Copper Dithiocarbamates: Coordination Chemistry and Applications in Materials Science, Biosciences and Beyond
Abstract
:1. Introduction
2. Copper(II) Bis(dithiocarbamate) Complexes
3. Copper(III) Dithiocarbamate Complexes
4. Copper(I) Dithiocarbamate Complexes
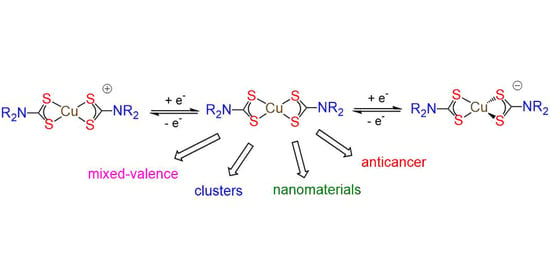
5. Mixed-Valence Copper Dithiocarbamate Complexes
6. Other Copper Dithiocarbamate Complexes
7. Applications as Single-Source Precursors (SSPs) to Semi-Conducting Nanomaterials
7.1. Binary Copper Sulfides
7.2. Ternary Metal Sulfides
7.3. Quaternary Metal Sulfides
8. Biological Applications
8.1. Anticancer Agents
8.2. Antimicrobial, Antibacterial, Antioxidant and SOD-like Activity
8.3. Applications in Medical Imaging
9. Other Applications
9.1. Removal of Cu(II) and Environmental Remediation
9.2. Photovoltaic Cells
9.3. Applications in Organic Transformations and Homogeneous Catalysis
10. Summary and Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Havlík, T. Chapter 3—Phase Equilibrium of Copper Iron Sulphides. In Hydrometallurgy; Havlík, T., Ed.; Woodhead Publishing: Sawston, UK, 2008; pp. 29–59. [Google Scholar]
- Mathys, Z.K.; White, A.R. Copper and Alzheimer’s disease. In Neurotoxicity of Metals; Springer: Berlin/Heidelberg, Germany, 2017; pp. 199–216. [Google Scholar]
- Hogarth, G. Transition metal dithiocarbamates: 1978–2003. Prog. Inorg. Chem. 2005, 53, 71–561. [Google Scholar]
- Heard, P.J. Main group dithiocarbamate complexes. Prog. Inorg. Chem. 2005, 53, 1–69. [Google Scholar]
- Delépine, M. Experiments on Copper and Iron. Bull. Société Chim. Fr. 1908, 3, 652–654. [Google Scholar]
- Delépine, M. Properties of the Metallic Salts of Dithiocarbamic Acid. Comptes Rendus 1908, 146, 981–985. [Google Scholar]
- Ngo, S.C.; Banger, K.K.; DelaRosa, M.J.; Toscano, P.J.; Welch, J.T. Thermal and structural characterization of a series of homoleptic Cu(II) dialkyldithiocarbamate complexes: Bigger is only marginally better for potential MOCVD performance. Polyhedron 2003, 22, 1575–1583. [Google Scholar] [CrossRef]
- Jian, F.; Wang, Z.; Bai, Z.; You, X.; Fun, H.-K.; Chinnakali, K.; Razak, I.A. The crystal structure, equilibrium and spectroscopic studies of bis(dialkyldithiocarbamate) copper(II) complexes [Cu2(R2dtc)4] (dtc=dithiocarbamate). Polyhedron 1999, 18, 3401–3406. [Google Scholar] [CrossRef]
- Monser, L.; Adhoum, N. Modified activated carbon for the removal of copper, zinc, chromium and cyanide from wastewater. Sep. Purif. Technol. 2002, 26, 137–146. [Google Scholar] [CrossRef]
- Bai, L.; Hu, H.; Fu, W.; Wan, J.; Cheng, X.; Zhuge, L.; Xiong, L.; Chen, Q. Synthesis of a novel silica-supported dithiocarbamate adsorbent and its properties for the removal of heavy metal ions. J. Hazard. Mater. 2011, 195, 261–275. [Google Scholar] [CrossRef]
- Gallagher, W.P.; Vo, A. Dithiocarbamates: Reagents for the removal of transition metals from organic reaction media. Org. Process. Res. Dev. 2015, 19, 1369–1373. [Google Scholar] [CrossRef]
- Casey, A.T.; Vecchio, A.M. The electrochemical synthesis of ethylxanthate and dimethyldithiocarbamate complexes of iron, cobalt, nickel and copper. J. Coord. Chem. 1988, 16, 375–381. [Google Scholar] [CrossRef]
- Victoriano, L.I. The reaction of copper and iron species with thiuram sulfides: Copper and iron dithiocarbamate derivatives. Polyhedron 2000, 19, 2269–2275. [Google Scholar] [CrossRef]
- Ferreira, I.P.; de Lima, G.M.; Paniago, E.B.; Takahashi, J.A.; Krambrock, K.; Pinheiro, C.B.; Wardell, J.L.; Visentin, L.C. Synthesis, characterization, structural and biological aspects of copper(II) dithiocarbamate complexes—Part II, [Cu{S2CN(Me)(R1)}2], [Cu{S2CN(Me)(R2)}2] and [Cu{S2CN(R3)(R4)}2] {R1=CH2CH(OMe)2, R2=2-methyl-1,3-dioxolane, R3=CH2(CH2)2NCHPhOCH2Ph and R4=CH2CH2OH}. J. Mol. Struct. 2013, 1048, 357–366. [Google Scholar]
- Anastasiadis, C.; Hogarth, G.; Wilton-Ely, J.D.E.T. Functionalised dithiocarbamate complexes: Complexes based on indoline, indole and substituted piperazine backbones—X-ray crystal structure of [Ni(S2CNC3H6C6H4)2]. Inorg. Chim. Acta 2010, 363, 3222–3228. [Google Scholar] [CrossRef]
- De Lima, G.M.; Menezes, D.C.; Cavalcanti, C.A.; dos Santos, J.A.F.; Ferreira, I.P.; Paniago, E.B.; Wardell, J.L.; Wardell, S.M.S.V.; Krambrock, K.; Mendes, I.C.; et al. Synthesis, characterisation and biological aspects of copper(II) dithiocarbamate complexes, [Cu{S2CNR(CH2CH2OH)}2], (R=Me, Et, Pr and CH2CH2OH). J. Mol. Struct. 2011, 988, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Gupta, A.; Singh, V.; Kumar, V.; Rajput, A.; Singh, L.; Drew, M.; Singh, N. Syntheses, crystal structures and conducting properties of new homoleptic copper (II) dithiocarbamate complexes. Inorg. Chim. Acta 2013, 408, 145–151. [Google Scholar] [CrossRef]
- Gurumoorthy, G.; Thirumaran, S.; Ciattini, S. Synthesis and characterization of copper(II) dithiocarbamate complexes involving pyrrole and ferrocenyl moieties and their utility for sensing anions and preparation of copper sulfide and copper–iron sulfide nanoparticles. Appl. Organomet. Chem. 2018, 32, e4363. [Google Scholar] [CrossRef]
- Hayat, F.; Faryad Ali, R.; Rehman, Z.u.; Bélanger-Gariepy, F. Molecular, supramolecular, DNA-binding and biological studies of piperazine and piperidine based dithiocarbamates of biocompatible copper. Inorg. Chem. Commun. 2020, 121, 108190. [Google Scholar] [CrossRef]
- Macías, B.; Villa, M.a.V.; Chicote, E.; Martín-Velasco, S.; Castiñeiras, A.; Borrás, J.N. Copper complexes with dithiocarbamates derived from natural occurring amino acids. Crystal and molecular structure of [Cu(en)(EtOH)(H2O)3][Cu(dtc-pro)2]. Polyhedron 2002, 21, 1899–1904. [Google Scholar] [CrossRef]
- Manar, K.K.; Neetu; Kumari, K.; Anamika; Yadav, C.L.; Srivastava, P.; Drew, M.G.B.; Singh, N. Preparation, Characterization and Photosensitizing Activities of Homoleptic Cu(II) Dithiocarbamates in TiO2-Based DSSC. ChemistrySelect 2019, 4, 11140–11148. [Google Scholar] [CrossRef]
- Ekennia, A.C.; Onwudiwe, D.C.; Osowole, A.A. Spectral, thermal stability and antibacterial studies of copper, nickel and cobalt complexes of N-methyl-N-phenyl dithiocarbamate. J. Sulfur Chem. 2015, 36, 96–104. [Google Scholar] [CrossRef]
- Martin, J.; Newman, P.; Robinson, B.; White, A. Crystal structures of bis-(N-methyl-N-phenyldithiocarbamato)-nickel-(II) and-copper (II). J. Chem. Soc. Dalton Trans. 1972, 2233–2238. [Google Scholar] [CrossRef]
- Onwudiwe, D.C.; Ekennia, A.C. Synthesis, characterization, thermal, antimicrobial and antioxidant studies of some transition metal dithiocarbamates. Res. Chem. Intermed. 2017, 43, 1465–1485. [Google Scholar] [CrossRef]
- Sarker, J.C.; Hogarth, G. Diaryldithiocarbamate Complexes and Their Use as Single Source Precursors. King’s College: London, UK, Unpublished work. 2021. [Google Scholar]
- Granell, J.; Green, M.L.H.; Lowe, V.J.; Marder, S.R.; Mountford, P.; Saunders, G.C.; Walker, N.M. Studies on the synthesis and electrochemistry of crown ether dithiocarbamates and the molecular dynamics of bis(aza-15-crown-5)thiuram disulphide. Crystal structure of cobalt tris[(aza-15-crown-5)dithiocarbamate]. J. Chem. Soc. Dalton Trans. 1990, 605–614. [Google Scholar] [CrossRef]
- Mnqiwu, K.; Xaba, T.; Moloto, M.J.; Mubiayi, P.K.; Nyamukamba, P.; Sibokoza, S.B. Plasmonic electron deficient Cu2−xS semiconductor nanoparticles from cyclohexylamine-N-dithiocarbamate ligand. Mater. Lett. 2017, 199, 28–31. [Google Scholar] [CrossRef]
- Kaushik, N.K.; Bhushan, B.; Sharma, A.K. Bis [n(chlorophenyl) dithiocarbamato] complexes of Cu(II), Zn(II), Cd(II) and Sn(II). Thermochim. Acta 1984, 76, 345–357. [Google Scholar] [CrossRef]
- Muthuswamy, S.; Venkappayya, D. A study on the thermal-decomposition of some metal dithiocarbamate complexes. J. Ind. Chem. Soc. 1987, 64, 571–573. [Google Scholar]
- Cachapa, A.; Mederos, A.; Gili, P.; Hernández-Molina, R.; Domínguez, S.; Chinea, E.; Rodríguez, M.L.; Feliz, M.; Llusar, R.; Brito, F. Studies of the interaction between bis (dithiocarbamato) copper(II) complexes with nitric oxide in aqueous solution and biological applications. Polyhedron 2006, 25, 3366–3378. [Google Scholar] [CrossRef]
- Mann, P.B.; McGregor, I.J.; Bourke, S.; Burkitt-Gray, M.; Fairclough, S.; Ma, M.T.; Hogarth, G.; Thanou, M.; Long, N.; Green, M. An atom efficient, single-source precursor route to plasmonic CuS nanocrystals. Nanoscale Adv. 2019, 1, 522–526. [Google Scholar] [CrossRef] [Green Version]
- Beer, P.D.; Berry, N.; Drew, M.G.; Fox, O.D.; Padilla-Tosta, M.E.; Patell, S. Self-assembled dithiocarbamate–copper (II) macrocycles for electrochemical anion recognition. Chem. Commun. 2001, 199–200. [Google Scholar] [CrossRef]
- Berry, N.G.; Shimell, T.W.; Beer, P.D. Heteroditopic transition metal dithiocarbamate receptors for binding cation-anion ion pairs. J. Supramol. Chem. 2002, 2, 89–92. [Google Scholar] [CrossRef]
- Cookson, J.; Evans, E.A.; Maher, J.P.; Serpell, C.J.; Paul, R.L.; Cowley, A.R.; Drew, M.G.; Beer, P.D. Metal-directed assembly of large dinuclear copper (II) dithiocarbamate macrocyclic complexes. Inorg. Chim. Acta 2010, 363, 1195–1203. [Google Scholar] [CrossRef]
- Cao, R., Jr.; Díaz, A.; Cao, R.; Otero, A.; Cea, R.; Rodríguez-Argüelles, M.C.; Serra, C. Building layer-by-layer a bis(dithiocarbamato)copper(II) complex on Au[111] surfaces. J. Am. Chem. Soc. 2007, 129, 6927–6930. [Google Scholar] [CrossRef]
- Brustolin, L.; Nardon, C.; Pettenuzzo, N.; Fantoni, N.Z.; Quarta, S.; Chiara, F.; Gambalunga, A.; Trevisan, A.; Marchio, L.; Pontisso, P. Synthesis, chemical characterization and cancer cell growth-inhibitory activities of Cu(II) and Ru(III) aliphatic and aromatic dithiocarbamato complexes. Dalton Trans. 2018, 47, 15477–15486. [Google Scholar] [CrossRef]
- Pettenuzzo, N.; Brustolin, L.; Coltri, E.; Gambalunga, A.; Chiara, F.; Trevisan, A.; Biondi, B.; Nardon, C.; Fregona, D. CuII and AuIII Complexes with Glycoconjugated Dithiocarbamato Ligands for Potential Applications in Targeted Chemotherapy. ChemMedChem 2019, 14, 1162–1172. [Google Scholar] [CrossRef]
