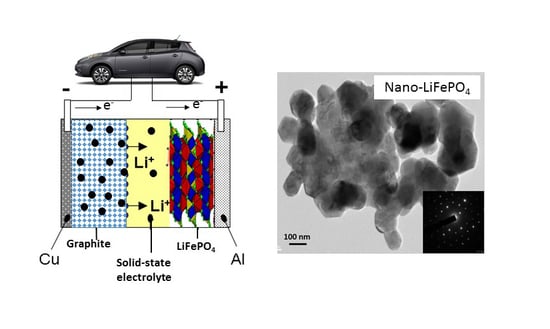
Nanotechnology of Positive Electrodes for Li-Ion Batteries
Abstract
:1. Introduction
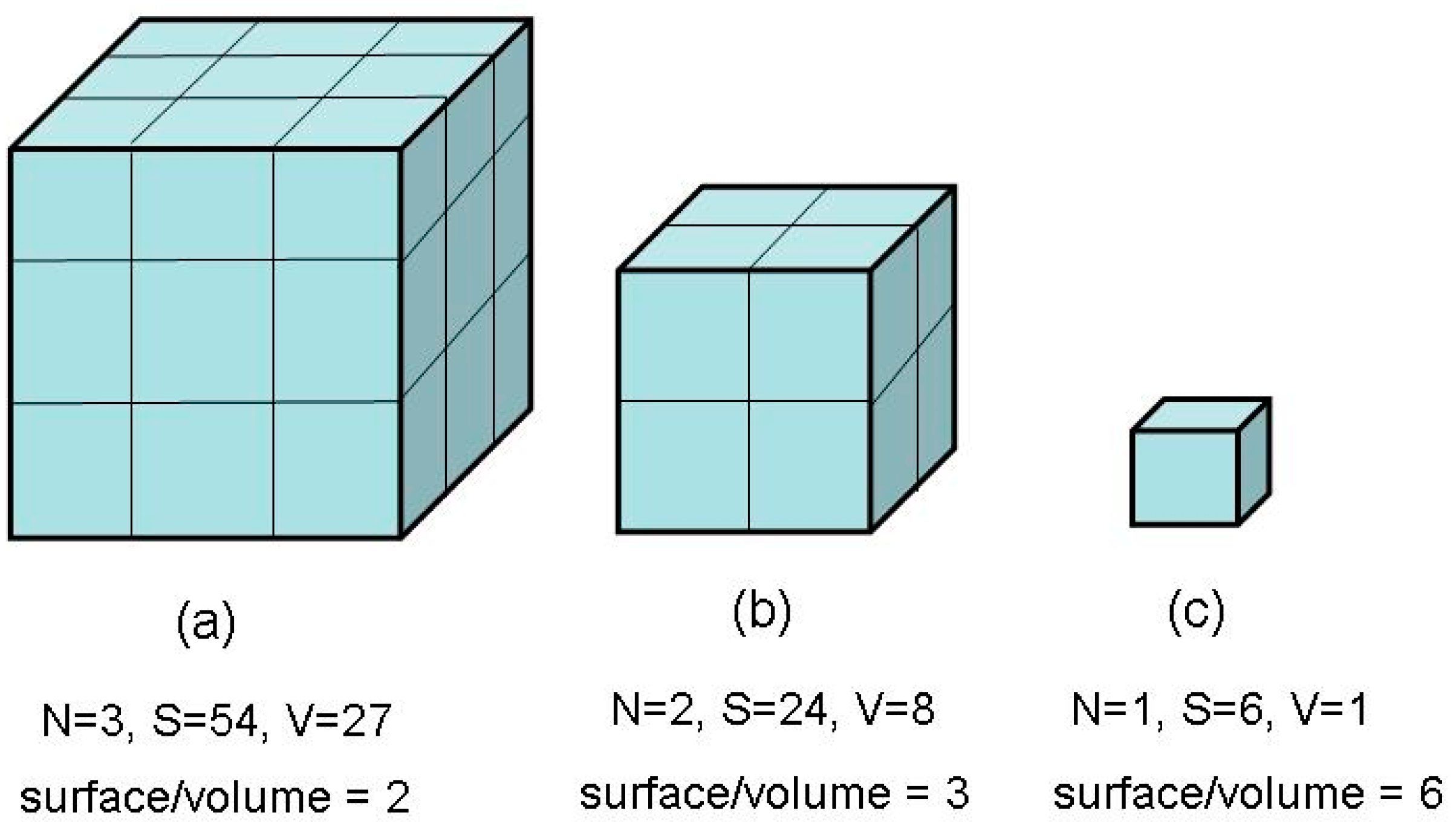
2. Synthesis of Nanomaterials
3. Electrochemistry of Nanostructured Cathodes
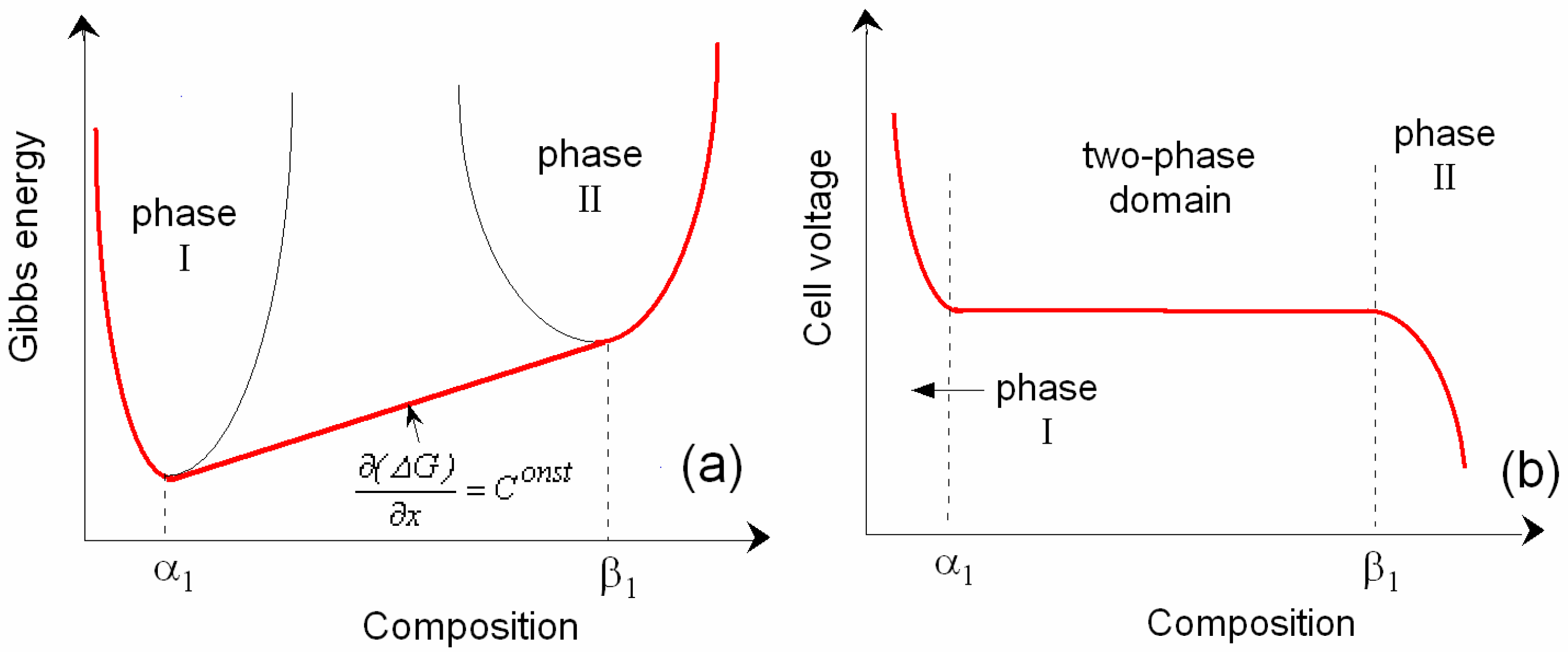
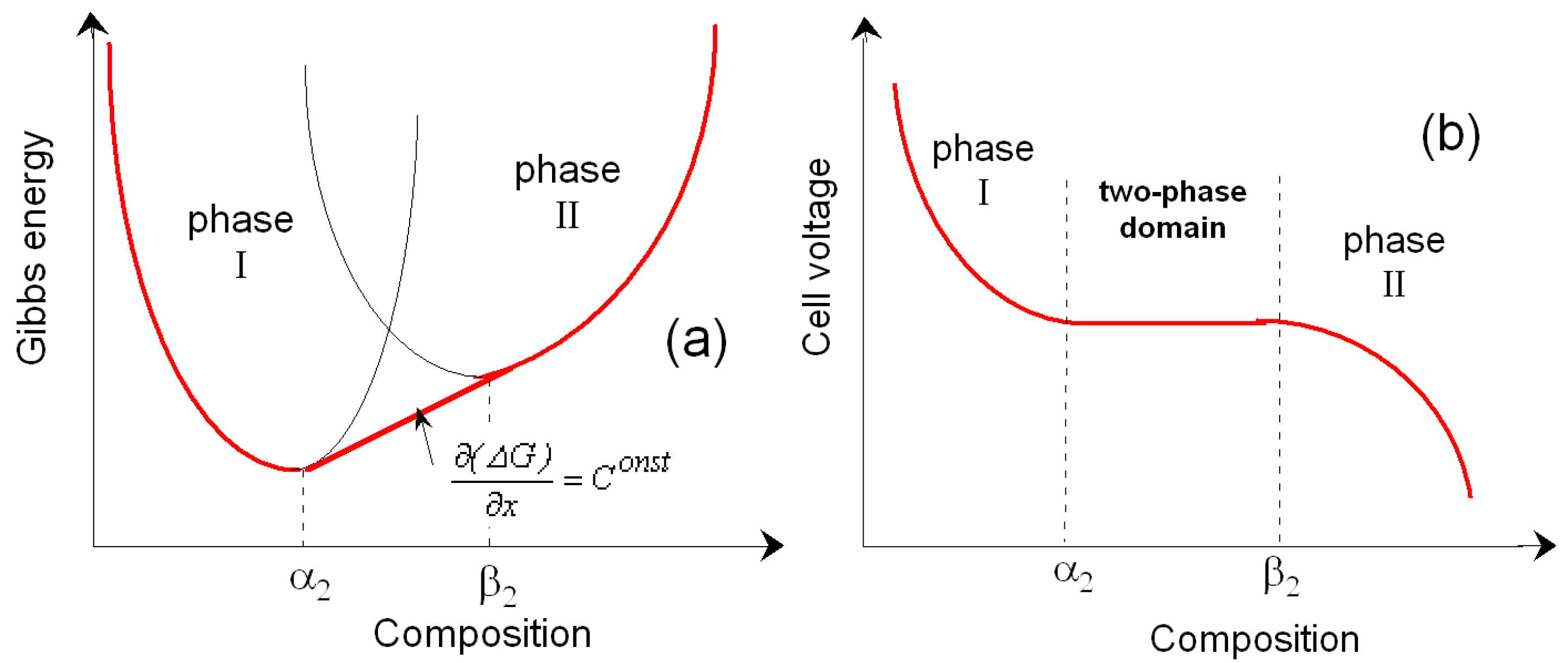
3.1. Thermodynamic Approach
3.2. Transition Metal Oxides
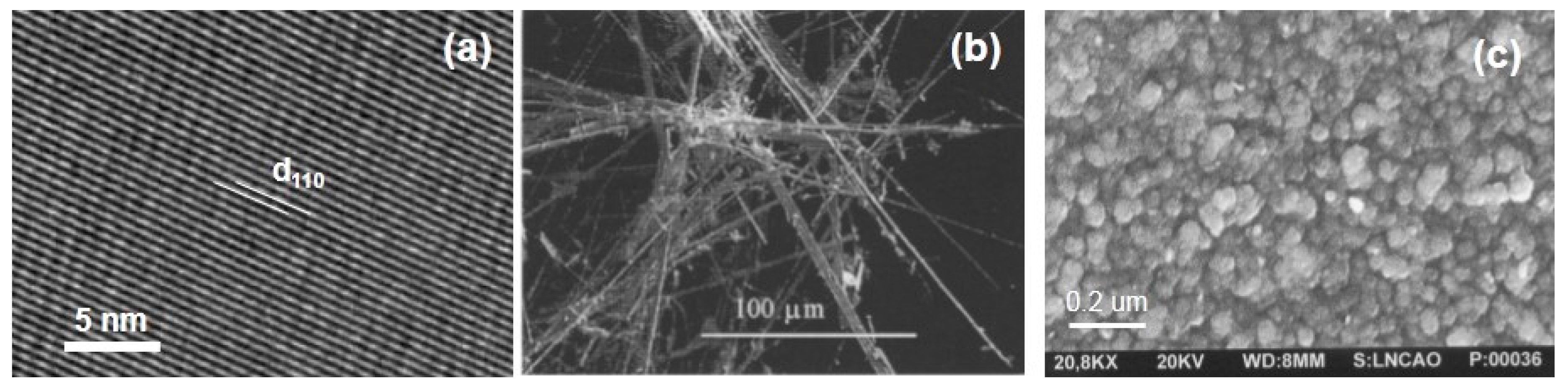
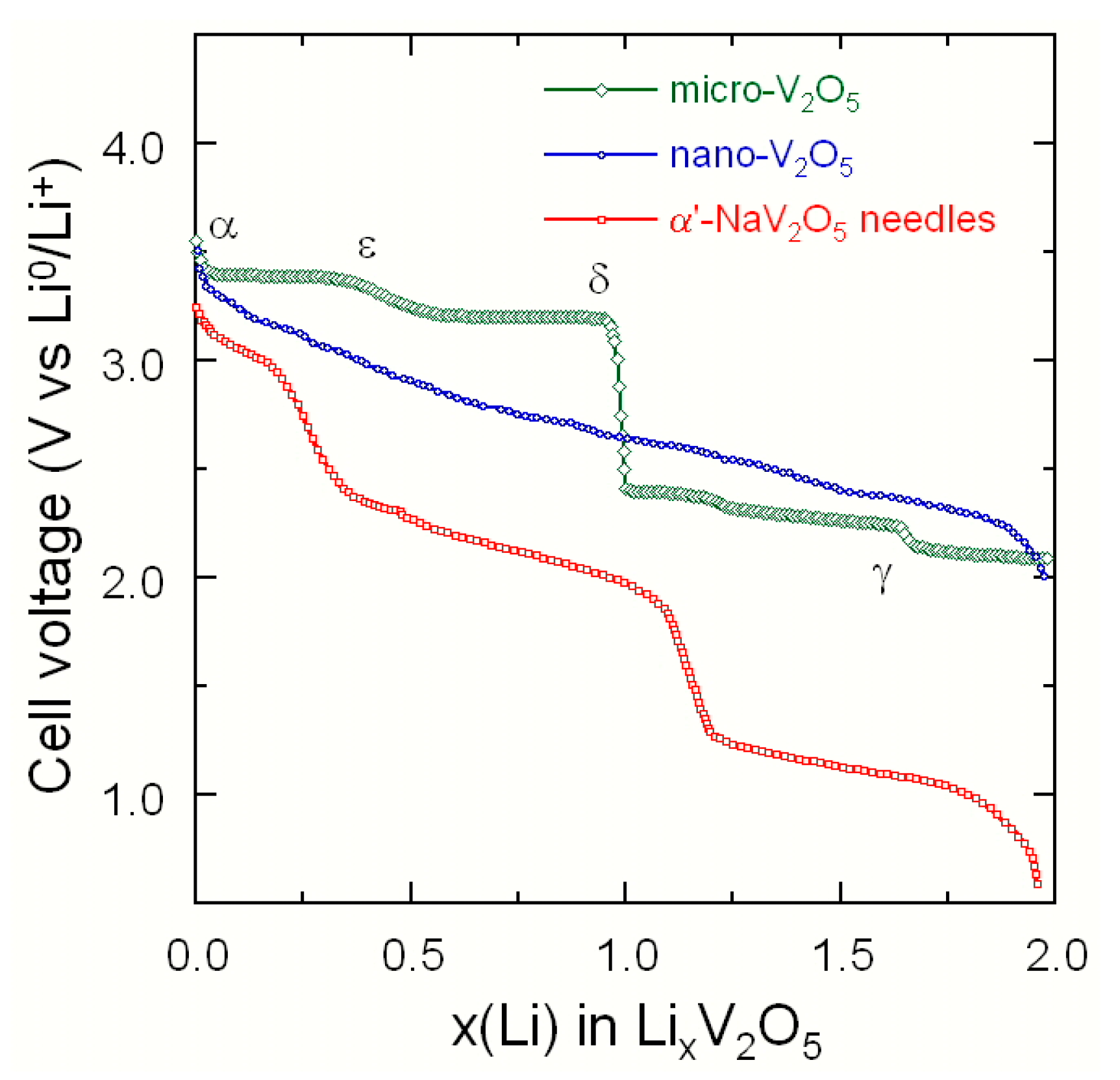
3.2.1. Vanadium Pentoxide
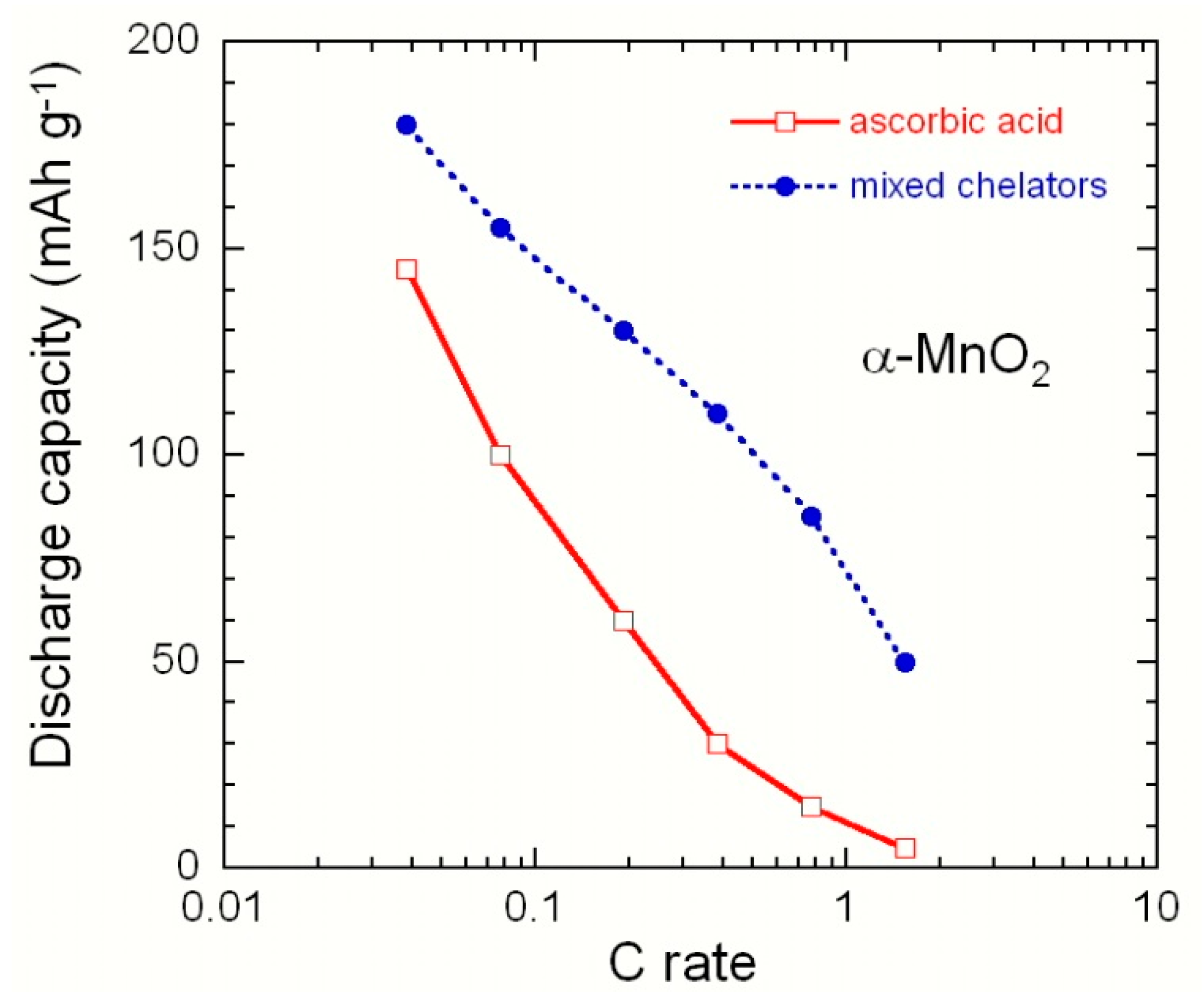
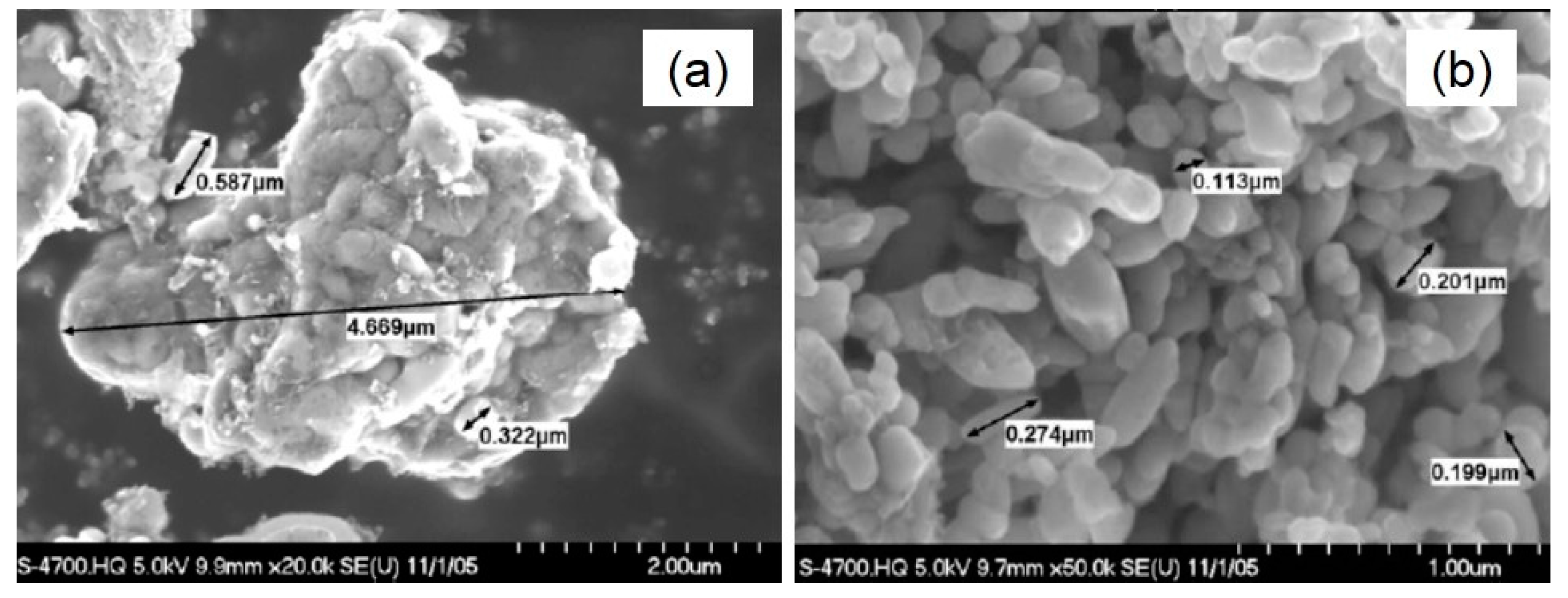
3.2.2. Manganese Dioxide
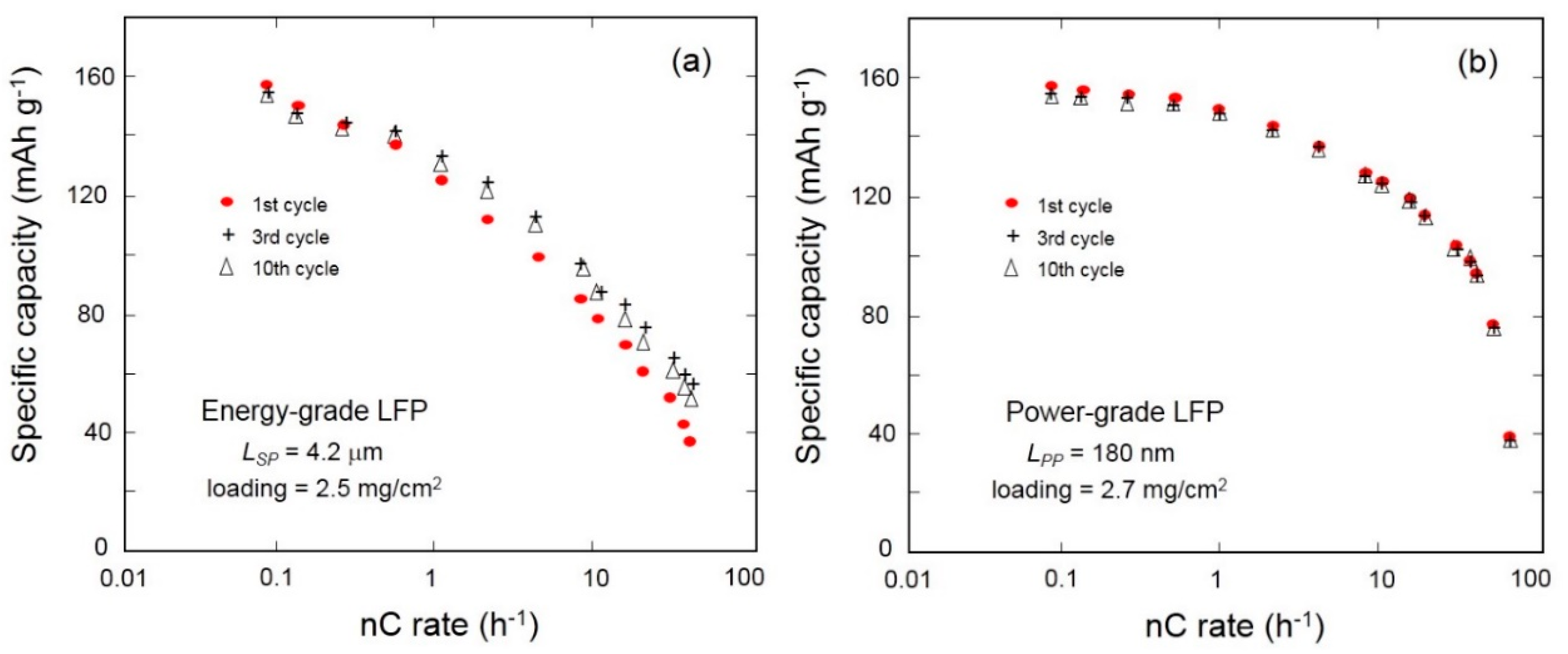
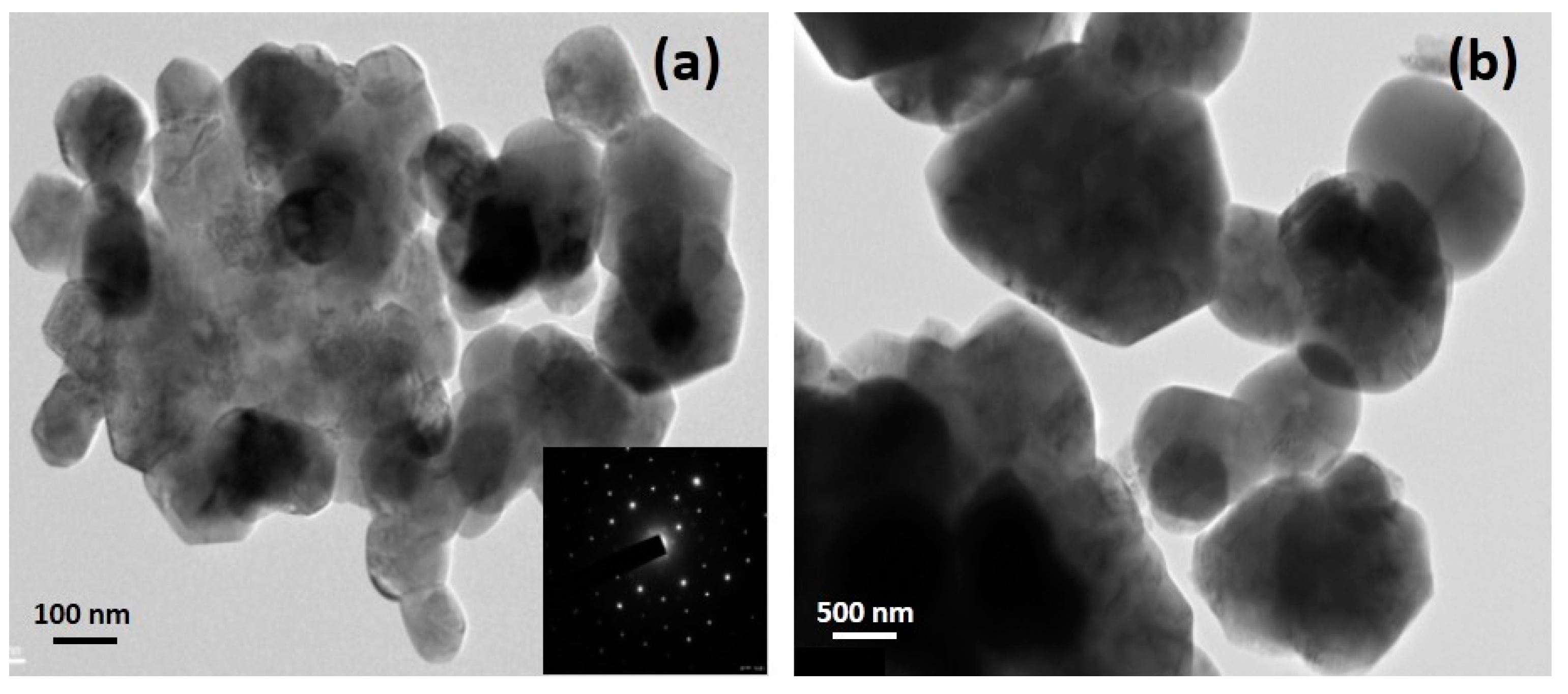
3.3. Olivine-Like Materials
3.4. Layered Structures
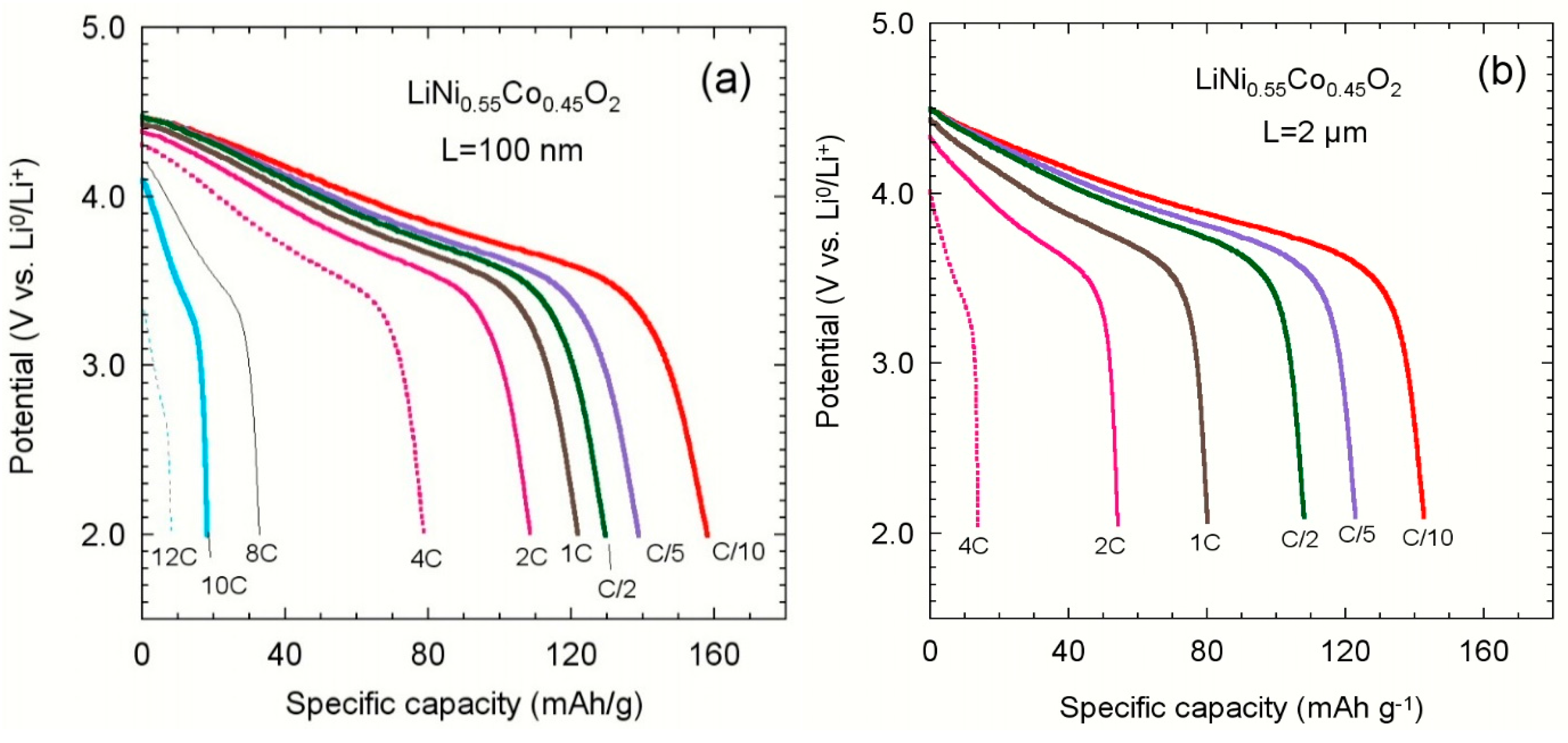
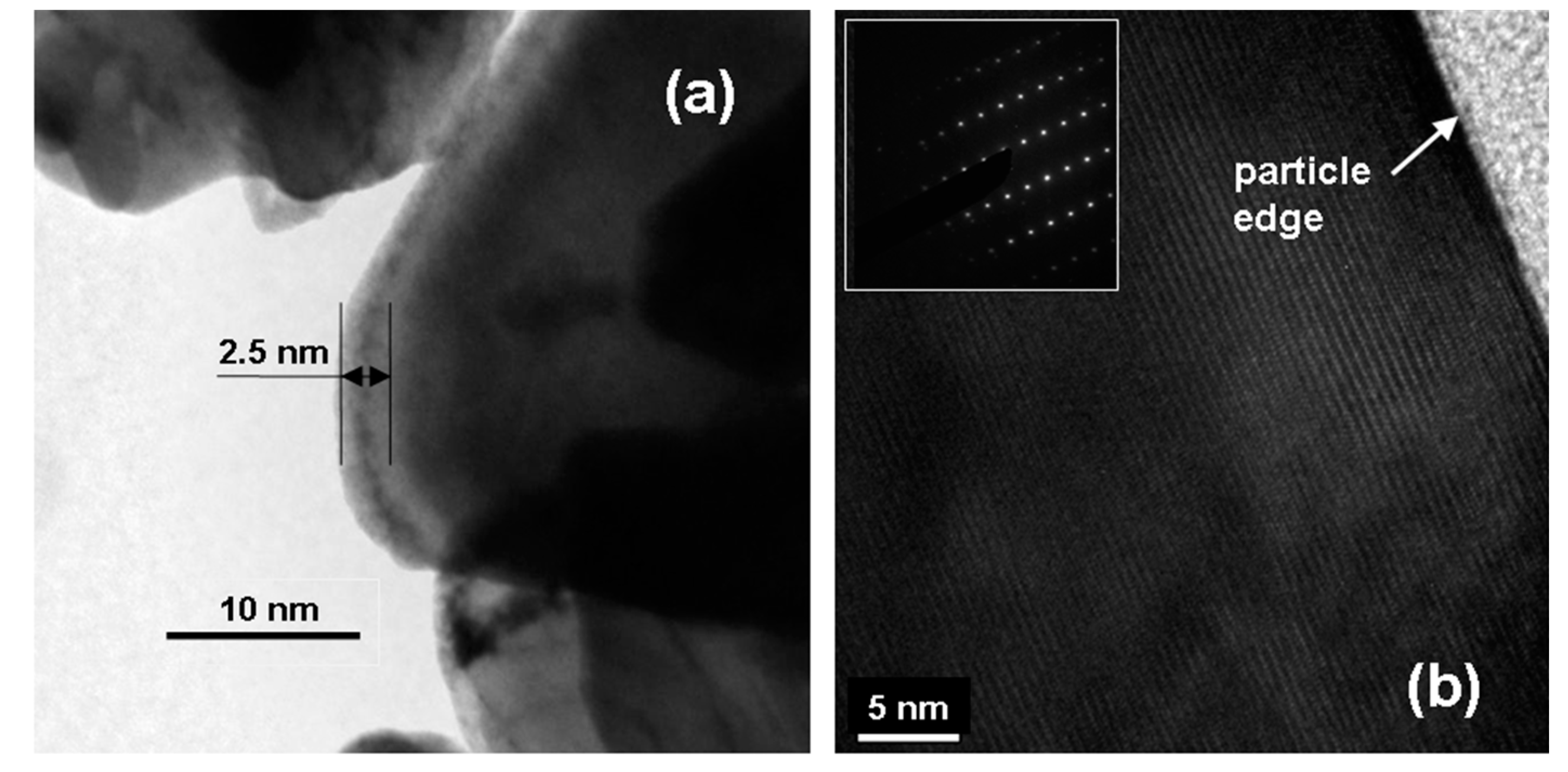
3.4.1. Layered LiNi0.55Co0.45O2
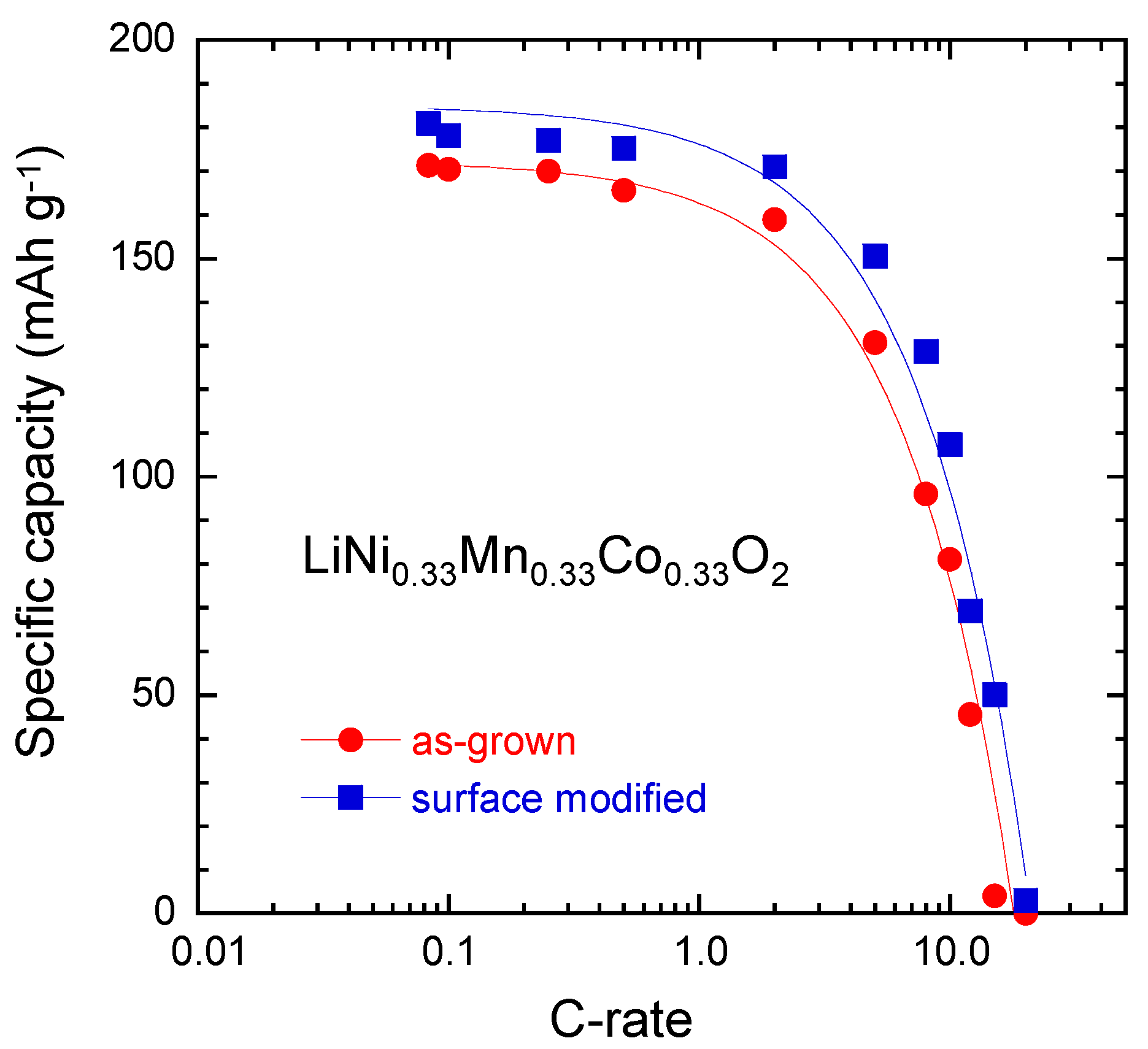
3.4.2. Layered LiNi1/3Mn1/3Co1/3O2
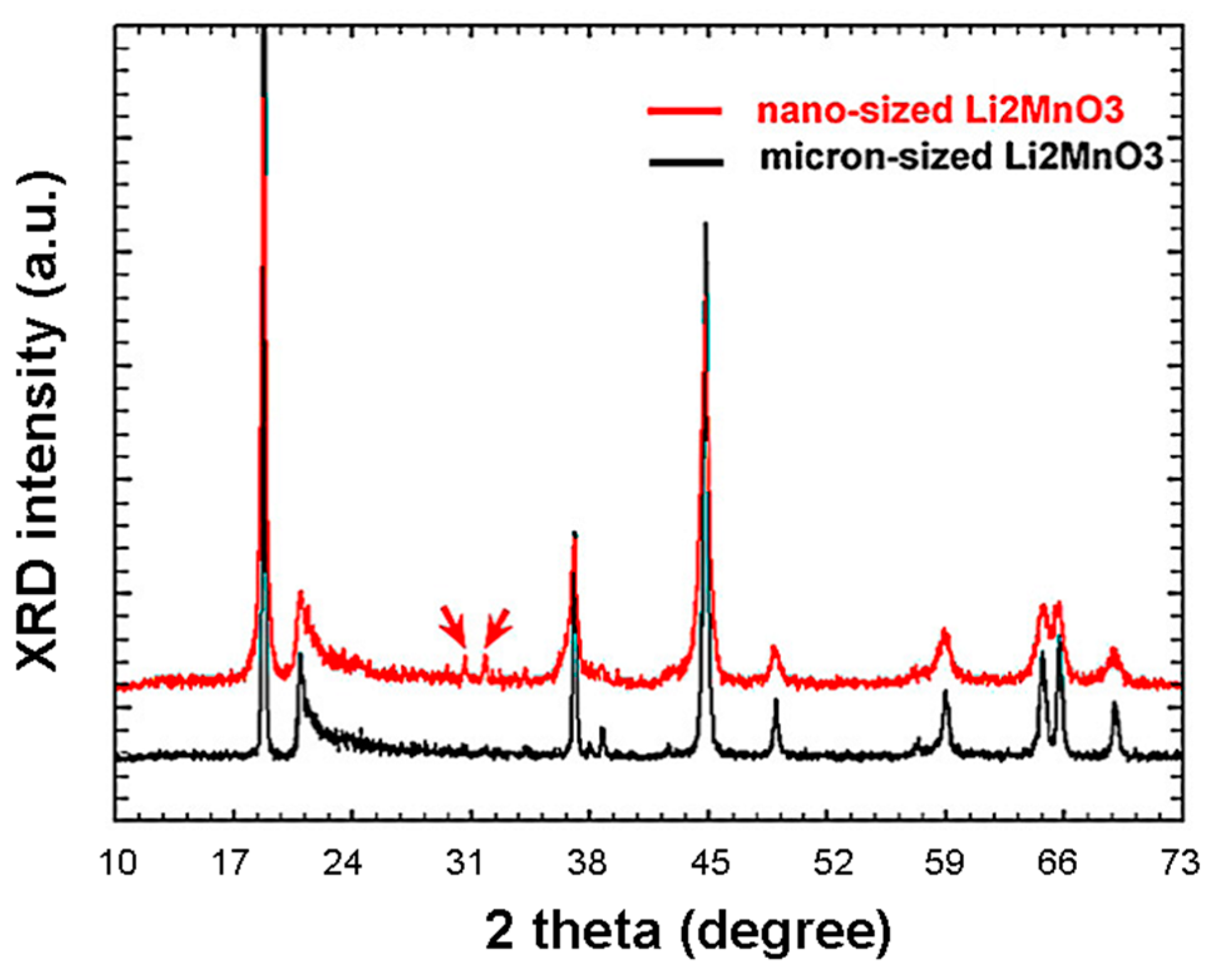
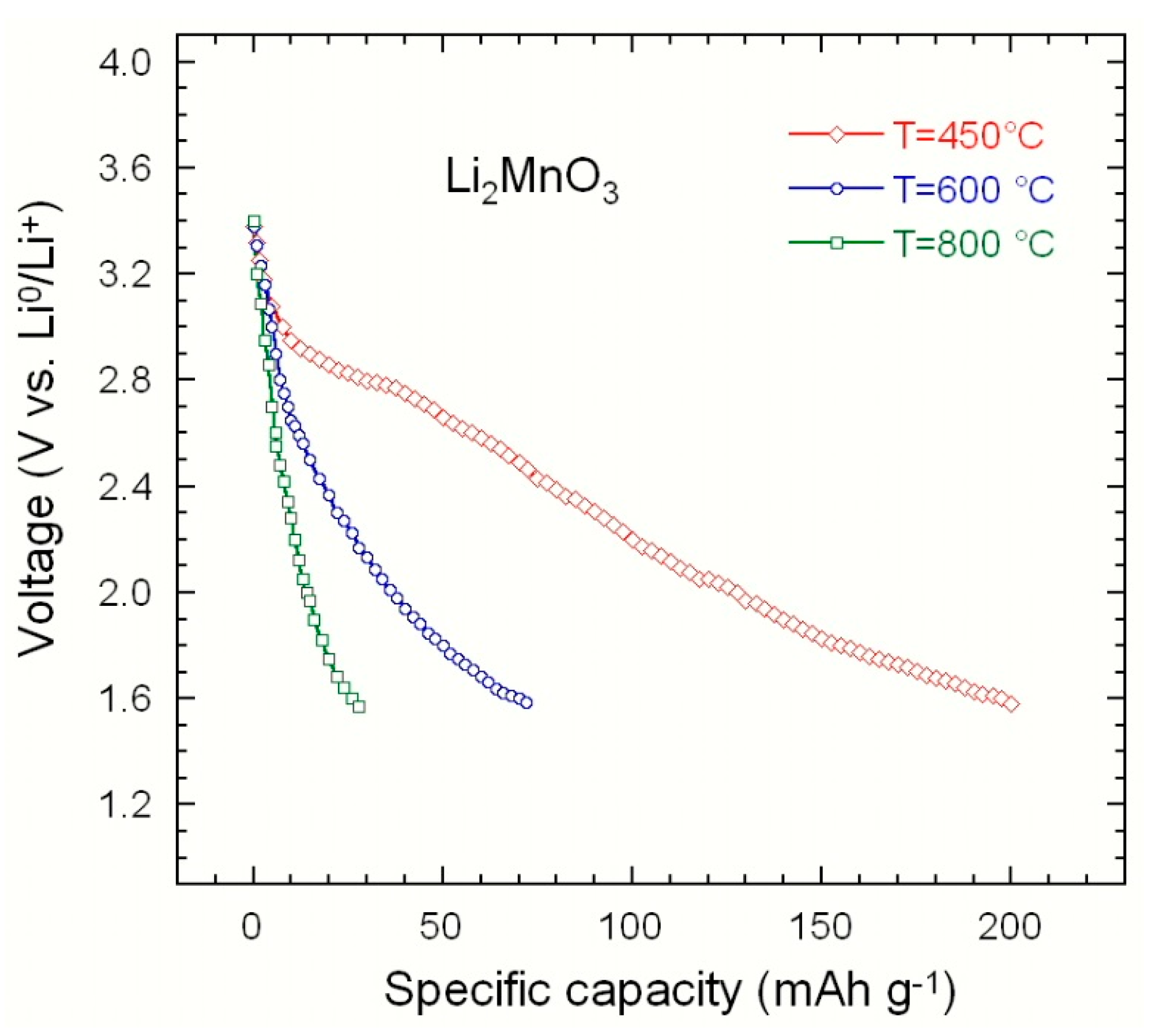
3.4.3. Rock-Salt Li2MnO3
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Julien, C.M.; Mauger, A.; Vijh, A.; Zaghib, K. Lithium Batteries: Science and Technology; Springer: Heidelberg, Germany, 2016. [Google Scholar]
- Zaghib, K.; Mauger, A.; Julien, C.M. Rechargeable Batteries; Zhang, Z., Zhang, S.S., Eds.; Springer: Heidelberg, Germany, 2015. [Google Scholar]
- Yamada, A.; Chung, S.C.; Hinokuma, K. Optimized LiFePO4 for lithium battery cathodes. J. Electrochem. Soc. 2001, 148, A224–A229. [Google Scholar] [CrossRef]
