development. Annu. Rev. Environ. Resour. 2011, 36, 271–293. [Google Scholar] [CrossRef]
- Ehiowemwenguan, G.; Emoghene, A.O.; Inetianbor, J.E. Antibacterial and Phytochemical Analysis of Banana Fruit Peel. IOSR J. Pharm. 2014, 4, 18–25. [Google Scholar]
- Lin, C.-C.; You, Y.-C. Mass-Production of ZnO Nanoparticles by Precipitation in a Rotating Packed Bed: Effect of Zinc Salt. J. Mater. Res. Technol. 2020, 9, 8451–8458. [Google Scholar] [CrossRef]
- Rueden, C.T.; Schindelin, J.; Hiner, M.C.; DeZonia, B.E.; Walter, A.E.; Arena, E.T.; Eliceiri, K.W. ImageJ2: ImageJ for the next Generation of Scientific Image Data. BMC Bioinform. 2017, 18, 529. [Google Scholar] [CrossRef]
- Sandhu, R.; Singh, N.; Dhankhar, J.; Kama, G.; Sharma, R. Dynamic Light Scattering (DLS) Technique, Principle, Theoretical Considerations and Applications. In Nanotechnological and Biochemical Techniques for Assessing the Quality and Safety of Milk and Milk Products; ICAR-NDRI: Karnal, India, 2018; pp. 135–137. Available online: https://www.researchgate.net/publication/331022012 (accessed on 14 October 2021).
- Qaiyumi, S. Macro-and Microdilution Methods of Antimicrobial Susceptibility Testing. In Antimicrobial Susceptibility Testing Protocols; Taylor & Francis: Boca Raton, FL, USA, 2007; pp. 75–79. [Google Scholar]
- Khashan, K.S.; Sulaiman, G.M.; Abdulameer, F.A.; Albukhaty, S.; Ibrahem, M.A.; Al-Muhimeed, T.; AlObaid, A.A. Antibacterial Activity of TiO2 Nanoparticles Prepared by One-Step Laser Ablation in Liquid. Appl. Sci. 2021, 11, 4623. [Google Scholar] [CrossRef]