- Torres Martin de Rosales, R.; Tavaré, R.; Paul, R.L.; Jauregui-Osoro, M.; Protti, A.; Glaria, A.; Varma, G.; Szanda, I.; Blower, P.J. Synthesis of 64CuII–Bis(dithiocarbamatebisphosphonate) and Its Conjugation with Superparamagnetic Iron Oxide Nanoparticles: In Vivo Evaluation as Dual-Modality PET–MRI Agent. Angew. Chem. Int. Ed. 2011, 50, 5509–5513. [Google Scholar] [CrossRef] [Green Version]
- Wilton-Ely, J.D.E.T.; Solanki, D.; Knight, E.R.; Holt, K.B.; Thompson, A.L.; Hogarth, G. Multimetallic Assemblies Using Piperazine-Based Dithiocarbamate Building Blocks. Inorg. Chem. 2008, 47, 9642–9653. [Google Scholar] [CrossRef] [PubMed]
- Akerström, S.; Lindahl, P.B. A convenient method for determination of tetramethylthiuram disulphide. Acta Chem. Scand. 1962, 16, 1206–1211. [Google Scholar] [CrossRef]
- Cao, R., Jr.; Villalonga, R.; Díaz-García, A.M.; Cao, R.; Rojo, T.; Rodríguez-Argüelles, M.C. Gold Nanoparticles Enhancing Dismutation of Superoxide Radical by Its Bis(dithiocarbamato)copper(II) Shell. Inorg. Chem. 2011, 50, 4705–4712. [Google Scholar] [CrossRef]
- Konarev, D.V.; Kovalevsky, A.Y.; Khasanov, S.S.; Saito, G.; Lopatin, D.V.; Umrikhin, A.V.; Otsuka, A.; Lyubovskaya, R.N. Synthesis, crystal structures, magnetic properties and photoconductivity of C60 and C70 complexes with metal dialkyldithiocarbamates M (R2dtc) x, where M= CuII, CuI, AgI, ZnII, CdII, HgII, MnII, NiII, and PtII; R= Me, Et, and nPr. Eur. J. Inorg. Chem. 2006, 1881–1895. [Google Scholar] [CrossRef]
- Hagen, K.; Holwill, C.J.; Rice, D.A. Gas-phase electron diffraction study of bis (dimethyldithiocarbamato) copper (II), [Cu(S2CNMe2)2], and bis (dimethyldithiocarbamato) zinc (II),[Zn(S2CNMe2)2]. Inorg. Chem. 1989, 28, 3239–3242. [Google Scholar] [CrossRef]
- Einstein, F.; Field, J. Copper (II) bis (N, N-dimethyldithiocarbamate). Acta Crystallogr. Sect. B Struct. Crystallogr. Cryst. Chem. 1974, 30, 2928–2930. [Google Scholar] [CrossRef]
- Boyd, P.D.; Mitra, S.; Raston, C.L.; Rowbottom, G.L.; White, A.H. Magnetic and structural studies on copper (II) dialkyldithiocarbamates. J. Chem. Soc. Dalton Trans. 1981, 13–22. [Google Scholar] [CrossRef]
- Hatfield, W.E.; Singh, P.; Nepveu, F. Structure and magnetic properties of bis (N, N-diisopropyldithiocarbamato) copper (II). Inorg. Chem. 1990, 29, 4214–4217. [Google Scholar] [CrossRef]
- Hogarth, G.; Faulkner, S. The crystal structure of [Cu(S2CNC4H8O)2]: An interesting structural motif constructed by intermolecular S⋯ S and C–H⋯ Cu interactions. Inorg. Chim. Acta 2013, 408, 222–224. [Google Scholar] [CrossRef]
- Nieke, C.; Reinhold, J. NDDO study of the coordination structure of M(S2CNH2)2 complexes (M= Ni, Cu). J. Mol. Struct. 1986, 139, 241–245. [Google Scholar] [CrossRef]
- Tiekink, E.R. The remarkable propensity for the formation of C–H⋯ π (chelate ring) interactions in the crystals of the first-row transition metal dithiocarbamates and the supramolecular architectures they sustain. CrystEngComm 2020, 22, 7308–7333. [Google Scholar] [CrossRef]
- Konarev, D.V.; Kovalevsky, A.Y.; Lopatin, D.V.; Umrikhin, A.V.; Yudanova, E.I.; Coppens, P.; Lyubovskaya, R.N.; Saito, G. Synthesis, crystal structure and photoconductivity of the first [60]fullerene complex with metal diethyldithiocarbamate:{Cu(dedtc)2}2·C60. Dalton Trans. 2005, 1821–1825. [Google Scholar] [CrossRef]
- Konarev, D.V.; Kovalevsky, A.Y.; Otsuka, A.; Saito, G.; Lyubovskaya, R.N. Neutral and Ionic Complexes of C60 with Metal Dibenzyldithiocarbamates. Reversible Dimerization of C60•-in Ionic Multicomponent Complex [CrI(C6H6) 2•+]⊙(C60•-)⊙ 0.5 [Pd(dbdtc)2]. Inorg. Chem. 2005, 44, 9547–9553. [Google Scholar] [CrossRef]
- Fox, O.D.; Cookson, J.; Wilkinson, E.J.; Drew, M.G.; MacLean, E.J.; Teat, S.J.; Beer, P.D. Nanosized polymetallic resorcinarene-based host assemblies that strongly bind fullerenes. J. Am. Chem. Soc. 2006, 128, 6990–7002. [Google Scholar] [CrossRef] [PubMed]
- Newton, W.J.; Tabner, B.J. Electron spin resonance study of some copper(II) dithiocarbamates and their mixed-ligand complexes. J. Chem. Soc. Dalton Trans. 1981, 466–471. [Google Scholar] [CrossRef]
- Weeks, M.; Fackler, J.P. Single-crystal electron paramagnetic resonance studies of copper diethyldithiocarbamate. Inorg. Chem. 1968, 7, 2548–2553. [Google Scholar] [CrossRef]
- Yordanov, N.D.; Dimitrova, A. Solvent effect on the ligand exchange between bis (diethyldithiocarbamato) copper (II) and bis (diethyldiselenocarbamato) copper (II). Z. Für Anorg. Und Allg. Chem. 2005, 631, 956–960. [Google Scholar] [CrossRef]
- Jeliazkova, B.; Dimitrova, A.; Yordanov, N. Charge-transfer photochemistry of the ternary complex (dithio-diseleno-carbamato) copper (II). Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2002, 58, 1163–1170. [Google Scholar] [CrossRef]
- Geurts, P.; Bouten, P.; Van der Avoird, A. Hartree–Fock–Slater–LCAO calculations on the Cu(II) bis (dithiocarbamate) complex; Magnetic coupling parameters and optical spectrum. J. Chem. Phys. 1980, 73, 1306–1312. [Google Scholar] [CrossRef] [Green Version]
- Suzuki, Y.; Fujii, S.; Tominaga, T.; Yoshimoto, T.; Yoshimura, T.; Kamada, H. The origin of an EPR signal observed in dithiocarbamate-loaded tissues: Copper (II)-dithiocarbamate complexes account for the narrow hyperfine lines. Biochim. Biophys. Acta Gen. Subj. 1997, 1335, 242–245. [Google Scholar] [CrossRef]
- Cvek, B. Targeting malignancies with disulfiram (Antabuse): Multidrug resistance, angiogenesis, and proteasome. Curr. Cancer Drug Targets 2011, 11, 332–337. [Google Scholar] [CrossRef]
- Hogarth, G. Metal-dithiocarbamate complexes: Chemistry and biological activity. Mini Rev. Med. Chem. 2012, 12, 1202–1215. [Google Scholar] [CrossRef]
- Skrott, Z.; Cvek, B. Diethyldithiocarbamate complex with copper: The mechanism of action in cancer cells. Mini Rev. Med. Chem. 2012, 12, 1184–1192. [Google Scholar] [CrossRef]
- Skrott, Z.; Mistrik, M.; Andersen, K.K.; Friis, S.; Majera, D.; Gursky, J.; Ozdian, T.; Bartkova, J.; Turi, Z.; Moudry, P. Alcohol-abuse drug disulfiram targets cancer via p97 segregase adaptor NPL4. Nature 2017, 552, 194–199. [Google Scholar] [CrossRef]
- Zhao, Z.; Fan, J.; Deng, X.; Liu, J. One-step synthesis of phosphorus-doped g-C3N4/Co3O4 quantum dots from vitamin B12 with enhanced visible-light photocatalytic activity for metronidazole degradation. Chem. Eng. J. 2019, 360, 1517–1529. [Google Scholar] [CrossRef]
- Larionov, S.; Kosareva, L.; Malikova, A.; Shklyaev, A. Thermal-properties of copper (II) complexes with dithiocarbamic acid-derivatives. Zhurnal Neorg. Khimii 1977, 22, 2401–2406. [Google Scholar]
- Hendrickson, A.; Martin, R.; Rohde, N. Dithiocarbamates of copper (I), copper (II), and copper (III). An electrochemical study. Inorg. Chem. 1976, 15, 2115–2119. [Google Scholar] [CrossRef]
- Furneaux, R.H.; Sinn, E. Antiferromagnetic copper (II) halide adducts of copper (II) dithiocarbamates. Inorg. Chem. 1977, 16, 1809–1812. [Google Scholar] [CrossRef]
- Sarova, G.H.; Jeliazkova, B.G. Effect of solvent and remote ligand substituents on the photochemical behaviour of copper (II) dithiocarbamates and dithiophosphates. Transit. Met. Chem. 2001, 26, 388–394. [Google Scholar] [CrossRef]
- Golding, R.; Rae, A.; Sulligoi, L. New series of polynuclear copper dithiocarbamate-copper halide polymers. Inorg. Chem. 1974, 13, 2499–2504. [Google Scholar] [CrossRef]
- Jeliazkova, B.; Doicheva, M. Charge-transfer photochemistry of copper(II) dithiocarbamate mixed-ligand complexes. Polyhedron 1996, 15, 1277–1282. [Google Scholar] [CrossRef]
- Plyusnin, V.F.; Kolomeets, A.V.; Grivin, V.P.; Larionov, S.V.; Lemmetyinen, H. Photochemistry of dithiocarbamate Cu(II) complex in CCl4. J. Phys. Chem. A 2011, 115, 1763–1773. [Google Scholar] [CrossRef] [PubMed]
- Ivanov, A.V.; Lutsenko, I.; Forsling, W. Bis (dimethyldithiocarbamato)(pyridine) zinc and-copper (II) and Their Benzene Solvates: EPR and Solid-State Natural Abundance (13C,15N) CP/MAS NMR. Russ. J. Coord. Chem. 2002, 28, 57–63. [Google Scholar] [CrossRef]
- Díaz, A.; Ortiz, M.; Sánchez, I.; Cao, R.; Mederos, A.; Sanchiz, J.; Brito, F. Interactions of nitric oxide with copper (II) dithiocarbamates in aqueous solution. J. Inorg. Biochem. 2003, 95, 283–290. [Google Scholar] [CrossRef]
- Mederos, A.; Cachapa, A.; Hernández-Molina, R.; Armas, M.T.; Gili, P.; Sokolov, M.; González-Platas, J.; Brito, F. Theoretical and spectrophotometrical study of the interaction of nitric oxide with copper (II) dithiocarbamates. Inorg. Chem. Commun. 2003, 6, 498–502. [Google Scholar] [CrossRef]
- Hogarth, G.; Holman, K.T.; Pateman, A.; Sella, A.; Steed, J.W.; Richards, I. Multiple nitrene insertions into metal–sulfur bonds of dithiocarbamate complexes: Synthesis of sulfido-amido and zwitterionic tetraamido complexes. Dalton Trans. 2005, 2688–2695. [Google Scholar] [CrossRef]
- Hogarth, G.; Pateman, A.; Sella, A. Multiple nitrene insertions into the copper–sulfur bonds ofdithiocarbamate ligands: Synthesis and molecular structure of thetetraamido complex [Cu{η2-RNSC(NMe2)SNR}2](R= SO2C6H4Me-p). Chem. Commun. 1997, 1029–1030. [Google Scholar] [CrossRef]
- Melník, M.; Kabešová, M. Copper (III) coordination compounds: Classification and analysis of crystallographic and structural data. J. Coord. Chem. 2000, 50, 323–338. [Google Scholar] [CrossRef]
- DiMucci, I.M.; Lukens, J.T.; Chatterjee, S.; Carsch, K.M.; Titus, C.J.; Lee, S.J.; Nordlund, D.; Betley, T.A.; MacMillan, S.N.; Lancaster, K.M. The myth of d8 copper (III). J. Am. Chem. Soc. 2019, 141, 18508–18520. [Google Scholar] [CrossRef] [PubMed]
- Ainscough, E.W.; Brodie, A.M. Sulphur-ligand–metal complexes. Part 7. The interaction of some diphosphine dichalcogenides and tetra-alkylthiuram disulphides with halogens and some first-row transition-metal salts. J. Chem. Soc. Dalton Trans. 1977, 565–570. [Google Scholar] [CrossRef]
- Fox, O.D.; Drew, M.G.; Beer, P.D. Resorcarene-based nanoarchitectures: Metal-directed assembly of a molecular loop and tetrahedron. Angew. Chem. Int. Ed. 2000, 39, 135–140. [Google Scholar] [CrossRef]
- Larin, G.; Zvereva, G.; Kozmin, P.; Larina, T.; Surazhskaya, M. Synthesis, structure, and properties of copper (III) bis (N, N-diethyldithiocarbamato) triiodide. Inorg. Mater. 1984, 20, 451–454. [Google Scholar]
