Halogen Substituent Effect on the Spin-Transition Temperature in Spin-Crossover Fe(III) Compounds Bearing Salicylaldehyde 2-Pyridyl Hydrazone-Type Ligands and Dicarboxylic Acids
Abstract
:1. Introduction
2. Results and Discussion
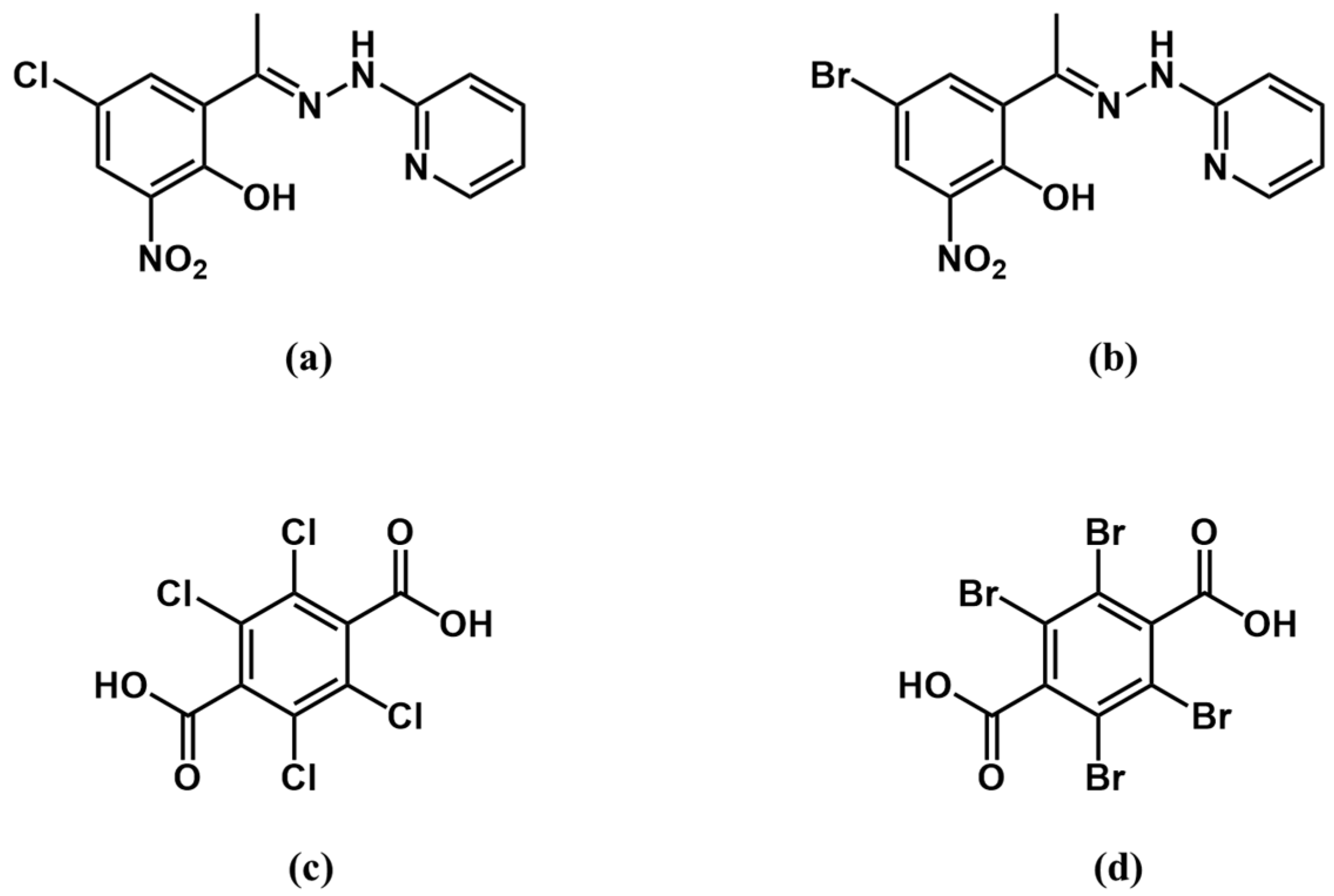
2.1. Synthesis and Characterization
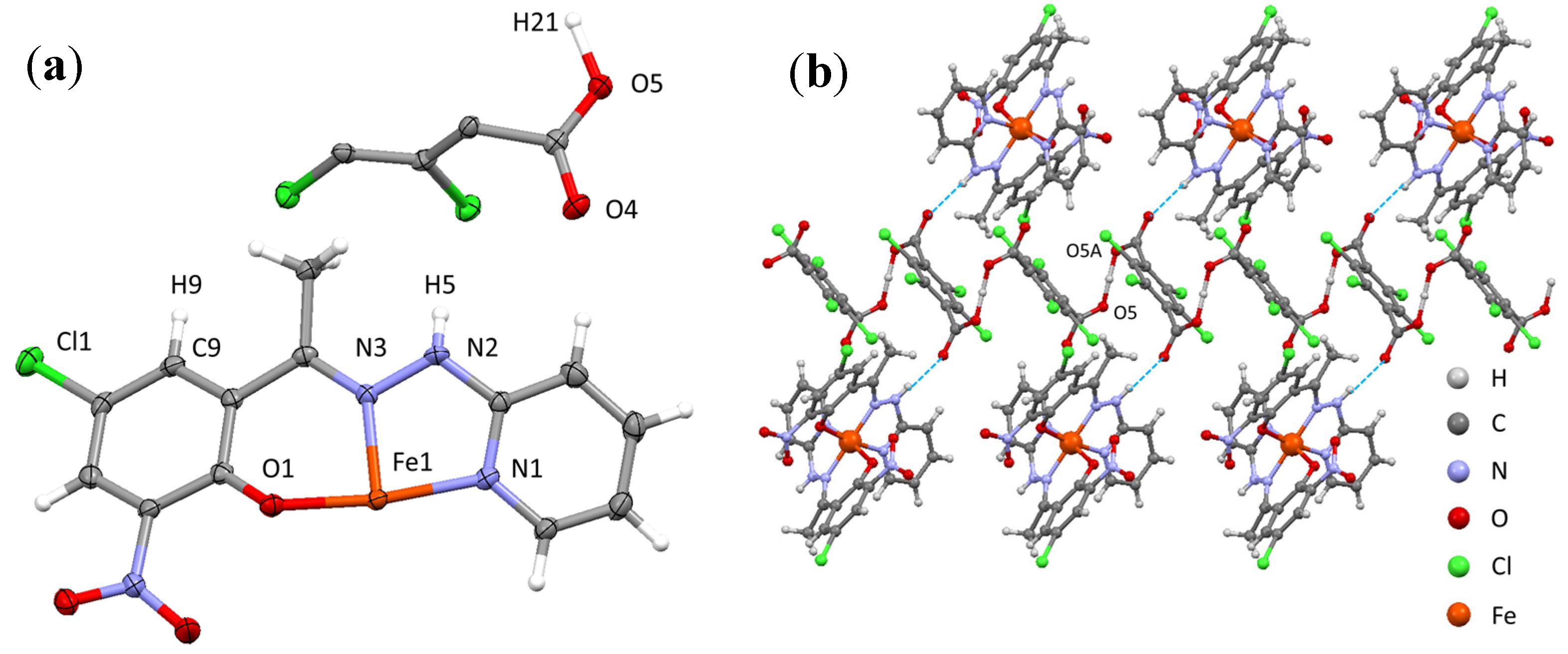
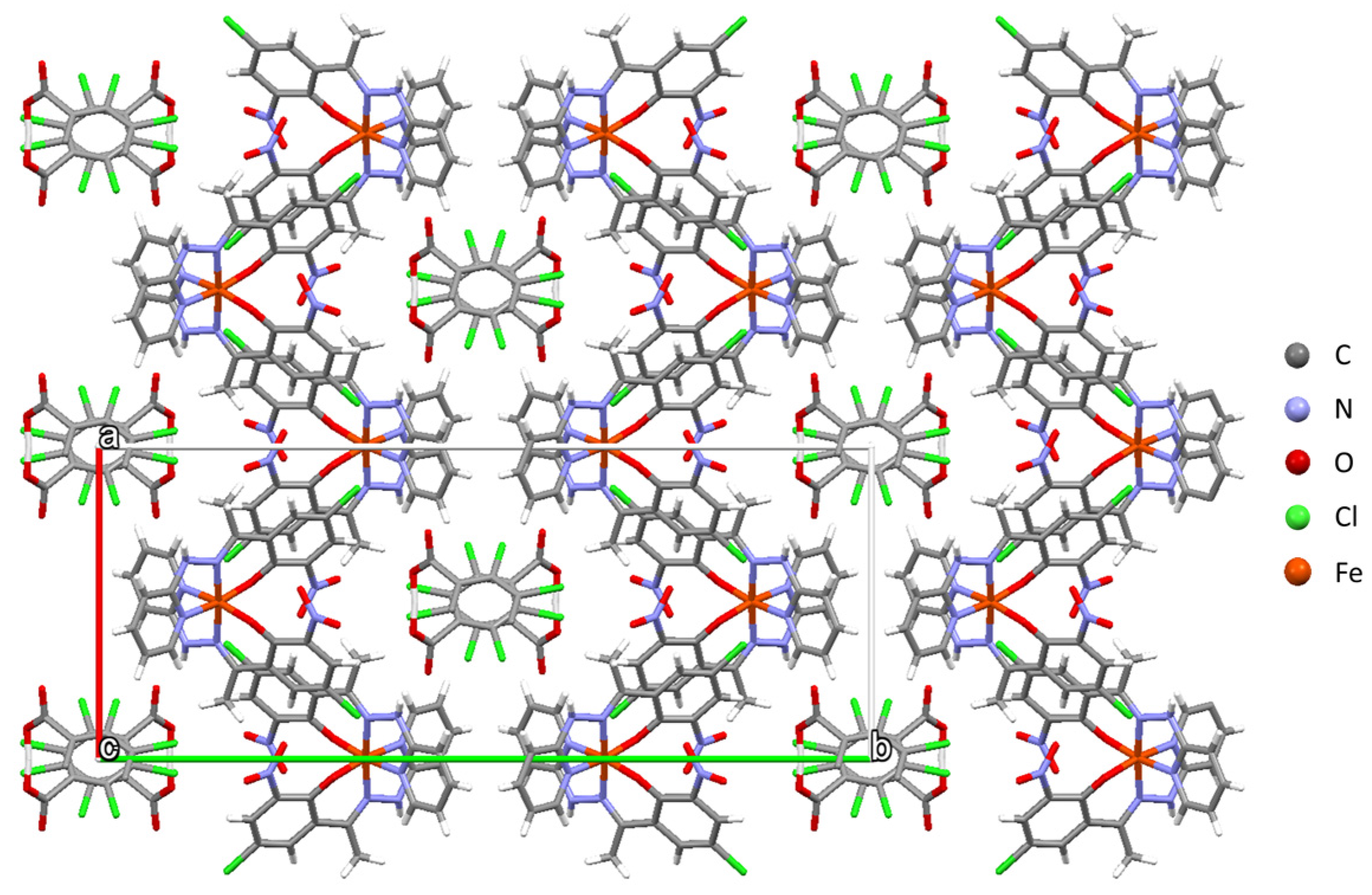
2.2. Crystal Structure
2.3. Mössbauer Spectroscopy
2.4. Magnetic Property
3. Materials and Methods
3.1. Compound Synthesis
3.1.1. Synthesis of Ligand HL1
3.1.2. Synthesis of Ligand HL2
3.1.3. Synthesis of 1-Cl
3.1.4. Synthesis of 1-Br
3.1.5. Synthesis of 2-Cl
3.1.6. Synthesis of 2-Br
3.2. X-ray Structure Determination
3.3. 57Fe Mössbauer Spectroscopy
3.4. Magnetic Property Measurements
4. Conclusions
Supplementary Materials
Author Contributions
Conflicts of Interest
References
- Gülich, P.; Hauser, A. Thermal and light-induced spin crossover in iron(II) complexes. Coord. Chem. Rev. 1990, 97, 1–22. [Google Scholar] [CrossRef]
- Sato, O. Optically switchable molecular solids: Photoinduced spin-crossover, photochromism, and photoinduced magnetization. Acc. Chem. Res. 2003, 36, 692–700. [Google Scholar] [CrossRef] [PubMed]
- Real, J.A.; Gaspar, A.B.; Munoz, M.C. Thermal, pressure and light switchable spin-crossover materials. Dalton Trans. 2005, 2062–2079. [Google Scholar] [CrossRef] [PubMed]