- Mahdy, S.A.; Raheed, Q.J.; Kalaichelvan, P.T. Antimicrobial Activity of Zero-Valent Iron Nanoparticles. Int. J. Mod. Eng. Res. 2012, 2, 578–581. [Google Scholar]
- Alekish, M.; Ismail, Z.B.; Albiss, B.; Nawasrah, S. In Vitro Antibacterial Effects of Zinc Oxide Nanoparticles on Multiple Drug-Resistant Strains of Staphylococcus Aureus and Escherichia Coli: An Alternative Approach for Antibacterial Therapy of Mastitis in Sheep. Vet. World 2018, 11, 1428. [Google Scholar] [CrossRef] [PubMed]
- Emami-Karvani, Z.; Chehrazi, P. Antibacterial Activity of ZnO Nanoparticle on Gram-Positive and Gram-Negative Bacteria. Afr. J. Microbiol. Res. 2011, 5, 1368–1373. [Google Scholar]


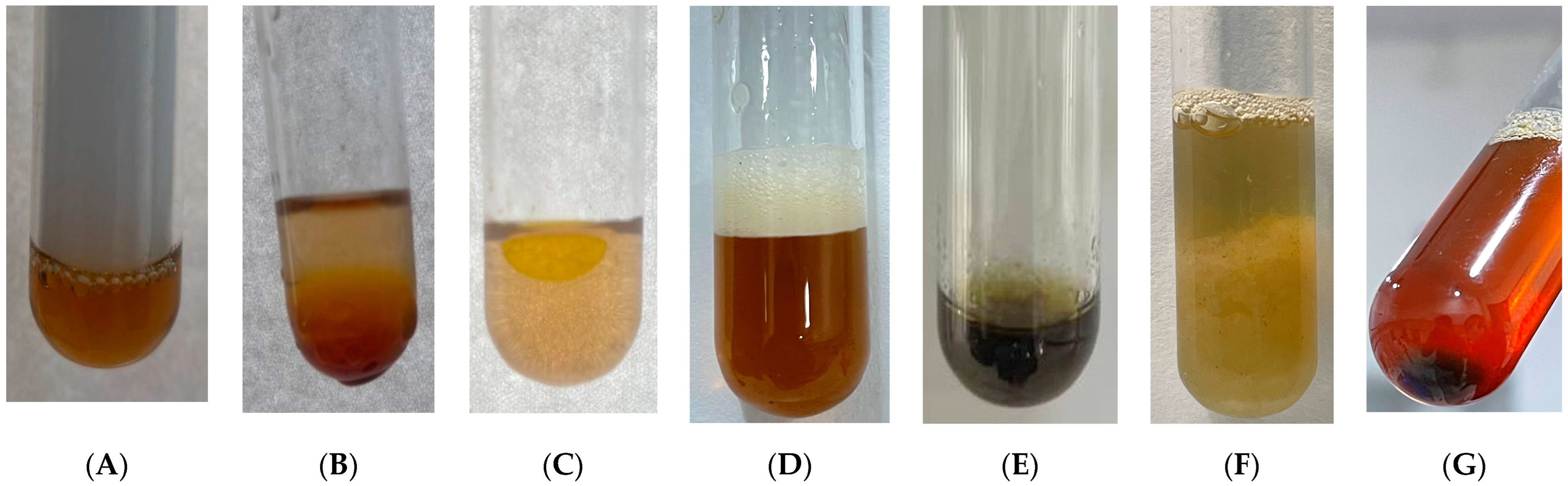



| Phytochemicals | Test | Result | Presence or Absence of Result | Figure |
|---|---|---|---|---|
| Flavonoids | Ammonia test | Pale brown color | + | Figure 3A |
| Glycosides | Glycosides test | Formation of an oil layer on the top | + | Figure 3B |
| Saponins | Emulsion test | Formation of a stable emulsion | + | Figure 3C |
| Froth test | Formation of stable froth | + | Figure 3D | |
| Phenols | Ferric chloride test | Dirty-green color | + | Figure 3E |
| Lead acetate test | Bulky white precipitate | + | Figure 3F | |
| Phlobatannins | Phlobatannins test | Red precipitate | + | Figure 3G |
