Design and Construction of a Mixed-Ligand Coordinated Fluorescent Complex and Its Application for Sensing Ions, Antibiotics, and Pesticides in Aqueous Solution
Abstract
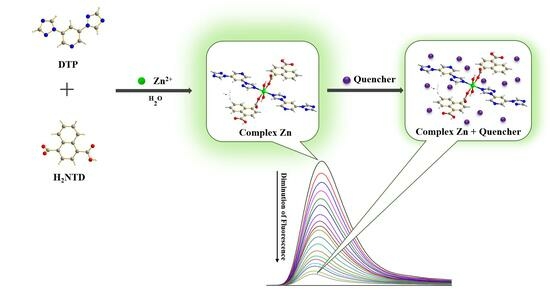
:1. Introduction
2. Experimental Section
2.1. Synthesis of Complex Zn
2.2. Analyte Quenching Test
2.3. Fluorescence Kinetic Titration
2.4. Recyclability Experiments
2.5. pH Stability Test
3. Results and Discussion
3.1. Structural Description of Complex Zn
3.2. PXRD, FT-IR, and TGA
3.3. Fluorescence of the Complex
3.4. Ionic Sensing
3.5. Anti-Interference Experiments
3.6. Antibiotics Sensing
3.7. Pesticide Sensing
3.8. Recyclability
3.9. Resistance to pH
3.10. Discussion and Possible Mechanism for Fluorescent Sensing
4. Materials and Methods
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yang, Q.Q.; Li, Z.Y.; Lu, X.N.; Duan, Q.N.; Huang, L.; Bi, J. A review of soil heavy metal pollution from industrial and agricultural regions in China: Pollution and risk assessment. Sci. Total Environ. 2018, 642, 690–700. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, P.; Gojenko, B.; Yu, J.J.; Wei, L.Z.; Luo, D.G.; Xiao, T.F. A review of water pollution arising from agriculture and mining activities in central asia: Facts, causes and effects. Environ. Pollut. 2021, 291, 118209. [Google Scholar] [CrossRef] [PubMed]
- Souza, H.D.O.; Costa, R.D.S.; Quadra, G.R.; Fernandez, M.A.D.S. Pharmaceutical pollution and sustainable development goals: Going the right way? Sustain. Chem. Pharm. 2021, 21, 100428. [Google Scholar] [CrossRef]
- Chen, Y.G.; He, X.L.S.; Huang, J.H.; Luo, R.; Ge, H.Z.; Wołowicz, A.; Wawrzkiewicz, M.; Gładysz-Płaska, A.; Li, B.; Yu, Q.X.; et al. Impacts of heavy metals and medicinal crops on ecological systems, environmental pollution, cultivation, and production processes in China. Ecotoxicol. Environ. Saf. 2021, 219, 112336. [Google Scholar] [CrossRef] [PubMed]
- Fanton, H.; Affre, L.; Franquet, E.; Bertrand, C.; Cavalli, L.; Dumas, E.; Guiller, C.; Kaldonski, N.; Meineri, E.; Mutillod, C.; et al. Heavy ionic pollution disrupts assemblages of algae, macroinvertebrates and riparian vegetation. Environ. Pollut. 2023, 331, 121791. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Wang, B.; Li, S.; Xiao, H.L.; Liu, G.; Zhang, Z.; Wang, X. Metal-/carboxylate-directed four d10 piperazine-amide-based coordination polymers for the fluorescent detection of nitrophenol and nitroaniline in various water environments. J. Mol. Struct. 2024, 1297, 136929. [Google Scholar] [CrossRef]
- Qadeer, A.; Rui, G.; Ya, Q.L.; Ran, D.; Liu, C.Y.; Jing, D.; Anis, M.; Liu, M.Y.; Wang, S.H.; Jiang, X.; et al. A mega study of antibiotics contamination in eastern aquatic ecosystems of China: Occurrence, interphase transfer processes, ecotoxicological risks, and source modeling. J. Hazard. Mater. 2023, 458, 131980. [Google Scholar] [CrossRef]
- Wei, Y.L.; Wang, L.P.; Liu, J. The diabetogenic effects of pesticides: Evidence based on epidemiological and toxicological studies. Environ. Pollut. 2023, 331, 121927. [Google Scholar] [CrossRef]
