Direct Energy Supply to the Reaction Mixture during Microwave-Assisted Hydrothermal and Combustion Synthesis of Inorganic Materials
Abstract
:1. Introduction
2. Microwave Energy Transfer Fundamentals

2.1. Volumetric and Selective Nature of MW Heating


2.2 Energy Efficiency Issues
3. Microwave-Assisted Hydrothermal Synthesis of Engineered Nanomaterials


| Class of materials | Main examples | Some crystal shapes observed | Ref. |
|---|---|---|---|
| Transition metal oxides | TiO2, ZnO, ZrO2, Fe2O3, Fe3O4 | Nanoparticles, nanotubes, nanowires, nanorods, nanocubes, nanoribbons | [20,59,64,65] |
| Multimetal oxides | BaTiO3, La1- xSrxMnO3, BiFeO3 | Nanoparticles, polyhedrons, nanoplatelets, nanocubes | [20,64,65] |
| Metal sulfides | CdS, ZnS, CuS, SnS | Nanoparticles, nanotubes, | [59,64,65,66,67,68] |
| Biomaterials | Hydroxyapatite | Needle-like (frequently), nanospheres, nanorods, nanowires, whiskers, platelets | [59,69] |
| Carbon-based nanostructured materials | Carbon nanotubes, nanospheres, nanofibers, graphene-based materials | [59,70,71] | |
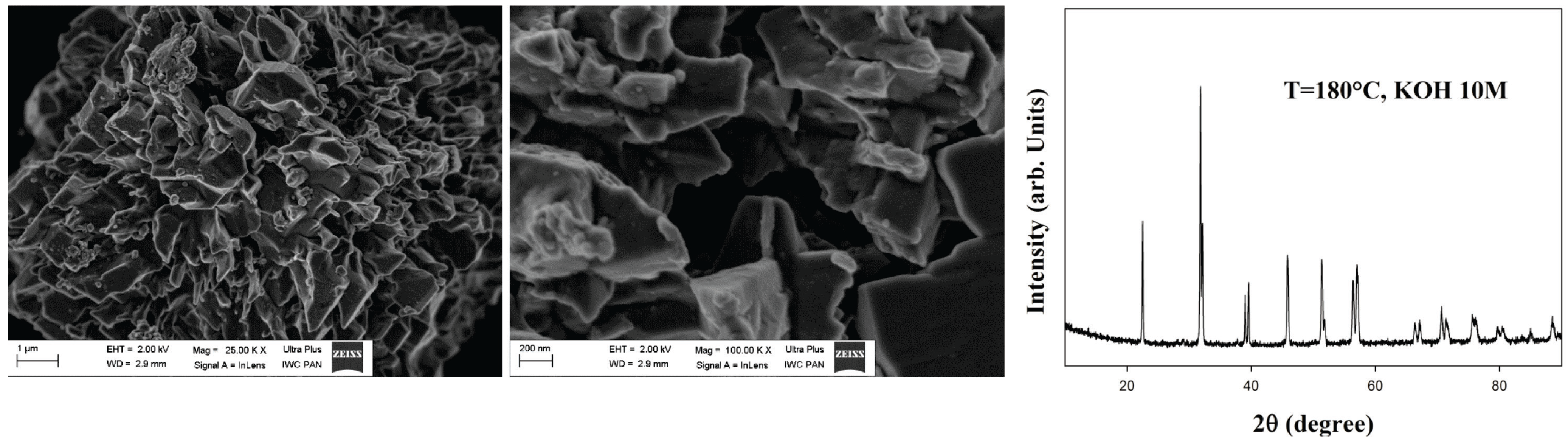
| Temperature (°C) | Time (min) | Product | Ref. |
|---|---|---|---|
| ca. 194 | 120 | agglomerated rombohedral BiFeO3 particles | [80] |
| 200 | 30 | polyhedral BiFeO3 nano-structured particles | [81] |
| 180 | 30 | nanocubic BiFeO3 aggregates | [82] |
4. Microwave Energy Transfer in the Ignition of Solution Combustion Synthesis

| Synthetic approach | Distinctive characteristics | Main classes of materials obtainable | Examples of synthesized compounds | Ref. |
|---|---|---|---|---|
| Solution combustion synthesis | The solution of the metal precursor(s) nitrate(s) and the fuel is slowly evaporated and then ignited locally or volumetrically. The exothermic reaction occurs between fuel and oxygen-containing species derived from the decomposition of nitrates | - Binary and more complex metal oxide nanopowders- metal nanopowders | - TiO2, ZnO, LaFeO3, BiFeO3 - Ni, Cu and their alloys | [88,91,92,93,94] |
| Impregnated layer and combustion | The reactive solution is impregnated with an inert porous oxide support or with a reactive cellulose paper also acting as a fuel. - a productivity of 0.5-2 kg/h of nanoparticles has been reached by a continuous synthesis approach | - binary and more complex oxides | ZnO, MgO, Ce1-xPtxO2, CuO/ZnO/ZrO2/Pd based catalysts | [95,96] |
| Carbon combustion synthesis | The exothermic oxidation reaction of carbon to carbon dioxide generates a reaction wave that propagates through the solid reactant mixture. The product of the exothermic reaction is not incorporated into the final product, leading to several advantages (e.g., smaller particles). | - Perovskite oxides | BaTiO3, SrTiO3, LiNbO3, CoFe2O4 | [97,98] |
5. Concluding Remarks
Acknowledgments
Conflicts of Interest
References
- Rao, K.J.; Vaidhyanathan, B.; Ganguli, M.; Ramakrishnan, P.A. Synthesis of inorganic solids using microwaves. Chem. Mater. 1999, 11, 882–895. [Google Scholar]
- Jansen, M. A concept for synthesis planning in solid-state chemistry. Angew. Chem. Int. Ed. 2002, 41, 3746–3766. [Google Scholar] [CrossRef]
