Prioritizing the Risk Factors of Severe Early Childhood Caries
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sampling and Sample Size
2.2. Study Design and Ethical Approval
2.3. Inclusion and Exclusion Criteria
2.4. Data Collection
2.4.1. Intra-Oral Examination
2.4.2. Saliva Sample Collection and Estimation of Streptococcus mutans (S. mutans) Level
2.4.3. Oral Health Questionnaire
2.5. Statistical Analysis
- Both the questionnaire and oral examination forms were manually checked for completion of the required information. Data was collected, recorded in a standardized form, and then entered into the SPSS software for statistical analysis.
- Spearman correlation was used to find the correlation between the different risk factors and the log streptococcal count (Table 1).
- A logistic regression model was used to identify risk factors for caries development among children by considering independent variables simultaneously (Table 2). A p-value < 0.05 was considered statistically significant.
- The odds ratio was calculated for all possible risk factors associated with the prevalence of EEC and arranged in descending order after omitting all the insignificant variables, which was the main idea of the study (Table 3).
3. Results
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Nobile, C.G.; Fortunato, L.; Bianco, A.; Pileggi, C.; Pavia, M. Pattern and severity of early childhood caries in Southern Italy: A preschool-based cross-sectional study. BMC Public Health 2014, 14. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Lin, H.C.; Lo, E.C.M.; Wong, M.C.M. Risk indicators for early childhood caries in 2-year-old children in southern China. Aust. Dent. J. 2011, 56, 33–39. [Google Scholar] [CrossRef] [PubMed]
- Olatosi, O.; Inem, V.; Sofola, O.; Prakash, P.; Sote, E. The prevalence of early childhood caries and its associated risk factors among preschool children referred to a tertiary care institution. Niger. J. Clin. Pract. 2015, 18, 493–501. [Google Scholar] [CrossRef] [PubMed]
- Carino, K.M.; Shinada, K.; Kawaguchi, Y. Early childhood caries in Northern Philippines. Community Dent. Oral Epidemiol. 2003, 3, 81–89. [Google Scholar] [CrossRef]
- American Academy of Pediatric Dentistry (AAPD). Definition of Early Childhood Caries (ECC); AAPD: Chicago, IL, USA, 2008; Volume 4, p. 15. [Google Scholar]
- Lee, G.H.; McGrath, C.; Yiu, C.K.; King, N.M. A comparison of a generic and oral health–specific measure in assessing the impact of early childhood caries on quality of life. Community Dent. Oral Epidemiol. 2010, 38, 333–339. [Google Scholar] [CrossRef] [PubMed]
- Casamassimo, P.S.; Thikkurissy, S.; Edelstein, B.L.; Maiorini, E. Beyond the dmft: The human and economic cost of early childhood caries. J. Am. Dent. Assoc. 2009, 140, 650–657. [Google Scholar] [CrossRef] [PubMed]