- Li, W.T.; Xin, S.H.; Deng, W.J.; Wang, B.B.; Liu, X.X.; Yuan, Y.; Wang, S.L. Occurrence, spatiotemporal distribution patterns, partitioning and risk assessments of multiple pesticide residues in typical estuarine water environments in eastern China. Water Res. 2023, 245, 120570. [Google Scholar] [CrossRef]
- Li, S.; Ondon, B.S.; Ho, S.H.; Li, F.X. Emerging soil contamination of antibiotics resistance bacteria (ARB) carrying genes (Args): New challenges for soil remediation and conservation. Environ. Res. 2023, 219, 115132. [Google Scholar] [CrossRef]
- Broom, A.; Peterie, M.; Ridge, D.; Lafferty, L.; Kenny, K.; Broom, J.; Kelly, H.A.; Treloar, C.; Applegate, T. Sex, drugs and superbugs: The rise of drug resistant STIs. SSM-Qual. Res. Health 2023, 4, 100310. [Google Scholar] [CrossRef]
- Anthony, A.A.; Adekunle, C.F.; Thor, A.S. Residual antibiotics, antibiotic resistant superbugs and antibiotic resistance genes in surface water catchments: Public health impact. Phys. Chem. Earth 2018, 105, 177–183. [Google Scholar] [CrossRef]
- Yadav, S.; Jijoe, S.P.; Thinley, T.; Hosakote, A.; Anil, K.K.M.; Shivaraju, H.P. Hybrid ZnFe2O4/Ag2S nanocomposite for enhanced photocatalytic and bacterial activity towards targeted superbugs. Mater. Today Proc. 2023, 75, 46–57. [Google Scholar] [CrossRef]
- Yadav, N.; Maurya, B.M.; Chettri, D.; Pooja; Pulwani, C.; Jajula, M.; Kanda, S.S.; Babu, H.W.S.; Elangovan, A.; Velusamy, P.; et al. Artificial intelligence in heavy metals detection: Methodological and ethical challenges. Hyg. Environ. Health Adv. 2023, 7, 100071. [Google Scholar] [CrossRef]
- Wang, S.Q.; Chen, H.T.; Sun, B.G. Recent progress in food flavor analysis using gas chromatography-ion mobility spectrometry (GC-IMS). Food Chem. 2020, 315, 126158. [Google Scholar] [CrossRef] [PubMed]
- Ocampo, A.Y.D.; Salazar, R.E.; Ramírez, C.M.Á.; Rios, M.Y. Comprehensive review of liquid chromatography methods for fumonisin determination, a 2006–2022 update. Arab. J. Chem. 2023, 16, 104716. [Google Scholar] [CrossRef]
- Amini, S.; Ebrahimzadeh, H.; Seidi, S.; Jalilian, N. Preparation of polyacrylonitrile/Ni-MOF electrospun nanofiber as an efficient fiber coating material for headspace solid-phase microextraction of diazinon and chlorpyrifos followed by CD-IMS analysis. Food Chem. 2021, 350, 129242. [Google Scholar] [CrossRef] [PubMed]
- Pallavi, M.S.; Harischandra, N.R.; Pavankumar, K.; Ratnamma, N.; Shwetha, A.; Naveenkumar, P.; Paramasivam, M.; Udaykumar, N.R.; Prabhuraj, A.; Bheemanna, M. Determination of 73 multi-class pesticides in okra (Abelmoschus esculentus L.) fruits using LC-MS/MS and GC-MS/MS and estimation of analytical uncertainty of measurement. Food Chem. X 2023, 19, 100814. [Google Scholar] [CrossRef] [PubMed]
- Jia, Q.; Liao, G.Q.; Chen, L.; Qian, Y.Z.; Yan, X.; Qiu, J. Pesticide residues in animal-derived food: Current state and perspectives. Food Chem. 2024, 438, 137974. [Google Scholar] [CrossRef]
