Modification of Cooperativity and Critical Temperatures on a Hofmann-Like Template Structure by Modular Substituent
Abstract
:1. Introduction
2. Results
2.1. X-ray Structural Analysis
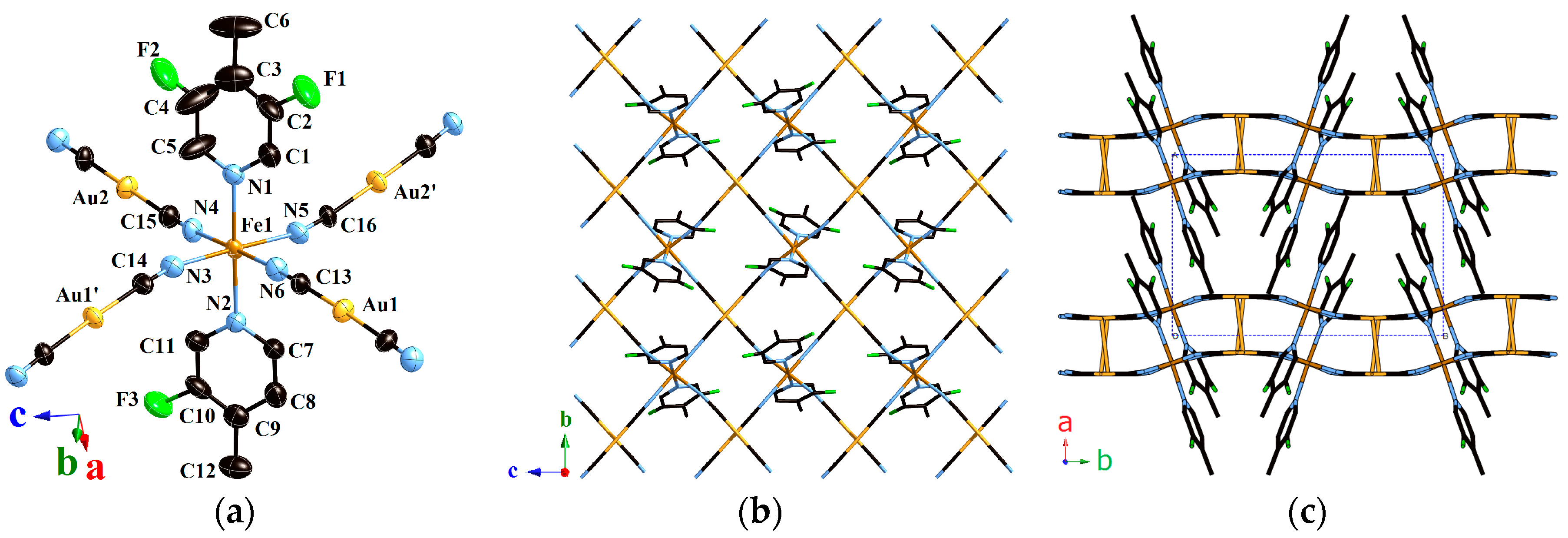
2.1.1. Structure of Compound 1 (T = 293 K)
2.1.2. Structure of Compound 1 (T = 180 K)
2.1.3. Structure of Compound 1 (T = 90 K)
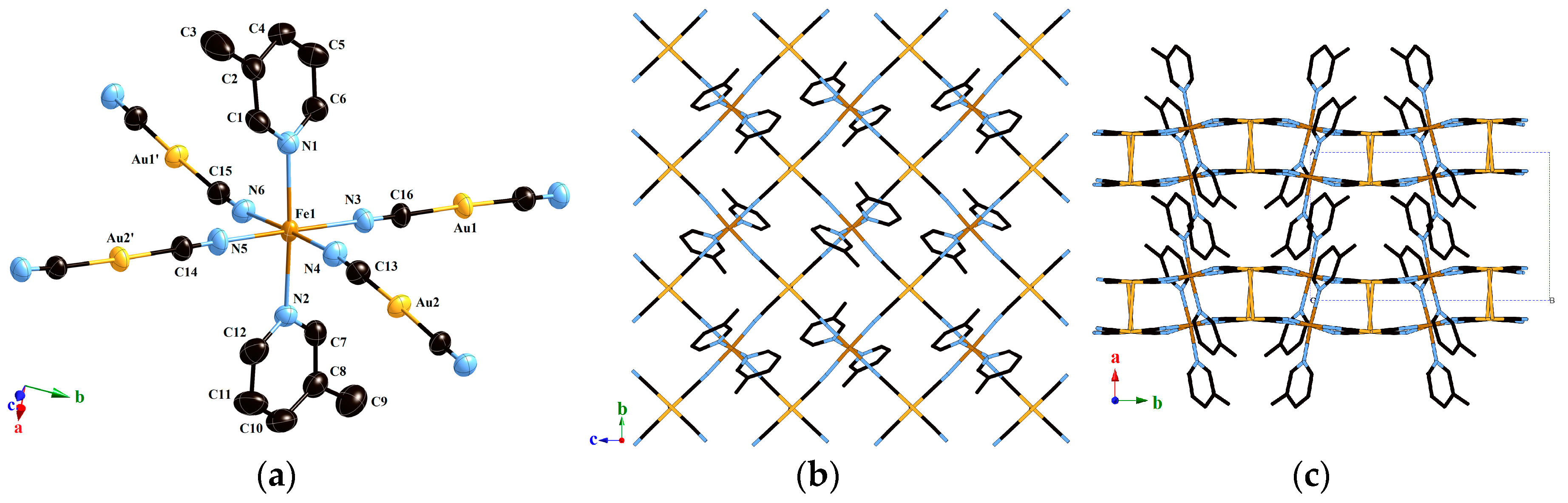
2.1.4. Structure of Compound 2 (T = 293 K)
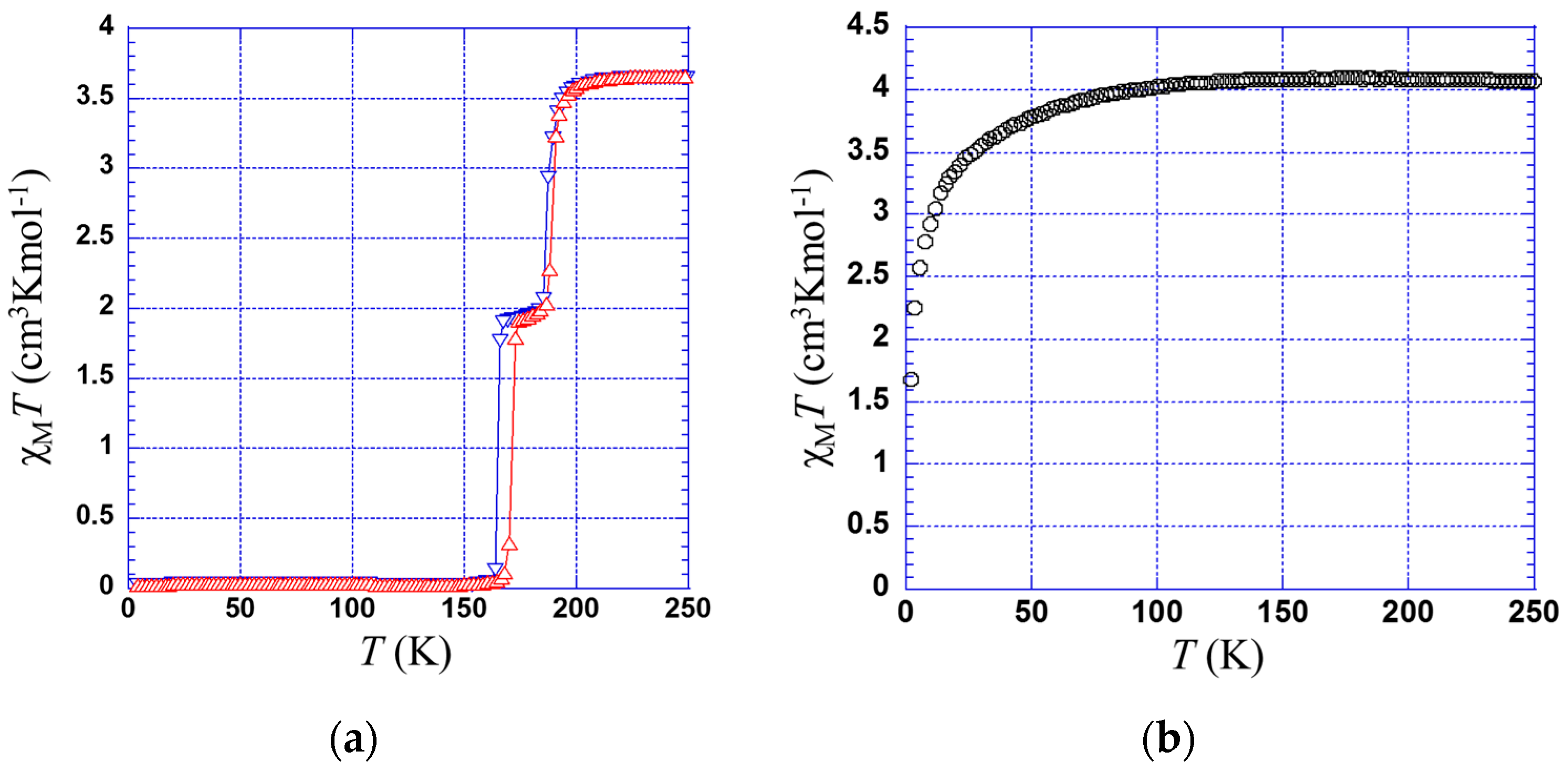
2.2. Magnetic Properties
2.2.1. Thermal Dependence Magnetic Behavior of Compound 1
2.2.2. Thermal Dependence Magnetic Behavior of Compound 2
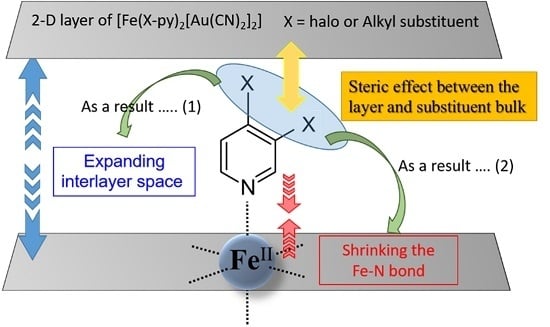
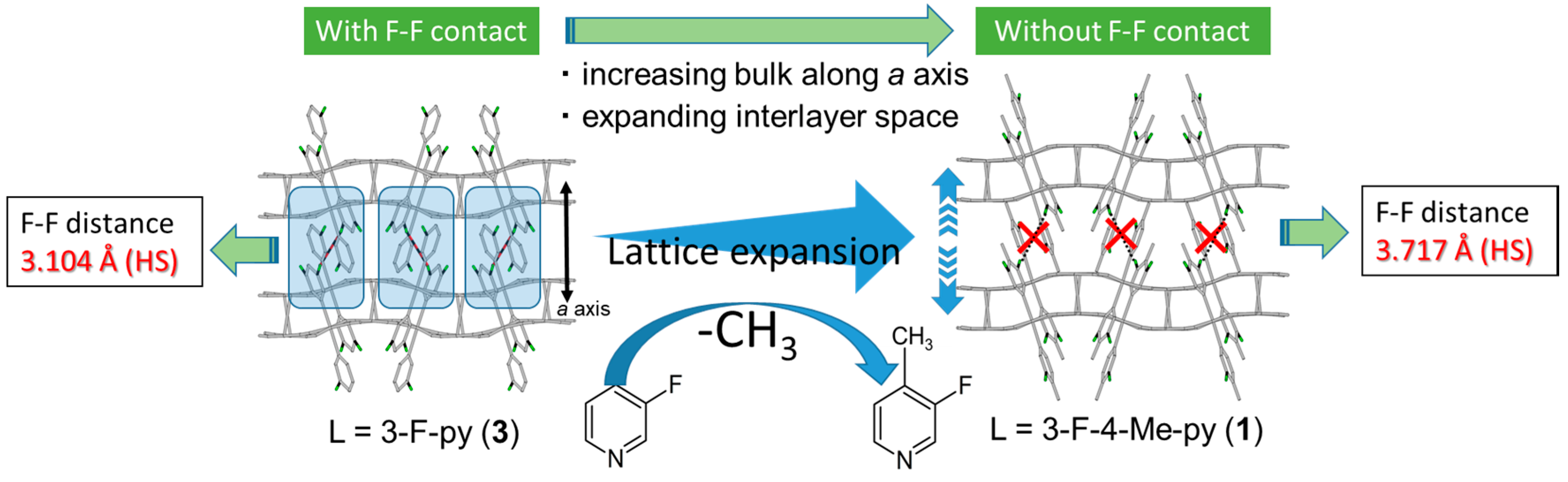
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Preparation of Compounds 1 and 2
4.3. Magnetic Measurements
4.4. X-ray Crystallography
5. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Real, J.A.; Andrés, E.; Munoz, M.C.; Julve, M.; Granier, T.; Bousseksou, A.; Varret, F. Spin crossover in a catenane supramolecular system. Science 1995, 268, 265–268. [Google Scholar] [CrossRef] [PubMed]
- Gütlich, P.; Garcia, Y.; Goodwin, H.A. Spin crossover phenomena in Fe(II) complexes. Chem. Soc. Rev. 2000, 29, 419–427. [Google Scholar] [CrossRef]
- Kitazawa, T.; Gomi, Y.; Takahashi, M.; Takeda, M.; Enomoto, M.; Miyazaki, A.; Enoki, T. Spin-crossover behaviour of the coordination polymer FeII(C5H5N)2NiII(CN)4. J. Mater. Chem. 1996, 6, 119–121. [Google Scholar] [CrossRef]
