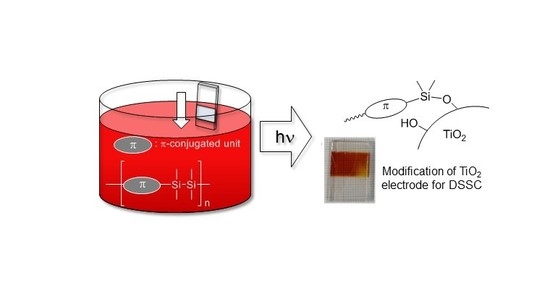
Modification of TiO2 Surface by Disilanylene Polymers and Application to Dye-Sensitized Solar Cells
Abstract
:1. Introduction
2. Results and Discussion
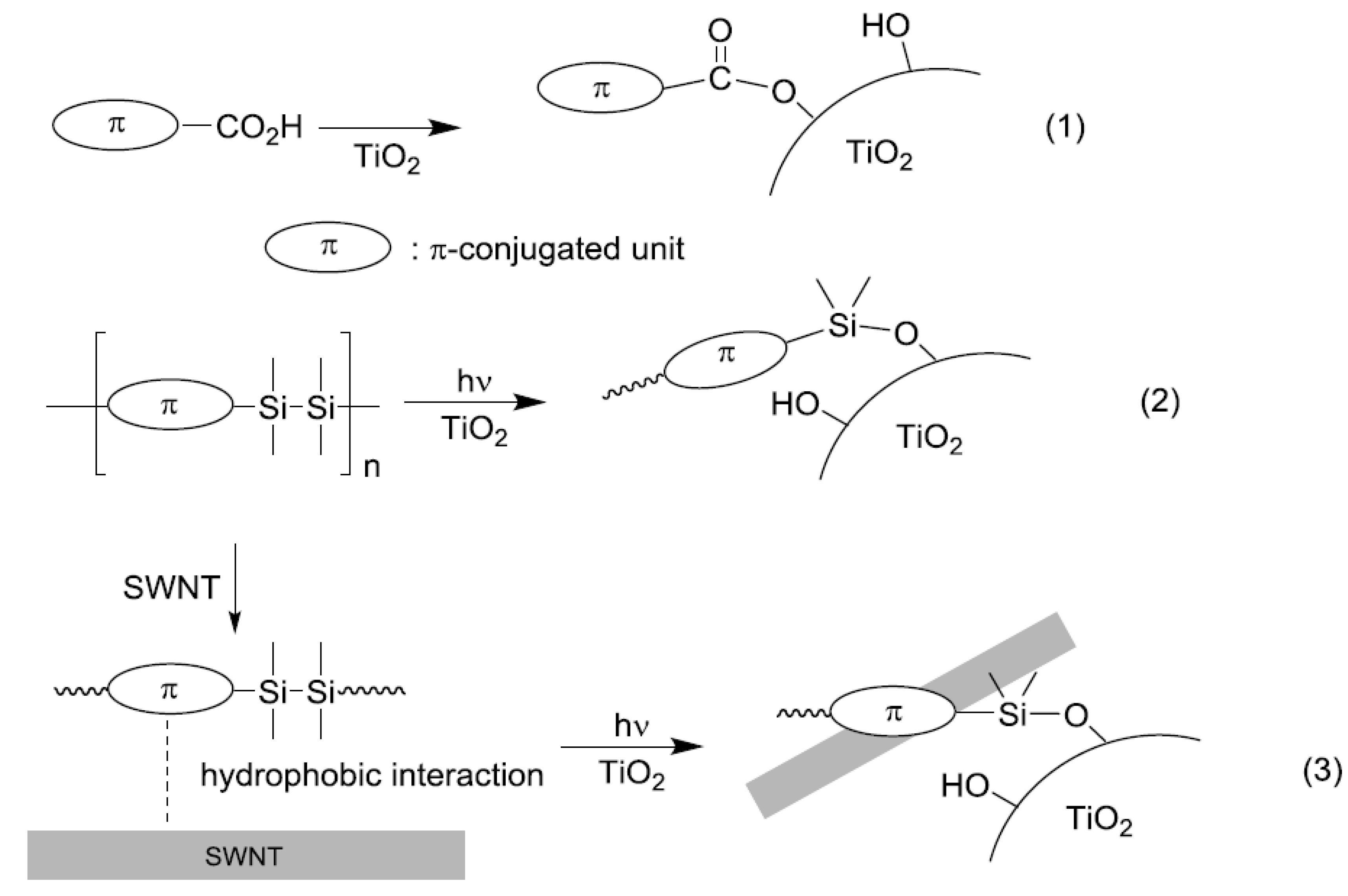
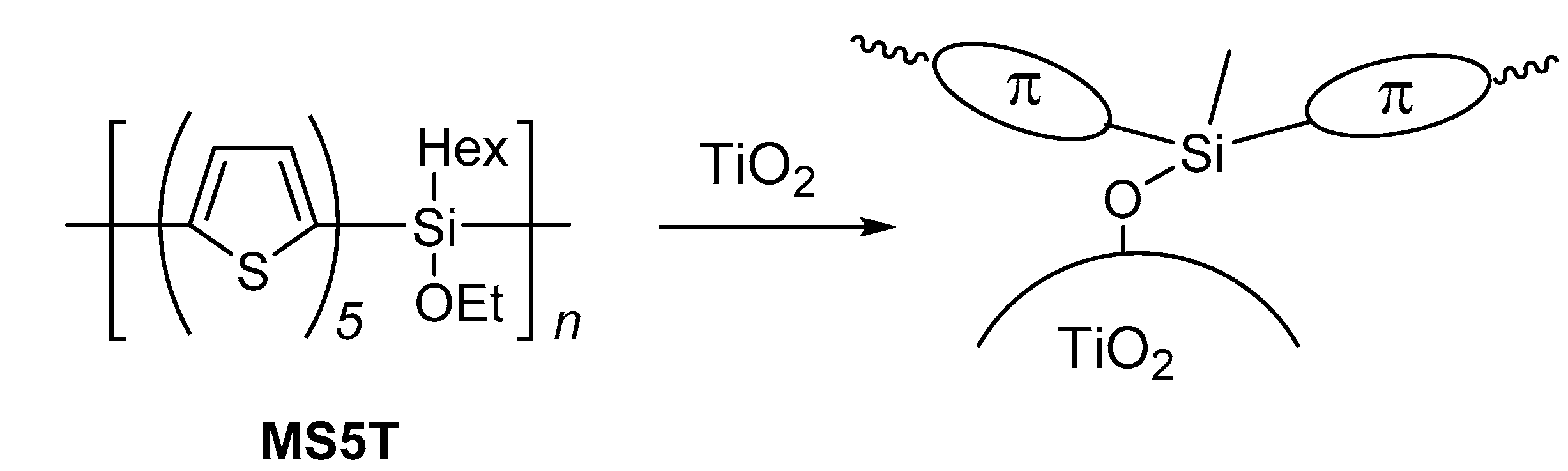
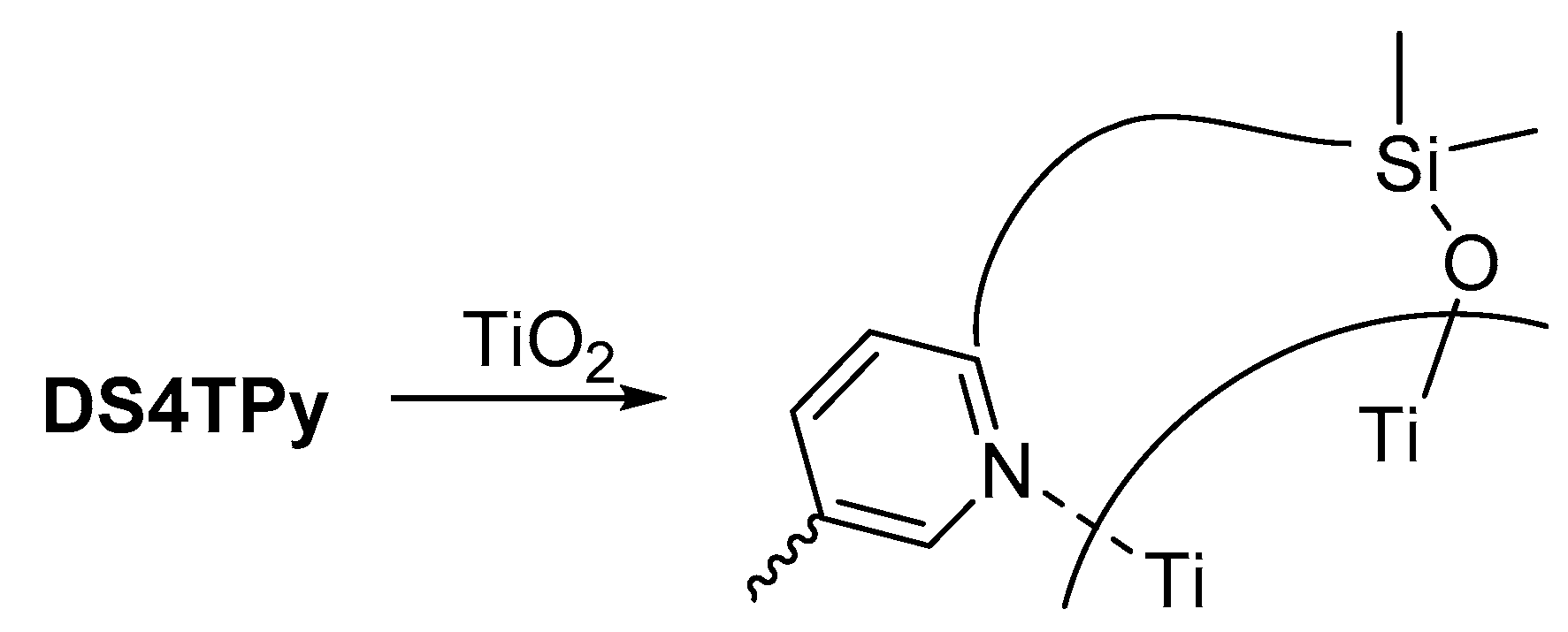
2.1. Photochemical Attachment of Si–Si–π Polymers to TiO2 Surface
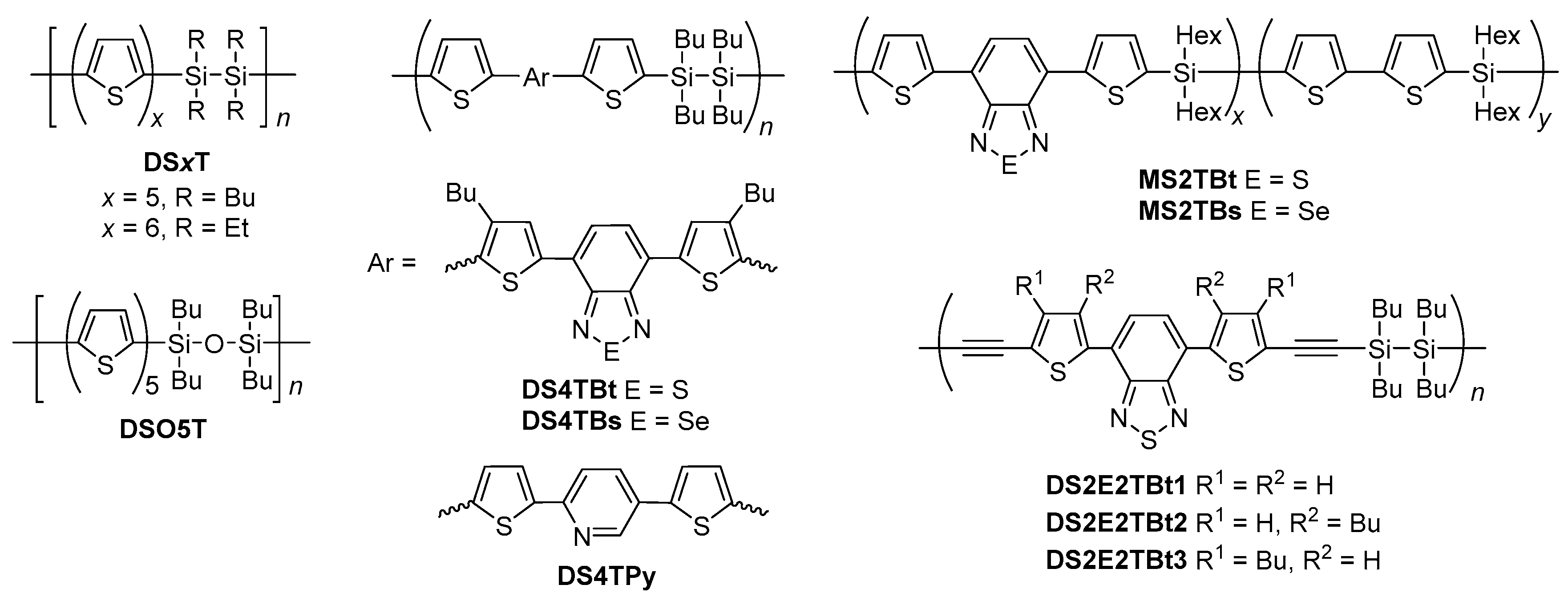
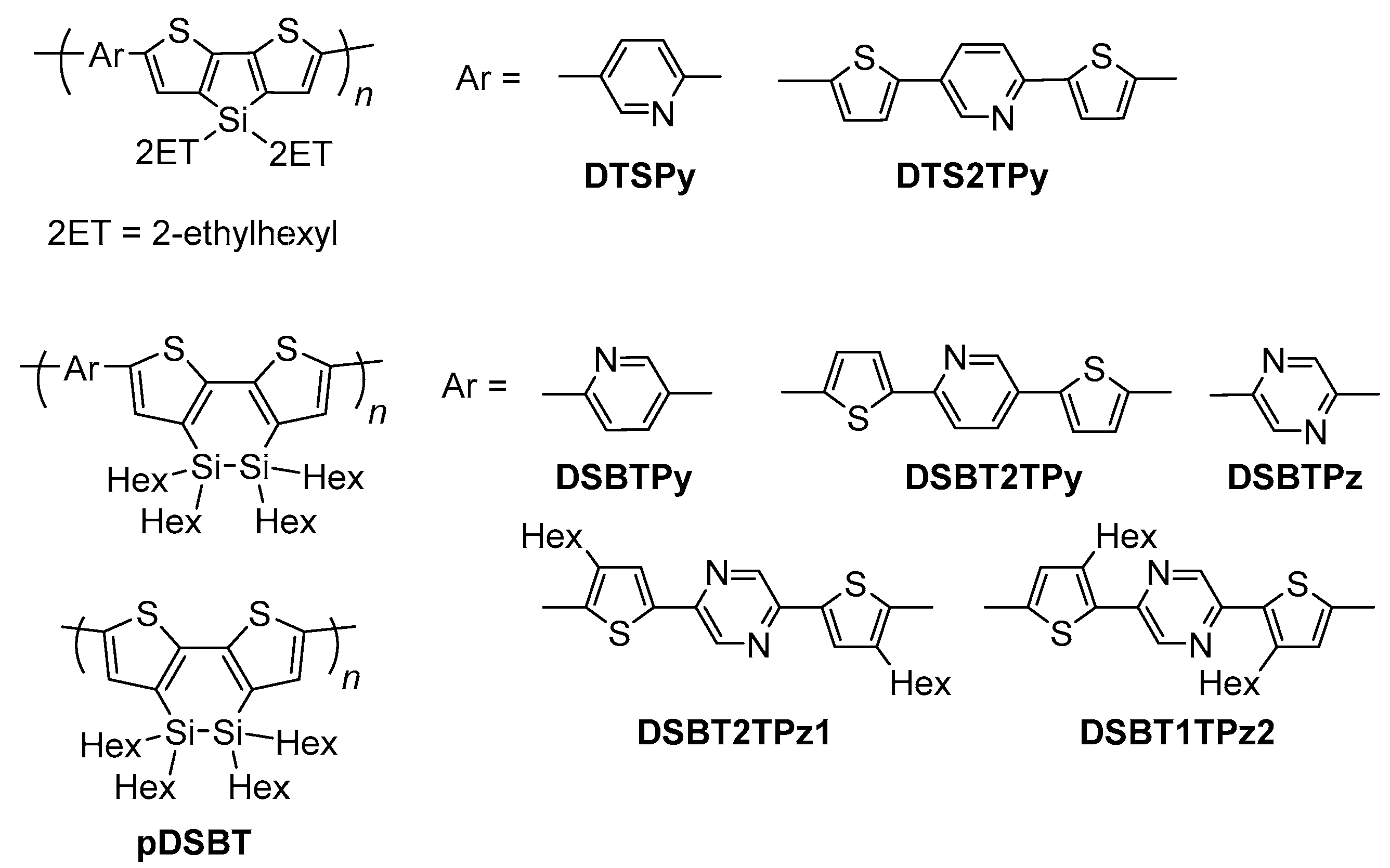
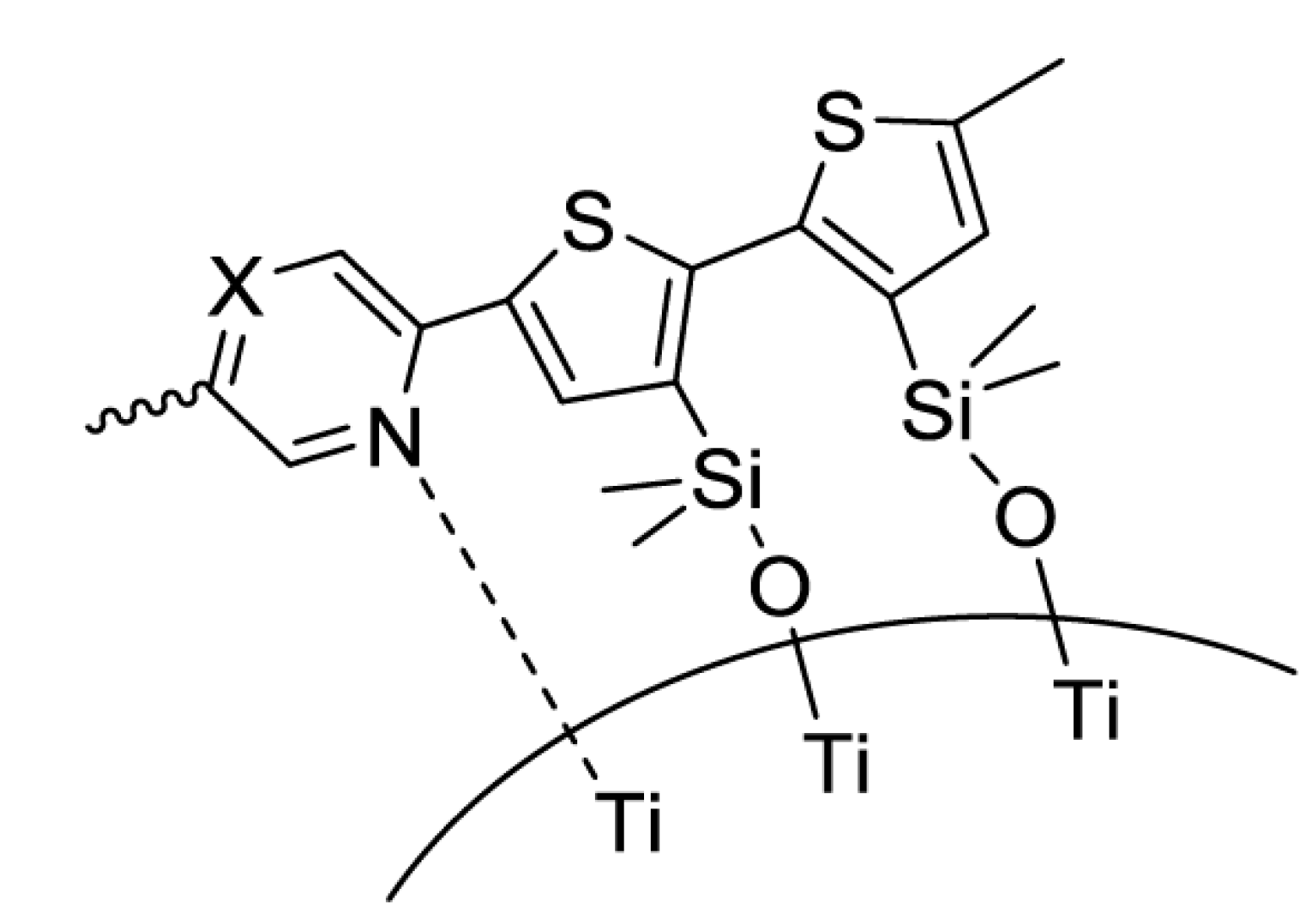
2.2. Dithienosilole- and Disilanobithiophene-Containing π-Conjugated Polymers as Modifiers of the TiO2 Surface
3. Conclusions
Acknowledgments
Conflicts of Interest
References
- Ooyama, Y.; Harima, Y. Molecular designs and syntheses of organic dyes for dye-sensitized solar cells. Eur. J. Org. Chem. 2009, 2903–2934. [Google Scholar] [CrossRef]
- Ohshita, J.; Kunai, A. Polymers with alternating organosilicon and π–conjugated units. Acta Polym. 1998, 49, 379–403. [Google Scholar] [CrossRef]
- Uhlig, W. Synthesis, functionalization, and cross-linking reactions of organosilicon polymers using silyl triflate intermediates. Prog. Polym. Sci. 2002, 27, 255–305. [Google Scholar] [CrossRef]
- Ponomarenko, S.A.; Kirchmeyer, S. Conjugated Organosilicon Materials for Organic Electronics and Photonics. Adv. Polym. Sci. 2011, 235, 33–110. [Google Scholar]