- Pervukhina, N.; Podberezskaya, N.; Patrina, L.; Larionov, S. Crystal and molecular structure of bis (di-n-propyldithiocarbamato) copper (III) penta-iodide Cu[S2CN(C3H7)2]2I5. J. Struct. Chem. 1989, 30, 694–697. [Google Scholar] [CrossRef]
- Bond, A.M.; Colton, R.; D—Agostino, A.; Harvey, J.; Traeger, J.C. Electrospray mass spectrometric study of the nature and lability of cationic complexes generated by the reaction of solutions of neutral iron (III), cobalt (III), nickel (II) and copper (II) dithiocarbamates with nitrosonium tetrafluoroborate. Inorg. Chem. 1993, 32, 3952–3956. [Google Scholar] [CrossRef]
- Jung, S.; Nam, D.; Choe, W. Trivalent copper and indium heterometallic complex with dithiocarbamate and iodide ligands. J. Mol. Struct. 2020, 1204, 127478. [Google Scholar]
- Barbier, J.-P. Copper(III) and nickel(III) diethyldithiocarbamates: An example of copper (II) disproportionation. Inorg. Chim. Acta 1983, 77, 117–118. [Google Scholar] [CrossRef]
- Farhadi, S.; Dusek, M.; Siadatnasab, F.; Eigner, V. First organic–inorganic hybrid nanomaterial constructed from a Keggin-type polyoxometallate and a copper-dithiocarbamate complex: Sonochemical synthesis, crystal structure and its adsorption performance for organic dye pollutants. Polyhedron 2017, 126, 227–238. [Google Scholar] [CrossRef]
- Siadatnasab, F.; Farhadi, S.; Dusek, M.; Eigner, V.; Hoseini, A.-A.; Khataee, A. Sonochemical synthesis and structural characterization of an organic-inorganic nanohybrid based on a copper-dithiocarbamate complex and PMo12O403− polyanion as a novel sonocatalyst. Ultrason. Sonochemistry 2020, 64, 104727. [Google Scholar] [CrossRef] [PubMed]
- Brown, K. Bis(N-pyrrolidyldithiocarbamato) copper(III) perchlorate C10H16ClCuN2O4S4. Cryst. Struct. Commun. 1979, 8, 157–158. [Google Scholar]
- Hogarth, G.; Ebony-Jewel, C.-R.; Richards, I. Functionalised dithiocarbamate complexes: Synthesis and molecular structures of bis(2-methoxyethyl) dithiocarbamate complexes [M{S2CN(CH2CH2OMe)2}2] (M = Ni, Cu, Zn) and [Cu{S2CN(CH2CH2OMe)2}2][ClO4]. Inorg. Chim. Acta 2009, 362, 1361–1364. [Google Scholar] [CrossRef]
- Kaul, B.B.; Pandeya, K. Some Cu(III) dithiocarbamates. J. Inorg. Nucl. Chem. 1981, 43, 1942–1944. [Google Scholar] [CrossRef]
- Pandeya, K.; Waraich, T.; Gaur, R.; Singh, R. Synthesis and Characterisation of Some Bis(dithiocarbamato) copper(III) Perchlorate Complexes. Synth. React. Inorg. Met. Org. Chem. 1982, 12, 493–500. [Google Scholar] [CrossRef]
- Hogarth, G.; Pateman, A.; Redmond, S.P. Crystal structures of copper(III) dithiocarbamate complexes [Cu(k2-S2CNMe2)2][ClO4] and [Cu(k2-S2CNEt2)2][FeCl4] with and without anion–cation interactions. Inorg. Chim. Acta 2000, 306, 232–236. [Google Scholar] [CrossRef]
- Shtyrlin, V.G.; Zakharov, A.V.; Kuznetsov, A.M.; Kukushkina, O.G.V.; Chernov, P.P. Thermodynamics of Redox Processes and Kinetics and Mechanism of Electron Self-Exchange Reactions in the Bis(N,N-diethyldithiocarbamato) copper (II)/Iodine/Dichloromethane System. Eur. J. Inorg. Chem. 2002, 2002, 2947–2955. [Google Scholar] [CrossRef]
- Yusuff, K.M.; Mathew, E. Reaction of Thionyl Chloride with bis(dithiocarbamato)-Copper(II) Complexes. Synth. React. Inorg. Met. Org. Nano-Met. Chem. 1992, 22, 575–583. [Google Scholar] [CrossRef]
- Victoriano, L.I. Copper (III) Dithiocarbamates. An Undergraduate Experimental Project with Unexpected Challenges. J. Chem. Educ. 2002, 79, 1252–1253. [Google Scholar] [CrossRef]
- Beurskens, P.; Cras, J.; Steggerda, J.J. Structure and properties of dibromo-N, N-dibutyldithiocarbamato complexes of copper (III) and gold (III). Inorg. Chem. 1968, 7, 810–813. [Google Scholar] [CrossRef]
- Willert-Porada, M.A.; Burton, D.J.; Baenziger, N.C. Synthesis and X-ray structure of bis (trifluoromethyl)(N, N-diethyldithiocarbamato)-copper; a remarkably stable perfluoroalkylcopper (III) complex. J. Chem. Soc. Chem. Commun. 1989, 1633–1634. [Google Scholar] [CrossRef]
- Naumann, D.; Roy, T.; Caeners, B.; Hutten, D.; Tebbe, K.; Gilles, T. Syntheses and properties of pentafluoroethylcopper (I) and-copper (III) compounds. Z. Fur Anorg. Und Allg. Chem. 2000, 626, 999–1003. [Google Scholar] [CrossRef]
- Hesse, R. Crystal structure of copper (I) diethyldithiocarbamate and its interpretation-an application of chemical topology. Ark. Kemi 1963, 20, 481. [Google Scholar]
- Nguyen, L.M.; Dellinger, M.E.; Lee, J.T.; Quinlan, R.A.; Rheingold, A.L.; Pike, R.D. Convenient synthesis of copper (I) thiolates and related compounds. Inorg. Chim. Acta 2005, 358, 1331–1336. [Google Scholar] [CrossRef] [Green Version]
- Cardell, D.; Hogarth, G.; Faulkner, S. A dithiocarbamate-stabilized copper (I) cube. Inorg. Chim. Acta 2006, 359, 1321–1324. [Google Scholar] [CrossRef]
- Victoriano, L.I.; Cortés, H.B. Cuprous dithiocarbamates. Syntheses and reactivity. J. Coord. Chem. 1996, 39, 231–239. [Google Scholar] [CrossRef]
- Victoriano, L.I.; Cortés, H.B.; Yuseff, M.I.S.; Fuentealba, L.C. Copper (III) dithiocarbamate complexes from thiuram disulfides and copper (I) halides. J. Coord. Chem. 1996, 39, 241–251. [Google Scholar] [CrossRef]
- Lane, A.C.; Vollmer, M.V.; Laber, C.H.; Melgarejo, D.Y.; Chiarella, G.M.; Fackler Jr, J.P.; Yang, X.; Baker, G.A.; Walensky, J.R. Multinuclear copper (I) and silver (I) amidinate complexes: Synthesis, luminescence, and CS2 insertion reactivity. Inorg. Chem. 2014, 53, 11357–11366. [Google Scholar] [CrossRef] [PubMed]
- Kita, H.; Miyake, S.-i.; Tanaka, K.; Tanaka, T. Kinetics and Mechanism of the Oxidation Reactions of Dialkyldithiocarbamatocopper (I) Tetramer and-silver (I) Hexamer with Tetraalkylthiuram Disulfide. Bull. Chem. Soc. Jpn. 1979, 52, 3532–3538. [Google Scholar] [CrossRef] [Green Version]
- Chakrahari, K.K.; Silalahi, R.P.B.; Chiu, T.H.; Wang, X.; Azrou, N.; Kahlal, S.; Liu, Y.C.; Chiang, M.H.; Saillard, J.Y.; Liu, C. Synthesis of Bimetallic Copper-Rich Nanoclusters Encapsulating a Linear Palladium Dihydride Unit. Angew. Chem. 2019, 131, 4997–5001. [Google Scholar] [CrossRef]
- Edwards, A.J.; Dhayal, R.S.; Liao, P.K.; Liao, J.H.; Chiang, M.H.; Piltz, R.O.; Kahlal, S.; Saillard, J.Y.; Liu, C. Chinese puzzle molecule: A 15 hydride, 28 copper atom nanoball. Angew. Chem. 2014, 126, 7342–7346. [Google Scholar] [CrossRef]
- Kishore, P.V.; Liao, J.-H.; Hou, H.-N.; Lin, Y.-R.; Liu, C. Ferrocene-functionalized Cu(I)/Ag(I) dithiocarbamate clusters. Inorg. Chem. 2016, 55, 3663–3673. [Google Scholar] [CrossRef] [PubMed]
- Liao, P.-K.; Fang, C.-S.; Edwards, A.J.; Kahlal, S.; Saillard, J.-Y.; Liu, C. Hydrido copper clusters supported by dithiocarbamates: Oxidative hydride removal and neutron diffraction analysis of [Cu7(H){S2C(aza-15-crown-5)}6]. Inorg. Chem. 2012, 51, 6577–6591. [Google Scholar] [CrossRef]
- Chakrahari, K.K.; Liao, J.; Silalahi, R.P.B.; Chiu, T.H.; Liao, J.H.; Wang, X.; Kahlal, S.; Saillard, J.Y.; Liu, C. Isolation and Structural Elucidation of 15-Nuclear Copper Dihydride Clusters: An Intermediate in the Formation of a Two-Electron Copper Superatom. Small 2020, 2002544. [Google Scholar] [CrossRef] [PubMed]
- Chakrahari, K.K.; Liao, J.H.; Kahlal, S.; Liu, Y.C.; Chiang, M.H.; Saillard, J.Y.; Liu, C. [Cu13{S2CNnBu2}6(acetylide) 4]+: A Two-Electron Superatom. Angew. Chem. 2016, 128, 14924–14928. [Google Scholar] [CrossRef]
- Chakrahari, K.K.; Silalahi, R.P.B.; Liao, J.-H.; Kahlal, S.; Liu, Y.-C.; Lee, J.-F.; Chiang, M.-H.; Saillard, J.-Y.; Liu, C. Synthesis and structural characterization of inverse-coordination clusters from a two-electron superatomic copper nanocluster. Chem. Sci. 2018, 9, 6785–6795. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Silalahi, R.P.B.; Chakrahari, K.K.; Liao, J.H.; Kahlal, S.; Liu, Y.C.; Chiang, M.H.; Saillard, J.Y.; Liu, C. Synthesis of Two-Electron Bimetallic Cu–Ag and Cu–Au Clusters by using [Cu13(S2CNnBu2)6(C≡CPh)4]+ as a Template. Chem. Asian J. 2018, 13, 500–504. [Google Scholar] [CrossRef]
- Silalahi, R.P.B.; Chiu, T.-H.; Kao, J.-H.; Wu, C.-Y.; Yin, C.-W.; Liu, Y.-C.; Chen, Y.J.; Saillard, J.-Y.; Chiang, M.-H.; Liu, C.W. Synthesis and Luminescence Properties of Two-Electron Bimetallic Cu-Ag and Cu-Au Nanoclusters via Copper Hydride Precursors. Inorg. Chem. 2021, 60, 10799–10807. [Google Scholar] [CrossRef]
- Teske, C.L. On Ammonium-bis(dithiocarbamato)-copper(I)-monohydrate and Mono(dithiocarbamato)-copper(I). Z. Für Anorg. Und Allg. Chem. 2013, 639, 2767–2773. [Google Scholar] [CrossRef]
- Kowala, C.; Swan, J. Coordination compounds of Group IB metals. III. Triethyl- and triphenylphosphine complexes of cuprous, argentous, and aurous NN-dialkyldithiocarbamates. Aust. J. Chem. 1966, 19, 555–559. [Google Scholar] [CrossRef]
- Brinkhoff, H.C.; Matthijssen, A.G.; Oomes, C.G. Triphenylphosphine complexes of Cu(I), Ag(I) and Au(I) N,N-dialkyldithiocarbamates. Inorg. Nucl. Chem. Lett. 1971, 7, 87–89. [Google Scholar] [CrossRef] [Green Version]
- Victoriano, L.; Cortes, H. The reaction of thiuram disulfides and copper metal. Copper (I) dithiocarbamates. Bol. Soc. Chil. Quim. 1996, 41, 27–31. [Google Scholar]
- Kumar, A.; Mayer-Figge, H.; Sheldrick, W.S.; Singh, N. Synthesis, Structure, Conductivity, and Calculated Nonlinear Optical Properties of Two Novel Bis(triphenylphosphane)copper(I) Dithiocarbamates. Eur. J. Inorg. Chem. 2009, 2009, 2720–2725. [Google Scholar] [CrossRef]
- Afzaal, M.; Rosenberg, C.L.; Malik, M.A.; White, A.J.P.; O’Brien, P. Phosphine stabilized copper(i) complexes of dithiocarbamates and xanthates and their decomposition pathways. New J. Chem. 2011, 35, 2773–2780. [Google Scholar] [CrossRef]
- Shono, T.; Fujii, Y.; Shinra, K. Syntheses of copper (I) triphenylphosphine complexes. Chem. Lett. 1972, 1, 163–164. [Google Scholar] [CrossRef] [Green Version]
- Xu, L. N,N-Bispropyldithiocarbamato Bis (triphenylphosphine) Copper (I) Dichloromethane Solvate:[(n-Pr)2dtc(PPh3) 2}Cu.CH2Cl2, (dtc= Dithiocarbamate). Pol. J. Chem. 2001, 75, 755–757. [Google Scholar]
- Rajput, G.; Yadav, M.K.; Drew, M.G.B.; Singh, N. Impact of Ligand Framework on the Crystal Structures and Luminescent Properties of Cu(I) and Ag(I) Clusters and a Coordination Polymer Derived from Thiolate/Iodide/dppm Ligands. Inorg. Chem. 2015, 54, 2572–2579. [Google Scholar] [CrossRef]
- Kociok-Kohn, G.; Molloy, K.; Sudlow, A. Molecular Routes to Cu2ZnSnS4: A Comparison of Approaches to Bulk and Thin-Film Materials. Can. J. Chem. 2014, 92, 514–524. [Google Scholar] [CrossRef]