| Compound | Chemical Formula | Phytochemical Class | Molecular Wt. g/mol | Mass to Charge (m/z) | Retention Time (min) | Concentration (ppm) | Figure |
|---|---|---|---|---|---|---|---|
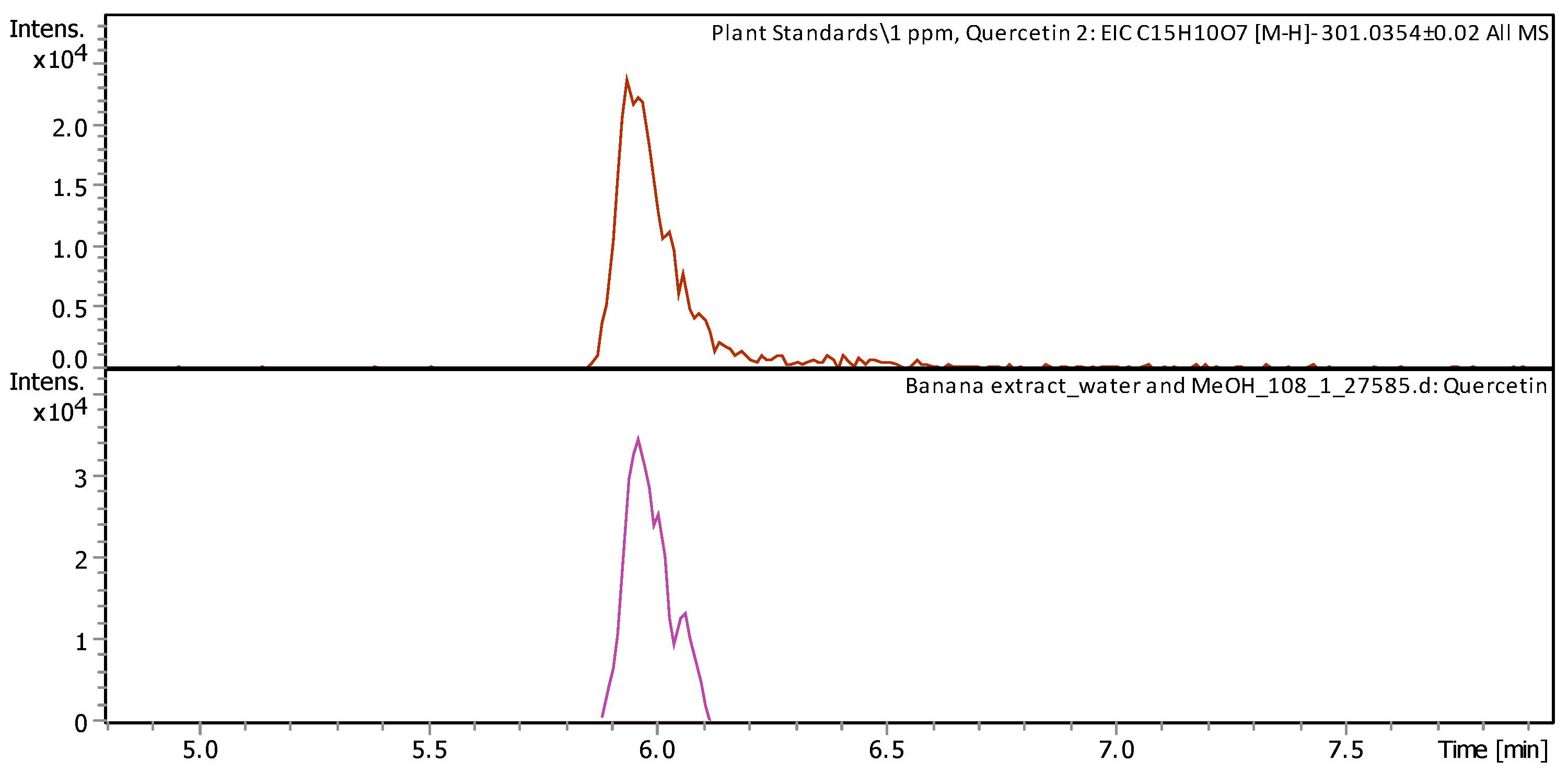
| Quercetin (3,3′,4′,5,7-pentahydroxyflavone) | C15H10O7 | Flavonol from the flavonoid group of polyphenols | 302.0342 | 301.0342 | 5.97 | 1.279 ± 0.086 | Figure A1 |
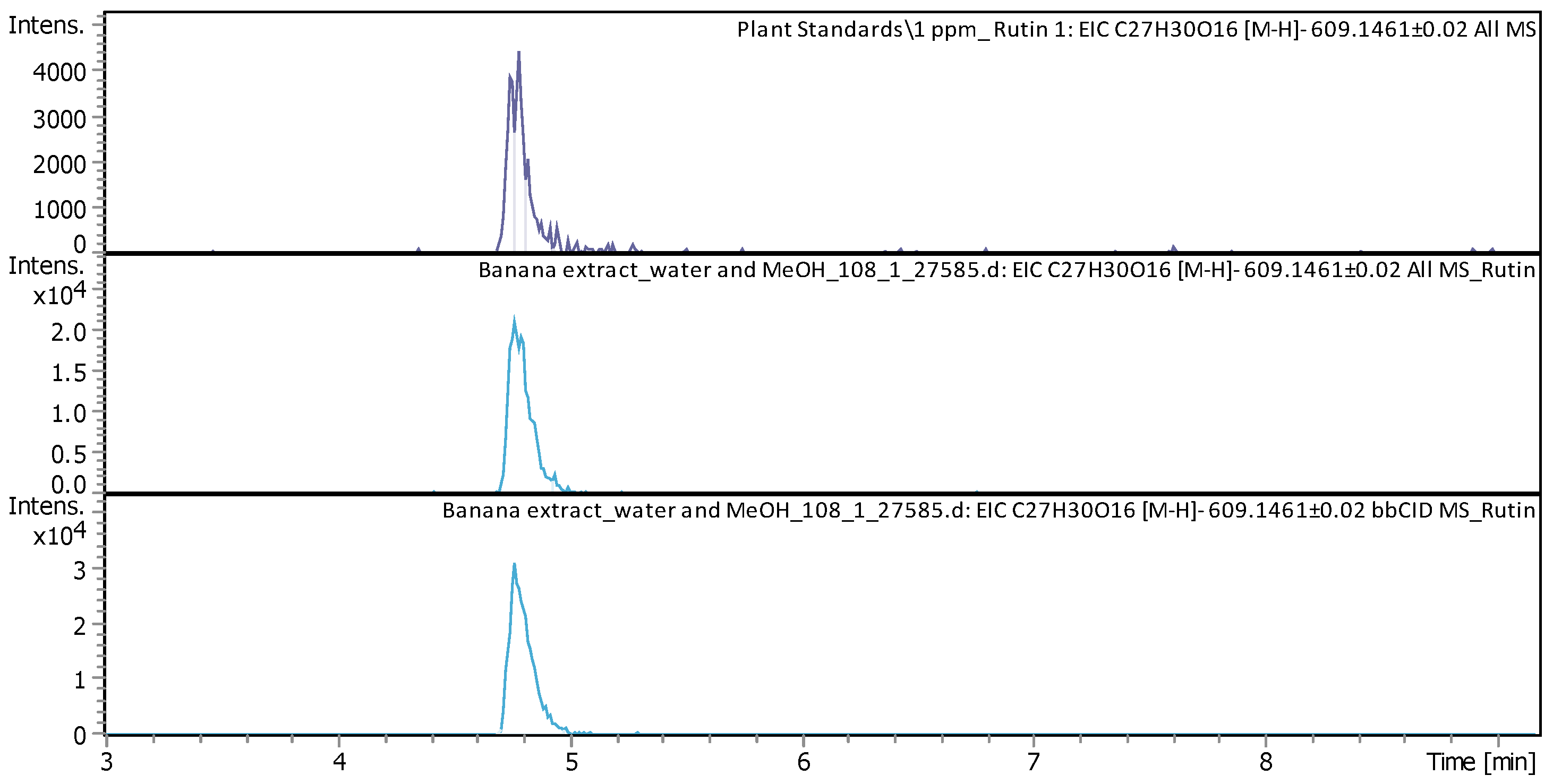
| Rutin (3′,4′,5,7-Tetrahydroxy-3-[α-L-rhamnopyranosyl-(1→6)-β-D-glucopyranosyloxy]flavone) | C27H30O16 | glycoside Flavonol from the flavonoid group of polyphenols | 610.1519 | 609.1447 | 4.77 | 5.877 ± 0.197 | Figure A2 |
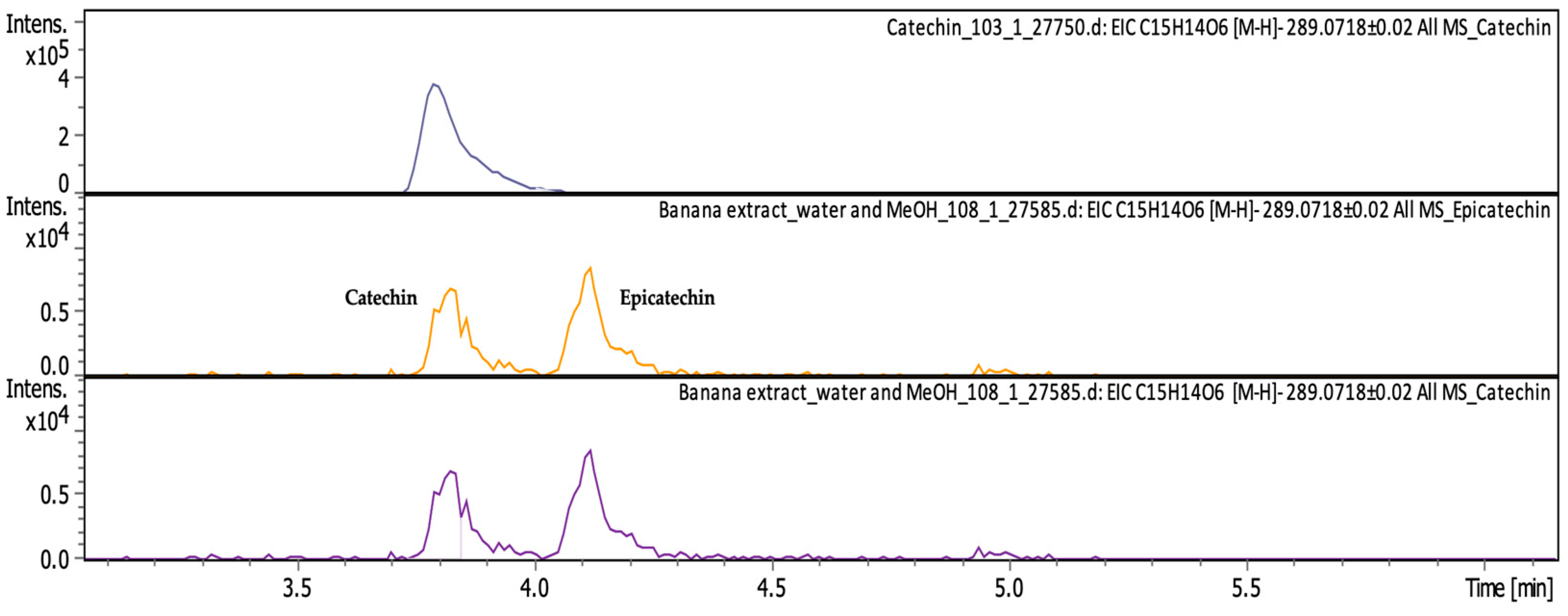
| Catechin ((2R,3S)-2-(3,4-Dihydroxyphenyl)-3,4-dihydro-2H-chromene-3,5,7-triol) | C15H14O6 | flavonols from the flavonoid group of polyphenols | 290.0786 | 289.0713 | 4.11 | 0.476 ± 0.087 | Figure A3 |
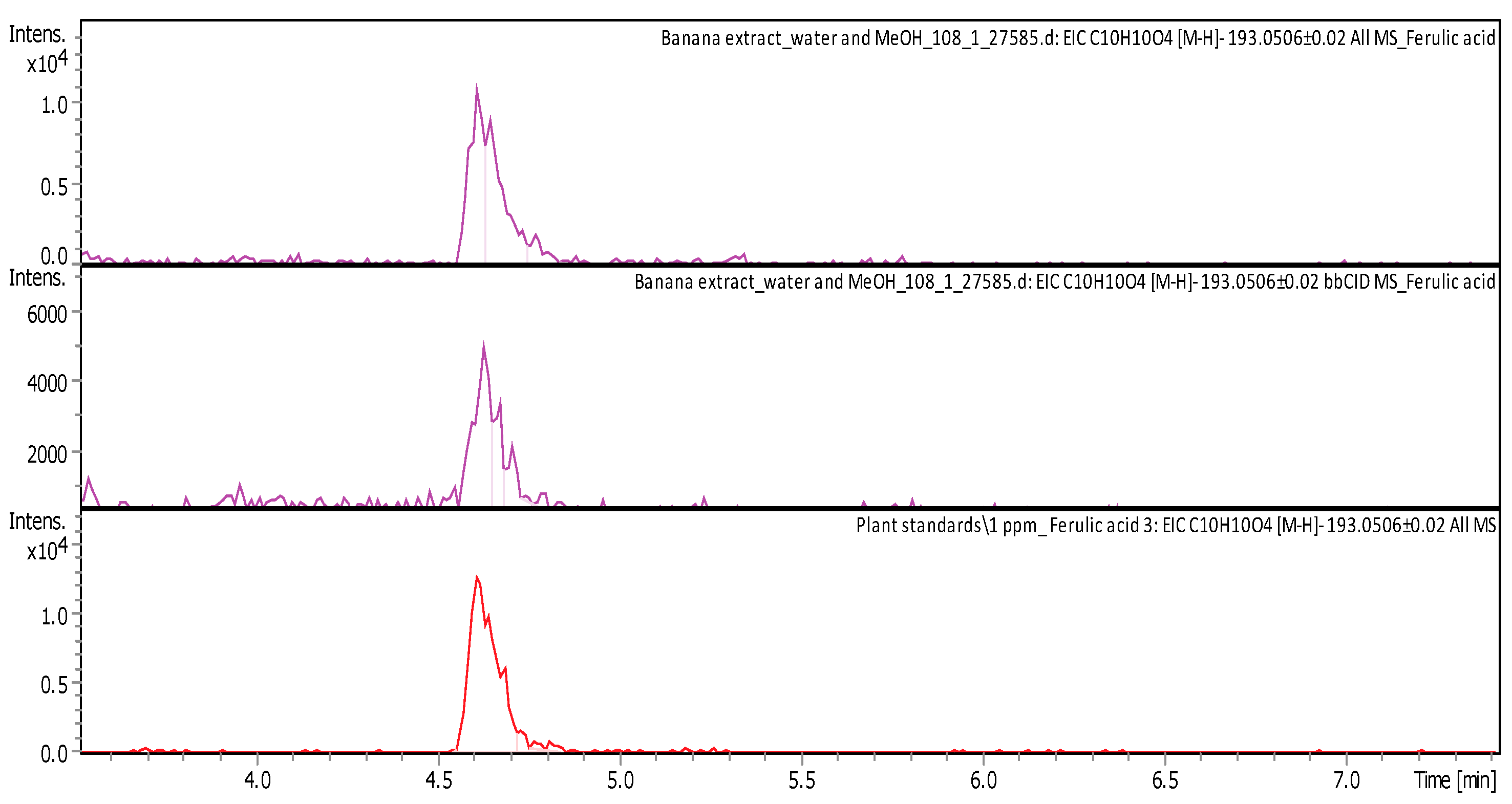
| Ferulic acid ((2E)-3-(4-hydroxy-3-methoxyphenyl) prop-2-enoic acid) | C10H10O4 | flavonols from the flavonoid group of polyphenols | 194.0574 | 193.0501 | 4.61 | 1.068 ± 0.086 | Figure A4 |
| Caffeic acid (3,4-Dihydroxybenzeneacrylicacid) | C9H8O4 | Intermediate in the production of lignin | 180.0419 | 179.0346 | 4.19 | 5.446 ± 0.508 | Figure A5 |
| Vanillic acid (4-hydroxy-3-methoxybenzoic acid) | C8H8O4 | Intermediate in the production of vanillin from ferulic acid | 168.0419 | 167.0347 | 5.97 | 1.496 ± 0.263 | Figure A6 |
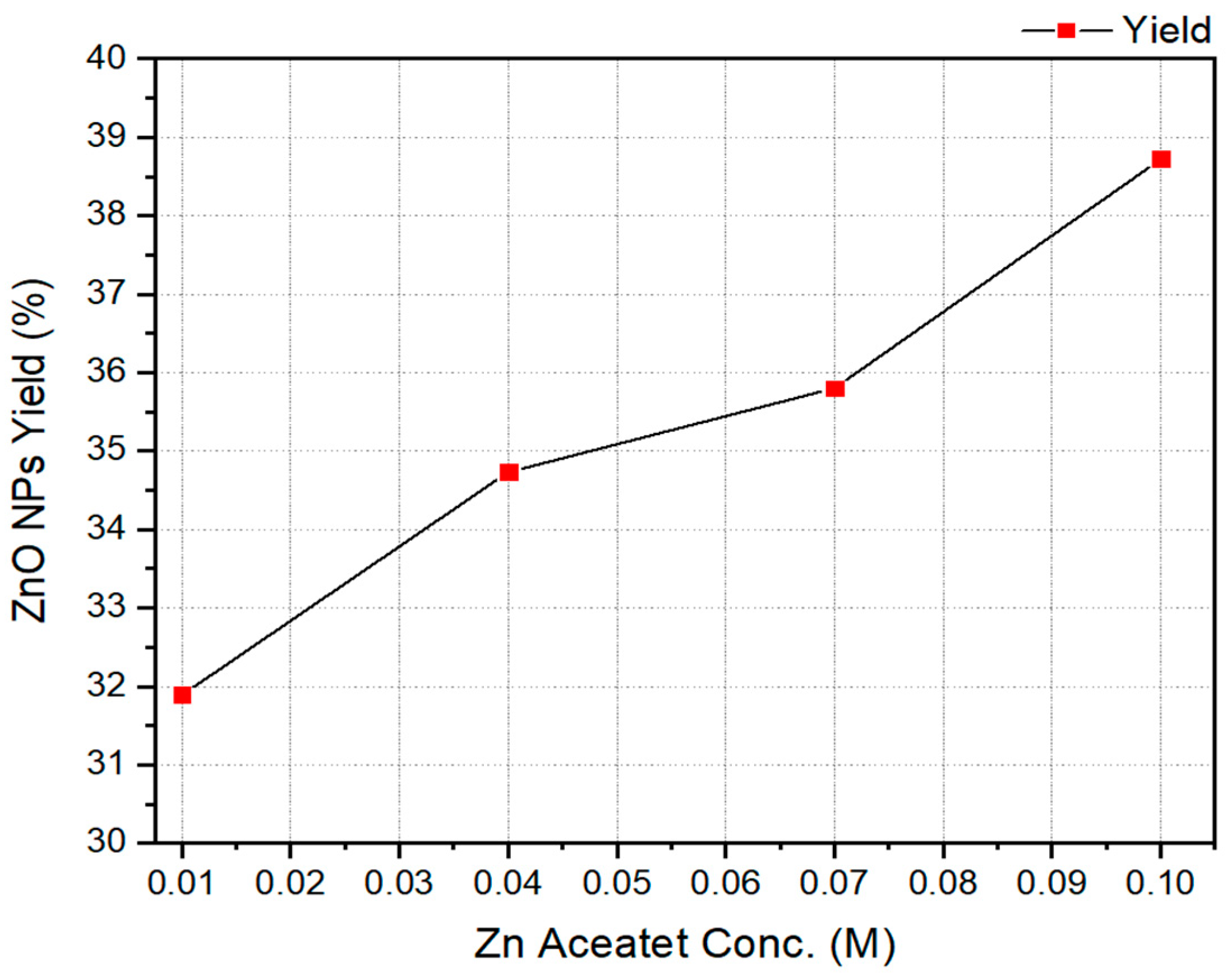