- Harris, R.; Nicoll, A.D.; Adair, P.M.; Pine, C.M. Risk factors for dental caries in young children: A systematic review of the literature. Community Dent. Health 2004, 21, 71–85. [Google Scholar] [PubMed]
- Kolker, J.L.; Yuan, Y.; Burt, B.A.; Sandretto, A.M.; Sohn, W.; Lang, S.W.; Ismail, A.I. Dental caries and dietary patterns in low-income African-American children. Paediatr. Dent. 2007, 29, 257–264. [Google Scholar]
- Gibson, S.; Williams, S. Dental caries in pre-school children: Associations with social class, toothbrushing habit and consumption of sugars and sugar-containing foods. Caries Res. 1999, 33, 101–113. [Google Scholar] [CrossRef] [PubMed]
- Schroth, R.J.; Halchuk, S.; Star, L.A.C. Prevalence and risk factors of caregiver reported Severe Early Childhood Caries in Manitoba First Nations children: Results from the RHS Phase 2 (2008–2010). Int. J. Circumpolar Health 2013, 72, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Warren, J.J.; Weber-Gasparoni, K.; Marshall, T.A.; Drake, D.R.; Dehkordi-Vakil, F.; Dawson, D.V.; Tharp, K.M. A longitudinal study of dental caries risk among very young low SES children. Community Dent. Oral Epidemiol. 2009, 37, 116–122. [Google Scholar] [CrossRef] [PubMed]
- Van Palenstein Helderman, W.H.; Soe, W.; Van’t Hof, M.A. Risk factors of early childhood caries in a Southeast Asian population. J. Dent. Res. 2006, 85, 85–88. [Google Scholar] [CrossRef] [PubMed]
- Hamila, N. Early Childhood Caries and Certain Risk Factors in a Sample of Children 1–3.5 Years in Tanta. Dentistry 2013, 4, 1–7. [Google Scholar] [CrossRef]
- Nascimento Filho, E.; Mayer, M.P.; Pontes, P.; Pignatari, A.C.; Weckx, L.L. Caries prevalence, levels of mutans streptococci, and gingival and plaque indices in 3.0 to 5.0 old mouth breathing children. Caries Res. 2004, 38, 572–575. [Google Scholar] [CrossRef] [PubMed]
- Axelsson, P. Diagnosis and Risk Prediction of Dental Caries; Quintessence Publishing Co. Inc.: Varmland, Sweden, 2000; Volume 2, pp. 156–168. [Google Scholar]
- Jenen, B.; Brathall, D. A new method for the estimation of mutans streptococci in human saliva. J. Dent. Res. 1989, 68, 468–471. [Google Scholar] [CrossRef]
- Pidamale, R.; Sowmya, B.; Thomas, A.; Jose, T.; Madhusudan, K.K.; Prasad, G. Association between early childhood caries, streptococcus mutans level and genetic sensitivity levels to the bitter taste of, 6-N propylthiouracil among the children below 71 months of age. Dent. Res. J. 2012, 9, 730–734. [Google Scholar]
- Almushayt, A.S.; Sharaf, A.A.; El-Meligy, O.A.; Tallab, H.Y. Dietary and Feeding Habits in a Sample of Preschool Children in Severe Early Childhood Caries (S-ECC). JKAU Med. Sci. 2009, 16, 13–36. [Google Scholar] [CrossRef]
- Mahesh, R.; Muthu, M.S.; Rodrigues, S.J.L. Risk factors for early childhood caries: A case–control study. Eur. Arch. Paediatr. Dent. 2013, 14, 331–337. [Google Scholar] [CrossRef] [PubMed]
- Bagramian, R.A.; Garcia-Godoy, F.; Volpe, A.R. The global increase in dental caries. A pending public health crisis. Am. J. Dent. 2009, 22, 3–8. [Google Scholar] [PubMed]