- Okubo, M.; Hosono, E.; Kim, J.; Enomoto, M.; Kojima, N.; Kudo, T.; Zhou, H.; Honma, I. Nanosize effect on high-rate Li-ion intercalation in LiCoO2 electrode. J. Am. Chem. Soc. 2007, 129, 7444–7452. [Google Scholar] [CrossRef] [PubMed]
- Castro-Couceiro, A.; Castro-Garcia, S.; Senaris-Rodriguez, M.A.; Soulette, F.; Julien, C. Effects of the aluminum doping on the microstructure and morphology of LiNi0.5Co0.5O2 oxides. Ionics 2002, 8, 192–200. [Google Scholar] [CrossRef]
- Vediappan, K.; Guerfi, A.; Gariépy, V.; Demopoulos, G.P.; Hovington, P.; Trottier, J.; Mauger, A.; Julien, C.M.; Zaghib, K. Stirring effect in hydrothermal synthesis of nano C-LiFePO4. J. Power Sources 2014, 266, 99–106. [Google Scholar] [CrossRef]
- Dominko, R.; Bele, M.; Gaberscek, M.; Remskar, M.; Hanzel, D.; Goupil, J.M.; Pejovnik, S.; Jamnik, J. Prorous olivine composites synthesized by sol–gel technique. J. Power Sources 2006, 153, 274–280. [Google Scholar] [CrossRef]
- Wang, Y.; Sun, B.; Park, J.; Kim, W.S.; Kim, H.-S.; Wang, G. Morphology control and electrochemical properties of nanosize LifePO4 cathode material synthesized by co-precipitation combined with in situ polymerization. J. Alloys Compd. 2011, 509, 1040–1044. [Google Scholar] [CrossRef]
- Brochu, F.; Guerfi, A.; Trottier, J.; Kopec, M.; Mauger, A.; Groult, H.; Julien, C.M.; Zaghib, K. Structure and electrochemistry of scalling nano C-LiFePO4 synthesized by hydrothermal route: complexing agent effect. J. Power Sources 2012, 214, 1–6. [Google Scholar] [CrossRef]