| ZnO NPs Samples | Zn Acetate Conc. (M) | SEM Size Range (nm) | Band Gap (eV) | ZnO NPs Yield % |
|---|---|---|---|---|
| A | 0.1 | 89 ± 22 | 3.36 | 38.72 ± 1.56 |
| B | 0.07 | 83 ± 16 | 3.41 | 35.8 ± 0.87 |
| C | 0.04 | 45 ± 7 | 3.44 | 34.73 ± 0.66 |
| D | 0.01 | 27 ± 4 | 3.61 | 31.89 ± 0.72 |
| ZnO NPs Samples | Size Range (nm) by DLS | Zeta Potential (mV) | PDI |
|---|---|---|---|
| A (0.1 M) | 609.5 ± 60.40 | −7.43 ± 0.35 | 0.694 ± 0.173 |
| B (0.07 M) | 554.7 ± 33.95 | −10.69 ± 20 | 0.618 ± 0.082 |
| C (0.04 M) | 435 ± 23.43 | −14.72 ± 0.77 | 0.253 ± 0.09 |
| D (0.01 M) | 278 ± 5.60 | −13.18 ± 1.15 | 0.28 ± 0.036 |
| Bacterial Strain | ZnO NPs | BPE | |
|---|---|---|---|
| MIC µg.mL−1 | MBC µg.mL−1 | MIC and MBC µg.mL−1 | |
| S. aureus ATCC no. 25913 | 500 | 500 | N.D. |
| S. epidermidis ATCC no. 12228 | 500 | 500 | N.D. |
| E. coli ATCC no. 25922 | 500 | N.D. * | N.D. |
| P. aeruginosa ATCC no. 27853 | 600 | N.D. | N.D. |
| Plant Used | Extract Type | Method | Particle Size | Potential Therapeutic Effect | MIC | MBC | Microorganism Strains | Bacterial Conc. (CFU/mL) | Annealing | Reference |
|---|---|---|---|---|---|---|---|---|---|---|
| Eriobotrya japonica leaves | Aqueous | Green | 13.4 nm by XRD | Antibacterial | 364–194 μg/mL | Not stated | E. coli, P. multocida, B. subtilis and S. aureus | 5.0 × 106 | calcined at 300 °C for 2 h | [62] |
| Prosopis farcta fruit | Aqueous | Green | 40–50 nm | Antifungal | 64 µg/mL | 512 µg/mL | C. parapsilosis and C. albicans | 1.0 × 105 | calcined at 500 °C, 600 °C, and 700 °C | [63] |