- Mani, S.; John, J.; Ping, W.; Ismail, N. Early Childhood Caries: Parent’s Knowledge, Attitude and Practice towards Its Prevention in Malaysia; INTECH Open Access Publisher: Rijeka, Croatia, 2012; pp. 3–19. [Google Scholar]
- El-Nadeef, M.I.; Hassab, H.; Al-Hosani, E. National survey of the oral health of 5-year-old children in the United Arab Emirates. East. Mediterr. Health J. 2010, 16, 51–55. [Google Scholar] [PubMed]
- Farooki, F.A.; Khabeer, A.; Moheet, I.A.; Khan, S.Q.; Farooq, I.; ArRejaie, A.S. Prevalence of dental caries in primary and permanent teeth and its relation with tooth brushing habits among schoolchildren in eastern Saudi Arabia. Saudi Med. J. 2015, 36, 737–742. [Google Scholar] [CrossRef] [PubMed]
- Schroth, R.J.; Cheba, V. Determining the Prevalence and Risk Factors for Early Childhood Caries in a Community Dental Health Clinic. Pediatr. Dent. 2007, 5, 387–396. [Google Scholar]
- Maciel, S.; Marcenes, W.; Watt, R.; Sheiham, A. The relationship between sweetness preference and dental caries in mother/child pairs from Maringá-Pr, BraziLac. Int. Dent. J. 2001, 51, 83–88. [Google Scholar] [CrossRef] [PubMed]
- Corrêa-Faria, P.; Martins-Júnior, P.A.; Vieira-Andrade, R.G.; Marques, L.S.; Ramos-Jorge, M.L.A.C. Factors associated with the development of early childhood caries among Brazilian preschoolers. Braz. Oral Res. 2013, 27, 356–362. [Google Scholar] [CrossRef] [PubMed]
- Tiberia, M.; Milnes, A.R.; Feigal, R.; Morley, K.; Richardson, D.; Croft, W.; Cheung, W. Risk factors for early childhood caries in Canadian preschool children seeking care. Pediatr. Dent. 2007, 29, 201–208. [Google Scholar] [PubMed]
- Manchanda, K.; Sampath, N.; De Sarkar, A. Evaluating the effectiveness of oral health education program among mothers with 6–18 months children in prevention of early childhood caries. Contemp. Clin. Dent. 2014, 5, 478–483. [Google Scholar] [CrossRef] [PubMed]
- Plutzer, K.; Keirse, M.J.N.C. Influence of first-time mothers’ early employment on severe early childhood caries in their child. Int. J. Pediatr. 2012, 8, 26–32. [Google Scholar] [CrossRef] [PubMed]
- Stephen, C.; John, S. Maternal Transmission of Mutans Streptococci in Severe-Early Childhood Caries. Pediatr. Dent. 2009, 29, 997–1003. [Google Scholar]
- Ghazal, T.; Levy, S.; Childers, N.; Broffitt, B.; Cutter, G.; Wiener, H.; Kempf, M.; Warren, J.; Cavanaugh, J. Factors associated with early childhood caries incidence among high caries-risk children. Community Dent. Oral Epidemiol. 2015, 43, 366–374. [Google Scholar] [CrossRef] [PubMed]
- Perera, P.J.; Fernando, M.P.; Warnakulasooriya, T.D.; Ranathunga, N. Effect of feeding practices on dental caries among preschool children: A hospital-based analytical cross-sectional study. Asia Pac. J. Clin. Nutr. 2014, 23, 272–277. [Google Scholar] [PubMed]