- Niel, V.; Martinez-Agudo, J.M.; Muñoz, M.C.; Gaspar, A.B.; Real, J.A. Cooperative Spin Crossover Behavior in Cyanide-Bridged Fe(II)–M(II) Bimetallic 3D Hofmann-like Networks (M = Ni, Pd, and Pt). Inorg. Chem. 2001, 40, 3838–3839. [Google Scholar] [CrossRef] [PubMed]
- Agusti, G.; Cobo, S.; Gaspar, A.B.; Molnár, G.; Moussa, N.O.; Szilágyi, P.Á.; Pálfi, V.; Vieu, C.; Carmen Muñoz, M.; Real, J.A.; et al. Thermal and light-induced spin crossover phenomena in new 3D Hofmann-like microporous metalorganic frameworks produced as bulk materials and nanopatterned thin films. Chem. Mater. 2008, 20, 6721–6732. [Google Scholar] [CrossRef]
- Martínez, V.; Gaspar, A.B.; Muñoz, M.C.; Bukin, G.V.; Levchenko, G.; Real, J.A. Synthesis and Characterisation of a New Series of Bistable Iron(II) Spin-Crossover 2D Metal–Organic Frameworks. Chem. Eur. J. 2009, 15, 10960–10971. [Google Scholar] [CrossRef] [PubMed]
- Bartual-Murgui, C.; Ortega-Villar, N.A.; Shepherd, H.J.; Muñoz, M.C.; Salmon, L.; Molnár, G.; Bousseksou, A.; Real, J.A. Enhanced porosity in a new 3D Hofmann-like network exhibiting humidity sensitive cooperative spin transitions at room temperature. J. Mater. Chem. 2011, 21, 7217. [Google Scholar] [CrossRef]
- Ohtani, R.; Arai, M.; Hori, A.; Takata, M.; Kitao, S.; Seto, M.; Kitagawa, S.; Ohba, M. Modulation of Spin-Crossover Behavior in an Elongated and Flexible Hofmann-Type Porous Coordination Polymer. J. Inorg. Organomet. Polym. Mater. 2013, 23, 104–110. [Google Scholar] [CrossRef]
- Sciortino, N.F.; Zenere, K.A.; Corrigan, M.E.; Halder, G.J.; Chastanet, G.; Létard, J.-F.; Kepert, C.J.; Neville, S.M. Four-step iron(ii) spin state cascade driven by antagonistic solid state interactions. Chem. Sci. 2017, 8, 701–707. [Google Scholar] [CrossRef] [PubMed]