- Gupta, A.N.; Singh, V.; Kumar, V.; Prasad, L.B.; Drew, M.G.B.; Singh, N. Syntheses, crystal structures and optical properties of heteroleptic copper(I) dithio/PPh3 complexes. Polyhedron 2014, 79, 324–329. [Google Scholar] [CrossRef]
- Mothes, R.; Petzold, H.; Jakob, A.; Rüffer, T.; Lang, H. Dithiocarbamate copper(I) and silver(I) complexes: Synthesis, structure and thermal behavior. Inorg. Chim. Acta 2015, 429, 227–236. [Google Scholar] [CrossRef]
- Singh, A.K.; Yadav, C.L.; Mishra, K.B.; Singh, S.K.; Gupta, A.N.; Tiwari, V.K.; Drew, M.G.B.; Singh, N. Highly efficient and recyclable pre-catalysts based on mono- and dinuclear heteroleptic Cu(I) dithio- PPh3 complexes to produce variety of glycoconjugate triazoles. Mol. Catal. 2019, 470, 152–163. [Google Scholar] [CrossRef]
- Jamaludin, N.S.; Halim, S.N.A.; Khoo, C.-H.; Chen, B.-J.; See, T.-H.; Sim, J.-H.; Cheah, Y.-K.; Seng, H.-L.; Tiekink, E.R. Bis(phosphane) copper(I) and silver(I) dithiocarbamates: Crystallography and anti-microbial assay. Z. Für Krist. Cryst. Mater. 2016, 231, 341–349. [Google Scholar] [CrossRef] [Green Version]
- Huang, T.-H.; Yang, H.; Zhu, S.-L.; Zhao, B.; Yang, Y. Synthesis, structures and fluorescent properties of metal complexes based on polyphosphine ligands. J. Mol. Struct. 2017, 1127, 138–144. [Google Scholar] [CrossRef]
- Bianchini, C.; Ghilardi, C.A.; Meli, A.; Midollini, S.; Orlandini, A. Reactivity of copper(I) tetrahydroborates toward carbon disulfide and phenyl isothiocyanate. Structures of (PPh3)2Cu(m-S2CSCH2SCS2)Cu(PPh3)2, (PPh3)2Cu(S2COEt), and (PPh3)2Cu(S2CNHPh).CHCl3. Inorg. Chem. 1985, 24, 932–939. [Google Scholar] [CrossRef]
- Tan, Y.J.; Yeo, C.I.; Halcovitch, N.R.; Jotani, M.M.; Tiekink, E.R. μ3-Chlorido-μ2-chlorido-(μ3-pyrrolidine-1-carbodithioato-κ4S:S,S′:S′) tris [(triethylphosphane-κP)copper (I)]: Crystal structure and Hirshfeld surface analysis. Acta Crystallogr. Sect. E Crystallogr. Commun. 2017, 73, 720–725. [Google Scholar] [CrossRef] [Green Version]
- Huang, S.-B.; Situ, Y. Synthesis and crystal structure of a dicopper(I) complex with bis(diphenylphosphino)methane and diethyldithiocarbamate. Jiegou Huaxue 2003, 3, 260–264. [Google Scholar]
- Langer, R.; Wünsche, L.; Fenske, D.; Fuhr, O. Copper Chalcogenide Cluster Compounds with Bromo-functionalized Ligand Shell. Z. Fuer Anorg. Allg. Chem. 2009, 635, 2488–2494. [Google Scholar]
- Okubo, T.; Kawajiri, R.; Mitani, T.; Shimoda, T. A mixed-valence coordination polymer featuring two-dimensional ferroelectric order: {[Cu(I)4Cu(II)(Et2dtc)2Cl3][Cu(II)(Et2dtc)2]2(FeCl4)}n (Et2dtc- = diethyldithiocarbamate). J. Am. Chem. Society. 2005, 127, 17598–17599. [Google Scholar] [CrossRef]
- Okubo, T.; Tanaka, N.; Kim, K.H.; Anma, H.; Seki, S.; Saeki, A.; Maekawa, M.; Kuroda-Sowa, T. Crystal structure and carrier transport properties of a new 3D mixed-valence Cu(I)–Cu(II) coordination polymer including pyrrolidine dithiocarbamate ligand. Dalton Trans. 2011, 40, 2218–2224. [Google Scholar] [CrossRef]
- Kawajiri, R.; Okubo, T.; Mitani, T. Structural and magnetic studies on a new mixed-valence Cu(I)–Cu(II) octanuclear cluster with a dithiocarbamate derivative. Polyhedron 2006, 25, 2650–2654. [Google Scholar] [CrossRef]
- Okubo, T.; Tanaka, N.; Kim, K.H.; Yone, H.; Maekawa, M.; Kuroda-Sowa, T. Magnetic and Conducting Properties of New Halide-Bridged Mixed-Valence CuI−CuII 1D Coordination Polymers Including a Hexamethylene Dithiocarbamate Ligand. Inorg. Chem. 2010, 49, 3700–3702. [Google Scholar] [CrossRef]
- Ho, K.K.; Takashi, U.; Takashi, O.; Shinya, H.; Haruho, A.; Kazuya, K.; Tetsuya, S.; Jyunji, F.; Masahiko, M.; Takayoshi, K.-S. Synthesis and Conducting Properties of a New Mixed-valence Cu(I)–Cu(II) 1-D Coordination Polymer Bridged by Morpholine Dithiocarbamate. Chem. Lett. 2011, 40, 1184–1186. [Google Scholar]
- Okubo, T.; Anma, H.; Tanaka, N.; Himoto, K.; Seki, S.; Saeki, A.; Maekawa, M.; Kuroda-Sowa, T. Crystal structure and carrier transport properties of a new semiconducting 2D coordination polymer with a 3,5-dimethylpiperidine dithiocarbamate ligand. Chem. Commun. 2013, 49, 4316–4318. [Google Scholar] [CrossRef]
- Tanaka, N.; Okubo, T.; Anma, H.; Kim, K.H.; Inuzuka, Y.; Maekawa, M.; Kuroda-Sowa, T. Halido-Bridged 1D Mixed-Valence CuI–CuII Coordination Polymers Bearing a Piperidine-1-carbodithioato Ligand: Crystal Structure, Magnetic and Conductive Properties, and Application in Dye-Sensitized Solar Cells. Eur. J. Inorg. Chem. 2013, 2013, 3384–3391. [Google Scholar] [CrossRef]
- Okubo, T.; Anma, H.; Maekawa, M.; Kuroda-Sowa, T. Tris([mu]4-azepane-1-carbodithioato)bis([mu]3-azepane-1-carbodithioato)-[mu]9-bromido-tetra-[mu]2-bromido-octacopper(I)copper(II). Acta Crystallogr. Sect. E 2013, 69, m275–m276. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Okubo, T.; Anma, H.; Nakahashi, Y.; Maekawa, M.; Kuroda-Sowa, T. New one-dimensional mixed-valence coordination polymers including an iodine-bridged pentanuclear copper(I) cluster unit. Polyhedron 2014, 69, 103–109. [Google Scholar] [CrossRef]
- Nakatani, K.; Himoto, K.; Kono, Y.; Nakahashi, Y.; Anma, H.; Okubo, T.; Maekawa, M.; Kuroda-Sowa, T. Synthesis, Crystal Structure, and Electroconducting Properties of a 1D Mixed-Valence Cu(I)–Cu(II) Coordination Polymer with a Dicyclohexyl Dithiocarbamate Ligand. Crystals 2015, 5, 215–225. [Google Scholar] [CrossRef] [Green Version]
- Himoto, K.; Suzuki, S.; Okubo, T.; Maekawa, M.; Kuroda-Sowa, T. A new semiconducting 1D Cu(I)–Cu(II) mixed-valence coordination polymer with Cu(II) dimethylpiperidine–dithiocarbamate and a tetranuclear Cu(I)–Br cluster unit. New J. Chem. 2018, 42, 3995–3998. [Google Scholar] [CrossRef]
- Mensforth, E.; Hill, M.; Batten, S. Coordination polymers of sulphur-donor ligands. Inorg. Chim. Acta 2013, 403, 9–24. [Google Scholar] [CrossRef]
- Cras, J.; Willemse, J.; Gal, A.; Hummelink-Peters, B. Preparation, structure and properties of compounds containing the dipositive tri-copper hexa (N, N-di-n-butyldithiocarbamato) ion, compounds with copper in the oxidation states II and III. Recl. Des Trav. Chim. Des Pays Bas 1973, 92, 641–650. [Google Scholar] [CrossRef] [Green Version]
- van de Leemput, P.J.H.A.M.; Cras, J.A.; Willemse, J.; Beurskens, P.T.; Menger, E. Preparation, structure and properties of dicopper tris(N,N-dialkyldithiocarbamato)dihalogeno compounds, Cu2(R2dtc)3X2, with copper in the oxidation states II and III. Recl. Des Trav. Chim. Des Pays Bas 1976, 95, 191–194. [Google Scholar] [CrossRef]
- Cras, J.A.; Willemse, J. Reactions of bisdithiocarbamato Cu(II) and Ni(II) with zinc halides. J. Inorg. Nucl. Chem. 1977, 39, 1225–1226. [Google Scholar] [CrossRef]
- Spek, A. Bis [bis (N, N-di-normal-butyldithiocarbamato) copper (III)] bis(dibutyldithiocarbamato) copper (II) tetrabromo-di-mu-bromo-di-mercurate (II)(−80°C), C54H108Br6Cu3Hg2N6S12. Cryst. Struct. Commun. 1979, 8, 577–582. [Google Scholar]
- Hogarth, G.; Faulkner, S. The mixed-valence coordination polymer [Cu(S2CNPr2)2]2[ClO4] containing alternating square-planar Cu(II) and Cu(III) centres. Inorg. Chem. Commun. 2013, 35, 65–68. [Google Scholar] [CrossRef]
- Padilla-Tosta, M.E.; Fox, O.D.; Drew, M.G.B.; Beer, P.D. Self-Assembly of a Mixed-Valence Copper(II)/Copper(III) Dithiocarbamate Catenane. Angewadte Chem. Int. Ed. Engl. 2001, 40, 4235–4239. [Google Scholar] [CrossRef]
- Okubo, T.; Kuwamoto, H.; Kim, K.H.; Hayami, S.; Yamano, A.; Shiro, M.; Maekawa, M.; Kuroda-Sowa, T. Intervalence Charge-Transfer System by 1D Assembly of New Mixed-Valence Octanuclear CuI/CuII/CuIII Cluster Units. Inorg. Chem. 2011, 50, 2708–2710. [Google Scholar] [CrossRef]
- Zhang, Y.; Schröder, K.; Kwak, Y.; Krys, P.; Morin, A.N.; Pintauer, T.; Poli, R.; Matyjaszewski, K. Reversible-Deactivation Radical Polymerization of Methyl Methacrylate and Styrene Mediated by Alkyl Dithiocarbamates and Copper Acetylacetonates. Macromolecules 2013, 46, 5512–5519. [Google Scholar] [CrossRef]
- Zhang, Q.; Chen, J.; Hong, M.; Xin, X.; Fun, H.-K. Crystal and molecular structure of the copper (I)-thiolate-selenide complex [Ph4P][Cu(SeS2CNC4H8)(S2CN2C4H8)] with an unusual Se-S bond. Z. Für Nat. B 1999, 54, 1313–1317. [Google Scholar] [CrossRef]
- Fan, L.; Wu, J. Crystal structure of (2,2′-bipyridine-κ2N,N′)-(N,N-dimethyldithiocarbamato-κ2S,S′)copper(II) iodide, CuI(C10H8N2)(C3H6NS2). Z. Für Krist. New Cryst. Struct. 2010, 225, 347–348. [Google Scholar] [CrossRef]
- Fan, L.-Q.; Ji-Hua, W.; Lin, J.; Huang, Y. Synthesis and Crystal Structure of a New Cu(II) Dithiocarbamate Complex CuI(prdtc)(Phen). Chin. J. Struct. Chem. 2009, 28, 580–584. [Google Scholar]
- Fan, L.-Q.; Ji-Huai, W.; Yun-Fang, H.; Ng, S. (N,N-Diethyldithiocarbamato-κ2S,S′)iodido(1,10-phenanthroline-κ2N,N′)copper(II). Acta Crystallogr. Sect. E 2009, 63, m1209. [Google Scholar] [CrossRef] [PubMed]
- Nobuo, O.; Munehiro, T.; Mamiko, O.; Masahiro, Y. Chloro(N,N’-dimethyldithiocarbamato-[kappa]2S,S’)(1,10-phenanthroline-[kappa]2N,N’)copper(II). Acta Crystallogr. Sect. E 2006, 62, m1589–m1591. [Google Scholar] [CrossRef]
- Fan, L.; Wu, J.; Huang, Y.; Lin, J. Synthesis, structure and characterization of a new trinuclear magnetic semiconductor PbI4[Cu(Me2dtc)(bipy)]2. Solid State Sci. 2010, 12, 558–562. [Google Scholar] [CrossRef]
- Liu, Q.; Yang, Y.; Huang, L.; Wu, D.; Kang, B.; Chen, C.; Deng, Y.; Lu, J. Study on an Assembly System Including Tetrathiovanadate. Syntheses and Structural Characterizations of V2Cu2S4 Cubane-Like Clusters and VS4Cu4 Bimetallic Aggregates. Inorg. Chem. 1995, 34, 1884–1893. [Google Scholar] [CrossRef]
- Yang, Y.; Liu, Q.; Huang, L.; Wu, D.; Kang, B.; Lu, J. Heterobimetallic aggregates of copper(I) with thiovanadate. Syntheses and characterizations of (Et4N)3[VS4Cu4(OC4H8dtc)n(PhS)4-n] (n = 0, 1, 2). Inorg. Chem. 1993, 32, 5431–5432. [Google Scholar] [CrossRef]
- Cao, R.; Bao, M.; Wu, D.; Su, W.; Hong, M. Syntheses and structures of cluster compounds containing WSe4Cun (n = 3,4) Cores with dialkyldithiocarbamate ligands. J. Coord. Chem. 2000, 49, 227–238. [Google Scholar] [CrossRef]