- Kershaw Cook, L.J.; Kulmaczewski, R.; Mohammed, R.; Dudley, S.; Barrett, S.A.; Little, M.A.; Deeth, R.J.; Halcrow, M.A. A unified treatment of the relationship between ligand substituents and spin state in a family of iron(II) complexes. Angew. Chem. Int. Ed. 2016, 55, 4327–4331. [Google Scholar] [CrossRef] [PubMed]
- Halcrow, M.A. The effect of ligand design on metal ion spin state-lessons from spin crossover complexes. Crystals 2016, 6, 20. [Google Scholar] [CrossRef]
- Sato, O. Dynamic molecular crystals with switchable physical properties. Nat. Chem. 2016, 8, 644–656. [Google Scholar] [CrossRef] [PubMed]
- Lemercier, G.; Brefuel, N.; Shova, S.; Wolny, J.A.; Dahan, F.; Verelst, M.; Paulsen, H.; Trautwein, A.X.; Tuchagues, J.P. A range of spin-crossover temperature T1/2 > 300 k results from out-of-sphere anion exchange in a series of ferrous materials based on the 4-(4-imidazolylmethyl)-2-(2-imidazolylmethyl)imidazole (trim) ligand, [Fe(trim)2]X2 (X=F, Cl, Br, I): Comparison of experimental results with those derived from density functional theory calculations. Chem. Eur. J. 2006, 12, 7421–7432. [Google Scholar] [PubMed]
- Paulsen, H.; Duelund, L.; Zimmermann, A.; Averseng, F.; Gerdan, M.; Winkler, H.; Toftlund, H.; Trautwein, A.X. Substituent effects on the spin-transition temperature in complexes with tris(pyrazolyl) ligands. Mon. Chem. 2003, 134, 295–306. [Google Scholar] [CrossRef]
- Wei, R.-J.; Tao, J.; Huang, R.-B.; Zheng, L.-S. Anion-dependent spin crossover and coordination assembly based on [Fe(tpa)]2+[tpa = tris(2-pyridylmethyl)amine] and [N(CN)2]−: Square, zigzag, dimeric, and [4 + 1]-cocrystallized complexes. Eur. J. Inorg. Chem. 2013, 2013, 916–926. [Google Scholar] [CrossRef]