| Flower of Nyctanthes arbor-tristis | Aqueous | Green | 12–32 nm | Antifungal | 128–16 μg/mL | Not stated | A. alternata, A. niger, B. cinerea, F. oxysporum, P. expansum | 1.0 × 105 | Not conducted | [13] |
| Banana peel | Aqueous | Green | 27 ± 4 nm by SEM | Antibacterial | 500–600 μg/mL | 500 μg/mL | S. aureus S. epidermidis E. coli P. aeruginosa | 6.0 × 106 | 400 °C for 2 h | Current study |
| Banana peel | Aqueous | Green | 20–40 nm | Antibacterial | one concentration used only 250 μg/mL | Not stated | P. aeruginosa, S. aureus, Candida albicans | 1.5 × 106 | Not conducted | [24] |
| Date seed and Banana peel | Aqueous | Green | 72.6–54.4 nm | Antibacterial activity | 750 µg/mL | 3000–1500 µg/mL | E.coli, Salmonella enteritidis, B. subtilis, S. aureus | 5.0 × 105 | Not conducted | [64] |
| Banana peel | Aqueous | Green | Nanosheet length 345.61 nm, width 81.22 nm | Antibacterial activity and anti-cancer activity | IC50 of 0.1 M = 11,810 µg/mL, IC50 of 0.2 M = 11,920 µg/mL | Not stated | B. subtilis, S. epidermidis, E.coli, Enterobacter aerogenes | 1.0 × 106 | Not conducted | [26] |
| Artabotrys hexapetalu, Bambusa vulgaris | Aqueous | Green | 33–24 nm by XRD | antibacterial activity-Zone inhibition | Not stated | Not stated | Streptococcus Serratia | Not stated | calcination at 500 °C for 3 h | [65] |