- Ramesh, P.D.; Vaidhyanathan, B.; Ganguli, M.; Rao, K.J. Synthesis of β-SiC powder by use of microwave radiation. J. Mater. Res. 1994, 9, 3025–3027. [Google Scholar] [CrossRef]
- Ramesh, P.D.; Rao, K.J. Microwave assisted synthesis of aluminum nitride. Adv. Mater. 1995, 7, 177–179. [Google Scholar] [CrossRef]
- Rizzuti, A.; Leonelli, C. Crystallization of aragonite particles from solution under microwave irradiation. Powder Technol. 2008, 186, 255–262. [Google Scholar] [CrossRef]
- Mastrovito, C.; Lekse, J.W.; Aitken, J.A. Rapid solid-state synthesis of binary group 15 chacogenides using microwave irradiation. J. Solid State Chem. 2007, 180, 3262–3270. [Google Scholar] [CrossRef]
- Bhunia, S.; Bose, D.N. Microwave synthesis, single crystal growth and characterization of ZnTe. J. Crystal Growth 1998, 186, 535–542. [Google Scholar] [CrossRef]
- Vaidhyanathan, B.; Rao, K.J. Microwave assisted synthesis of technologically important transition metal silicides. J. Mater. Res. 1997, 12, 3225–3229. [Google Scholar] [CrossRef]
- Vaidhyanathan, B.; Raizada, P.; Rao, K.J. Microwave assisted fast solid state synthesis of niobates and titanates. J. Mater. Sci. Lett. 1997, 16, 2022–2025. [Google Scholar] [CrossRef]
- Wang, Y.; Herron, N. Nanometer-sized semiconductor clusters: materials synthesis, quantum size effects, and photophysical properties. J. Phys. Chem. 1991, 95, 525–532. [Google Scholar] [CrossRef]
- Berry, C.R. Structure and optical absorption of AgI microcrystals. Phys. Rev. 1967, 161, 848–851. [Google Scholar] [CrossRef]
- Hischier, R.; Walser, T. Life cycle assessment of engineered nanomaterials: state of the art and strategies to overcome existing gaps. Sci. Total Environ. 2012, 425, 271–282. [Google Scholar] [CrossRef]
- Gopalakrishnan, J. Chimie douce approaches to the synthesis of metastable oxide materials. Chem. Mater. 1995, 7, 1265–1275. [Google Scholar] [CrossRef]
- Hubert-Pfalzgraf, L.G. To what extent can design of molecular precursors control the preparation of high tech oxides? J. Mater. Chem. 2004, 14, 3113–3123. [Google Scholar] [CrossRef]
- Roy, R. Ceramics by the solution-sol-gel route. Science 1987, 238, 1664–1669. [Google Scholar]
- Vioux, A. Nonhydrolytic sol-gel routes to oxides. Chem. Mater. 1997, 9, 2292–2299. [Google Scholar]
- Niederberger, M.; Garnweitner, G. Organic reaction pathways in the nonaqueous synthesis of metal oxide nanoparticles. Chem. Eur. J. 2006, 12, 7282–7302. [Google Scholar] [CrossRef]
- Giordano, C.; Antonietti, M. Synthesis of crystalline metal nitride and metal carbide nanostructures by sol-gel chemistry. Nano Today 2011, 6, 366–380. [Google Scholar] [CrossRef]
- Valizadeh, A.; Mikaeili, H.; Samiei, M.; Farkhani, S.M.; Zarghami, N.; Kouhi, M.; Akbarzadeh, A.; Davaran, S. Quantum dots: Synthesis, bioapplications, and toxicity. Nanoscale Res. Lett. 2012, 7, 480. [Google Scholar] [CrossRef]
- Patzke, G.R.; Zhou, Y.; Kontic, R.; Conrad, F. Oxide nanomaterials: Synthetic developments, mechanistic studies, and technological innovations. Angew. Chem. Int. Ed. 2011, 50, 826–859. [Google Scholar] [CrossRef]