- Ishikawa, M.; Nate, K. Photochemical behavior of organosilicon polymers bearing phenyldisilanyl units. ACS Symp. Ser. 1988, 360, 209–223. [Google Scholar]
- Nate, K.; Ishikawa, M.; Ni, H.; Watanabe, H.; Saheki, Y. Photolysis of polymeric organosilicon systems. 4. photochemical behavior of poly[p-(disilanylene)phenylene]. Organometallics 1987, 6, 1673–1679. [Google Scholar] [CrossRef]
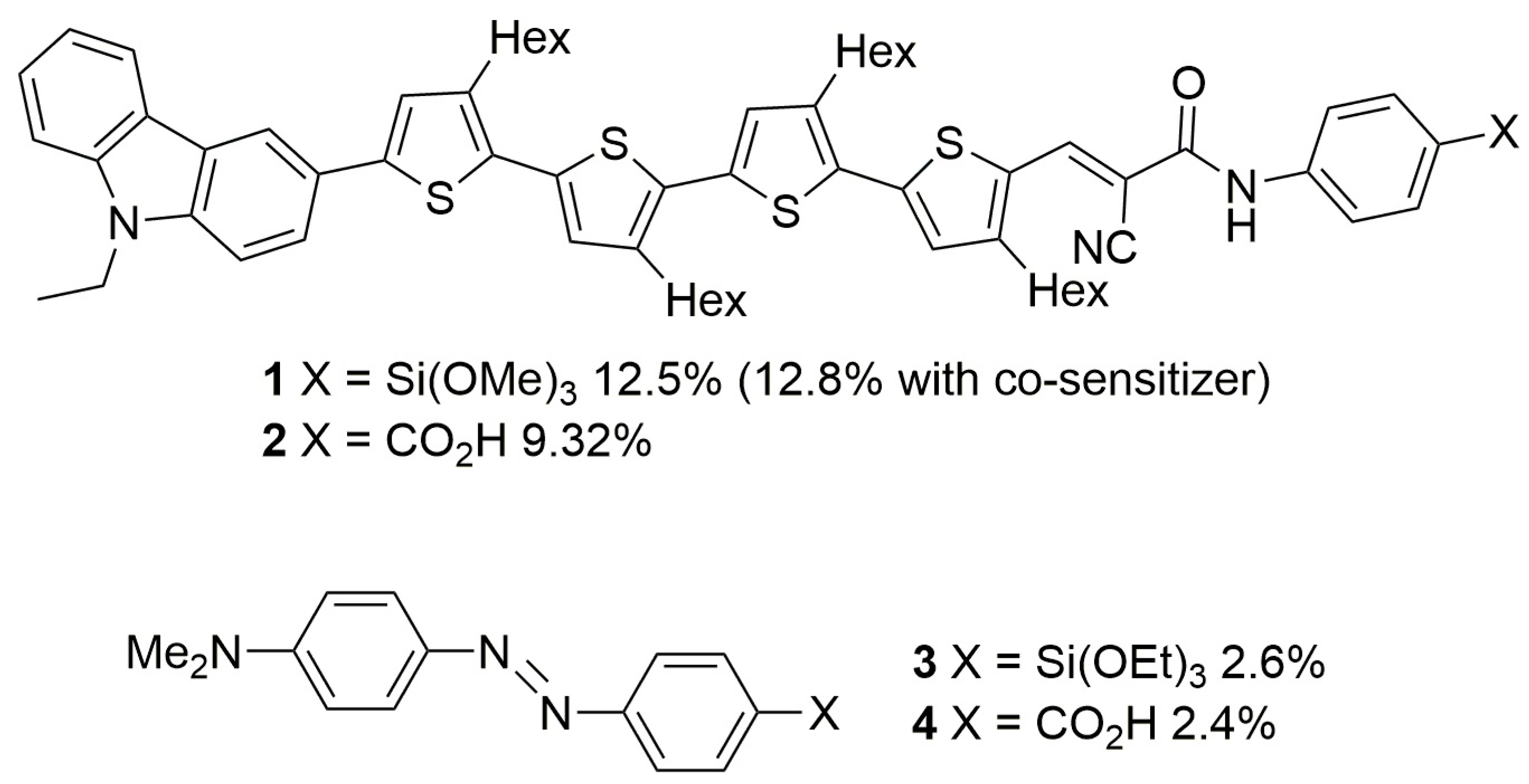
- Kakiage, K.; Yamamura, M.; Fujimura, E.; Kyomen, T.; Unno, M.; Hanaya, M. High performance of Si–O–Ti bonds for anchoring sensitizing dyes on TiO2 electrodes in dye-sensitized solar cells evidenced by using alkoxysilylazobenzenes. Chem. Lett. 2010, 39, 260–262. [Google Scholar] [CrossRef]
- Unno, M.; Kakiage, K.; Yamamura, M.; Kogure, T.; Kyomen, T.; Hanaya, M. Silanol dyes for solar cells: Higher efficiency and significant durability. Appl. Organometal. Chem. 2010, 24, 247–250. [Google Scholar] [CrossRef]
- Kakiage, K.; Aoyama, Y.; Yano, T.; Otsuka, T.; Kyomen, T.; Unno, M.; Hanaya, M. An achievement of over 12 percent efficiency in an organic dye-sensitized solar cell. Chem. Commun. 2014, 50, 6379–6381. [Google Scholar] [CrossRef] [PubMed]
- Matta, S.K.; Kakiage, K.; Makuta, S.; Veamatshau, A.; Aoyama, Y.; Yano, T.; Hanaya, M.; Tachibana, Y. Dye-anchoring functional groups on the performance of dye-sensitized solar cells: Comparison between alkoxysilyl and carboxyl groups. J. Phys. Chem. C 2014, 118, 28425–28434. [Google Scholar] [CrossRef]