- Bin, D.N.; Kurdi, A.; Alshihri, S.; Tabbakh, T. Organic heterocyclic-based colorimetric and fluorimetric chemosensors for the detection of different analytes: A review (from 2015 to 2022). Mater. Today Chem. 2023, 27, 101347. [Google Scholar]
- Kumar, G.; Guda, R.; Husain, A.; Bodapati, R.; Das, S.K. A functional Zn(II) metallacycle formed from an n-heterocyclic carbene precursor: A molecular sensor for selective recognition of Fe3+ and IO4− ions. Inorg. Chem. 2017, 56, 5017–5025. [Google Scholar] [CrossRef] [PubMed]
- Pathan, I.R.; Patel, M.K. A comprehensive review on the synthesis and applications of Schiff base ligand and metal complexes: A comparative study of conventional heating, microwave heating, and sonochemical methods. Inorg. Chem. Commun. 2023, 158, 111464. [Google Scholar] [CrossRef]
- Retnam, C.T.G.; Rose, S.V.; Kumari, B.S. Synthesis, characterization, biological activity and molecular docking study of transition metal complexes from heterocyclic ligand system. J. Mol. Struct. 2023, 1282, 135162. [Google Scholar] [CrossRef]
- Ziółkowska, A.; Doroszuk, J.; Ponikiewski, Ł. Overview of the synthesis and catalytic reactivity of transition metal complexes based on C=P bond systems. Organometallics 2023, 42, 505–537. [Google Scholar] [CrossRef]
- Li, B.; Xu, Z.Q.; Xu, Y.B.; Yong, G.P. Effects of substituent groups on the crystal structures and luminescence properties of zero-/two-dimensional Zn(II) complexes. Inorg. Chem. Commun. 2019, 102, 57–60. [Google Scholar] [CrossRef]
- Li, S.; Sun, J.Y.; Liu, G.C.; Zhang, S.; Zhang, Z.; Wang, X.L. A new keggin-type polyoxometallate-based bifunctional catalyst for trace detection and pH-universal photodegradation of phenol. Chin. Chem. Lett. 2023, 21, 109148. [Google Scholar] [CrossRef]
- Fang, W.Q.; Zhang, L.; Feng, H.R.; Meng, J.X.; Zhang, Z.P.; Liu, Z.B. Research progress of fluorescent-substance@MOFs. Microchem. J. 2023, 185, 108265. [Google Scholar] [CrossRef]
- Das, R.; Pal, R.; Bej, S.; Mondal, M.; Kundu, K.; Banerjee, P. Recent progress in 0D optical nanoprobes for applications in the sensing of (bio)analytes with the prospect of global health monitoring and detailed mechanistic insights. Adv. Mater. 2022, 3, 4421–4459. [Google Scholar] [CrossRef]
- Zhou, Z.D.; Li, S.Q.; Liu, Y.; Du, B.; Shen, Y.Y.; Yu, B.Y.; Wang, C.C. Two bis-ligand-coordinated Zn(II)-MOFs for luminescent sensing of ions, antibiotics and pesticides in aqueous solutions. RSC Adv. 2022, 12, 7780–7788. [Google Scholar] [CrossRef]
- Wang, C.L.; Song, C.Q.; Shen, W.H.; Qi, Y.Y.; Xue, Y.; Shi, Y.C.; Yu, H.G.; Feng, L.G. A two-dimensional Ni(II) coordination polymer based on a 3,5-bis(1′,2′,4′-triazol-1′-yl)pyridine ligand for water electro-oxidation. Catal. Sci. Technol. 2019, 9, 1769. [Google Scholar] [CrossRef]
- Hadjiivanov, K.I.; Panayotov, D.A.; Mihaylov, M.Y.; Ivanova, E.Z.; Chakarova, K.K.; Andonova, S.M.; Drenchev, N.L. Power of infrared and raman spectroscopies to characterize metalorganic frameworks and investigate their interaction with guest molecules. Chem. Rev. 2021, 121, 1077–1980. [Google Scholar] [CrossRef]