- Dias, A.; Ciminelli, V.S.T. Electroceramic materials of tailored phase and morphology by hydrothermal technology. Chem. Mater. 2003, 15, 1344–1352. [Google Scholar] [CrossRef]
- Hayashi, H.; Hakuta, Y. Hydrothermal synthesis of metal oxide nanoparticles in supercritical water. Materials 2010, 3, 3794–3817. [Google Scholar] [CrossRef]
- Demazeau, G. Solvothermal reactions: An original route for the synthesis of novel materials. J. Mater. Sci. 2008, 43, 2104–2114. [Google Scholar] [CrossRef]
- Namratha, K.; Byrappa, K. Novel solution routes of synthesis of metal oxide and hybrid metal oxide nanocrystals. Progr. Crystal Growth Charact. Mater. 2012, 58, 14–42. [Google Scholar] [CrossRef]
- Kingsley, J.J.; Patil, K.C. A novel combustion process for the synthesis of fine particle alpha-alumina and related oxide materials. Mater. Lett. 1988, 6, 427–432. [Google Scholar] [CrossRef]
- Manoharan, S.S.; Patil, K.C. Combustion route to fine particle perovskite oxides. J. Solid State Chem. 1993, 102, 267–276. [Google Scholar] [CrossRef]
- Patil, K.C.; Aruna, S.T.; Ekambaram, S. Combustion synthesis. Curr. Opin. Solid State Mater. Sci. 1997, 2, 158–165. [Google Scholar] [CrossRef]
- Patil, K.C.; Aruna, S.T.; Mimani, T. Combustion synthesis: an update. Curr. Opin. Solid State Mater. Sci. 2002, 6, 507–512. [Google Scholar] [CrossRef]
- Mukasyan, A.S.; Epstein, P.; Dinka, P. Solution combustion synthesis of nanomaterials. Proc. Comb. Inst. 2007, 31, 1789–1795. [Google Scholar] [CrossRef]
- Aruna, S.T.; Mukasyan, A.S. Combustion synthesis and nanomaterials. Curr. Opin. Solid State Mater. Sci. 2008, 12, 44–50. [Google Scholar] [CrossRef]
- Ruiz-Gomez, M.A.; Gomez-Solis, C.; Zarazua-Morin, M.E.; Torres-Martinez, L.M.; Juarez-Ramirez, I.; Sanchez-Martinez, D.; Figueroa-Torres, M.Z. Innovative solvo-combustion route for the rapid synthesis of MoO3 and Sm2O3 materials. Ceram. Int. 2014, 40, 1893–1899. [Google Scholar]
- Gedye, R.; Smith, F.; Westaway, K.; Ali, H.; Baldisera, L.; Laberge, L.; Rousell, J. The use of microwave ovens for rapid organic synthesis. Tetrahedron Lett. 1986, 27, 279–282. [Google Scholar] [CrossRef]
- Giguere, R.J.; Bray, T.L.; Duncan, S.M.; Majetich, G. Application of commercial microwave ovens to organic synthesis. Tetrahedron Lett. 1986, 27, 4945–4948. [Google Scholar] [CrossRef]
- Adam, D. Microwave chemistry: out of the kitchen. Nature 2003, 421, 571–572. [Google Scholar] [CrossRef]
- Loupy, A. Microwaves in Organic Synthesis; Wiley-VCH: Weinheim, Germany, 2002. [Google Scholar]
- Kappe, C.O. Controlled microwave heating in modern organic synthesis. Angew. Chem. Int. Ed. 2004, 43, 6250–6284. [Google Scholar]
- Corradi, A.; Leonelli, C.; Rizzuti, A.; Rosa, R.; Veronesi, P.; Grandi, R.; Baldassari, S.; Villa, C. New “green” approaches to the synthesis of pyrazole derivatives. Molecules 2007, 12, 1482–1495. [Google Scholar] [CrossRef]
- Leonelli, C.; Lojkowski, W. Main development directions in the application of microwave irradiation to the synthesis of nanopowders. Chem. Today 2007, 25, 34–38. [Google Scholar]
- Anastas, P.T.; Warner, J.C. Green Chemistry: Theory and Practice; Oxford University Press: New York, NY, USA, 1998. [Google Scholar]