| Mango and Soursop Leaf | Aqueous | Green | 23–17 nm | Not stated | Not stated | Not stated | Not stated | Not stated | 400 °C-3 h | [12] |
| Cherry fruit | Aqueous | Green | 20.18 nm | Not stated | Not stated | Not stated | Not stated | Not stated | Not conducted | [31] |
| Phoenix Dactylifera L. leaves | Aqueous | Green | 19.77–26.28 nm | Not stated | Not stated | Not stated | Not stated | Not stated | 450 °C-3 h | [14] |
| Laurus nobilis L. leaves | Aqueous | Green | 21.49, 25.26 nm | Not stated | Not stated | Not stated | Not stated | Not stated | Not conducted | [15] |
| Nephelium lappaceum L. peels | Aqueous | Green | 20 nm by XRD | Not stated | Not stated | Not stated | Not stated | Not stated | 450 °C | [66] |
| Hibiscus rosa-sinensis | Aqueous | Green | 30–35 nm | Not stated | Not stated | Not stated | Not stated | Not stated | Not conducted | [67] |
| Plant not used | Extract not used | Chemical method | 47–33 nm | Antibacterial activity of ZnO and Nd doped ZnO NPs | 800 µg/mL | 800 µg/mL | E. coli, K. pneumoniae | Not stated | 700 °C for 5 h. | [68] |
| Plant not used | Extract not used | Commercial ZnO-NPs | Not stated | Antibacterial Activity | 1000–8000 µg/mL | 4000, 8000, 16,000 µg/mL | E. coli, K. pneumoniae, P. aeruginosa | 2.5 × 105 | Not conducted | [69] |
| Plant not used | Extract not used | Chemical method | 50 nm | Antibacterial activity | 1250, 625, 1250, 156 μg/mL | 1250, 2500, 5000, 312.5 μg/mL | E. coli, S. epidermidis, S. aureus, P. aurugenosa | 1.0 × 108 | Not conducted | [70] |