- Galet, A.; Muñoz, M.C.; Martinez, V.; Real, J.A. Supramolecular isomerism in spin crossover networks with aurophilic interactions. Chem. Commun. 2004, 2268–2269. [Google Scholar] [CrossRef] [PubMed]
- Muñoz, M.C.; Gaspar, A.B.; Galet, A.; Real, J.A. Spin-Crossover Behavior in Cyanide-Bridged Iron(II)–Silver(I) Bimetallic 2D Hofmann-like Metal–Organic Frameworks. Inorg. Chem. 2007, 46, 8182–8192. [Google Scholar] [CrossRef] [PubMed]
- Agustí, G.; Muñoz, M.C.; Gaspar, A.B.; Real, J.A. Spin-Crossover Behavior in Cyanide-bridged Iron(II)–Gold(I) Bimetallic 2D Hofmann-like Metal–Organic Frameworks. Inorg. Chem. 2008, 47, 2552–2561. [Google Scholar] [CrossRef] [PubMed]
- Kosone, T.; Kachi-Terajima, C.; Kanadani, C.; Saito, T.; Kitazawa, T. A two-step and hysteretic spin-crossover transition in new cyano-bridged hetero-metal FeIIAuI 2-dimensional assemblage. Chem. Lett. 2008, 37, 422–423. [Google Scholar] [CrossRef]
- Kosone, T.; Kachi-Terajima, C.; Kanadani, C.; Saito, T.; Kitazawa, T. Isotope Effect on Spin-crossover Transition in a New Two-dimensional Coordination Polymer [FeII(C5H5N)2][AuI(CN)2]2,[FeII(C5D5N) 2][AuI(CN)2]2, and [FeII(C5H515N)2][AuI(CN)2]2. Chem. Lett. 2008, 37, 754–755. [Google Scholar] [CrossRef]
- Kosone, T.; Kanadani, C.; Saito, T.; Kitazawa, T. Synthesis, crystal structures, magnetic properties and fluorescent emissions of two-dimensional bimetallic coordination frameworks FeII(3-fluoropyridine)2[AuI(CN)2]2 and MnII(3-fluoropyridine)2[AuI(CN)2]2. Polyhedron 2009, 28, 1930–1934. [Google Scholar] [CrossRef]