- Doherty, C.M.; Caruso, R.A.; Smarsly, B.M.; Drummond, C.J. Colloidal crystal templating to produce hierarchically porous LiFePO4 electrode materials for high power lithium ion batteries. Chem. Mater. 2009, 21, 2895–2903. [Google Scholar] [CrossRef]
- Zaghib, K.; Charest, P.; Dontigny, M.; Guerfi, A.; Lagacé, M.; Mauger, A.; Kopec, M.; Julien, C.M. LiFePO4: From molten ingot to nanoparticles with high-rate performance in Li-ion batteries. J. Power Sources 2010, 195, 8280–8288. [Google Scholar] [CrossRef]
- Wang, Z.; Wu, F.; Su, Y.F.; Bao, L.-Y.; Chen, L.; Li, N.; Chen, S. Preparation and characterization of xLi2MnO3·(1–x) Li[Ni1/3Mn1/3Co1/3]O2 cathode materials for lithium-ion batteries. Acta Phys. Chim. Sin. 2012, 28, 823–830. [Google Scholar]
- Hashem, A.M.; Abdel-Ghany, A.E.; El-Tawil, R.; Bhaskar, A.; Hunzinger, B.; Ehrenberg, H.; Mauger, A.; Julien, C.M. Urchin-like α-MnO2 formed of nano-needles for high-performance lithium batteries. Ionics 2016, 22, 2263–2271. [Google Scholar] [CrossRef]
- Feng, L.; Xuan, Z.; Zhao, H.; Bai, Y.; Guo, J.; Su, C.-W.; Chen, X. MnO2 prepared by hydrothermal method and electrochemical performance as anode for lithium-ion battery. Nanoscale Res. Lett. 2014, 9, 290. [Google Scholar] [CrossRef] [PubMed]
- Jarvis, K.A.; Deng, Z.; Allard, L.F.; Manthiram, A.; Ferreira, P.J. Atomic structure of a lithium-rich layered oxide material for lithium-ion batteries: Evidence of a solid solution. Chem. Mater. 2011, 23, 3614–3621. [Google Scholar] [CrossRef]