- Hong, M.; Zhang, Q.; Cao, R.; Wu, D.; Chen, J.; Zhang, W.; Liu, H.; Lu, J. Chemistry of the Tetraselenomolybdate Anion: Syntheses, Spectroscopic Results, and Structural Characterizations of Polynuclear Mo−Cu−Se Compounds Containing Thiolate Ligands. Inorg. Chem. 1997, 36, 6251–6260. [Google Scholar] [CrossRef]
- Zhang, Q.-F.; Bao, M.-T.; Hong, M.-C.; Cao, R.; Song, Y.-L.; Xin, X.-Q. Syntheses, crystal structures and non-linear optical responses of two new heteroselenometallic cluster compounds containing dithiocarbamate ligands. J. Chem. Soc. Dalton Trans. 2000, 605–610. [Google Scholar] [CrossRef]
- Zhiying, H.; Xinjian, L.; Beisheng, K.; Jiangnan, L.; Qiutian, L.; Maochun, H.; Hanqin, L. Heteronuclear W(Mo)/CuS complexes of dialkyldithiocarbamate. Syntheses of (Et4N)2[MCu3S4(dtcR2)3] and structures of (Et4N)2[MCu3S4(dtcC5H10)3]· DMF (M = W or Mo). Inorg. Chim. Acta 1990, 169, 25–29. [Google Scholar] [CrossRef]
- Zhu, H.; Huang, X.; Deng, Y.; Wu, D.; Chen, C.; Liu, Q. Synthesis and structural characterization of a linear copper(I) tetrathiomolybdate complex containing the Me2dtc− ligand, (Et4N)2[MoS4(CuMe2dtc)2]. Inorg. Chim. Acta 1997, 256, 29–34. [Google Scholar] [CrossRef]
- Lei, X.; Huang, Z.; Liu, Q.; Hong, M.; Liu, H. A novel polynuclear molybdenum-copper cluster from tetrathiomolybdate: Preparation and structure of (Et4N)2[Mo2Cu5S8(S2CNMe2)3].2H2O. Inorg. Chem. 1989, 28, 4302–4304. [Google Scholar] [CrossRef]
- Scott, J.A.; Angeloski, A.; Aharonovich, I.; Lobo, C.J.; McDonagh, A.; Toth, M. In situ study of the precursor conversion reactions during solventless synthesis of Co9S8, Ni3S2, Co and Ni nanowires. Nanoscale 2018, 10, 15669–15676. [Google Scholar] [CrossRef]
- Roffey, A.; Hollingsworth, N.; Islam, H.U.; Mercy, M.; Sankar, G.; Catlow, C.R.; Hogarth, G.; de Leeuw, N.H. Phase control during the synthesis of nickel sulfide nanoparticles from dithiocarbamate precursors. Nanoscale 2016, 8, 11067–11075. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Breviglieri, S.T.; Cavalheiro, É.T.G.; Chierice, G.O. Correlation between ionic radius and thermal decomposition of Fe(II), Co(II), Ni(II), Cu(II) and Zn(II) diethanoldithiocarbamates. Thermochim. Acta 2000, 356, 79–84. [Google Scholar] [CrossRef]
- Roy, P.; Srivastava, S.K. Nanostructured copper sulfides: Synthesis, properties and applications. CrystEngComm 2015, 17, 7801–7815. [Google Scholar] [CrossRef]
- Kim, Y.-Y.; Walsh, D. Metal sulfidenanoparticles synthesized via enzyme treatment of biopolymer stabilized nanosuspensions. Nanoscale 2010, 2, 240–247. [Google Scholar] [CrossRef]
- McCain, M.N.; Metz, A.W.; Yang, Y.; Stern, C.L.; Marks, T.J. Bis[di(2,2,2-trifluoroethyl) dithiocarbamato] CuII: A Volatile Precursor for the Efficient Growth of Cuprous Sulfide Films by MOCVD. Chem. Vap. Depos. 2005, 11, 291–294. [Google Scholar] [CrossRef]
- Chen, Y.-B.; Chen, L.; Wu, L.-M. Water-induced thermolytic formation of homogeneous core− shell CuS microspheres and their shape retention on desulfurization. Cryst. Growth Des. 2008, 8, 2736–2740. [Google Scholar] [CrossRef]
- Jen-La Plante, I.; Zeid, T.W.; Yang, P.; Mokari, T. Synthesis of metal sulfide nanomaterials via thermal decomposition of single-source precursors. J. Mater. Chem. 2010, 20, 6612–6617. [Google Scholar] [CrossRef] [Green Version]
- Lou, Y.; Samia, A.C.; Cowen, J.; Banger, K.; Chen, X.; Lee, H.; Burda, C. Evaluation of the photoinduced electron relaxation dynamics of Cu 1.8 S quantum dots. Phys. Chem. Chem. Phys. 2003, 5, 1091–1095. [Google Scholar] [CrossRef]
- Liu, Z.; Xu, D.; Liang, J.; Shen, J.; Zhang, S.; Qian, Y. Growth of Cu2S ultrathin nanowires in a binary surfactant solvent. J. Phys. Chem. B 2005, 109, 10699–10704. [Google Scholar] [CrossRef]
- Wu, Y.; Wadia, C.; Ma, W.; Sadtler, B.; Alivisatos, A.P. Synthesis and photovoltaic application of copper (I) sulfide nanocrystals. Nano Lett. 2008, 8, 2551–2555. [Google Scholar] [CrossRef]
- Luther, J.M.; Jain, P.K.; Ewers, T.; Alivisatos, A.P. Localized surface plasmon resonances arising from free carriers in doped quantum dots. Nat. Mater. 2011, 10, 361–366. [Google Scholar] [CrossRef]
- Tian, Q.; Jiang, F.; Zou, R.; Liu, Q.; Chen, Z.; Zhu, M.; Yang, S.; Wang, J.; Wang, J.; Hu, J. Hydrophilic Cu9S5 nanocrystals: A photothermal agent with a 25.7% heat conversion efficiency for photothermal ablation of cancer cells in vivo. ACS Nano 2011, 5, 9761–9771. [Google Scholar] [CrossRef]
- Li, B.; Wang, Q.; Zou, R.; Liu, X.; Xu, K.; Li, W.; Hu, J. Cu7.2S4 nanocrystals: A novel photothermal agent with a 56.7% photothermal conversion efficiency for photothermal therapy of cancer cells. Nanoscale 2014, 6, 3274–3282. [Google Scholar] [CrossRef]
- Botha, N.L.; Ajibade, P.A. Optical and structural characterization of copper sulphide nanoparticles from copper(II) piperidine dithiocarbamate. Opt. Quantum Electron. 2020, 52, 337. [Google Scholar] [CrossRef]
- ul Ain, N.; Zia ur, R.; Aamir, A.; Khan, Y.; Rehman, M.-u.; Lin, D.-J. Catalytic and photocatalytic efficacy of hexagonal CuS nanoplates derived from copper(II) dithiocarbamate. Mater. Chem. Phys. 2020, 242, 122408. [Google Scholar] [CrossRef]
- Solomane, N.; Ajibade, P.A. Synthesis and crystal structure of bis(thiomorpholinyldithiocarbamato)Cu(II) complex and its use as precursor for CuS nanoparticles photocatalyst for the degradation of organic dyes. J. Sulfur Chem. 2020, 42, 167–179. [Google Scholar] [CrossRef]
- Motaung, M.P.; Osuntokun, J.; Onwudiwe, D.C. The heat-up synthesis of monodispersed Bi2S3 and Cu7S4 nanoparticles from novel precursor complexes and their characterizations. Mater. Sci. Semicond. Process. 2019, 99, 92–98. [Google Scholar] [CrossRef]
- Cui, J.; Xu, S.; Guo, C.; Jiang, R.; James, T.D.; Wang, L. Highly efficient photothermal semiconductor nanocomposites for photothermal imaging of latent fingerprints. Anal. Chem. 2015, 87, 11592–11598. [Google Scholar] [CrossRef]
- Estrada, A.C.; Silva, F.M.; Soares, S.F.; Coutinho, J.A.; Trindade, T. An ionic liquid route to prepare copper sulphide nanocrystals aiming at photocatalytic applications. RSC Adv. 2016, 6, 34521–34528. [Google Scholar] [CrossRef]
- Olalekan, O.C.; Onwudiwe, D.C. Temperature controlled evolution of pure phase Cu9S5 nanoparticles by solvothermal process. Front. Mater. 2021, 8, 211. [Google Scholar]
- Khalid, S.; Ahmed, E.; Azad Malik, M.; Lewis, D.J.; Abu Bakar, S.; Khan, Y.; O’Brien, P. Synthesis of pyrite thin films and transition metal doped pyrite thin films by aerosol-assisted chemical vapour deposition. New J. Chem. 2015, 39, 1013–1021. [Google Scholar] [CrossRef] [Green Version]
- Majid, S.; Ahmad, K.S. Optical and morphological properties of environmentally benign Cu-Tin sulphide thin films grown by physical vapor deposition technique. Mater. Res. Express 2018, 6, 036406. [Google Scholar] [CrossRef]
- Hollingsworth, N.; Roffey, A.; Islam, H.-U.; Mercy, M.; Roldan, A.; Bras, W.; Wolthers, M.; Catlow, C.R.A.; Sankar, G.; Hogarth, G.; et al. Active Nature of Primary Amines during Thermal Decomposition of Nickel Dithiocarbamates to Nickel Sulfide Nanoparticles. Chem. Mater. 2014, 26, 6281–6292. [Google Scholar] [CrossRef]
- Sarker, J.C.; Hogarth, G. Dithiocarbamate Complexes as Single Source Precursors to Nanoscale Binary, Ternary and Quaternary Metal Sulfides. Chem. Rev. 2021, 121, 6057–6123. [Google Scholar] [CrossRef]
- Roffey, A.; Hollingsworth, N.; Islam, H.-U.; Bras, W.; Sankar, G.; De Leeuw, N.H.; Hogarth, G. Fe(II) and Fe(III) dithiocarbamate complexes as single source precursors to nanoscale iron sulfides: A combined synthetic and in situ XAS approach. Nanoscale Adv. 2019, 1, 2965–2978. [Google Scholar] [CrossRef] [Green Version]
- Cui, Y.; Ren, J.; Chen, G.; Qian, Y.; Xie, Y. A simple route to synthesize MInS2 (M= Cu, Ag) nanorods from single-molecule precursors. Chem. Lett. 2001, 30, 236–237. [Google Scholar] [CrossRef] [Green Version]
- Park, J.-H.; Afzaal, M.; Kemmler, M.; O’Brien, P.; Otway, D.J.; Raftery, J.; Waters, J. The deposition of thin films of CuME2 by CVD techniques (M= In, Ga and E= S, Se). J. Mater. Chem. 2003, 13, 1942–1949. [Google Scholar] [CrossRef]
- Pan, D.; An, L.; Sun, Z.; Hou, W.; Yang, Y.; Yang, Z.; Lu, Y. Synthesis of Cu− In− S ternary nanocrystals with tunable structure and composition. J. Am. Chem. Soc. 2008, 130, 5620–5621. [Google Scholar] [CrossRef]
- Hao, Z.; Cui, Y.; Wang, G. Colloidal synthesis of wurtzite CuInS2 nanocrystals and their photovoltaic application. Mater. Lett. 2015, 146, 77–80. [Google Scholar] [CrossRef]
- Shin, S.J.; Koo, J.-J.; Lee, J.-K.; Chung, T.D. Unique Luminescence of Hexagonal Dominant Colloidal Copper Indium Sulphide Quantum Dots in Dispersed Solutions. Sci. Rep. 2019, 9, 1–8. [Google Scholar] [CrossRef]
- Motaung, M.P.; Onwudiwe, D.C.; Wei, L.; Lou, C. CuS, In2S3 and CuInS2 nanoparticles by microwave-assisted solvothermal route and their electrochemical studies. J. Phys. Chem. Solids 2021, 160, 110319. [Google Scholar] [CrossRef]
- Li, Q.; Zhai, L.; Zou, C.; Huang, X.; Zhang, L.; Yang, Y.; Huang, S. Wurtzite CuInS2 and CuInxGa1− xS2 nanoribbons: Synthesis, optical and photoelectrical properties. Nanoscale 2013, 5, 1638–1648. [Google Scholar] [CrossRef]
- Li, Q.; Zou, C.; Zhai, L.; Zhang, L.; Yang, Y.; Huang, S. Synthesis of wurtzite CuInS2 nanowires by Ag2S-catalyzed growth. CrystEngComm 2013, 15, 1806–1813. [Google Scholar] [CrossRef]
- Nomura, R.; Kanaya, K.; Matsuda, H. Preparation of Copper-Indium-Sulfide thin films by solution Pyrolysis of organometallic sources. Chem. Lett. 1988, 17, 1849–1850. [Google Scholar] [CrossRef]
- Nomura, R.; Fujii, S.; Kanaya, K.; Matsuda, H. Oxygen-or sulphur-containing organoindium compounds for precursors of indium oxide and sulphide thin films. Polyhedron 1990, 9, 361–366. [Google Scholar] [CrossRef]
- Nowotny, M.; Foro, S.; Heinschke, S.; Hoffmann, R.C.; Schneider, J.J. 1,2-Dithiooxalato-Bridged Heterobimetallic Complexes as Single-Source Precursors for Ternary Metal Sulfide Semiconductors. Eur. J. Inorg. Chem. 2015, 2015, 512–519. [Google Scholar] [CrossRef]
- Pan, D.; Weng, D.; Wang, X.; Xiao, Q.; Chen, W.; Xu, C.; Yang, Z.; Lu, Y. Alloyed semiconductor nanocrystals with broad tunable band gaps. Chem. Commun. 2009, 4221–4223. [Google Scholar] [CrossRef] [PubMed]