- Kosone, T.; Kanadani, C.; Saito, T.; Kitazawa, T. Spin crossover behavior in two-dimensional bimetallic coordination polymer FeII(3-bromo-4-picoline)2[AuI(CN)2]2: Synthesis, crystal structures, and magnetic properties. Polyhedron 2009, 28, 1991–1995. [Google Scholar] [CrossRef]
- Kosone, T.; Tomori, I.; Kanadani, C.; Saito, T.; Mochida, T.; Kitazawa, T. Unprecedented three-step spin-crossover transition in new 2-dimensional coordination polymer {FeII(4-methylpyridine)2[AuI(CN)2]2}. Dalton Trans. 2010, 39, 1719–1721. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Velamazán, J.A.; Carbonera, C.; Castro, M.; Palacios, E.; Kitazawa, T.; Létard, J.-F.; Burriel, R. Two-Step Thermal Spin Transition and LIESST Relaxation of the Polymeric Spin-Crossover Compounds Fe(X-py)2[Ag(CN)2]2 (X=H, 3-methyl, 4-methyl, 3,4-dimethyl, 3-Cl). Chem. Eur. J. 2010, 16, 8785–8796. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kosone, T.; Kitazawa, T. Guest-dependent spin transition with long range intermediate state for 2-dimensional Hofmann-like coordination polymer. Inorg. Chim. Acta 2016, 439, 159–163. [Google Scholar] [CrossRef]
- Chiruta, D.; Linares, J.; Garcia, Y.; Dimian, M.; Dahoo, P.R. Analysis of multi-step transitions in spin crossover nanochains. Phys. B Condens. Matter 2014, 434, 134–138. [Google Scholar] [CrossRef]
- Okabayashi, J.; Ueno, S.; Kawasaki, T.; Kitazawa, T. Ligand 4-X pyridine (X = Cl, Br, I) dependence in Hofmann-type spin crossover complexes: Fe(4-Xpyridine)2[Au(CN)2]2. Inorg. Chim. Acta 2016, 445, 17–21. [Google Scholar] [CrossRef]