- Zhang, X.; Mauger, A.; Lu, Q.; Groult, H.; Perrigaud, L.; Gendron, F.; Julien, C.M. Synthesis and characterization of LiNi1/3Mn1/3Co1/3O2 by wet chemical method. Electrochim. Acta 2010, 55, 6440–6449. [Google Scholar] [CrossRef]
- Wu, H.M.; Rao, C.V.; Rambabu, B. Electrochemical performance of LiNi0.5Mn1.5O4 prepared by improved solid state method as cathode in hybrid supercapacitor. Mater. Chem. Phys. 2009, 116, 532–535. [Google Scholar] [CrossRef]
- Suchanek, W.L.; Riman, R.E. Hydrothermal synthesis of advanced ceramic powders. Adv. Sci. Technol. 2006, 45, 184–193. [Google Scholar] [CrossRef]
- Cao, G.; Liu, D. Template-based synthesis of nanorod, nanowire, and nanotube arrays. Adv. Colloid. Interface Sci. 2008, 136, 45–64. [Google Scholar] [CrossRef] [PubMed]
- Hwang, B.-J.; Hsu, K.-F.; Hu, S.-K.; Cheng, M.-Y.; Chou, T.-C.; Tsay, S.-Y.; Santhanam, R. Template-free reverse micelle process for the synthesis of a rod-like LiFePO4/C composite cathode material for lithium batteries. J. Power Sources 2009, 194, 515–519. [Google Scholar] [CrossRef]
- Kwon, S.J.; Kim, C.W.; Jeong, W.T.; Lee, K.S. Synthesis and electrochemical properties of olivine LiFePO4 as a cathode material prepared by mechanical alloying. J. Power Sources 2004, 137, 93–99. [Google Scholar] [CrossRef]
- Liao, X.Z.; Ma, Z.F.; Wang, L.; Zhang, X.-M.; Jiang, Y.; He, Y.-S. A novel synthesis route for LiFePO4/C cathode materials for lithium-ion batteries. Electrochem. Solid-State Lett. 2004, 7, A522–A525. [Google Scholar] [CrossRef]