- Zhang, W.; Zhao, F.; Liu, T.; Yuan, M.; Wang, Z.M.; Gao, S. Spin crossover in a series of iron(II) complexes of 2-(2-Alkyl-2H-tetrazol-5-yl)-1,10-phenanthroline: Effects of alkyl side chain, solvent, and anion. Inorg. Chem. 2007, 46, 2541–2555. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.Y.; Li, H.Q.; Ding, Z.Y.; Lu, X.J.; Zhao, L.; Meng, Y.S.; Liu, T.; Gao, S. Spin transitions in a series of [Fe(pybox)2]2+ complexes modulated by ligand structures, counter anions, and solvents. Inorg. Chem. Front. 2016, 3, 1624–1636. [Google Scholar] [CrossRef]
- Ivanova, T.A.; Ovchinnikov, I.V.; Turanov, A.N. Influence of the outersphere anion on the properties of the spin transition in Fe(4-OCH3-SalEen)2 Y (Y = PF6, NO3). Phys. Solid State 2007, 49, 2132–2137. [Google Scholar] [CrossRef]
- Nemec, I.; Herchel, R.; Travnicek, Z. The relationship between the strength of hydrogen bonding and spin crossover behaviour in a series of iron(III) Schiff base complexes. Dalton Trans. 2015, 44, 4474–4484. [Google Scholar] [CrossRef] [PubMed]
- Phonsri, W.; Harding, D.J.; Harding, P.; Murray, K.S.; Moubaraki, B.; Gass, I.A.; Cashion, J.D.; Jameson, G.N.; Adams, H. Stepped spin crossover in Fe(III) halogen substituted quinolylsalicylaldimine complexes. Dalton Trans. 2014, 43, 17509–17518. [Google Scholar] [CrossRef] [PubMed]