- Kakiage, K.; Aoyama, Y.; Yano, T.; Oya, K.; Kyomen, T.; Hanaya, M. Fabrication of a high-performance dye-sensitized solar cell with 12.8% conversion efficiency using organic silyl-anchor dyes. Chem. Commun. 2015, 51, 6315–6317. [Google Scholar] [CrossRef] [PubMed]
- Ohshita, J.; Matsukawa, J.; Hara, M.; Kunai, A.; Kajiwara, S.; Ooyama, Y.; Harima, Y.; Kakimoto, M. Attachment of disilanylene–oligothienylene polymers on TiO2 surface by photochemical cleavage of the Si–Si bonds. Chem. Lett. 2008, 37, 316–317. [Google Scholar] [CrossRef]
- Kira, M.; Miyazawa, T.; Sugiyama, H.; Yamaguchi, M.; Sakurai, H. σπ* Orthogonal intramolecular charge-transfer (OICT) excited states and photoreaction mechanism of trifluoromethyl-substituted phenyldisilanes. J. Am. Chem. Soc. 1993, 115, 3116–3124. [Google Scholar] [CrossRef]
- Ohshita, J.; Ohsaki, H.; Ishikawa, M.; Minato, A. Silicon–carbon unsaturated compounds. 26. Photochemical behavior of 1,4- and 1,5-bis(pentamethyldisilanyl)naphthalene. Organometallics 1991, 10, 880–887. [Google Scholar] [CrossRef]
- Ishikawa, M.; Watanabe, K.; Sakamoto, H.; Kunai, A. Silicon carbon unsaturated-compounds. 40. Photolysis of 1,4-bis(2-phenyltetramethyldisilanyl)benzene. J. Organomet. Chem. 1992, 435, 249–256. [Google Scholar] [CrossRef]