- Franger, S.; Bourbon, C.; Le Cras, F. Optimized lithium iron phosphate for high-rate electrochemical applicatioons. J. Electrochem. Soc. 2004, 151, A1024–A1027. [Google Scholar] [CrossRef]
- Kim, J.-K.; Choi, J.-W.; Cheruvally, G.; Kim, J.-U.; Ahn, J.-H.; Cho, G.-B.; Ahn, H.-J. A modified mechanical activation synthesis for carbon-coated LiFePO4 cathode in lithium batteries. Mater. Lett. 2007, 61, 3822–3825. [Google Scholar] [CrossRef]
- Kosova, N.V.; Devyatkina, E.T. Synthesis of nanosized materials for lithium-ion batteries by mechanical activation. Studies of their structure and properties. Russian J. Electrochem. 2012, 48, 320–329. [Google Scholar] [CrossRef]
- Kosova, N.V.; Devyatkina, E.T. A new approach to prepare nanosized cathode materials. ECS Trans. 2010, 25, 19–25. [Google Scholar]
- Liu, D.; Trottier, J.; Charest, P.; Fréchette, J.; Guerfi, A.; Mauger, A.; Julien, C.M.; Zaghib, K. Effect of nano LiFePO4 coating on LiMn1.5Ni0.5O4 5 V cathode for lithium ion batteries. J. Power Sources 2012, 204, 127–132. [Google Scholar] [CrossRef]
- Whittingham, M.S. Lithium batteries and cathode materials. Chem. Rev. 2004, 104, 4271–4301. [Google Scholar] [CrossRef] [PubMed]
- Julien, C.; Haro-Poniatowski, E.; Camacho-Lopez, M.A.; Escobar-Alarcon, L.; Jimenez-Jarquin, J. Growth of V2O5 thin films by pulsed laser deposition and their applications in lithium microbatteries. Mater. Sci. Eng. B 1999, 65, 170–176. [Google Scholar] [CrossRef]