- Phonsri, W.; Macedo, D.S.; Vignesh, K.R.; Rajaraman, G.; Davies, C.G.; Jameson, G.N.L.; Moubaraki, B.; Ward, J.S.; Kruger, P.E.; Chastanet, G.; et al. Halogen substitution effects on N2O Schiff base ligands in unprecedented abrupt FeII spin crossover complexes. Chem. Eur. J. 2017, 23, 7052–7065. [Google Scholar] [CrossRef] [PubMed]
- Ueno, T.; Miyano, K.; Hamada, D.; Ono, H.; Fujinami, T.; Matsumoto, N.; Sunatsuki, Y. Abrupt spin transition and chiral hydrogen-bonded one-dimensional structure of iron(III) complex [FeIII(Him)2(hapen)]SbF6 (Him = imidazole, H2hapen = N,N′-bis(2-hydroxyacetophenylidene)ethylenediamine). Magnetochemistry 2015, 1, 72. [Google Scholar] [CrossRef]
- Nakanishi, T.; Sato, O. Synthesis, structure, and magnetic properties of new spin crossover Fe(II) complexes forming short hydrogen bonds with substituted dicarboxylic acids. Crystals 2016, 6, 8. [Google Scholar] [CrossRef]
- Koningsbruggen, P.J.; Maeda, Y.; Oshio, H. Iron(III) spin crossover compounds. Spin Crossover Transit. Metal Compd. I 2004, 233, 259–324. [Google Scholar]
- Nihei, M.; Shiga, T.; Maeda, Y.; Oshio, H. Spin crossover iron(III) complexes. Coord. Chem. Rev. 2007, 251, 2606–2621. [Google Scholar] [CrossRef]
- Hayami, S.; Gu, Z.; Yoshiki, H.; Fujishima, A.; Sato, O. Iron(III) spin-crossover compounds with a wide apparent thermal hysteresis around room temperature. J. Am. Chem. Soc. 2001, 123, 11644–11650. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.Y.; Dai, J.W.; Shiota, Y.; Yoshizawa, K.; Kanegawa, S.; Sato, O. Multi-step spin crossover accompanied by symmetry breaking in an Fe(III) complex: Crystallographic evidence and DFT studies. Chem. Eur. J. 2013, 19, 12948–12952. [Google Scholar] [CrossRef] [PubMed]