- Yam, V.W.W.; Wong, K.M.C. Luminescent metal complexes of d6, d8 and d10 transition metal centres. Chem. Commun. 2011, 47, 11579–11592. [Google Scholar] [CrossRef] [PubMed]
- Curiel, D.; Más-Montoya, M.; Sánchez, G. Complexation and sensing of dicarboxylate anions and dicarboxylic acids. Coord. Chem. Rev. 2015, 284, 19–66. [Google Scholar] [CrossRef]
- Li, Y.M.; Xiao, C.Y.; Zhang, X.D.; Xu, Y.H.; Li, J.R.; Lun, H.J.; Chen, Q. Two new coordination polymers constructed by naphthalene-1,4-dicarboxylic acid and 2,4-diamino-6-methyl-triazine. J. Solid State Chem. 2013, 204, 190–196. [Google Scholar] [CrossRef]
- Han, S.D.; Hu, J.X.; Wang, G.M. Recent advances in crystalline hybrid photochromic materials driven by electron transfer. Coord. Chem. Rev. 2022, 452, 214304. [Google Scholar] [CrossRef]
- Shao, Z.C.; Wu, Q.; Han, X.; Zhao, Y.J.; Xie, Q.; Wang, H.F.; Hou, H.W. Proton coupled electron transfer: Novel photochromic performance in a host–guest collaborative MOF. Chem. Commun. 2019, 55, 10948. [Google Scholar] [CrossRef] [PubMed]
- Jiang, M.; Weng, Y.G.; Zhou, Z.Y.; Ge, C.Y.; Zhu, Q.Y.; Dai, J. Cobalt metal−organic frameworks incorporating redox-active tetrathiafulvalene ligand: Structures and effect of LLCT within the MOF on photoelectrochemical properties. Inorg. Chem. 2020, 59, 10727–10735. [Google Scholar] [CrossRef] [PubMed]
- Zhu, G.S.; Cheng, S.L.; Zhou, Z.D.; Du, B.; Shen, Y.Y.; Yu, B.Y. Bisligand-coordinated cadmium organic frameworks as fluorescent sensors to detect ions, antibiotics and pesticides in aqueous solutions. Polyhedron 2022, 217, 115759. [Google Scholar] [CrossRef]
- Yu, B.Y.; Wang, Z.P.; Ding, H.; Miao, F.F.; Yang, A.Z.; Zhao, W.T.; Jia, Y.; Zhao, H.Q. Fluorescent metal-organic frameworks based on cadmium(II) coordinated by a mixture of ligands for sensitively sensing ions and pesticides. J. Mol. Struct. 2023, 1291, 135891. [Google Scholar] [CrossRef]
- Zhu, H.B.; Shen, Y.; Fu, Z.Z.; Yu, Y.Y.; Jiang, Y.F.; Zhao, Y. A multifunctional Zn(II)-based four-fold interpenetrated metal-organic framework for highly sensitive sensing 2,4,6-trinitrophenol (TNP), nitrofurazone (NFZ) and nitrofurantoin (NFT). Inorg. Chem. Commun. 2019, 103, 21–24. [Google Scholar] [CrossRef]
- Li, J.; Chen, T.J.; Han, S.; Song, L.F. Four Zn(II)-organic frameworks as luminescent probe for highly selectivity detection of Cr(VI) ions and antibiotics. J. Solid State Chem. 2019, 277, 107–114. [Google Scholar] [CrossRef]
- Zhang, Y.Q.; Wu, X.H.; Mao, S.; Tao, W.Q.; Li, Z. Highly luminescent sensing for nitrofurans and tetracyclines in water based on zeolitic imidazolate framework-8 incorporated with dyes. Talanta 2019, 204, 344–352. [Google Scholar] [CrossRef]
- He, H.; Zhu, Q.Q.; Li, C.P.; Du, M. Design of a highly-stable pillar-layer Zinc(II) porous framework for rapid, reversible, and multi-responsive luminescent sensor in water. Cryst. Growth Des. 2018, 19, 694–703. [Google Scholar] [CrossRef]