- Ishikawa, M.; Watanabe, K.; Sakamoto, H.; Kunai, A. Silicon-carbon unsaturated-compounds. 44. Photochemical behavior of permethylated p-(disilanylene)phenylene oligomers. J. Organomet. Chem. 1993, 455, 61–68. [Google Scholar] [CrossRef]
- Ohshita, J.; Watanabe, T.; Kanaya, D.; Ohsaki, H.; Ishikawa, M.; Ago, H.; Tanaka, K.; Yamabe, T. Polymeric organosilicon systems. 22. Synthesis and photochemical properties of poly[(disilanylene)oligophenylylenes] and poly[(silylene)biphenylylenes]. Organometallics 1994, 13, 5002–5012. [Google Scholar] [CrossRef]
- Kunai, A.; Ueda, T.; Horata, K.; Toyoda, E.; Nagamoto, I.; Ohshita, J.; Ishikawa, M. Polymeric organosilicon systems. 26. Synthesis and photochemical and conducting properties of poly[(tetraethyldisilanylene)oligo(2,5-thienylenes)]. Organometallics 1996, 15, 2000–2008. [Google Scholar] [CrossRef]
- Ohshita, J.; Matsukawa, J.; Iwawaki, T.; Matsui, S.; Ooyama, Y.; Harima, Y. Attachment of poly[(ethoxyhexylsilylene)oligothienylene]s to inorganic oxide surface. Synth. Met. 2009, 159, 817–820. [Google Scholar] [CrossRef]
- Ohshita, J.; Tanaka, D.; Matsukawa, J.; Mizumo, T.; Yoshida, H.; Ooyama, Y.; Harima, Y. Hybridization of carbon nanotubes with Si–π polymers and attachment of resulting hybrids to TiO2 surface. Chem. Lett. 2011, 40, 87–89. [Google Scholar] [CrossRef]