- Bouhedja, L.; Castro-Garcia, S.; Livage, J.; Julien, C. Lithium intercalation in α’-NayV2O5 synthesized via the hydrothermal route. Ionics 1998, 4, 227–233. [Google Scholar] [CrossRef]
- Tompsett, D.A.; Islam, M.S. Electrochemistry of hollandite α-MnO2: Li-ion and Na-ion insertion and Li2O incorporation. Chem. Mater. 2013, 25, 2515–2526. [Google Scholar] [CrossRef]
- Hashem, A.M.; Abuzeid, H.M.; Abdel-Latif, A.M.; Abbas, H.M.; Ehrenberg, H.; Indris, S.; Mauger, A.; Groult, H.; Julien, C.M. MnO2 nanorods prepared by redox reaction as cathodes in lithium batteries. ECS Trans. 2013, 50–24, 125–130. [Google Scholar] [CrossRef]
- Xing, L.; Cui, C.; Ma, C.; Xue, X. Facile synthesis of α-MnO2/graphene nanocomposites and their high performance as lithium-ion battery anode. Mater. Lett. 2011, 65, 2104–2106. [Google Scholar] [CrossRef]
- Li, B.; Rong, G.; Xie, Y.; Huang, L.; Feng, C. Low-temperature synthesis of α-MnO2 hollow urchins and their application in rechargeable Li+ batteries. Inorg. Chem. 2006, 45, 6404–6410. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Nan, C.; Lu, J.; Peng, Q.; Li, Y. α-MnO2 nanotubes: High surface area and enhanced lithium battery properties. Chem. Commun. 2012, 48, 6945–6947. [Google Scholar] [CrossRef] [PubMed]
- Cheng, F.Y.; Zhao, J.Z.; Song, W.; Li, C.; Ma, H.; Chen, J.; Shen, P. Facile controlled synthesis of MnO2 nanostructures of novel shapes and their applications in batteries. Inorg. Chem. 2006, 45, 2038–2044. [Google Scholar] [CrossRef] [PubMed]