- Krzystek, J.; Ozarowski, A.; Telser, J. Multi-frequency, high-field EPR as a powerful tool to accurately determine zero-field splitting in high-spin transition metal coordination complexes. Coord. Chem. Rev. 2006, 250, 2308–2324. [Google Scholar] [CrossRef]
- Nakano, K.; Suemura, N.; Yoneda, K.; Kawata, S.; Kaizaki, S. Substituent effect of the coordinated pyridine in a series of pyrazolato bridged dinuclear diiron(II) complexes on the spin-crossover behavior. Dalton Trans. 2005, 740. [Google Scholar] [CrossRef] [PubMed]
- Sheldrick, G.M. SADABS, Program for Empirical Absorption Correction for Area Detector Data; University of Göttingen: Göttingen, Germany, 1996. [Google Scholar]
- Sheldrick, G.M. SHELXL, Program for the Solution of Crystal Structures; University of Göttingen: Göttingen, Germany, 1997. [Google Scholar]
- Sakaida, S.; Otsubo, K.; Sakata, O.; Song, C.; Fujiwara, A.; Takata, M.; Kitagawa, H. Crystalline coordination framework endowed with dynamic gate-opening behaviour by being downsized to a thin film. Nat. Chem. 2016, 8, 377–383. [Google Scholar] [CrossRef] [PubMed]
| Substituents | Cell Volume (Å3) | SCO Behavior Type | Critical Temperature Tc (K) | Ref. |
|---|---|---|---|---|
| 3-Methyl (2) | 1976.30 Å3 | None | None | − |
| 4-Methyl (HS) (4) | 2112.5 Å3 | Steep (3-step) | 210 K (first step) | [17] |
| 3-Fuluoro (HS) (3) | 1915.7 Å3 | Steep (2-step) | 147.9 K (first step) | [12,13,15] |
| 3-Fuluoro-4-Methyl (HS) (1) | 2155.2 Å3 | Steep (2-step) | 188.5 K (first step) | − |
| 3-Bromo (5) | 1990.9 Å3 | None | None | [12] |