- Hehemann, D.G.; Lau, J.E.; Harris, J.D.; Hoops, M.D.; Duffy, N.V.; Fanwick, P.E.; Khan, O.; Jin, M.H.-C.; Hepp, A.F. Synthesis, characterization and decomposition studies of tris (N, N-dibenzyldithiocarbamato) indium (III): Chemical spray deposition of polycrystalline CuInS2 on copper films. Mater. Sci. Eng. B 2005, 116, 381–389. [Google Scholar] [CrossRef]
- Wang, X.; Pan, D.; Weng, D.; Low, C.-Y.; Rice, L.; Han, J.; Lu, Y. A general synthesis of Cu−In−S based multicomponent solid-Solution nanocrystals with tunable band gap, size, and structure. J. Phys. Chem. C 2010, 114, 17293–17297. [Google Scholar] [CrossRef]
- Zou, C.; Zhang, L.; Zhai, L.; Lin, D.; Gao, J.; Li, Q.; Yang, Y.; Chen, X.a.; Huang, S. Solution-based synthesis of quaternary Cu–In–Zn–S nanobelts with tunable composition and band gap. Chem. Commun. 2011, 47, 5256–5258. [Google Scholar] [CrossRef]
- Lin, Y.; Zhang, F.; Pan, D. A facile route to (ZnS)x(CuInS2)1−x hierarchical microspheres with excellent water-splitting ability. J. Mater. Chem. 2012, 22, 22619–22623. [Google Scholar] [CrossRef]
- Xu, D.; Shen, S.; Zhang, Y.; Gu, H.; Wang, Q. Selective synthesis of ternary copper-antimony sulfide nanocrystals. Inorg. Chem. 2013, 52, 12958–12962. [Google Scholar] [CrossRef]
- Deng, M.; Shen, S.; Zhang, Y.; Xu, H.; Wang, Q. A generalized strategy for controlled synthesis of ternary metal sulfide nanocrystals. New J. Chem. 2014, 38, 77–83. [Google Scholar] [CrossRef]
- Adekoya, J.A.; Khan, M.D.; Revaprasadu, N. Phase transition in Cu2+xSnS3+y (0 ≤ x ≤ 2; 0 ≤ y ≤ 1) ternary systems synthesized from complexes of coumarin derived thiocarbamate motifs: Optical and morphological properties. RSC Adv. 2019, 9, 35706–35716. [Google Scholar] [CrossRef] [Green Version]
- van Embden, J.; Mendes, J.O.; Jasieniak, J.J.; Chesman, A.S.R.; Della Gaspera, E. Solution-Processed CuSbS2 Thin Films and Superstrate Solar Cells with CdS/In2S3 Buffer Layers. ACS Appl. Energy Mater. 2020, 3, 7885–7895. [Google Scholar] [CrossRef]
- Matthews, P.D.; McNaughter, P.D.; Lewis, D.J.; O’Brien, P. Shining a light on transition metal chalcogenides for sustainable photovoltaics. Chem. Sci. 2017, 8, 4177–4187. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Olatunde, O.C.; Onwudiwe, D.C. Chapter 3—Copper-based ternary metal sulfide nanocrystals embedded in graphene oxide as photocatalyst in water treatment. In Nanotechnology in the Beverage Industry; Amrane, A., Rajendran, S., Nguyen, T.A., Assadi, A.A., Sharoba, A.M., Eds.; Elsevier: Amsterdam, The Netherlands, 2020; pp. 51–113. [Google Scholar]
- Tshabalala, T.W.; Onwudiwe, D.C.; Elemike, E.E.; Okpara, E.C. Structural and optical characterizations of Cu2SnS3 nanoparticles and the electrochemical studies. Results Chem. 2021, submitted. [Google Scholar]
- Wang, Y.-H.A.; Bao, N.; Gupta, A. Shape-controlled synthesis of semiconducting CuFeS2 nanocrystals. Solid State Sci. 2010, 12, 387–390. [Google Scholar] [CrossRef]
- Roffey, A.; Hollingsworth, N.; Hogarth, G. Synthesis of ternary sulfide nanomaterials using dithiocarbamate complexes as single source precursors. Nanoscale Adv. 2019, 1, 3056–3066. [Google Scholar] [CrossRef] [Green Version]
- Ramasamy, K.; Malik, M.A.; O’Brien, P. The chemical vapor deposition of Cu2ZnSnS4 thin films. Chem. Sci. 2011, 2, 1170–1172. [Google Scholar] [CrossRef]
- Chernomordik, B.D.; Béland, A.E.; Deng, D.D.; Francis, L.F.; Aydil, E.S. Microstructure evolution and crystal growth in Cu2ZnSnS4 thin films formed by annealing colloidal nanocrystal coatings. Chem. Mater. 2014, 26, 3191–3201. [Google Scholar] [CrossRef]
- Exarhos, S.; Bozhilov, K.; Mangolini, L. Spray pyrolysis of CZTS nanoplatelets. Chem. Commun. 2014, 50, 11366–11369. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Zhai, L.; He, N.; Zou, C.; Geng, X.; Cheng, L.; Dong, Y.; Huang, S. Solution-based synthesis of wurtzite Cu2ZnSnS4 nanoleaves introduced by α-Cu2S nanocrystals as a catalyst. Nanoscale 2013, 5, 8114–8121. [Google Scholar] [CrossRef] [PubMed]
- Liu, K.; Yao, B.; Li, Y.; Ding, Z.; Sun, H.; Jiang, Y.; Wang, G.; Pan, D. A versatile strategy for fabricating various Cu2ZnSnS4 precursor solutions. J. Mater. Chem. C 2017, 5, 3035–3041. [Google Scholar] [CrossRef]
- Wang, G.; Zhao, W.; Cui, Y.; Tian, Q.; Gao, S.; Huang, L.; Pan, D. Fabrication of a Cu2ZnSn(S,Se)4 photovoltaic device by a low-toxicity ethanol solution process. ACS Appl. Mater. Interfaces 2013, 5, 10042–10047. [Google Scholar] [CrossRef]
- Metzler-Nolte, N.; Kraatz, H. Concepts and Models in Bioinorganic Chemistry; Wiley-VCH: Weinheim, Germany, 2006. [Google Scholar]
- Horn, N.; Møller, L.B.; Nurchi, V.M.; Aaseth, J. Chelating principles in Menkes and Wilson diseases: Choosing the right compounds in the right combinations at the right time. J. Inorg. Biochem. 2019, 190, 98–112. [Google Scholar] [CrossRef] [PubMed]
- Kodama, H.; Sato, E.; Gu, Y.H.; Shiga, K.; Fujisawa, C.; Kozuma, T. Effect of copper and diethyldithiocarbamate combination therapy on the macular mouse, an animal model of Menkes disease. J. Inherit. Metab. Dis. Off. J. Soc. Study Inborn Errors Metab. 2005, 28, 971–978. [Google Scholar] [CrossRef]
- Nobel, C.S.I.; Kimland, M.; Lind, B.; Orrenius, S.; Slater, A.F.G. Dithiocarbamates Induce Apoptosis in Thymocytes by Raising the Intracellular Level of Redox-active Copper. J. Biol. Chem. 1995, 270, 26202–26208. [Google Scholar] [CrossRef] [Green Version]
- Buac, D.; Schmitt, S.; Ventro, G.; Rani Kona, F.; Ping Dou, Q. Dithiocarbamate-based coordination compounds as potent proteasome inhibitors in human cancer cells. Mini Rev. Med. Chem. 2012, 12, 1193–1201. [Google Scholar] [CrossRef] [Green Version]
- Neims, A.H.; Coffey, D.S.; Hellerman, L. Interaction between tetraethylthiuram disulfide and the sulfhydryl groups of D-amino acid oxidase and of hemoglobin. J. Biol. Chem. 1966, 241, 5941–5948. [Google Scholar] [CrossRef]
- Hosni, M.; Meskini, N.; Prigent, A.F.; Anker, G.; Joulain, C.; El Habib, R.; Lagarde, M. Diethyldithiocarbamate (ditiocarb sodium) effect on arachidonic acid metabolism in human mononuclear cells. Glutathione peroxidase-like activity. Biochem. Pharmacol. 1992, 43, 1319–1329. [Google Scholar] [CrossRef]
- Zemaitis, M.A.; Greene, F.E. In vivo and in vitro effects of thiuram disulfides and dithiocarbamates on hepatic microsomal drug metabolism in the rat. Toxicol. Appl. Pharmacol. 1979, 48, 343–350. [Google Scholar] [CrossRef]
- Dierickx, P.J. In vitro interaction of dithiocarb with rat liver glutathione s-transferases. Pharmacol. Res. Commun. 1984, 16, 135–143. [Google Scholar] [CrossRef]
- Eneanya, D.I.; Bianchine, J.R.; Duran, D.O.; Andresen, B.D. The actions and metabolic fate of disulfiram. Annu. Rev. Pharmacol. Toxicol. 1981, 21, 575–596. [Google Scholar] [CrossRef] [PubMed]
- Suh, J.J.; Pettinati, H.M.; Kampman, K.M.; O’Brien, C.P. The status of disulfiram: A half of a century later. J. Clin. Psychopharmacol. 2006, 26, 290–302. [Google Scholar] [CrossRef]
- Heilig, M.; Egli, M. Pharmacological treatment of alcohol dependence: Target symptoms and target mechanisms. Pharmacol. Ther. 2006, 111, 855–876. [Google Scholar] [CrossRef]
- Cen, D.; Brayton, D.; Shahandeh, B.; Meyskens, F.L.; Farmer, P.J. Disulfiram facilitates intracellular Cu uptake and induces apoptosis in human melanoma cells. J. Med. Chem. 2004, 47, 6914–6920. [Google Scholar] [CrossRef] [Green Version]
- Lewis, D.J.; Deshmukh, P.; Tedstone, A.A.; Tuna, F.; O’Brien, P. On the interaction of copper (II) with disulfiram. Chem. Commun. 2014, 50, 13334–13337. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Santini, C.; Pellei, M.; Gandin, V.; Porchia, M.; Tisato, F.; Marzano, C. Advances in Copper Complexes as Anticancer Agents. Chem. Rev. 2014, 114, 815–862. [Google Scholar] [CrossRef] [PubMed]
- Imran, M.; Liviu, M.; Latif, S.; Mahmood, Z.; Naimat, I.; Zaman, S.S.; Fatima, S. Antibacterial Co(II), Ni(II), Cu(II) and Zn(II) complexes with biacetyl-derived Schiff bases. J. Serb. Chem. Soc. 2010, 75, 1075–1084. [Google Scholar] [CrossRef]
- Anacona, J.; Rangel, V.; Lorono, M.; Camus, J. Tetradentate metal complexes derived from cephalexin and 2, 6-diacetylpyridine bis (hydrazone): Synthesis, characterization and antibacterial activity. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2015, 149, 23–29. [Google Scholar] [CrossRef]
- Johnstone, T.C.; Suntharalingam, K.; Lippard, S.J. The next generation of platinum drugs: Targeted Pt (II) agents, nanoparticle delivery, and Pt (IV) prodrugs. Chem. Rev. 2016, 116, 3436–3486. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wehbe, M.; Leung, A.W.Y.; Abrams, M.J.; Orvig, C.; Bally, M.B. A Perspective—can copper complexes be developed as a novel class of therapeutics? Dalton Trans. 2017, 46, 10758–10773. [Google Scholar] [CrossRef]
- Wang, T.; Fu, Y.; Huang, T.; Liu, Y.; Wu, M.; Yuan, Y.; Li, S.; Li, C. Copper Ion Attenuated the Antiproliferative Activity of Di-2-pyridylhydrazone Dithiocarbamate Derivative; However, There Was a Lack of Correlation between ROS Generation and Antiproliferative Activity. Molecules 2016, 21, 1088. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ronconi, L.; Marzano, C.; Zanello, P.; Corsini, M.; Miolo, G.; Macca, C.; Trevisan, A.; Fregona, D. Gold (III) dithiocarbamate derivatives for the treatment of cancer: Solution chemistry, DNA binding, and hemolytic properties. J. Med. Chem. 2006, 49, 1648–1657. [Google Scholar] [CrossRef]
- Milacic, V.; Chen, D.; Ronconi, L.; Landis-Piwowar, K.R.; Fregona, D.; Dou, Q.P. A novel anticancer gold (III) dithiocarbamate compound inhibits the activity of a purified 20S proteasome and 26S proteasome in human breast cancer cell cultures and xenografts. Cancer Res. 2006, 66, 10478–10486. [Google Scholar] [CrossRef] [Green Version]
- Schreck, R.; Meier, B.; Männel, D.N.; Dröge, W.; Baeuerle, P.A. Dithiocarbamates as potent inhibitors of nuclear factor kappa B activation in intact cells. J. Exp. Med. 1992, 175, 1181–1194. [Google Scholar] [CrossRef]
- Nobel, C.S.I.; Burgess, D.H.; Zhivotovsky, B.; Burkitt, M.J.; Orrenius, S.; Slater, A.F. Mechanism of dithiocarbamate inhibition of apoptosis: Thiol oxidation by dithiocarbamate disulfides directly inhibits processing of the caspase-3 proenzyme. Chem. Res. Toxicol. 1997, 10, 636–643. [Google Scholar] [CrossRef] [PubMed]