- Huang, J.; Xia, C.; Cao, L.; Zeng, X. Facile microwave hydrothermal synthesis of zinc oxide one-dimensional nanostructure with three-dimensional morphology. Mater. Sci. Eng. B 2008, 150, 187–193. [Google Scholar] [CrossRef]
- Shi, W.; Song, S.; Zhang, H. Hydrothermal synthetic strategies of inorganic semiconducting nanostructures. Chem. Soc. Rev. 2013, 42, 5714–5743. [Google Scholar] [CrossRef]
- Metaxas, A.C. Foundations of Electroheat: A Unified Approach; John Wiley and Sons: Chichester, UK, 1996. [Google Scholar]
- Gupta, M.; Eugene, W.W.L. Microwaves-Theory. In Microwaves and Metals; John Wiley and Sons: Singapore, 2007 and references therein.
- Metaxas, A.C.; Meredith, R.J. Industrial Microwave Heating; Peter Peregrinus: London, UK, 1983. [Google Scholar]
- Leonelli, C.; Mason, T.J. Microwave and ultrasonic processing: Now a realistic option for industry. Chem. Eng. Process. 2010, 49, 885–900. [Google Scholar] [CrossRef]
- Rosa, R.; Veronesi, P.; Leonelli, C. A review on combustion synthesis intensification by means of microwave energy. Chem. Eng. Process. 2013, 71, 2–18. [Google Scholar] [CrossRef]
- Moseley, J.D.; Kappe, C.O. A critical assessment of the greenness and energy efficiency of microwave-assisted organic synthesis. Green Chem. 2011, 13, 794–806. [Google Scholar] [CrossRef]
- Nüchter, M.; Müller, U.; Ondruschka, B.; Tied, A.; Lautenschläger, W. Microwave-assisted chemical reactions. Chem. Eng. Technol. 2003, 26, 1207–1216. [Google Scholar]
- Chan, T.V.C.T.; Reader, H.C. Understanding Microwave Heating Cavities; Artech House: Norwood, UK, 2000. [Google Scholar]
- For further details see for example: http://www.mksinst.com, www.sairem.com.
- Ferrero, M.A.; Kremsner, J.M.; Kappe, C.O. Nonthermal microwave effects revisited: on the importance of internal temperature monitoring and agitation in microwave chemistry. J. Org. Chem. 2008, 73, 36–47. [Google Scholar]
- Corradi, A.B.; Bondioli, F.; Ferrari, A.M.; Focher, B.; Leonelli, C. Synthesis of silica nanoparticles in a continuous-flow microwave reactor. Powder Technol. 2006, 167, 45–48. [Google Scholar] [CrossRef]
- Katsuki, H.; Furuta, S.; Komarneni, S. Semi-continuous and fast synthesis of nanophase cubic BaTiO3 using a single-mode home-built microwave reactor. Mater. Lett. 2012, 83, 8–10. [Google Scholar] [CrossRef]
- Wiles, C.; Watts, P. Continuous flow reactors: A perspective. Green Chem. 2012, 14, 38–54. [Google Scholar] [CrossRef]
- Nishioka, M.; Miyakawa, M.; Daino, Y.; Kataoka, H.; Koda, H.; Sato, K.; Suzuki, T.M. Single-mode microwave reactor used for continuous flow reactions under elevated pressure. Ind. Eng. Chem. Res. 2013, 52, 4683–4687. [Google Scholar]
- Bondioli, F.; Ferrari, A.M.; Braccini, S.; Leonelli, C.; Pellacani, G.C.; Opalińska, A.; Chudoba, T.; Grzanka, E.; Palosz, B.; Lojkowski, W. Microwave-hydrothermal synthesis of nanocrystalline Pr-doped zirconia powders at pressures up to 8 MPa, Interfacial Effects and Novel Properties of Nanomaterials. Solid State Phenom. 2003, 94, 193–196. [Google Scholar] [CrossRef]
- Riman, R.E. High Performance Ceramics: Surface Chemistry in Processing Technology; Pugh, R., Bergstrom, L., Eds.; Marcel-Dekker: New York, NY, USA, 1993. [Google Scholar]