- Tanaka, D.; Ohshita, J.; Ooyama, Y.; Mizumo, T.; Harima, Y. Synthesis of disilanylene polymers with donor–acceptor-type π–conjugated units and applications to dye-sensitized solar cells. J. Organomet. Chem. 2012, 719, 30–35. [Google Scholar] [CrossRef]
- Tanaka, D.; Ohshita, J.; Mizumo, T.; Ooyama, Y.; Harima, Y. Synthesis of donor–acceptor type new organosilicon polymers and their applications to dye-sensitized solar cells. J. Organomet. Chem. 2013, 741–742, 97–101. [Google Scholar] [CrossRef]
- Ooyama, Y.; Inoue, S.; Nagano, T.; Kushimoto, K.; Ohshita, J.; Imae, I.; Komaguchi, K.; Harima, Y. Dye-sensitized solar cells based on donor–acceptor π–conjugated fluorescent dyes with a pyridine ring as an electron-withdrawing anchoring group. Angew. Chem. Int. Ed. 2011, 50, 7429–7433. [Google Scholar] [CrossRef] [PubMed]
- Ohshita, J.; Kangai, S.; Yoshida, H.; Kunai, A.; Kajiwara, S.; Ooyama, Y.; Harima, Y. Synthesis of organosilicon polymers containing donor–acceptor type π–conjugated units and their applications to dye-sensitized solar cells. J. Organomet. Chem. 2007, 692, 801–805. [Google Scholar] [CrossRef]
- Tanaka, D.; Ohshita, J.; Ooyama, Y.; Morihara, Y. Synthesis and optical and photovoltaic properties of dithienosilole–dithienylpyridine and dithienosilole–pyridine alternate polymers and polymer–B(C6F5) complexes. Polym. J. 2013, 45, 1153–1158. [Google Scholar] [CrossRef]