- Ying, Y.M.; Tao, C.L.; Yu, M.; Xiong, Y.; Guo, C.R.; Liu, X.G.; Zhao, Z. In situ encapsulation of pyridine-substituted tetraphenylethene cations in metal-organic framework for the detection of antibiotics in aqueous medium. J. Mater. Chem. C 2019, 7, 8383–8388. [Google Scholar] [CrossRef]
- Fan, L.; Wang, F.; Zhao, D.; Peng, Y.; Deng, Y.; Luo, Y.; Zhang, X. A self-penetrating and chemically stable Zinc(II)-organic framework as multi-responsive chemo-sensor to detect pesticide and antibiotics in water. Appl. Organomet. Chem. 2020, 34, e5960. [Google Scholar] [CrossRef]
- Zhou, Z.D.; Wang, C.Y.; Zhu, G.S.; Du, B.; Yu, B.Y.; Wang, C.C. Water-stable europium(III) and terbium(III)-metal organic frameworks as fluorescent sensors to detect ions, antibiotics and pesticides in aqueous solutions. J. Mol. Struct. 2022, 1251, 132009. [Google Scholar] [CrossRef]
- Zhou, Z.D.; Xu, Z.L.; Jia, L.F.; Wang, D.; Zhao, H.Q.; Yu, B.Y.; Wang, C.C. Two bisligand-coordinated luminescent Zn(II)-coordination polymers for sensing of ions and pesticides in aqueous solutions. Chin. J. Struct. Chem. 2022, 41, 2209087–2209093. [Google Scholar]
- Yu, B.Y.; Wang, D.; Zhu, G.S.; Wei, C.J.; Jia, Y.; Song, C.L.; Cheng, J.; Zhao, H.Q. Synthesis, crystal structure of four 1D to 3D coordination polymers as potential sensor for the detection of ions, antibiotics and pesticides in water media. Polyhedron 2023, 230, 116242. [Google Scholar] [CrossRef]
- Mingke, Y.; Ying, X.; Xin, Y.W.; Yu, X.L.; Guang, M.L. Highly water-stable Dye@Ln-MOFs for sensitive and selective detection toward antibiotics in water. ACS Appl. Mater. Interfaces 2019, 11, 21201–21210. [Google Scholar]
- Feng, Z.; Hua, Y.; Tian, S.C.; Gao, W.Z.; Yi, W.; Yang, Y.Y. A lanthanide MOF thin-film fixed with Co3O4 nano-anchors as a highly efficient luminescent sensor for nitrofuran antibiotics. Chem. Eur. J. 2017, 23, 10293–10300. [Google Scholar]
- Wang, H.S.; Liu, H.L.; Wang, K.; Ding, Y.; Xu, J.J.; Xia, X.H.; Chen, H.Y. Insight into the unique fluorescence quenching property of metal-organic frameworks upon DNA binding. Anal. Chem. 2017, 21, 11366–11371. [Google Scholar] [CrossRef] [PubMed]
- Jing, L.; Fan, M.; Lei, G.; Jun, H.H.; Zheng, L.; Qin, X.; Hong, B.L. Ligand reduction and cation exchange on nanostructures for an elegant design of copper ions photoelectrochemical sensing. Sens. Actuators B Chem. 2021, 328, 129032. [Google Scholar]
- Wang, Z.Q.; Wang, C.; Zhang, H.; Liu, Z.M.; Zhao, B.; Li, W.L. The application of charge transfer host based exciplex and thermally activated delayed fluorescence materials in organic light-emitting diodes. Org. Electron. 2019, 66, 227–241. [Google Scholar] [CrossRef]
- Ma, S.Y.; Wang, Y.H.; Wang, C.; Wang, L.L.; Miao, Q.; Liu, Y.X.; Ma, Y.M.; Chen, G. Accurate photoactivation monitoring via the construction of an intramolecular synergistic counteracting mechanism of FRET and IFE. Chem. Comm. 2023, 59, 5599–5602. [Google Scholar] [CrossRef]