- Komarneni, S.; Roy, R.; Li, H.Q. Microwave-hydrothermal synthesis of ceramic powders. Mater. Res. Bull. 1992, 27, 1393–1405. [Google Scholar] [CrossRef]
- Byrappa, K.; Adschiri, T. Hydrotermal technology for nanotechnology. Prog. Cryst. Growth Charact. Mater. 2007, 57, 117–166. [Google Scholar] [CrossRef]
- Yoshimura, M.; Byrappa, K. Hydrothermal processing of materials: past, present and future. J. Mater. Sci. 2008, 43, 2085–2103. [Google Scholar] [CrossRef]
- Van Gerven, T.; Stankiewicz, A. Structure, energy, synergy, time-the fundamentals of process intensification. Ind. Eng. Chem. Res. 2009, 48, 2465–2474. [Google Scholar] [CrossRef]
- Schwalbe, T.; Autze, V.; Hohmann, M.; Stirner, W. Novel innovation systems for a cellular approach to continuous process chemistry from discovery to market. Org. Proc. Res. Devel. 2004, 8, 440–454. [Google Scholar] [CrossRef]
- Leonelli, C.; Rizzuti, A.; Rosa, R.; Corradi, A.B.; Veronesi, P. Numerical simulation of a microwave reactor used in synthesis of nanoparticles. In Proceedings of IMPI 44th Annual Symposium, Denver, CO, USA, 14–16 July 2010.
- Baghbanzadeh, M.; Carbone, L.; Cozzoli, P.D.; Kappe, C.O. Microwave-assisted synthesis of colloidal inorganic nanocrystals. Angew. Chem. Int. Ed. 2011, 50, 11312–11359. [Google Scholar] [CrossRef]
- Yao, W.T.; Yu, S.H. Recent advances in hydrothermal syntheses of low dimensional nanoarchitectures. Int. J. Nanotechnol. 2007, 4, 129–162. [Google Scholar] [CrossRef]
- Yan, S.; Wang, B.; Shi, Y.; Yang, F.; Hu, D.; Xu, X.; Wu, J. Hydrothermal synthesis of CdS nanoparticle/functionalized graphene sheet nanocomposites for visible-light photocatalytic degradation of methyl orange. Appl. Surf. Sci. 2013, 285P, 840–845. [Google Scholar]
- Wan, H.; Ji, X.; Jiang, J.; Yu, J.; Miao, L.; Zhang, L.; Bie, S.; Chen, H.; Ruan, Y. Hydrothermal synthesis of cobalt sulfide nanotubes: The size control and its application in supercapacitors. J. Power Sources 2013, 243, 396–402. [Google Scholar] [CrossRef]
- Yan, X.; Michael, E.; Komarneni, S.; Brownson, J.R.; Yan, Z.F. Microwave- and conventional-hydrothermal synthesis of CuS, SnS and ZnS: Optical properties. Ceram. Int. 2013, 39, 4757–4763. [Google Scholar]
- Shojai, M.S.; Khorasani, M.T.; Khoshdargi, E.D.; Jamshidi, A. Synthesis methods for nanosized hydroxyapatite with diverse structures. Acta Biomat. 2013, 9, 7591–7621. [Google Scholar] [CrossRef]
- Hu, B.; Wang, K.; Wu, L.; Yu, S.H.; Antonietti, M.; Titirici, M.M. Engineering carbon materials from the hydrothermal carbonization process of biomass. Adv. Mater. 2010, 22, 813–828. [Google Scholar] [CrossRef]
- Chen, P.; Yang, J.J.; Li, S.S.; Wang, Z.; Xiao, T.Y.; Qian, Y.H.; Yu, S.H. Hydrothermal synthesis of macroscopic nitrogen-doped graphene hydrogels for ultrafast supercapacitors. Nano Energy 2013, 2, 249–256. [Google Scholar] [CrossRef]
- Eerenstein, W.; Mathur, N.D.; Scott, J.F. Multiferroic and magnetoelectric materials. Nature 2006, 442, 759–765. [Google Scholar] [CrossRef]
- Wang, J.; Neaton, J.B.; Zheng, H.; Nagarajan, V.; Ogale, S.B.; Liu, B.; Viehland, D.; Vaidhyanathan, V.; Schlom, D.G.; Waghmare, U.V.; et al. Epitaxial BiFeO3 multiferroic thin film heterostructures. Science 2003, 299, 1719–1722. [Google Scholar] [CrossRef]