- Ohshita, J.; Nakashima, M.; Tanaka, D.; Morihara, Y.; Fueno, H.; Tanaka, K. Preparation of a D–A polymer with disilanobithiophene as a new donor component and application to high-voltage bulk heterojunction polymer solar cells. Polym. Chem. 2014, 5, 346–349. [Google Scholar] [CrossRef]
- Nakashima, M.; Otsura, T.; Naito, H.; Ohshita, J. Synthesis of new D–A polymers containing disilanobithiophene donor and application to bulk heterojunction polymer solar cells. Polym. J. 2015, 47, 733–738. [Google Scholar] [CrossRef]
- Nakashima, M.; Ooyama, Y.; Sugiyama, T.; Naito, H.; Ohshita, J. Synthesis of a Conjugated D–A Polymer with Bi(disilanobithiophene) as a New Donor Component. Molecules 2016, 21, 789. [Google Scholar] [CrossRef] [PubMed]
- Ohshita, J.; Adachi, Y.; Tanaka, D.; Nakashima, M.; Ooyama, Y. Synthesis of D–A polymers with a disilanobithiophene donor and a pyridine or pyrazine acceptor and their applications to dye-sensitized solar cells. RSC Adv. 2015, 5, 36673–36679. [Google Scholar] [CrossRef]
- Ooyama, Y.; Hagiwara, Y.; Oda, Y.; Mizumo, T.; Harima, Y.; Ohshita, J. Dye-sensitized solar cells based on a functionally separated D–π–A fluorescent dye with an aldehyde as an electron-accepting group. New J. Chem. 2013, 37, 2336–2340. [Google Scholar] [CrossRef]