| 3-Bromo-4-Methyl (HS) (6) | 2204.5 Å3 | Steep (1-step, 50% transition) | 109.1 K | [16] |
| Fe(X-py)2[Au(CN)2]2 | Hysteresis Width (K) | Closest Approach F···F Distances [Å] | Thermal Trapping After Cooling at 2 K/min |
|---|---|---|---|
| X = 3-F (3) | ca. 40 K | F(1)···F(1): 3.104 Å (HS) F(1)···F(1): 2.955 Å (HS–LS) F(3)···F(3): 3.264 Å (LS) | Observed (see SI) |
| X = 3-F-4-Me (1) | ca. 7 K | F(3)···F(3): 3.717 Å (HS) F(2)···F(3): 3.305 Å (LS) | None |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kosone, T.; Kawasaki, T.; Tomori, I.; Okabayashi, J.; Kitazawa, T. Modification of Cooperativity and Critical Temperatures on a Hofmann-Like Template Structure by Modular Substituent. Inorganics 2017, 5, 55. https://doi.org/10.3390/inorganics5030055
Kosone T, Kawasaki T, Tomori I, Okabayashi J, Kitazawa T. Modification of Cooperativity and Critical Temperatures on a Hofmann-Like Template Structure by Modular Substituent. Inorganics. 2017; 5(3):55. https://doi.org/10.3390/inorganics5030055
Chicago/Turabian StyleKosone, Takashi, Takeshi Kawasaki, Itaru Tomori, Jun Okabayashi, and Takafumi Kitazawa. 2017. "Modification of Cooperativity and Critical Temperatures on a Hofmann-Like Template Structure by Modular Substituent" Inorganics 5, no. 3: 55. https://doi.org/10.3390/inorganics5030055
APA StyleKosone, T., Kawasaki, T., Tomori, I., Okabayashi, J., & Kitazawa, T. (2017). Modification of Cooperativity and Critical Temperatures on a Hofmann-Like Template Structure by Modular Substituent. Inorganics, 5(3), 55. https://doi.org/10.3390/inorganics5030055