- Phonsri, W.; Davies, C.G.; Jameson, G.N.; Moubaraki, B.; Murray, K.S. Spin crossover, polymorphism and porosity to liquid solvent in heteroleptic iron(III) {quinolylsalicylaldimine/thiosemicarbazone-salicylaldimine} complexes. Chem. Eur. J. 2016, 22, 1322–1333. [Google Scholar] [CrossRef] [PubMed]
- Fujinami, T.; Ikeda, M.; Koike, M.; Matsumoto, N.; Oishi, T.; Sunatsuki, Y. Syntheses, hydrogen-bonded assembly structures, and spin crossover properties of [FeIII (Him)2(n-MeOhapen)]PF6 (Him = imidazole and n-MeOhapen = N,N′-bis(n-methoxy-2-oxyacetophenylidene)ethylenediamine); n = 4, 5, 6). Inorg. Chim. Acta 2015, 432, 89–95. [Google Scholar] [CrossRef]
- Tang, J.; Sanchez Costa, J.; Smulders, S.; Molnar, G.; Bousseksou, A.; Teat, S.J.; Li, Y.; van Albada, G.A.; Gamez, P.; Reedijk, J. Two-step spin-transition iron(III) compound with a wide [high spin-low spin] plateau. Inorg. Chem. 2009, 48, 2128–2135. [Google Scholar] [CrossRef] [PubMed]
- Anderson, R.G.; Nickless, G. Co-ordinating properties of some ligand systems related to 4-(2-pyridylazo)resorcinol. Talanta 1967, 14, 1221–1228. [Google Scholar] [CrossRef]
- Basu, C.; Chowdhury, S.; Banerjee, R.; Evans, H.S.; Mukherjee, S. A novel blue luminescent high-spin iron(III) complex with interlayer O–HCl bridging: Synthesis, structure and spectroscopic studies. Polyhedron 2007, 26, 3617–3624. [Google Scholar] [CrossRef]