- Tawari, P.E.; Wang, Z.; Najlah, M.; Tsang, C.W.; Kannappan, V.; Liu, P.; McConville, C.; He, B.; Armesilla, A.L.; Wang, W. The cytotoxic mechanisms of disulfiram and copper(II) in cancer cells. Toxicol. Res. 2015, 4, 1439–1442. [Google Scholar] [CrossRef] [Green Version]
- Chen, D.; Cui, Q.C.; Yang, H.; Dou, Q.P. Disulfiram, a clinically used anti-alcoholism drug and copper-binding agent, induces apoptotic cell death in breast cancer cultures and xenografts via inhibition of the proteasome activity. Cancer Res. 2006, 66, 10425–10433. [Google Scholar] [CrossRef] [Green Version]
- Lu, X.; Lin, B.; Xu, N.; Huang, H.; Wang, Y.; Lin, J.-M. Evaluation of the accumulation of disulfiram and its copper complex in A549 cells using mass spectrometry. Talanta 2020, 211, 120732. [Google Scholar] [CrossRef]
- Wang, F.; Jiao, P.; Qi, M.; Frezza, M.; Dou, Q.; Yan, B. Turning tumor-promoting copper into an anti-cancer weapon via high-throughput chemistry. Curr. Med. Chem. 2010, 17, 2685–2698. [Google Scholar] [CrossRef] [Green Version]
- Viola-Rhenals, M.; Rieber, M.S.; Rieber, M. Suppression of survival in human SKBR3 breast carcinoma in response to metal–chelator complexes is preferential for copper–dithiocarbamate. Biochem. Pharmacol. 2006, 71, 722–734. [Google Scholar] [CrossRef] [PubMed]
- Matias, A.C.; Manieri, T.M.; Cipriano, S.S.; Carioni, V.M.; Nomura, C.S.; Machado, C.M.; Cerchiaro, G. Diethyldithiocarbamate induces apoptosis in neuroblastoma cells by raising the intracellular copper level, triggering cytochrome c release and caspase activation. Toxicol. Vitr. 2013, 27, 349–357. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fujie, T.; Segawa, Y.; Yoshida, E.; Kimura, T.; Fujiwara, Y.; Yamamoto, C.; Satoh, M.; Naka, H.; Kaji, T. Induction of metallothionein isoforms by copper diethyldithiocarbamate in cultured vascular endothelial cells. J. Toxicol. Sci. 2016, 41, 225–232. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Viola-Rhenals, M.; Rieber, M.S.; Rieber, M. Role of peroxidases, thiols and Bak/Bax in tumor cell susceptibility to Cu[DEDTC] 2. Biochem. Pharmacol. 2007, 74, 841–850. [Google Scholar] [CrossRef]
- Wehbe, M.; Lo, C.; Leung, A.W.; Dragowska, W.H.; Ryan, G.M.; Bally, M.B. Copper(II) complexes of bidentate ligands exhibit potent anti-cancer activity regardless of platinum sensitivity status. Investig. New Drugs 2017, 35, 682–690. [Google Scholar] [CrossRef]
- Daniel, K.G.; Chen, D.; Orlu, S.; Cui, Q.C.; Miller, F.R.; Dou, Q.P. Clioquinol and pyrrolidine dithiocarbamate complex with copper to form proteasome inhibitors and apoptosis inducers in human breast cancer cells. Breast Cancer Res. 2005, 7, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Chen, D.; Peng, F.; Cui, Q.C.; Daniel, K.G.; Orlu, S.; Liu, J.; Dou, Q.P. Inhibition of prostate cancer cellular proteasome activity by a pyrrolidine dithiocarbamate-copper complex is associated with suppression of proliferation and induction of apoptosis. Front. Biosci. 2005, 10, 2932–2939. [Google Scholar] [CrossRef] [Green Version]
- Brustolin, L.; Pettenuzzo, N.; Nardon, C.; Quarta, S.; Montagner, I.; Pontisso, P.; Rosato, A.; Conte, P.; Merigliano, S.; Fregona, D. Labelled micelles for the delivery of cytotoxic Cu(II) and Ru(III) compounds in the treatment of aggressive orphan cancers: Design and biological in vitro data. Inorg. Biochem. 2020, 213, 111259. [Google Scholar] [CrossRef]
- Ajibade, P.A.; Andrew, F.P.; Botha, N.L.; Solomane, N. Synthesis, Crystal Structures and Anticancer Studies of Morpholinyldithiocarbamato Cu(II) and Zn(II) Complexes. Molecules 2020, 25, 3584. [Google Scholar] [CrossRef]
- Ajibade, P.A.; Fatokun, A.A.; Andrew, F.P. Synthesis, characterization and anti-cancer studies of Mn(II), Cu(II), Zn(II) and Pt(II) dithiocarbamate complexes—Crystal structures of the Cu(II) and Pt(II) complexes. Inorg. Chim. Acta 2020, 504, 119431. [Google Scholar] [CrossRef]
- Sedlacek, J.; Martins, L.M.D.R.S.; Danek, P.; Pombeiro, A.J.L.; Cvek, B. Diethyldithiocarbamate complexes with metals used as food supplements show different effects in cancer cells. J. Appl. Biomed. 2014, 12, 301–308. [Google Scholar] [CrossRef]
- Daniel, K.G.; Chen, D.; Yan, B.; Dou, Q.P. Copper-binding compounds as proteasome inhibitors and apoptosis inducers in human cancer. Front. Biosci. 2007, 12, 135–144. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, H.; Wu, J.-S.; Peng, F. Potent anticancer activity of pyrrolidine dithiocarbamate–copper complex against cisplatin-resistant neuroblastoma cells. Anti-Cancer Drugs 2008, 19, 125–132. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Zhai, S.; Liu, X.; Li, L.; Wu, S.; Dou, Q.P.; Yan, B. A novel dithiocarbamate analogue with potentially decreased ALDH inhibition has copper-dependent proteasome-inhibitory and apoptosis-inducing activity in human breast cancer cells. Cancer Lett. 2011, 300, 87–95. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yu, Z.; Wang, F.; Milacic, V.; Li, X.; Cui, Q.C.; Zhang, B.; Yan, B.; Dou, Q.P. Evaluation of copper-dependent proteasome-inhibitory and apoptosis-inducing activities of novel pyrrolidine dithiocarbamate analogues. Int. J. Mol. Med. 2007, 20, 919–925. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Turnaturi, R.; Oliveri, V.; Viale, M.; Monticone, M.; Vecchio, G. Antiproliferative and antioxidant activity of glycoconjugates of dithiocarbamates and their copper(II) and zinc(II) complexes. ChemPlusChem 2015, 80, 1786. [Google Scholar] [CrossRef] [PubMed]
- Wu, W.; Yu, L.; Jiang, Q.; Huo, M.; Lin, H.; Wang, L.; Chen, Y.; Shi, J. Enhanced tumor-specific disulfiram chemotherapy by in situ Cu2+ chelation-initiated nontoxicity-to-toxicity transition. J. Am. Chem. Soc. 2019, 141, 11531–11539. [Google Scholar] [CrossRef]
- Zhou, L.; Yang, L.; Yang, C.; Liu, Y.; Chen, Q.; Pan, W.; Cai, Q.; Luo, L.; Liu, L.; Jiang, S. Membrane loaded copper oleate PEGylated liposome combined with disulfiram for improving synergistic antitumor effect in vivo. Pharm. Res. 2018, 35, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Peng, X.; Pan, Q.; Zhang, B.; Wan, S.; Li, S.; Luo, K.; Pu, Y.; He, B. Highly stable, coordinated polymeric nanoparticles loading copper(II) diethyldithiocarbamate for combinational chemo/chemodynamic therapy of cancer. Biomacromolecules 2019, 20, 2372–2383. [Google Scholar] [CrossRef]
- Bakthavatsalam, S.; Wiangnak, P.; George, D.J.; Zhang, T.; Franz, K.J. Dithiocarbamate prodrugs activated by prostate specific antigen to target prostate cancer. Bioorganic Med. Chem. Lett. 2020, 30, 127148. [Google Scholar] [CrossRef]
- Bakthavatsalam, S.; Sleeper, M.L.; Dharani, A.; George, D.J.; Zhang, T.; Franz, K.J. Leveraging γ-Glutamyl Transferase To Direct Cytotoxicity of Copper Dithiocarbamates against Prostate Cancer Cells. Angew. Chem. Int. Ed. 2018, 57, 12780–12784. [Google Scholar] [CrossRef]
- Wehbe, M.; Anantha, M.; Shi, M.; Leung, A.W.-Y.; Dragowska, W.H.; Sanche, L.; Bally, M.B. Development and optimization of an injectable formulation of copper diethyldithiocarbamate, an active anticancer agent. Int. J. Nanomed. 2017, 12, 4129. [Google Scholar] [CrossRef] [Green Version]
- Peng, Y.; Liu, P.; Meng, Y.; Hu, S.; Ding, J.; Zhou, W. Nanoscale Copper (II)–Diethyldithiocarbamate Coordination Polymer as a Drug Self-Delivery System for Highly Robust and Specific Cancer Therapy. Mol. Pharm. 2020, 17, 2864–2873. [Google Scholar] [CrossRef]
- Marengo, A.; Forciniti, S.; Dando, I.; Dalla Pozza, E.; Stella, B.; Tsapis, N.; Yagoubi, N.; Fanelli, G.; Fattal, E.; Heeschen, C. Pancreatic cancer stem cell proliferation is strongly inhibited by diethyldithiocarbamate-copper complex loaded into hyaluronic acid decorated liposomes. Biochim. Et Biophys. Acta (BBA) Gen. Subj. 2019, 1863, 61–72. [Google Scholar] [CrossRef] [Green Version]
- Li, X.; Du, K.; Sun, J.; Feng, F. Apoferritin as a Carrier of Cu(II) Diethyldithiocarbamate and Biomedical Application for Glutathione-Responsive Combination Chemotherapy. ACS Appl. Bio Mater. 2019, 3, 654–663. [Google Scholar] [CrossRef]
- Chen, W.; Yang, W.; Chen, P.; Huang, Y.; Li, F. Disulfiram copper nanoparticles prepared with a stabilized metal ion ligand complex method for treating drug-resistant prostate cancers. ACS Appl. Mater. Interfaces 2018, 10, 41118–41128. [Google Scholar] [CrossRef]
- Ren, L.; Feng, W.; Shao, J.; Ma, J.; Xu, M.; Zhu, B.-Z.; Zheng, N.; Liu, S. Diethyldithiocarbamate-copper nanocomplex reinforces disulfiram chemotherapeutic efficacy through light-triggered nuclear targeting. Theranostics 2020, 10, 6384. [Google Scholar] [CrossRef]
- Shi, H.; Suo, Y.; Zhang, Z.; Liu, R.; Liu, H.; Cheng, Z. Copper (II)-disulfiram loaded melanin-dots for cancer theranostics. Nanomed. Nanotechnol. Biol. Med. 2021, 32, 102340. [Google Scholar] [CrossRef]
- Cvek, B.; Milacic, V.; Taraba, J.; Dou, Q.P. Ni(II), Cu(II), and Zn(II) diethyldithiocarbamate complexes show various activities against the proteasome in breast cancer cells. J. Med. Chem. 2008, 51, 6256–6258. [Google Scholar] [CrossRef] [PubMed]
- Cvek, B.; Dvorak, Z. Targeting of nuclear factor-κB and proteasome by dithiocarbamate complexes with metals. Curr. Pharm. Des. 2007, 13, 3155–3167. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Milacic, V.; Chen, D.; Giovagnini, L.; Diez, A.; Fregona, D.; Dou, Q.P. Pyrrolidine dithiocarbamate-zinc (II) and-copper (II) complexes induce apoptosis in tumor cells by inhibiting the proteasomal activity. Toxicol. Appl. Pharmacol. 2008, 231, 24–33. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fujie, T.; Murakami, M.; Yoshida, E.; Tachinami, T.; Shinkai, Y.; Fujiwara, Y.; Yamamoto, C.; Kumagai, Y.; Naka, H.; Kaji, T. Copper diethyldithiocarbamate as an activator of Nrf2 in cultured vascular endothelial cells. J. Biol. Inorg. Chem. 2016, 21, 263–273. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yeo, C.I.; Tiekink, E.R.T.; Chew, J. Insights into the Antimicrobial Potential of Dithiocarbamate Anions and Metal-Based Species. Inorganics 2021, 9, 48. [Google Scholar] [CrossRef]