- Ooyama, Y.; Uenaka, K.; Ohshita, J. Development of a functionally separated D–π–A fluorescent dye with a pyrazyl group as an electron-accepting group for dye-sensitized solar cells. Org. Chem. Front. 2015, 2, 552–559. [Google Scholar] [CrossRef]
- Zhang, L.; Cole, J.M. Anchoring groups for dye-sensitized solar cells. ACS Appl. Mater. Interfaces 2015, 7, 3427–3455. [Google Scholar] [CrossRef] [PubMed]

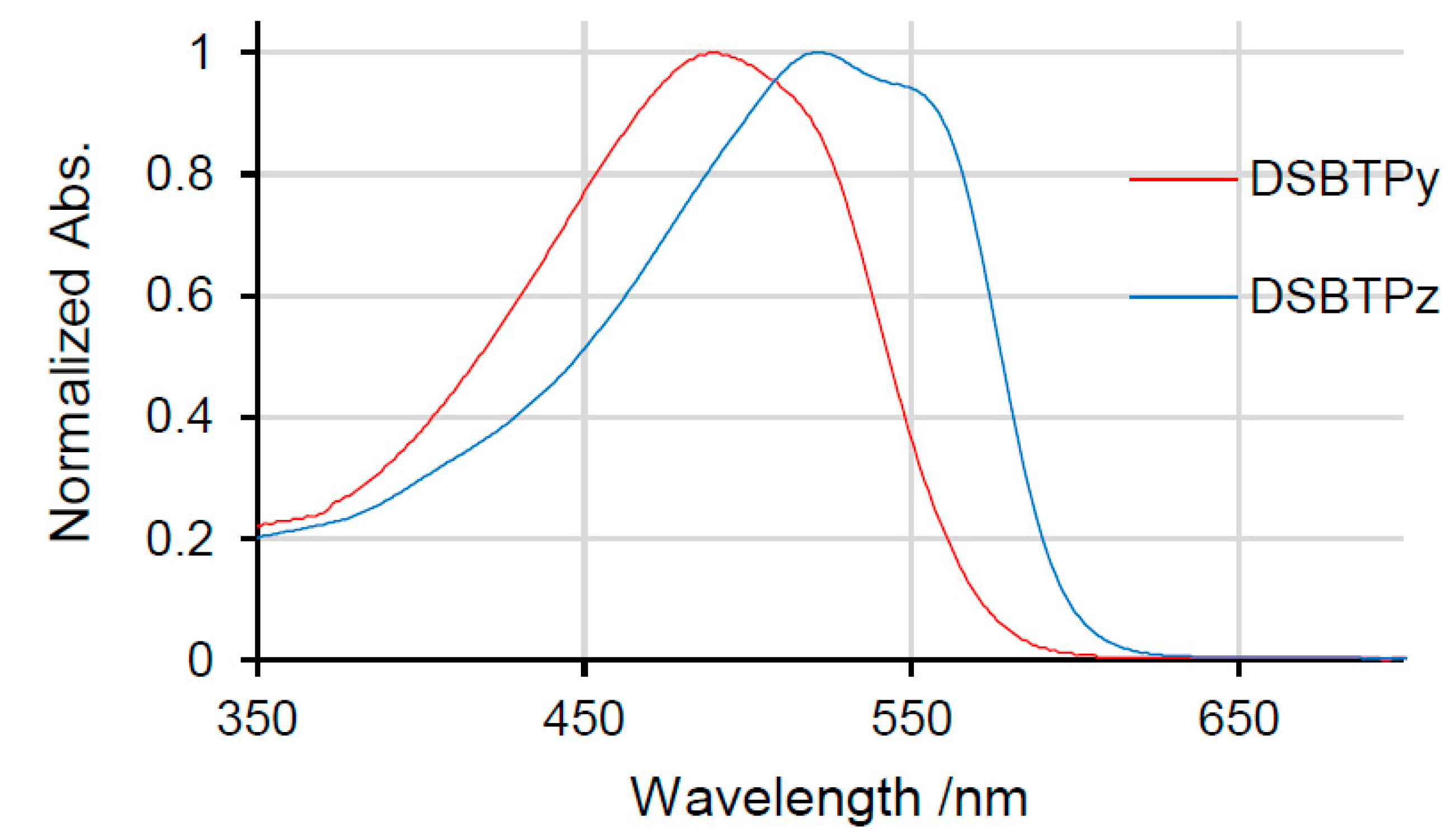
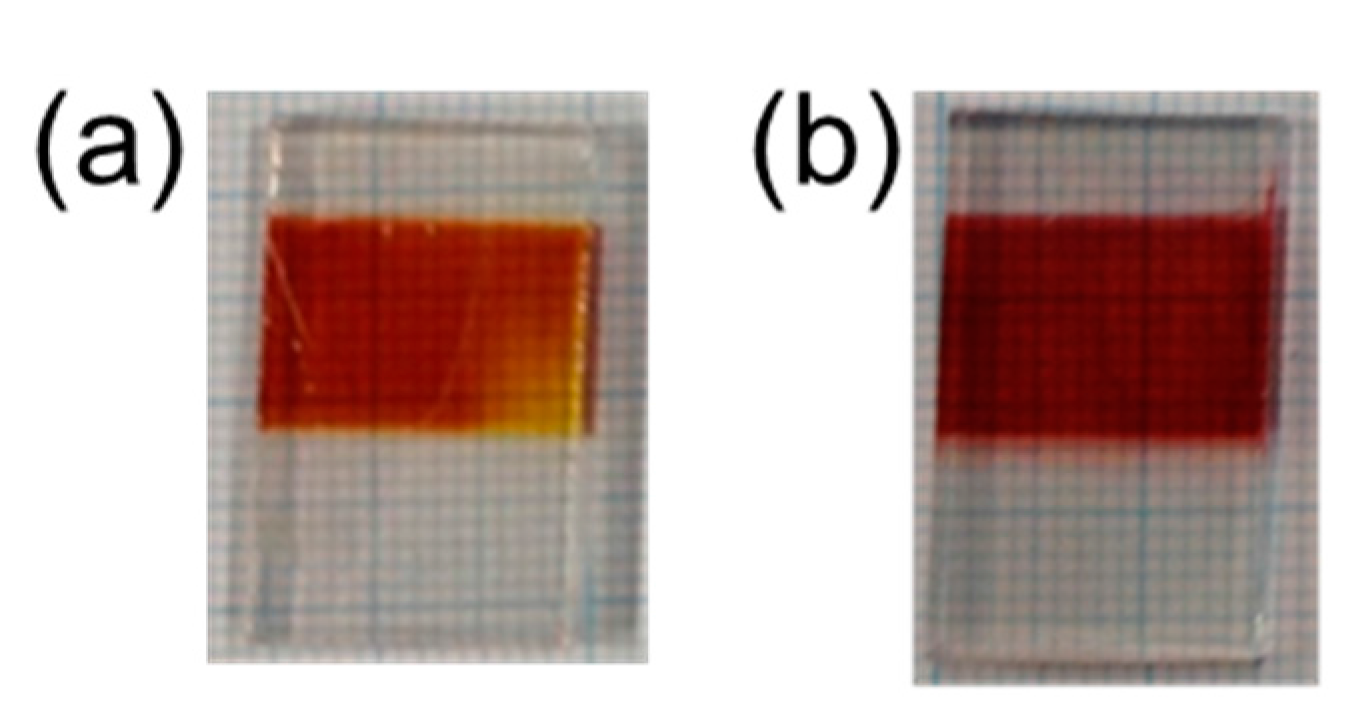
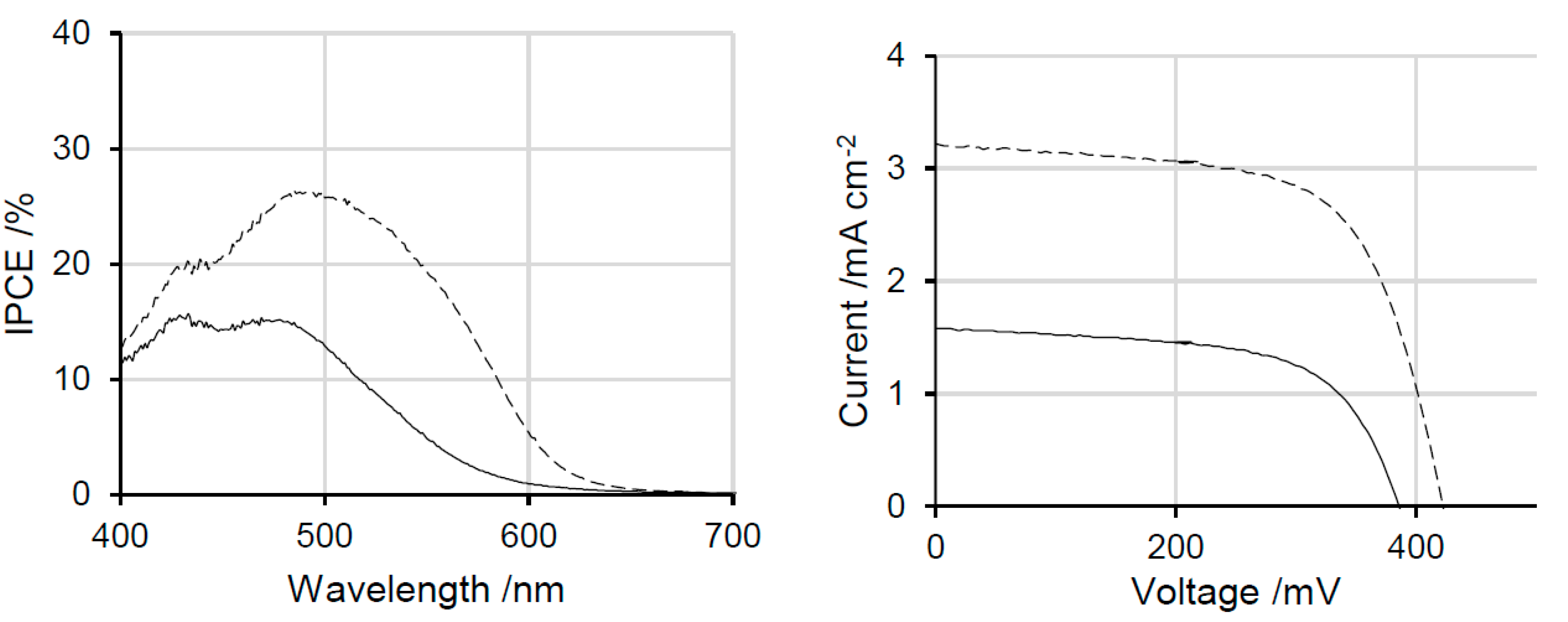
| Polymer | λmax a/nm | DSSC b Jsc/mA cm−2 | Voc/mV | FF | PCE/% |
|---|---|---|---|---|---|
| DS5T | 436 | 0.76 | 292 | 0.52 | 0.11 |
| DS6T | 418 | 0.86 | 296 | 0.48 | 0.12 |
| DSO5T c | 426 | 0.57 | 234 | 0.39 | 0.05 |
| DS5T/SWNT | - | 1.84 | 340 | 0.62 | 0.39 |
| DS2E2TBt1 | 487 | 1.30 | 308 | 0.61 | 0.25 |
| DS2E2TBt2 | 430 | 0.29 | 228 | 0.46 | 0.03 |
| DS2E2TBt3 | 504 | 1.08 | 392 | 0.60 | 0.26 |
| DS4TBt | 508 | 1.30 | 324 | 0.63 | 0.28 |
| DS4TBs | 546 | 0.61 | 324 | 0.57 | 0.11 |
| DS4TPy | 416 | 2.15 | 296 | 0.63 | 0.40 |
| MS2TBt c | 451 | 0.42 | 358 | 0.40 | 0.06 |
| MS2TBs c | 482 | 0.54 | 336 | 0.49 | 0.09 |
| Polymer | λmax a/nm | DSSC b Jsc/mA cm−2 | Voc/mV | FF | PCE/% |
|---|---|---|---|---|---|
| DTSPy c | - | 2.17 | 400 | 0.63 | 0.54 |
| DTS2TPy c | - | 2.03 | 390 | 0.69 | 0.55 |
| pDSBT | 451 | 2.10 | 308 | 0.61 | 0.39 |
| pDSBT c | 520 | 0.69 | 356 | 0.67 | 0.16 |
| DSBTPy | 439 | 1.91 | 344 | 0.63 | 0.41 |