- Hur, N.; Park, S.; Sharma, P.A.; Ahn, J.S.; Guha, S.; Cheong, S.W. Electric polarization reversal and memory in a multiferroic material induced by magnetic fields. Nature 2004, 429, 392–395. [Google Scholar] [CrossRef]
- Seidel, J.; Martin, L.W.; He, Q.; Zhan, Q.; Chu, Y.H.; Rother, A.; Hawkridge, M.E.; Maksymovych, P.; Yu, P.; Gajek, M.; et al. Conduction at domain walls in oxide multiferroics. Nat. Mater. 2009, 8, 229–234. [Google Scholar] [CrossRef]
- Choi, T.; Lee, S.; Choi, Y.J.; Kiryukhin, V.; Cheong, S.W. Switchable ferroelectric diode and photovoltaic effect in BiFeO3. Science 2009, 324, 63–66. [Google Scholar] [CrossRef]
- Han, S.H.; Kim, K.S.; Kim, H.-G.; Lee, H.-G.; Kang, H.-W.; Kim, J.S.; Cheon, C.I. Synthesis and characterization of multiferroic BiFeO3 powders fabricated by hydrothermal methods. Ceram. Int. 2010, 36, 1365–1375. [Google Scholar]
- Wang, Y.; Xu, G.; Ren, Z.; Wei, X.; Weng, W.; Du, P.; Shen, G.; Han, G. Mineralizer-assisted hydrothermal synthesis and characterization of BiFeO3 nanoparticles. J. Am. Ceram. Soc. 2007, 90, 2615–2617. [Google Scholar] [CrossRef]
- Chen, C.; Cheng, J.; Yu, S.; Che, L.; Meng, Z. Hydrothermal synthesis of perovskite bismuth ferrite crystallites. J. Cryst. Growth 2006, 291, 135–139. [Google Scholar] [CrossRef]
- Komarneni, S.; Menon, V.C.; Li, H.Q.; Roy, R.; Ainger, F. Microwave-hydrothermal processing of BiFeO3 and CsAl2PO6. J. Am. Ceram. Soc. 1996, 79, 1409–1412. [Google Scholar] [CrossRef]
- Prado-Gonjal, J.; Villafuerte-Castrejon, M.E.; Fuentes, L.; Moran, E. Microwave-hydrothermal synthesis of the multiferroic BiFeO3. Mater. Res. Bull. 2009, 44, 1734–1737. [Google Scholar] [CrossRef]
- Ponzoni, C.; Rosa, R.; Cannio, M.; Buscaglia, V.; Finocchio, E.; Nanni, P.; Leonelli, C. Optimization of BFO microwave-hydrothermal synthesis: Influence of process parameters. J. Alloys Compds. 2013, 558, 150–159. [Google Scholar] [CrossRef]
- Merzhanov, A.G.; Shkiro, V.M.; Borovinskaya, I.P. Synthesis of refractory inorganic compounds. US Patent 3726643, April 1973. [Google Scholar]
- Merzhanov, A.G.; Shkiro, V.M.; Borovinskaya, I.P. Synthesis of refractory inorganic compounds. Byull. Izobr. 1971, 10. [Google Scholar]
- Varma, A.; Lebrat, J.P. Combustion synthesis of advanced materials. Chem. Eng. Sci. 1992, 47, 2179–2194. [Google Scholar] [CrossRef]
- Merzhanov, A.G. Reviews: fundamentals, achievements, and perspectives for development of solid-flame combustion. Russ. Chem. Bull. 1997, 46, 1–27. [Google Scholar] [CrossRef]
- Morsi, K. The diversity of combustion synthesis processing: A review. J. Mater. Sci. 2012, 47, 68–92. [Google Scholar] [CrossRef]
- Rajeshwar, K.; de Tacconi, N.R. Solution combustion synthesis of oxide semiconductors for solar energy conversion and environmental remediation. Chem. Soc. Rev. 2009, 38, 1984–1998. [Google Scholar] [CrossRef]
- Nagaveni, K.; Hedge, M.S.; Ravishankar, N.; Subbanna, G.N.; Madras, G. Synthesis and structure of nanocrystalline TiO2 with lower band gap showing high photocatalytic activity. Langmuir 2004, 20, 2900–2907. [Google Scholar] [CrossRef]