- Kijima, N.; Takahashi, Y.; Akimoto, J.; Awaka, J. Lithium ion insertion and extraction reactions with hollandite-type manganese dioxide free from any stabilizing cations in its tunnel cavity. J. Solid State Chem. 2005, 178, 2741–2750. [Google Scholar] [CrossRef]
- Yang, Y.; Xiao, L.; Zhao, Y.; Wang, F. Hydrothermal synthesis and electrochemical characterization of α-MnO2 nanorods as cathode material for lithium batteries. Int. J. Electrochem. Sci. 2008, 3, 67–74. [Google Scholar]
- Hill, L.I.; Verbaere, A.; Guyomard, D. MnO2 (α-, β-, γ-) compounds prepared by hydrothermal-electrochemical synthesis: Characterization, morphology, and lithium insertion behavior. J. Power Sources 2003, 119–121, 226–231. [Google Scholar] [CrossRef]
- Wang, C.; Hong, J. Ionic/electronic conducting characteristics of LiFePO4 cathode materials. The determining factors for high rate performance. Electrochem. Solid-State Lett. 2007, 10, A65–A69. [Google Scholar] [CrossRef]
- Zaghib, K.; Mauger, A.; Gendron, F.; Julien, C.M. Surface effects on the physical and electrochemical properties of thin LiFePO4 particles. Chem. Mater. 2008, 20, 462–469. [Google Scholar] [CrossRef]
- Mauger, A.; Zaghib, K.; Groult, H.; Julien, C.M. Surface and bulk properties of LiFePO4: The magnetic analysis. ECS Trans. 2013, 50, 115–123. [Google Scholar] [CrossRef]
- Aurbach, D.; Gamolsky, K.; Markovsky, B.; Salitra, G.; Gofer, Y.; Heider, U.; Oesten, R.; Schmidt, M. The study of surface phenomena related to electrochemical lithium intercalation into LixMOy host materials (M = Ni, Mn). J. Electrochem. Soc. 2000, 147, 1322–1331. [Google Scholar] [CrossRef]