- Chen, C.; Yang, K.-W.; Wu, L.-Y.; Li, J.-Q.; Sun, L.-Y. Disulfiram as a potent metallo-β-lactamase inhibitor with dual functional mechanisms. Chem. Commun. 2020, 56, 2755–2758. [Google Scholar] [CrossRef]
- Wang, M.-M.; Chu, W.-C.; Yang, Y.; Yang, Q.-Q.; Qin, S.-S.; Zhang, E. Dithiocarbamates: Efficient metallo-β-lactamase inhibitors with good antibacterial activity when combined with meropenem. Bioorganic Med. Chem. Lett. 2018, 28, 3436–3440. [Google Scholar] [CrossRef]
- Balakrishnan, S.; Duraisamy, S.; Kasi, M.; Kandasamy, S.; Sarkar, R.; Kumarasamy, A. Syntheses, physicochemical characterization, antibacterial studies on potassium morpholine dithiocarbamate nickel (II), copper (II) metal complexes and their ligands. Heliyon 2019, 5, e01687. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oladipo, S.D.; Omondi, B.; Mocktar, C. Synthesis and structural studies of nickel(II)- and copper(II)-N,N′-diarylformamidine dithiocarbamate complexes as antimicrobial and antioxidant agents. Polyhedron 2019, 170, 712–722. [Google Scholar] [CrossRef]
- Oladipo, S.D.; Mocktar, C.; Omondi, B. In vitro biological studies of heteroleptic Ag(I) and Cu(I) unsymmetrical N,N′-diarylformamidine dithiocarbamate phosphine complexes; the effect of the metal center. Arab. J. Chem. 2020, 13, 6379–6394. [Google Scholar] [CrossRef]
- Tetteh, S.; Dodoo, D.K.; Appiah-Opong, R.; Tuffour, I. Cytotoxicity, antioxidant and glutathione S -transferase inhibitory activity of palladium(II) chloride complexes bearing nucleobase ligands. Transit. Met. Chem. 2014, 39, 667–674. [Google Scholar] [CrossRef]
- Corona-Bustamante, A.; Viveros-Paredes, J.M.; Flores-Parra, A.; Peraza-Campos, A.L.; Martínez-Martínez, F.J.; Sumaya-Martínez, M.T.; Ramos-Organillo, Á. Antioxidant Activity of Butyl- and Phenylstannoxanes Derivedfrom 2-, 3- and 4-Pyridinecarboxylic Acids. Molecules 2010, 15, 5445–5459. [Google Scholar] [CrossRef]
- Chen, W.; Sun, S.; Cao, W.; Liang, Y.; Song, J. Antioxidant property of quercetin–Cr(III) complex: The role of Cr(III) ion. J. Mol. Struct. 2009, 918, 194–197. [Google Scholar] [CrossRef]
- Fragoso, A.; Cao, R.; D’Souza, V.T. Influence of Positively-Charged Guests on the Superoxide Dismutase Mimetic Activity of Copper (II) β-Cyclodextrin Dithiocarbamates. J. Carbohydr. Chem. 1997, 16, 171–180. [Google Scholar] [CrossRef]
- Martín, R.; Fragoso, A.; Cao, R. Complexation of bis (morpholyldithiocarbamato) copper (II), a superoxide scavenger, in β-cyclodextrins. Supramol. Chem. 2003, 15, 171–175. [Google Scholar] [CrossRef]
- Cao, R.; Travieso, N.; Fragoso, A.; Villalonga, R.; Diaz, A.; Martinez, M.E.; Alpizar, J.; West, D.X. Determination of SOD-Like activity of Copper (II) complexes with α-Amino acid dithiocarbamates. J. Inorg. Biochem. 1997, 66, 213–217. [Google Scholar] [CrossRef]
- Cao, R.; Pérez, L.G.; Moya, R.; Díaz, A. Interaction of Copper (II) Dithiocarbamates with Hydrogen Peroxide. Rev. Soc. Química México 2000, 44, 158–162. [Google Scholar]
- Fragoso, A.; Cao, R.; Villalonga, R. Superoxide Dismutase Mimetic Activity of the Metal (II) Complexes of a Dithiocarbamate Derivative of β-Cyclodextrin1. Carbohydr. Chem. 1995, 14, 1379–1386. [Google Scholar] [CrossRef]
- Díaz, A.M.; Villalonga, R.; Cao, R. Antioxidative properties of copper (II) complexes. J. Coord. Chem. 2009, 62, 100–107. [Google Scholar] [CrossRef]
- Matsumoto, K.; Fujibayashi, Y.; Konishi, J.; Yokoyama, A. Radiolabeling and Biodistribution of 62Cu-Dithiocarbamate; An Application for the New 62Zn62Cu Generator. Radioisotopes 1990, 39, 482–486. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Southworth, R.; Torres Martin de Rosales, R.; Meszaros, L.K.; Ma, M.T.; Mullen, G.E.D.; Fruhwirth, G.; Young, J.D.; Imberti, C.; Bagunya-Torres, J.; Andreozzi, E.; et al. Opportunities and challenges for metal chemistry in molecular imaging: From gamma camera imaging to PET and multimodality imaging. Adv. Inorg. Chem. 2016, 68, 1–41. [Google Scholar]
- Rowshanfarzad, P.; Sabet, M.; Reza Jalilian, A.; Kamalidehghan, M. An overview of copper radionuclides and production of 61Cu by proton irradiation of natZn at a medical cyclotron. Appl. Radiat. Isot. 2006, 64, 1563–1573. [Google Scholar] [CrossRef]
- Dearling, J.L.J.; Mullen, G.E.D.; Lewis, J.S.; Welch, M.J.; Blower, P.J. Dithiocarbamate copper complexes as blood flow tracers. Label. Compd. Radiopharm. 1999, 42, 835. [Google Scholar]
- Charoenphun, P.; Paul, R.; Weeks, A.; Berry, D.; Shaw, K.; Mullen, G.; Ballinger, J.; Blower, P. PET tracers for cell labelling with the complexes of copper 64 with lipophilic ligands. Eur. J. Nucl. Med. Mol. Imaging 2011, 38, S294. [Google Scholar]
- Benyettou, F.; Lalatonne, Y.; Sainte-Catherine, O.; Monteil, M.; Motte, L. Superparamagnetic nanovector with anti-cancer properties: γFe2O3@ Zoledronate. Int. J. Pharm. 2009, 379, 324–327. [Google Scholar] [CrossRef]
- Lalatonne, Y.; Paris, C.; Serfaty, J.M.; Weinmann, P.; Lecouvey, M.; Motte, L. Bis-phosphonates–ultra small superparamagnetic iron oxide nanoparticles: A platform towards diagnosis and therapy. Chem. Commun. 2008, 2553–2555. [Google Scholar] [CrossRef]
- Ge, Y.; Xiao, D.; Li, Z.; Cui, X. Dithiocarbamate functionalized lignin for efficient removal of metallic ions and the usage of the metal-loaded bio-sorbents as potential free radical scavengers. J. Mater. Chem. A 2014, 2, 2136–2145. [Google Scholar] [CrossRef]
- Xiang, B.; Fan, W.; Yi, X.; Wang, Z.; Gao, F.; Li, Y.; Gu, H. Dithiocarbamate-modified starch derivatives with high heavy metal adsorption performance. Carbohydr. Polym. 2016, 136, 30–37. [Google Scholar] [CrossRef] [PubMed]
- Fu, F.; Xiong, Y.; Xie, B.; Chen, R. Adsorption of Acid Red 73 on copper dithiocarbamate precipitate-type solid wastes. Chemosphere 2007, 66, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Carella, A.; Borbone, F.; Centore, R. Research Progress on Photosensitizers for DSSC. Front. Chem. 2018, 6, 481. [Google Scholar] [CrossRef] [PubMed]
- Singh, V.; Chauhan, R.; Gupta, A.N.; Kumar, V.; Drew, M.G.; Bahadur, L.; Singh, N. Photosensitizing activity of ferrocenyl bearing Ni(II) and Cu(II) dithiocarbamates in dye sensitized TiO2 solar cells. Dalton Trans. 2014, 43, 4752–4761. [Google Scholar] [CrossRef]
- Yadav, R.; Waghadkar, Y.; Kociok-Köhn, G.; Kumar, A.; Rane, S.B.; Chauhan, R. Transition metal ferrocenyl dithiocarbamates functionalized dye-sensitized solar cells with hydroxy as an anchoring group. Opt. Mater. 2016, 62, 176–183. [Google Scholar] [CrossRef]
- Ma, D.; Lu, X.; Shi, L.; Zhang, H.; Jiang, Y.; Liu, X. Domino Condensation/S-Arylation/Heterocyclization Reactions: Copper-Catalyzed Three-Component Synthesis of 2-N-Substituted Benzothiazoles. Angew. Chem. 2011, 123, 1150–1153. [Google Scholar] [CrossRef]
- Ashirbaev, S.S.; Levin, V.V.; Struchkova, M.I.; Dilman, A.D. Copper-Catalyzed Coupling of Acyl Chlorides with gem-Difluorinated Organozinc Reagents via Acyl Dithiocarbamates. J. Org. Chem. 2018, 83, 478–483. [Google Scholar] [CrossRef] [PubMed]
- Gao, M.Y.; Xu, W.; Zhang, S.B.; Li, Y.S.; Dong, Z.B. Synthesis of Phenyl Dithiocarbamates Starting from Sodium Dialkyldithiocarbamates and Aryl Boronic Acids: A Copper Catalyzed S-Arylation. Eur. J. Org. Chem. 2018, 2018, 6693–6698. [Google Scholar] [CrossRef]
- Dong, Z.-B.; Liu, X.; Bolm, C. Copper-catalyzed C (sp2)–S coupling reactions for the synthesis of aryl dithiocarbamates with thiuram disulfide reagents. Org. Lett. 2017, 19, 5916–5919. [Google Scholar] [CrossRef]
- Zeng, M.T.; Xu, W.; Liu, X.; Chang, C.Z.; Zhu, H.; Dong, Z.B. Copper-Catalyzed S-Arylation of Tetraalkylthiuram Disulfides by Using Diaryliodonium Salts. Eur. J. Org. Chem. 2017, 2017, 6060–6066. [Google Scholar] [CrossRef]
- Xu, W.; Gao, F.; Dong, Z.B. Copper-Catalyzed S-Arylation Starting from Arylboronic Acids and Tetraalkylthiuram Disulfide. Eur. J. Org. Chem. 2018, 2018, 821–828. [Google Scholar] [CrossRef]
- Peng, K.; Zhu, H.; Liu, X.; Peng, H.Y.; Chen, J.Q.; Dong, Z.B. Chemoselective C–S/S–S Formation between Diaryl Disulfides and Tetraalkylthiuram Disulfides. Eur. J. Org. Chem. 2019, 2019, 7629–7634. [Google Scholar] [CrossRef]
- Li, P.; Qiu, K.Y. Cu(S2CNEt2)Cl-Catalyzed Reverse Atom-Transfer Radical Polymerization of Vinyl Monomers. Macromol. Rapid Commun. 2002, 23, 1124–1129. [Google Scholar] [CrossRef]
- Kwak, Y.; Nicolaÿ, R.; Matyjaszewski, K. A simple and efficient synthesis of RAFT chain transfer agents via atom transfer radical addition−fragmentation. Macromolecules 2009, 42, 3738–3742. [Google Scholar] [CrossRef]
- Nishimura, M.; Kamigaito, M.; Sawamoto, M. Living radical polymerization of styrene with transition metal dithiocarbamate/AIBN systems: Halogen-free living processes. Polym. Prepr. 1999, 40, 470–471. [Google Scholar]
- Pateman, A. Copper Catalysed Aziridination Reactions. Ph.D. Thesis, University College London, London, UK, 1999. [Google Scholar]
- Pateman, A.; Cardell, D.C.; Hogarth, G. Copper Dithiocarbamate Complexes as Catalysts for Alkene Aziridination. University College London: London, UK, Unpublished work. 2003. [Google Scholar]
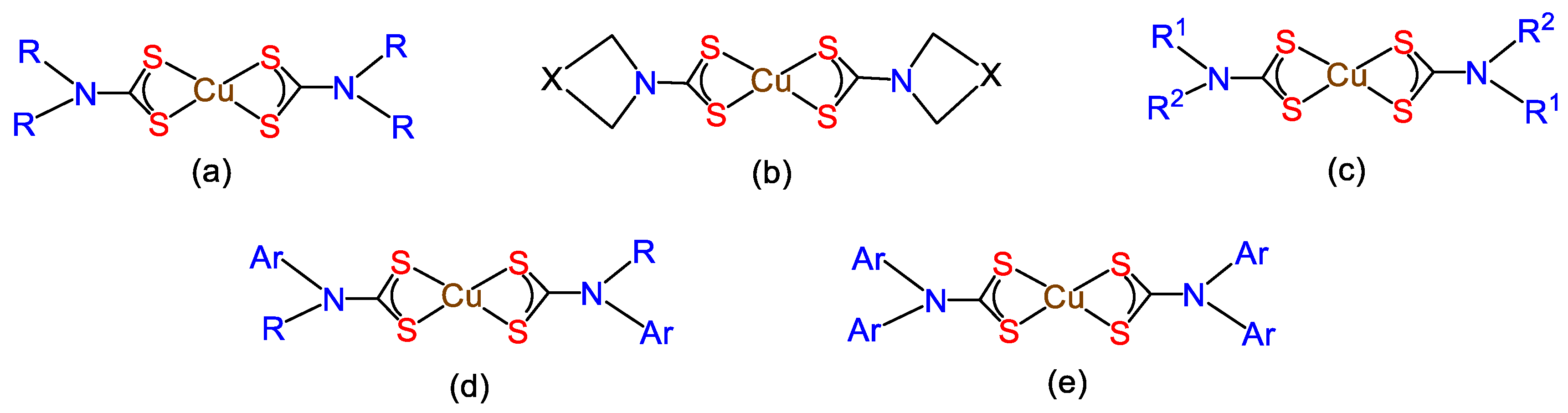





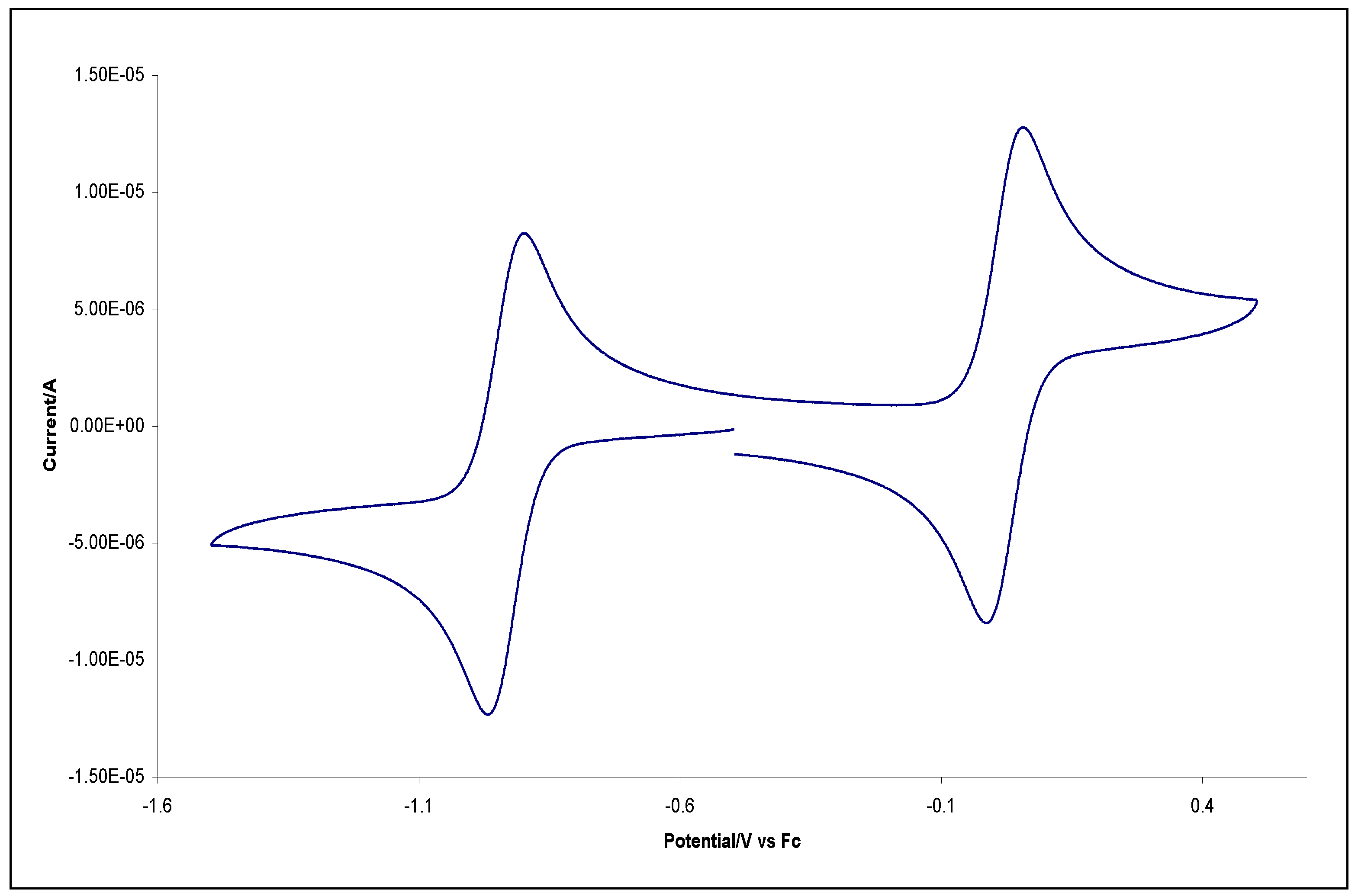

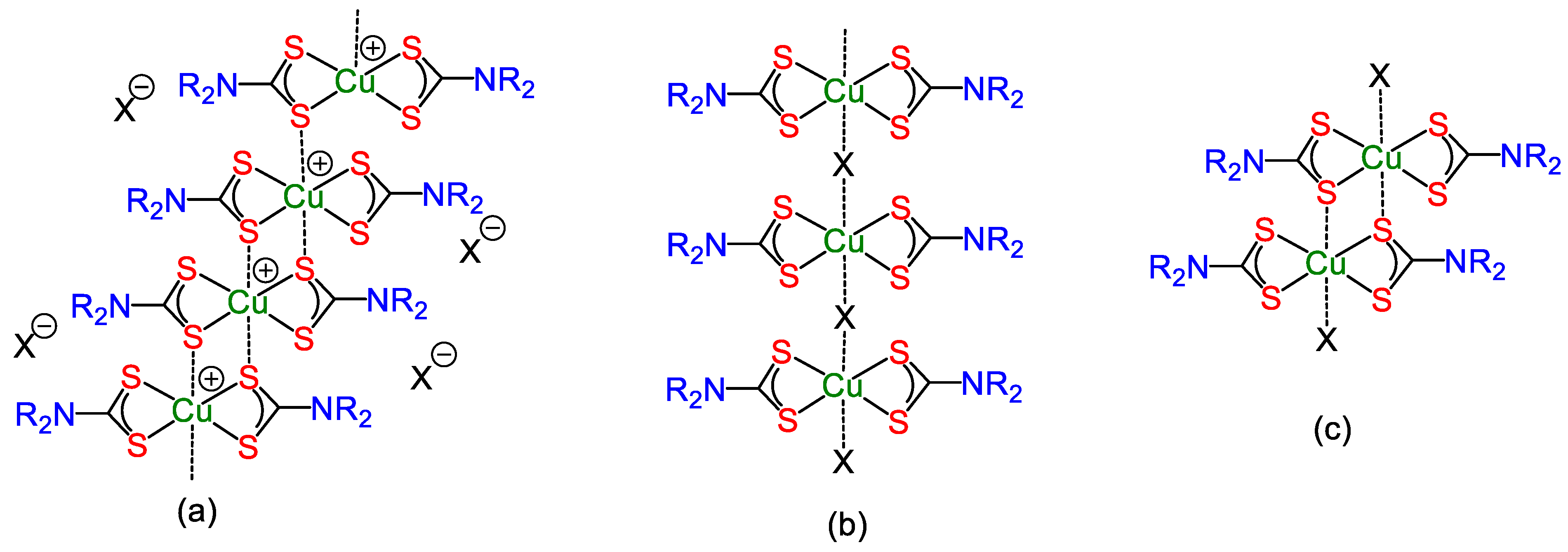
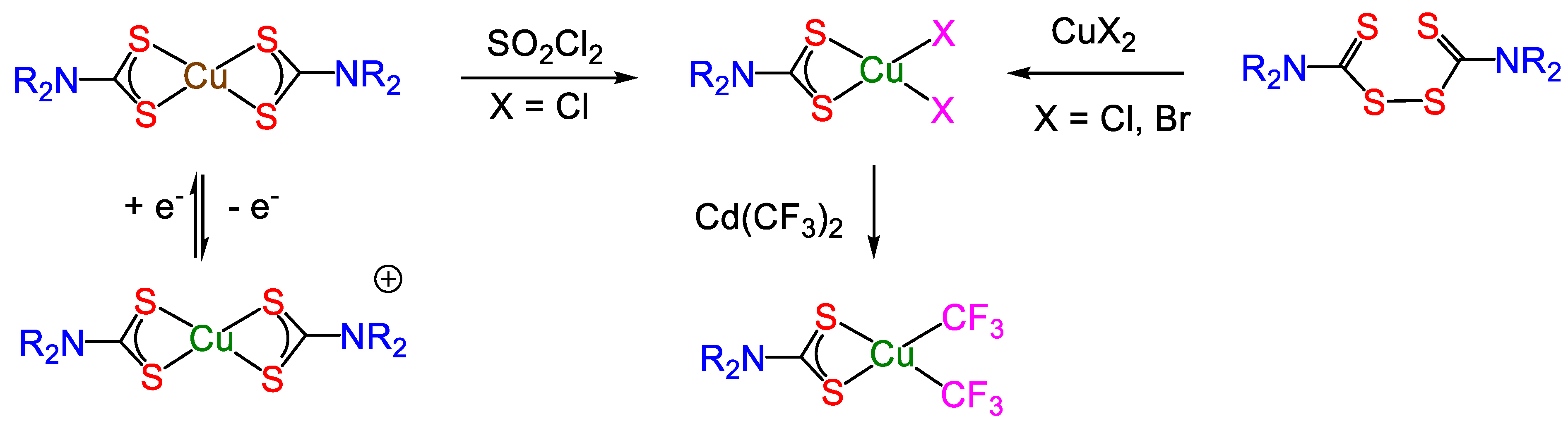



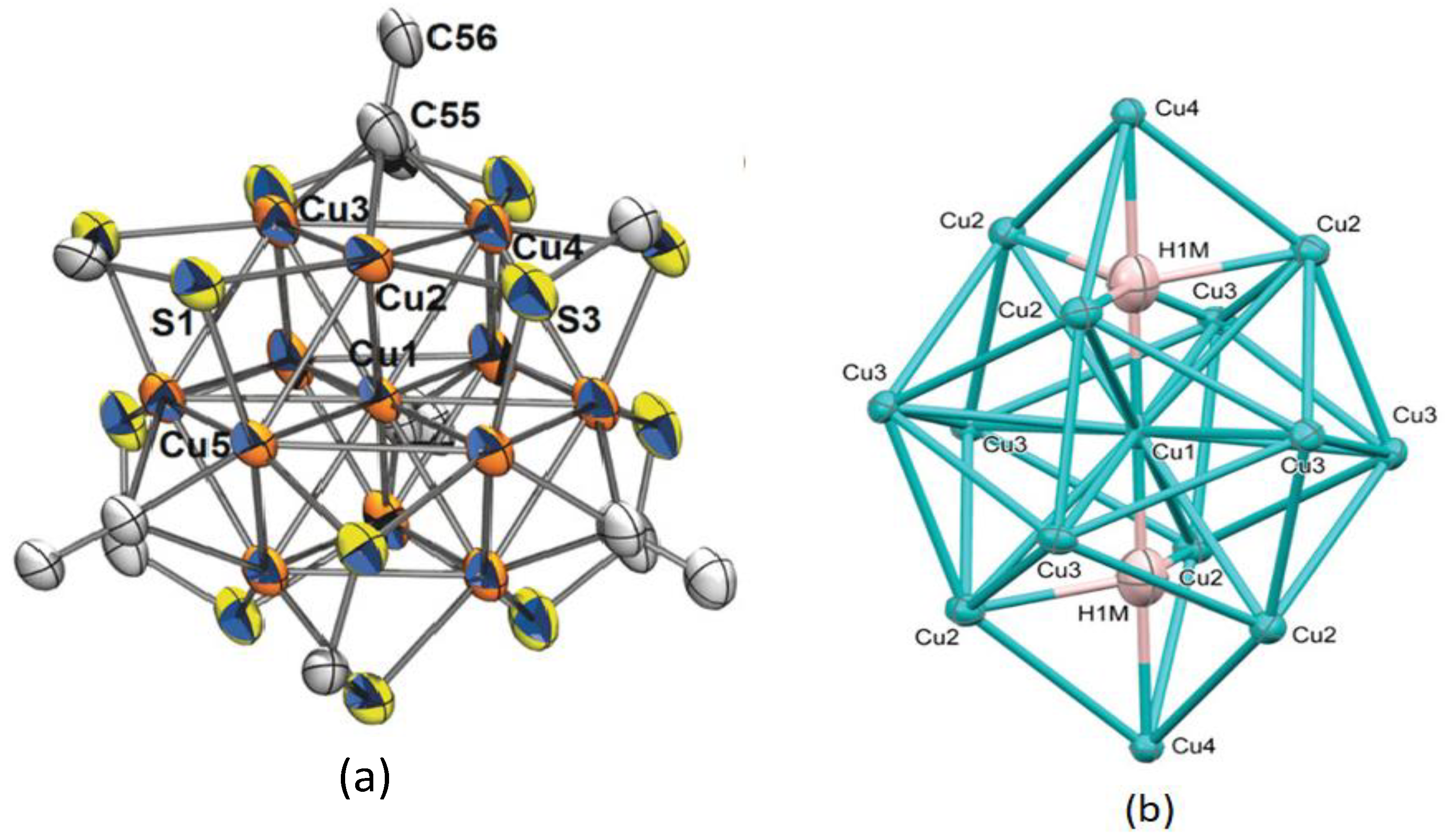

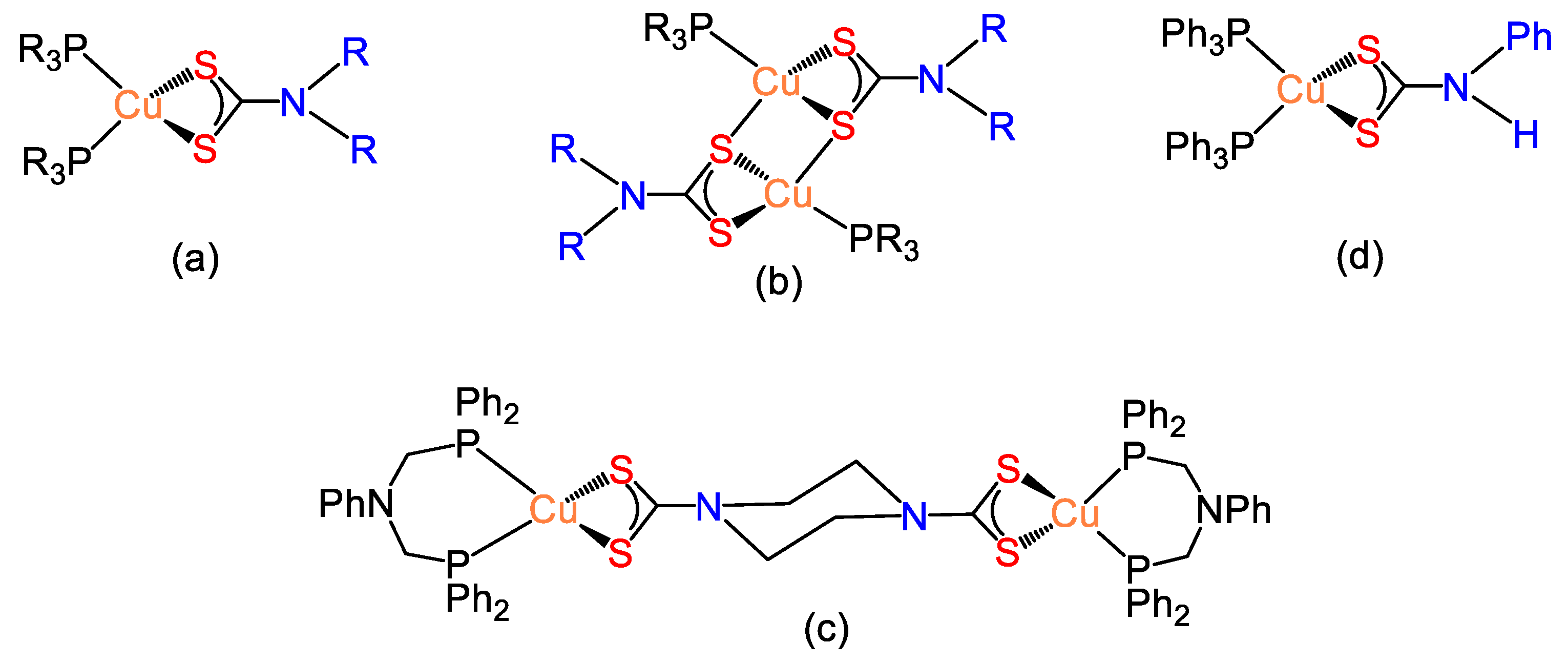

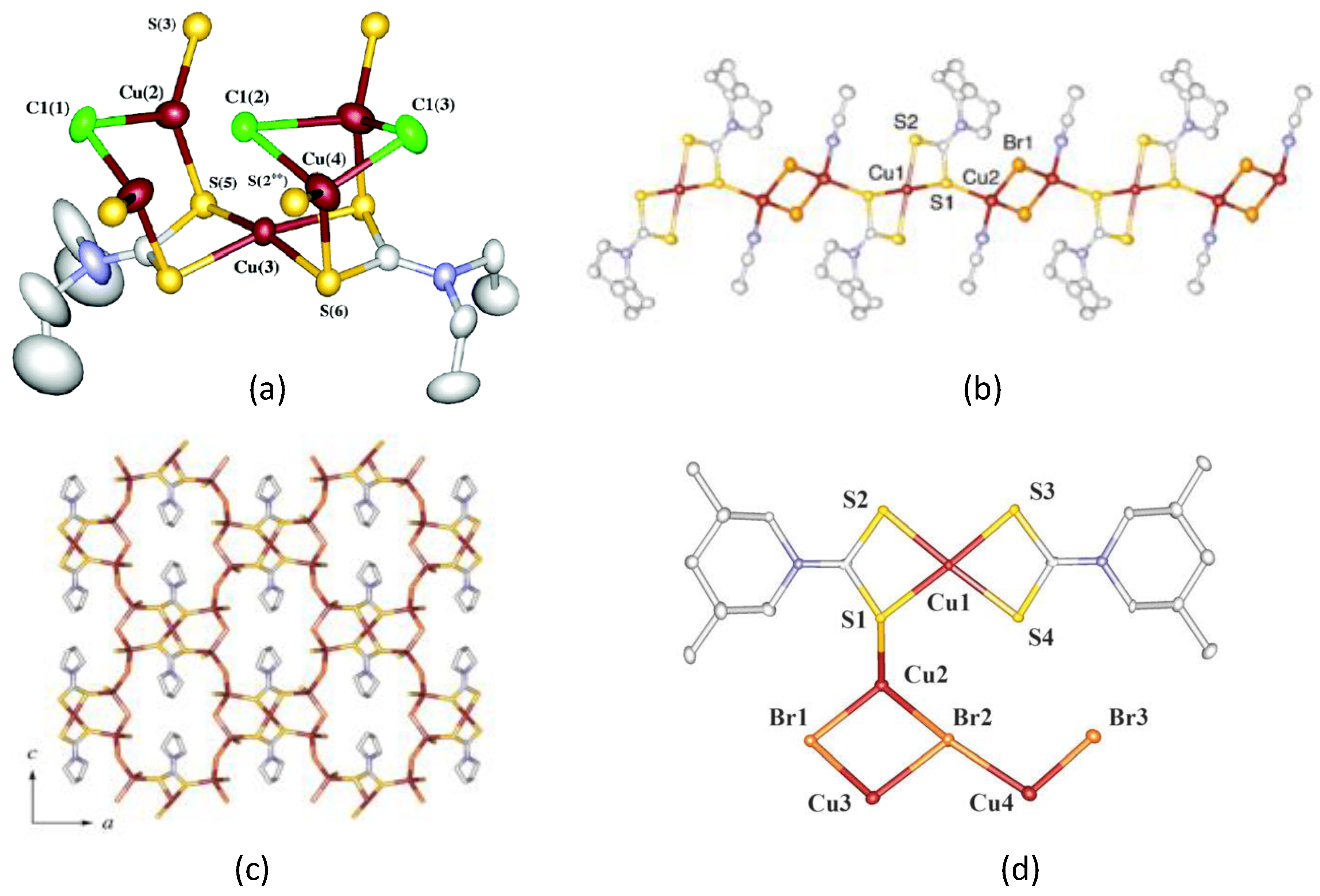



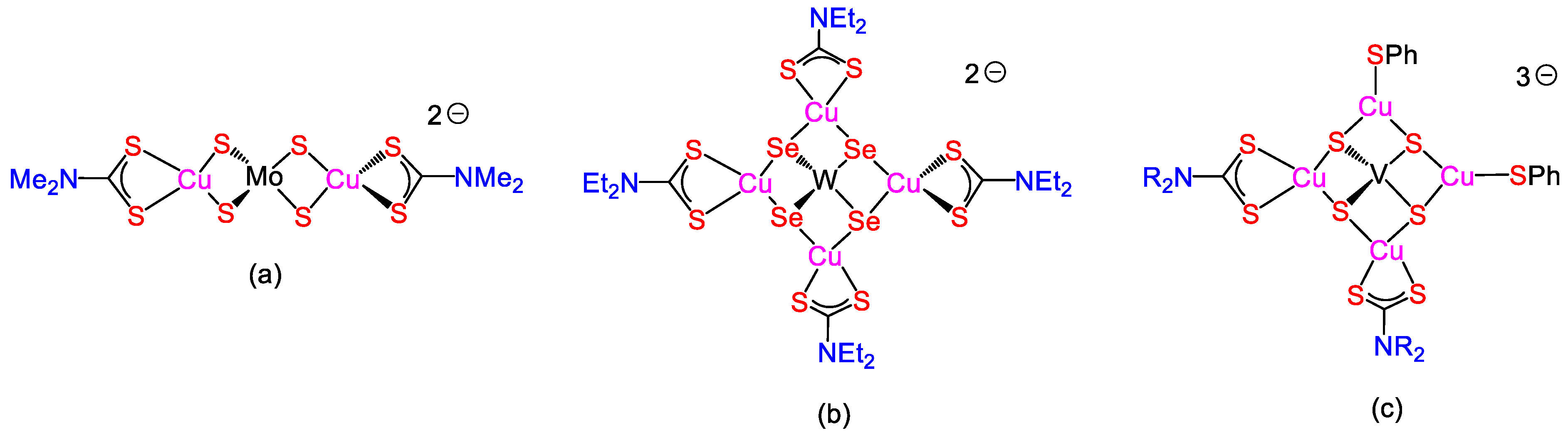
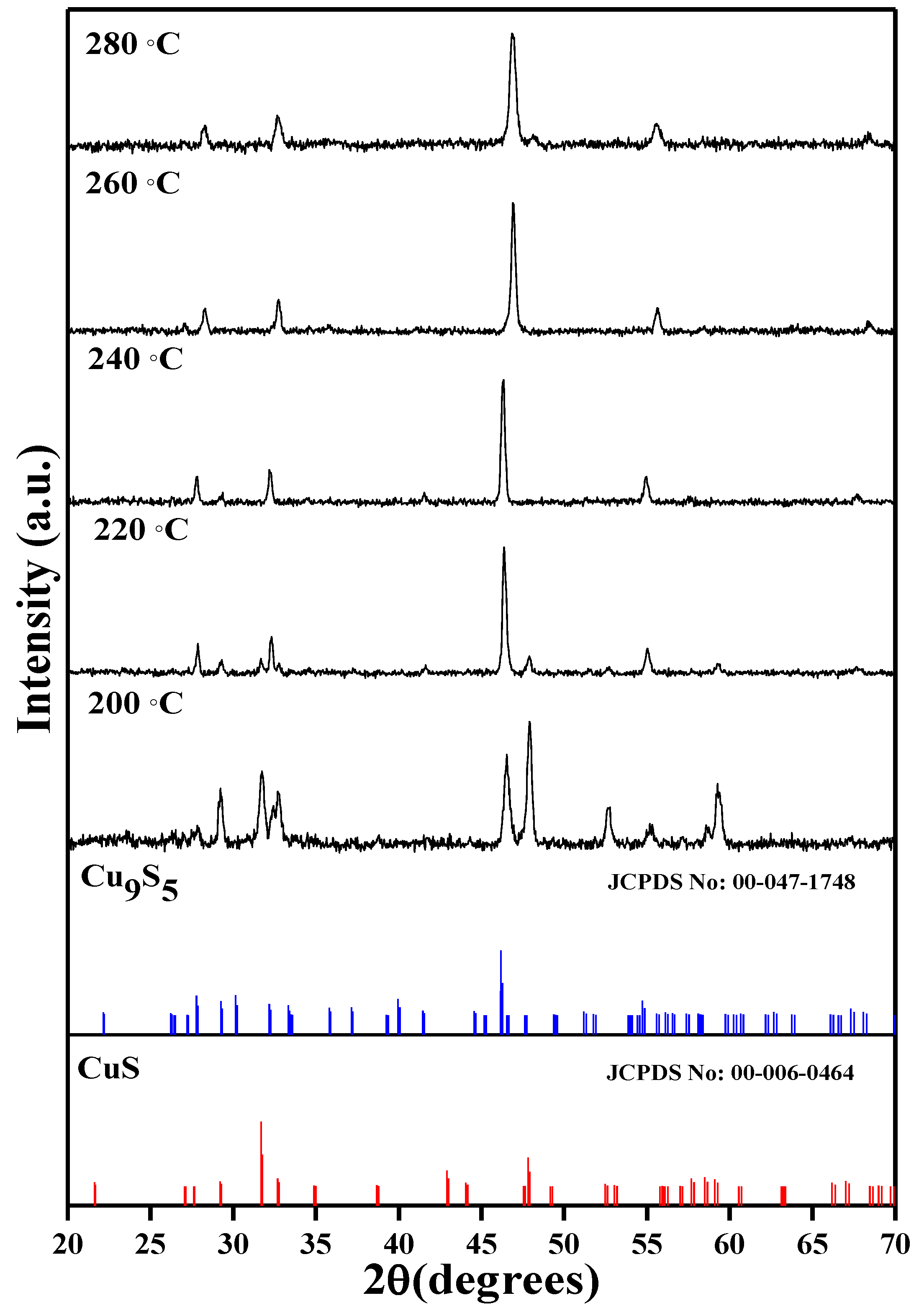
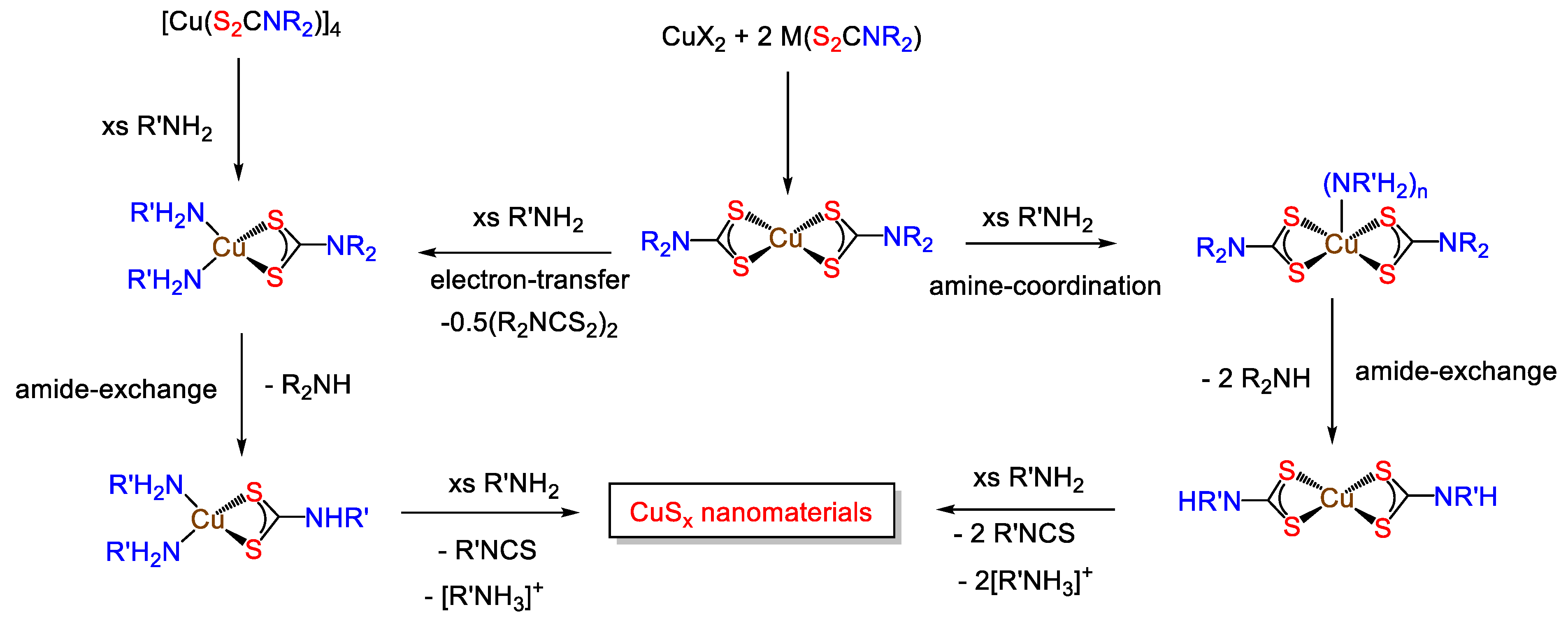


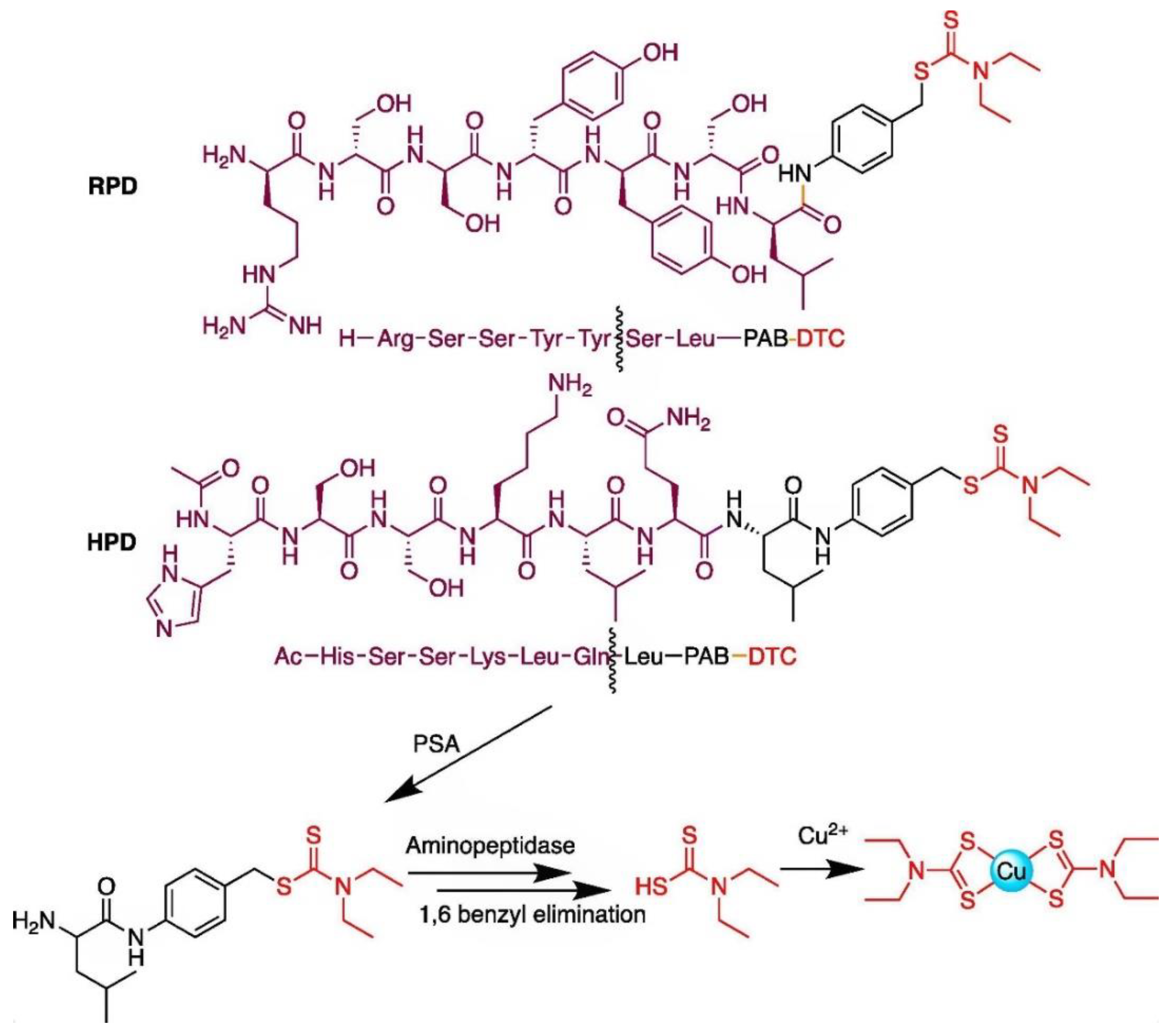



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hogarth, G.; Onwudiwe, D.C. Copper Dithiocarbamates: Coordination Chemistry and Applications in Materials Science, Biosciences and Beyond. Inorganics 2021, 9, 70. https://doi.org/10.3390/inorganics9090070
Hogarth G, Onwudiwe DC. Copper Dithiocarbamates: Coordination Chemistry and Applications in Materials Science, Biosciences and Beyond. Inorganics. 2021; 9(9):70. https://doi.org/10.3390/inorganics9090070
Chicago/Turabian StyleHogarth, Graeme, and Damian C. Onwudiwe. 2021. "Copper Dithiocarbamates: Coordination Chemistry and Applications in Materials Science, Biosciences and Beyond" Inorganics 9, no. 9: 70. https://doi.org/10.3390/inorganics9090070