| DSBTPy c | 484 | 1.67 | 396 | 0.63 | 0.42 |
| DSBT2Tpy | 468 | 3.11 | 380 | 0.63 | 0.74 |
| DSBT2TPy c | 475 | 1.34 | 392 | 0.66 | 0.35 |
| DSBTPz | 468 | 1.58 | 384 | 0.62 | 0.38 |
| DSBTPz c | 496 | 3.22 | 424 | 0.65 | 0.89 |
| DSBT2TPz1 | 482 | 2.21 | 396 | 0.64 | 0.56 |
| DSBT2TPz1 c | 489 | 2.28 | 432 | 0.68 | 0.67 |
| DSBT2TPz2 | 490 | 2.70 | 384 | 0.59 | 0.61 |
| DSBT2TPz2 c | 503 | 1.58 | 420 | 0.66 | 0.44 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Adachi, Y.; Tanaka, D.; Ooyama, Y.; Ohshita, J. Modification of TiO2 Surface by Disilanylene Polymers and Application to Dye-Sensitized Solar Cells. Inorganics 2018, 6, 3. https://doi.org/10.3390/inorganics6010003
Adachi Y, Tanaka D, Ooyama Y, Ohshita J. Modification of TiO2 Surface by Disilanylene Polymers and Application to Dye-Sensitized Solar Cells. Inorganics. 2018; 6(1):3. https://doi.org/10.3390/inorganics6010003
Chicago/Turabian StyleAdachi, Yohei, Daiki Tanaka, Yousuke Ooyama, and Joji Ohshita. 2018. "Modification of TiO2 Surface by Disilanylene Polymers and Application to Dye-Sensitized Solar Cells" Inorganics 6, no. 1: 3. https://doi.org/10.3390/inorganics6010003
APA StyleAdachi, Y., Tanaka, D., Ooyama, Y., & Ohshita, J. (2018). Modification of TiO2 Surface by Disilanylene Polymers and Application to Dye-Sensitized Solar Cells. Inorganics, 6(1), 3. https://doi.org/10.3390/inorganics6010003