- Hashem, A.M.A.; Abdel-Ghany, A.E.; Eid, A.E.; Trottier, J.; Zaghib, K.; Mauger, A.; Julien, C.M. Study of the surface modification of LiNi1/3Co1/3Mn1/3O2 cathode material for lithium ion battery. J. Power Sources 2011, 196, 8632–8637. [Google Scholar] [CrossRef]
- Amalraj, S.F.; Sharon, D.; Talianker, M.; Julien, C.M.; Burlaka, L.; Lavi, R.; Zhecheva, E.; Markovsky, B.; Zinigrad, E.; Kovacheva, D.; et al. Study of the nanosized Li2MnO3: Electrochemical behavior, structure, magnetic properties, and vibrational modes. Electrochim. Acta 2013, 97, 259–270. [Google Scholar] [CrossRef]
- Kalyani, P.; Chitra, S.; Mohan, T.; Gopukumar, S. Lithium metal rechargeable cells using Li2MnO3 as the positive electrode. J. Power Sources 1999, 80, 103–106. [Google Scholar] [CrossRef]




© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, X.; Porras-Gutierrez, A.-G.; Mauger, A.; Groult, H.; Julien, C.M. Nanotechnology of Positive Electrodes for Li-Ion Batteries. Inorganics 2017, 5, 25. https://doi.org/10.3390/inorganics5020025
Zhang X, Porras-Gutierrez A-G, Mauger A, Groult H, Julien CM. Nanotechnology of Positive Electrodes for Li-Ion Batteries. Inorganics. 2017; 5(2):25. https://doi.org/10.3390/inorganics5020025
Chicago/Turabian StyleZhang, Xiaoyu, Ana-Gabriela Porras-Gutierrez, Alain Mauger, Henri Groult, and Christian M. Julien. 2017. "Nanotechnology of Positive Electrodes for Li-Ion Batteries" Inorganics 5, no. 2: 25. https://doi.org/10.3390/inorganics5020025