- Liu, H.Y.; Gao, F.; Niu, D.Z. Synthesis and structure of iron(III) complex with N,N,O-donor aroylhydrazones: The chloride anion as hydrogen bond acceptor forming infinite chains. Asian J. Chem. 2011, 23, 2014–2016. [Google Scholar]
- Zhang, J.; Campolo, D.; Dumur, F.; Xiao, P.; Fouassier, J.P.; Gigmes, D.; Lalevee, J. Iron complexes as photoinitiators for radical and cationic polymerization through photoredox catalysis processes. J. Polym. Sci. Pol. Chem. 2015, 53, 42–49. [Google Scholar] [CrossRef]
- Mohan, M.; Gupta, N.S.; Chandra, L.; Jha, N.K. Synthesis, characterization and antitumor properties of some metal–complexes of 3-substituted and 5-substituted salicylaldehyde 2-pyridinylhydrazones. J. Inorg. Biochem. 1987, 31, 7–27. [Google Scholar] [CrossRef]
- Dega-Szafran, Z.; Katrusiak, A.; Szafran, M. Short and symmetrical OHO hydrogen bond in bis(quinuclidine betaine) hydrochloride. J. Mol. Struct. 2010, 971, 1–7. [Google Scholar] [CrossRef]
- Tweedle, M.F.; Wilson, L.J. Variable spin iron(III) chelates with hexadentate ligands derived from triethylenetetramine and various salicylaldehydes—Synthesis, characterization, and solution state studies of a new 2T reversible 6A spin equilibrium system. J. Am. Chem. Soc. 1976, 98, 4824–4834. [Google Scholar] [CrossRef]
- Klencsár, Z. MossWinn Manual. 2016. Available online: http://www.mosswinn.hu/downloads/mosswinn.pdf (accessed on 26 April 2017).