| Plant not used | Extract not used | Commercial ZnO-NPs | 50−70 nm | Antibacterial activity | 1200–80 μg/mL | Not stated | S. aureus, S. epidermidis, Streptococcus pyogenes, E. coli | Not stated | Not conducted | [71] |
| Comparison Aspect | Traditional Methods | Green Synthesis |
|---|---|---|
| Environmental Impact | Frequently entail the utilization of hazardous chemicals, solvents, and reducing agents. Produce substantial quantities of dangerous waste [73]. | Utilize natural, renewable, and non-toxic materials as reducing agents. Minimize the production of potentially dangerous waste [73]. |
| Energy Consumption | High temperatures, pressures, and extended reaction times are necessary, resulting in elevated energy consumption and the release of greenhouse gas emissions [74]. | Operate under milder reaction conditions, often at ambient temperatures and atmospheric pressure, resulting in lower energy requirements [75]. |
| Cost-Effectiveness | The high costs are attributed to the use of costly reagents, energy-intensive procedures, and restrictive waste management regulations [76]. | Utilize inexpensive and readily available biological materials as reducing and capping agents. Eliminate costly purification steps and reduce energy consumption [77]. |
| Product Quality and Purity | The production of nanoparticles results in the formation of particles that have a high level of purity and a consistent size distribution. However, it is possible for these particles to contain trace amounts of hazardous compounds or by-products, which can have an impact on their characteristics [3]. | The nanoparticles could show differences in both particle size and morphology, nevertheless, they demonstrate excellent biocompatibility and less toxicity [3]. |
| Regulatory Compliance | Concerns related to regulations governing occupational safety, waste disposal, and environmental protection arise from the use of hazardous chemicals. Compliance necessitates costly measures [78]. | Align well with emerging regulatory frameworks promoting sustainable manufacturing practices. Facilitate easier compliance with regulatory requirements [78]. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Al-Khaial, M.Q.; Chan, S.Y.; Abu-Zurayk, R.A.; Alnairat, N. Biosynthesis and Characterization of Zinc Oxide Nanoparticles (ZnO-NPs) Utilizing Banana Peel Extract. Inorganics 2024, 12, 121. https://doi.org/10.3390/inorganics12040121
Al-Khaial MQ, Chan SY, Abu-Zurayk RA, Alnairat N. Biosynthesis and Characterization of Zinc Oxide Nanoparticles (ZnO-NPs) Utilizing Banana Peel Extract. Inorganics. 2024; 12(4):121. https://doi.org/10.3390/inorganics12040121
Chicago/Turabian StyleAl-Khaial, Mohammed Qahtan, Siok Yee Chan, Rund A. Abu-Zurayk, and Nour Alnairat. 2024. "Biosynthesis and Characterization of Zinc Oxide Nanoparticles (ZnO-NPs) Utilizing Banana Peel Extract" Inorganics 12, no. 4: 121. https://doi.org/10.3390/inorganics12040121