- Qiu, H.M.; Yang, H.; Gao, X.; Nie, C.; Gu, Y.; Shen, Y.Z. Inner filter effect-based fluorescence assays toward environmental pesticides and antibiotics. Coord. Chem. Rev. 2023, 493, 215309. [Google Scholar] [CrossRef]
- Wu, L.L.; Huang, C.S.; Emery, B.P.; Sedgwick, A.C.; Bull, S.D.; He, X.P.; Tian, H.; Yoon, J.Y.; Sessler, J.L.; James, T.D. Förster resonance energy transfer (FRET)-based small-molecule sensors and imaging agents. Chem. Soc. Rev. 2020, 49, 5110–5139. [Google Scholar] [CrossRef]
- Ling, Q.H.; Cheng, T.Y.; Tan, S.Y.; Huang, J.H.; Xu, L. Fluorescence-resonance energy transfer (FRET) within the fluorescent metallacycles. Chin. Chem. Lett. 2020, 31, 2884–2890. [Google Scholar] [CrossRef]
- Marcelo, H.G. The centenary of the Stern-Volmer equation of fluorescence quenching: From the single line plot to the SV quenching map. J. Photochem. Photobiol. C 2020, 42, 100338. [Google Scholar]
- Krause, L.; Herbst, I.R.; Sheldrick, G.M.; Stalke, D. Comparison of silver and molybdenum microfocus X-ray sources for single-crystal structure determination. J. Appl. Crystallogr. 2015, 48, 3–10. [Google Scholar] [CrossRef]
- Burla, M.C.; Caliandro, R.; Camalli, M.; Carrozzini, B.; Cascarano, G.L.; De Caro, L.; Giacovazzo, C.; Polidori, G.; Siliqi, D.; Spagna, R. Il milione: A suite of computer programs for crystal structure solution of proteins. J. Appl. Crystallogr. 2007, 40, 609–613. [Google Scholar] [CrossRef]
- Dolomanov, O.V.; Bourhis, L.J.; Gildea, R.J.; Howard, J.A.K.; Puschmann, H. OLEX2: A complete structure solution, refinement and analysis program. J. Appl. Crystallogr. 2009, 42, 339–341. [Google Scholar] [CrossRef]
- Sheldrick, M.G. A short history of SHELX. Acta Crystallogr. A 2007, 64, 112–122. [Google Scholar] [CrossRef] [PubMed]
- Sheldrick, M.G. SHELXT-integrated space-group and crystal-structure determination. Acta Crystallogr. A 2015, 71, 3–8. [Google Scholar] [CrossRef] [PubMed]








Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhu, G.-S.; Jia, Y.; Ding, J.-Y.; Yin, H.; Chen, Y.; Yu, B.-Y.; Zheng, Y.-Y.; Verpoort, F. Design and Construction of a Mixed-Ligand Coordinated Fluorescent Complex and Its Application for Sensing Ions, Antibiotics, and Pesticides in Aqueous Solution. Inorganics 2024, 12, 93. https://doi.org/10.3390/inorganics12040093
Zhu G-S, Jia Y, Ding J-Y, Yin H, Chen Y, Yu B-Y, Zheng Y-Y, Verpoort F. Design and Construction of a Mixed-Ligand Coordinated Fluorescent Complex and Its Application for Sensing Ions, Antibiotics, and Pesticides in Aqueous Solution. Inorganics. 2024; 12(4):93. https://doi.org/10.3390/inorganics12040093
Chicago/Turabian StyleZhu, Gao-Sheng, Yi Jia, Jia-Yao Ding, Hao Yin, Yan Chen, Bao-Yi Yu, Yan-Ying Zheng, and Francis Verpoort. 2024. "Design and Construction of a Mixed-Ligand Coordinated Fluorescent Complex and Its Application for Sensing Ions, Antibiotics, and Pesticides in Aqueous Solution" Inorganics 12, no. 4: 93. https://doi.org/10.3390/inorganics12040093