| Crystallographic Data | |||||
|---|---|---|---|---|---|
| Compound | 1-Cl | 1-Br | 2-Cl | 2-Br | |
| Formula | C34H21Cl6FeN8O10 | C34H21Br4Cl2FeN8O10 | C34H21Br2Cl4FeN8O10 | C34H21Br6FeN8O10 | |
| Formula weight | 970.15 | 1147.96 | 1059.05 | 1236.86 | |
| Crystal system | Monoclinic | Monoclinic | Monoclinic | Monoclinic | |
| Space group | C2/c | C2/c | C2/c | C2/c | |
| T (K) | 123 | 330 | 123 | 123 | 123 |
| a (Å) | 12.479(5) | 12.665(3) | 12.595(3) | 12.627(4) | 12.683(7) |
| b (Å) | 30.593(11) | 31.287(6) | 30.851(7) | 30.551(9) | 30.984(14) |
| c (Å) | 9.469(3) | 9.569(2) | 9.607(2) | 9.519(3) | 9.633(5) |
| β (°) | 96.795(8) | 97.326(6) | 95.945(5) | 96.239(7) | 95.330(11) |
| V (Å3) | 3590(2) | 3760.4(13) | 3713.2(16) | 3650.6(19) | 3769.(3) |
| Z | 4 | 4 | 4 | 4 | 4 |
| Dcal (g/cm3) | 1.795 | 1.713 | 2.053 | 1.927 | 2.179 |
| F (000) | 1956.00 | 1956.00 | 2244.00 | 2100.00 | 2388.00 |
| Data collected | 15,405 | 16,406 | 16,626 | 16,224 | 17,268 |
| Unique data | 4069 | 4272 | 4242 | 4197 | 4309 |
| R(int) | 0.0699 | 0.0783 | 0.0699 | 0.0989 | 0.1157 |
| GOF on F2 | 1.115 | 1.104 | 1.130 | 1.106 | 1.116 |
| RI [I > 2σ(I)] | 0.0581 | 0.0659 | 0.0518 | 0.0636 | 0.0579 |
| Bond lengths Around Fe(III) (Å) | |||||
| Compound | 1-Cl | 1-Br | 2-Cl | 2-Br | |
| Temperature | 123 K | 330 K | 123 K | 123 K | 123 K |
| Fe1–N1 | 1.975(3) | 2.098(3) | 1.976(4) | 1.966(4) | 1.980(4) |
| Fe1–N3 | 1.925(3) | 2.112(3) | 1.926(4) | 1.923(4) | 1.929(4) |
| Fe1–O1 | 1.857(2) | 1.882(3) | 1.857(3) | 1.853(3) | 1.862(4) |
| Hydrogen Bond Distances (Å) | |||||
| O5···O5A | 2.458(3) | 2.464(5) | 2.463(4) | 2.464(5) | 2.458(6) |
| O5–H21 | 1.233(5) | 1.232(7) | 1.236(13) | 1.235(9) | 1.239(12) |
| N2···O4 | 2.892(4) | 2.890(5) | 2.892(5) | 2.886(5) | 2.872(6) |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nakanishi, T.; Okazawa, A.; Sato, O. Halogen Substituent Effect on the Spin-Transition Temperature in Spin-Crossover Fe(III) Compounds Bearing Salicylaldehyde 2-Pyridyl Hydrazone-Type Ligands and Dicarboxylic Acids. Inorganics 2017, 5, 53. https://doi.org/10.3390/inorganics5030053
Nakanishi T, Okazawa A, Sato O. Halogen Substituent Effect on the Spin-Transition Temperature in Spin-Crossover Fe(III) Compounds Bearing Salicylaldehyde 2-Pyridyl Hydrazone-Type Ligands and Dicarboxylic Acids. Inorganics. 2017; 5(3):53. https://doi.org/10.3390/inorganics5030053
Chicago/Turabian StyleNakanishi, Takumi, Atsushi Okazawa, and Osamu Sato. 2017. "Halogen Substituent Effect on the Spin-Transition Temperature in Spin-Crossover Fe(III) Compounds Bearing Salicylaldehyde 2-Pyridyl Hydrazone-Type Ligands and Dicarboxylic Acids" Inorganics 5, no. 3: 53. https://doi.org/10.3390/inorganics5030053