- Selvam, N.C.S.; Kumar, R.T.; Kennedy, L.J.; Vijaya, J.J. Comparative study of microwave and conventional methods for the preparation and optical properties of novel MgO-micro and nano-structures. J. Alloys Compds. 2011, 509, 9809–9815. [Google Scholar] [CrossRef]
- Nehru, L.C.; Swaminathan, V.; Sanjeeviraja, C. Rapid synthesis of nanocrystalline ZnO by a microwave-assisted combustion method. Powder Technol. 2012, 226, 29–33. [Google Scholar] [CrossRef]
- Rosa, R.; Ponzoni, C.; Veronesi, P.; Natali Sora, I.; Felice, V.; Leonelli, C. Solution combustion synthesis of perovskite oxides: Comparison between MWs and conventional ignition. In Proceedings of 14th International Conference on Microwave and High Frequency Heating, Nottingham, UK, 16–19 September 2013; The University of Nottingham: Nottingham, UK, 2013; pp. 250–253. [Google Scholar]
- Kumar, A.; Wolf, E.E.; Mukasyan, A.S. Solution combustion synthesis of metal nanopowders: Nickel reaction pathways. AIChE J. 2011, 57, 2207–2214. [Google Scholar] [CrossRef]
- Luo, W.; Wang, D.; Peng, X.; Wang, F. Microwave synthesis and phase transitions in nanoscale BiFeO3. J. Sol-Gel Sci. Technol. 2009, 51, 53–57. [Google Scholar] [CrossRef]
- Mukasyan, A.S.; Dinka, P. Apparatus and methods for combustion synthesis of nano-powders. US Patent WO2007019332-A1, February 2007. [Google Scholar]
- Kumar, A.; Mukasyan, A.S.; Wolf, E.E. Impregnated layer combustion synthesis method for preparation of multicomponent catalysts for the production of hydrogen from oxidative reforming of methanol. Appl. Catal. A 2010, 372, 175–183. [Google Scholar] [CrossRef]
- Martirosyan, K.S.; Luss, D. Carbon combustion synthesis of oxides. US Patent 0097419 A1, May 2006. [Google Scholar]
- Martirosyan, K.S.; Iliev, M.; Luss, D. Carbon combustion synthesis of nanostructured perovskites. Int. J. SHS 2007, 16, 36–45. [Google Scholar]
- Dinka, P.; Mukasyan, A.S. In situ preparation of oxide-based supported catalysts by solution combustion synthesis. J. Phys. Chem. B 2005, 109, 21627–21633. [Google Scholar] [CrossRef]
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Rosa, R.; Ponzoni, C.; Leonelli, C. Direct Energy Supply to the Reaction Mixture during Microwave-Assisted Hydrothermal and Combustion Synthesis of Inorganic Materials. Inorganics 2014, 2, 191-210. https://doi.org/10.3390/inorganics2020191
Rosa R, Ponzoni C, Leonelli C. Direct Energy Supply to the Reaction Mixture during Microwave-Assisted Hydrothermal and Combustion Synthesis of Inorganic Materials. Inorganics. 2014; 2(2):191-210. https://doi.org/10.3390/inorganics2020191
Chicago/Turabian StyleRosa, Roberto, Chiara Ponzoni, and Cristina Leonelli. 2014. "Direct Energy Supply to the Reaction Mixture during Microwave-Assisted Hydrothermal and Combustion Synthesis of Inorganic Materials" Inorganics 2, no. 2: 191-210. https://doi.org/10.3390/inorganics2020191
APA StyleRosa, R., Ponzoni, C., & Leonelli, C. (2014). Direct Energy Supply to the Reaction Mixture during Microwave-Assisted Hydrothermal and Combustion Synthesis of Inorganic Materials. Inorganics, 2(2), 191-210. https://doi.org/10.3390/inorganics2020191