- Choi, E.; Lee, S.; Kim, Y. Quantitative real-time polymerase chain reaction for Streptococcus mutans and Streptococcus sobrinus in dental plaque samples and its association with early childhood caries. Int. J. Paediatr. Dent. 2009, 19, 141–147. [Google Scholar] [CrossRef] [PubMed]
- Feldens, C.; Giugliani, E.; Vigo, A.; Vítolo, M. Early feeding practices and severe early childhood caries in four-year-old children from southern Brazil: A birth cohort study. Caries Res. 2010, 44, 445–452. [Google Scholar] [CrossRef] [PubMed]
- American Academy of Pediatric Dentistry (AAPD). Guideline on Perinatal Oral Health Care. AAPD: Chicago, IL, USA, 2011; Volume 38, pp. 140–145. [Google Scholar]
- Jiang, E.; Lo, E.; Chu, C.; Wong, M. Prevention of early childhood caries (ECC) through parental tooth brushing training and fluoride varnish application: A 24-month randomized controlled trial. J. Dent. 2014, 42, 1543–1550. [Google Scholar] [CrossRef] [PubMed]
- Retnakumari, N.; Cyriac, G. Childhood caries as influenced by maternal and child characteristics in preschool children of Kerala-an epidemiological study. Contemp. Clin. Dent. 2012, 3, 2–8. [Google Scholar] [CrossRef] [PubMed]
| Risk Factors | Log. Streptococcal Count | |
|---|---|---|
| Rho | p-Value | |
| Age | −0.034 | 0.693 |
| Male gender | 0.287 | 0.001 ** |
| Mother’s education | −0.483 | <0.001 ** |
| Father’s education | −0.198 | 0.019 * |
| Child’s first dental visit | −0.364 | <0.001 ** |
| Mother’s current caries experience | 0.530 | <0.001 ** |
| Siblings | 0.400 | <0.001 ** |
| Brushing frequency | −0.605 | <0.001 ** |
| Brushing time | −0.602 | <0.001 ** |
| Who brushes | −0.774 | <0.001 ** |
| Feeding on demand | 0.452 | <0.001 ** |
| Night feeding | 0.518 | <0.001 ** |
| Sharing utensils | 0.420 | <0.001 ** |
| Sugary food intake frequency | 0.584 | <0.001 ** |
| Risk Factors | p-Value | Exp. (β) | 95.0% C.I. for Exp. (β) | |
|---|---|---|---|---|
| Night feeding | No | Ref. | ||
| Yes | 0.012 * | 44.48 | 2.32–853.99 | |
| Mothers’ current caries experience | No | Ref. | ||
| Yes | <0.001 ** | 29.30 | 4.95–173.47 | |
| Gender | No | Ref. | ||
| Yes | 0.017 * | 11.54 | 1.56–85.54 | |
| Child’s first dental visit | None | 0.022 * | Ref. | |
| Late | 0.961 | 1.069 | 0.073–15.74 | |
| Early | 0.031 * | 0.068 | 0.006–0.78 | |
| Brushing time | Never | 0.294 | Ref. | |
| Others | 0.183 | 0.070 | 0.001–3.50 | |
| Night | 0.117 | 0.048 | 0.001–2.15 | |
| Brushing frequency | Never | 0.424 | Ref. | |
| 1/day | 0.194 | 11.02 | 0.30–409.85 | |
| 2/day | 0.552 | 1.72 | 0.29–10.18 | |
| Mother’s education | School | Ref. | ||
| University | 0.175 | 0.094 | 0.003–2.87 | |
| Father’s education | School | Ref. | ||
| University | 0.274 | 6.27 | 0.23–168.34 | |
| On demand feeding | No | Ref. | ||
| Yes | 0.177 | 4.08 | 0.53–31.44 | |
| Sharing utensils | No | Ref. | ||
| Yes | 0.648 | 0.607 | 0.71–5.16 |
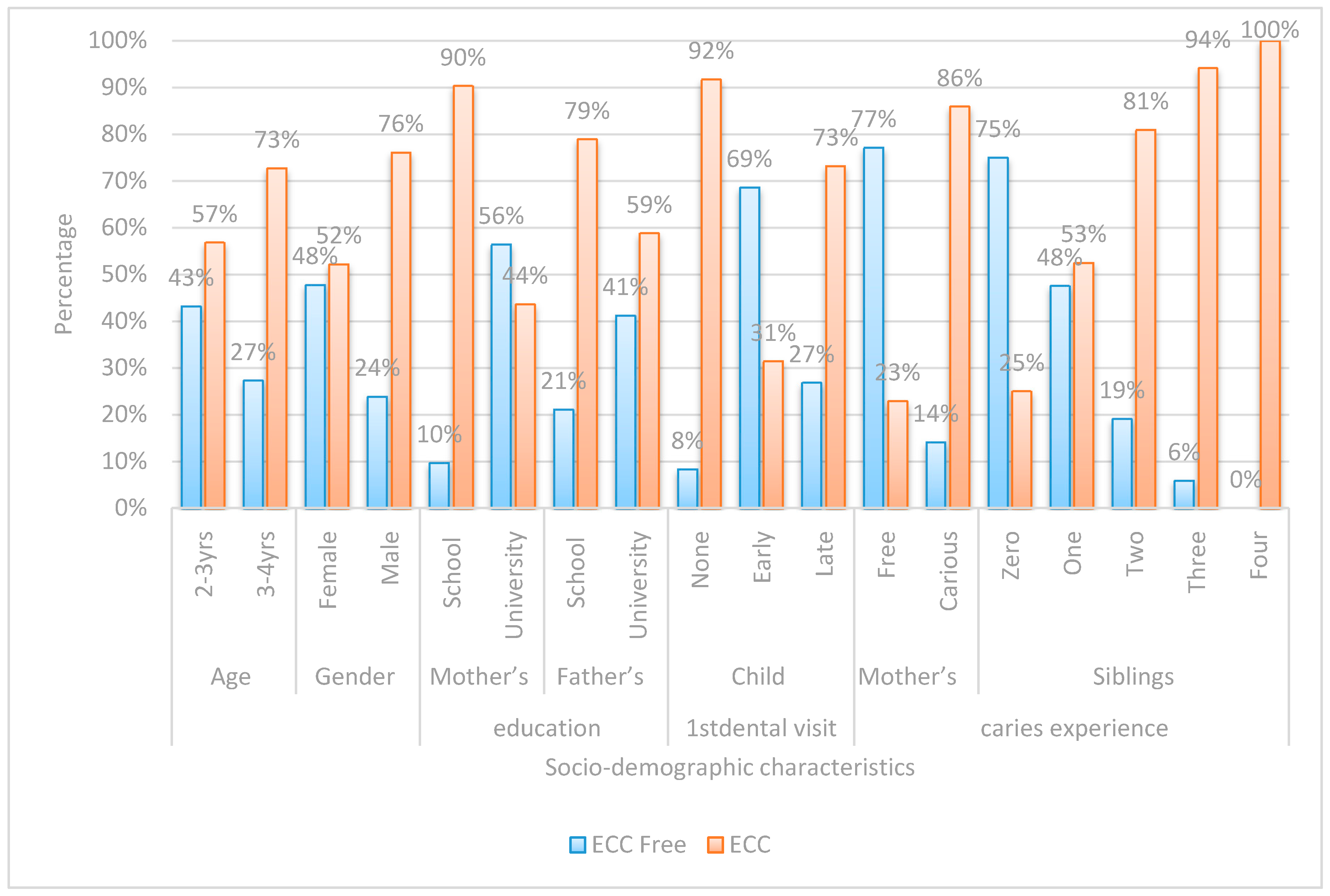
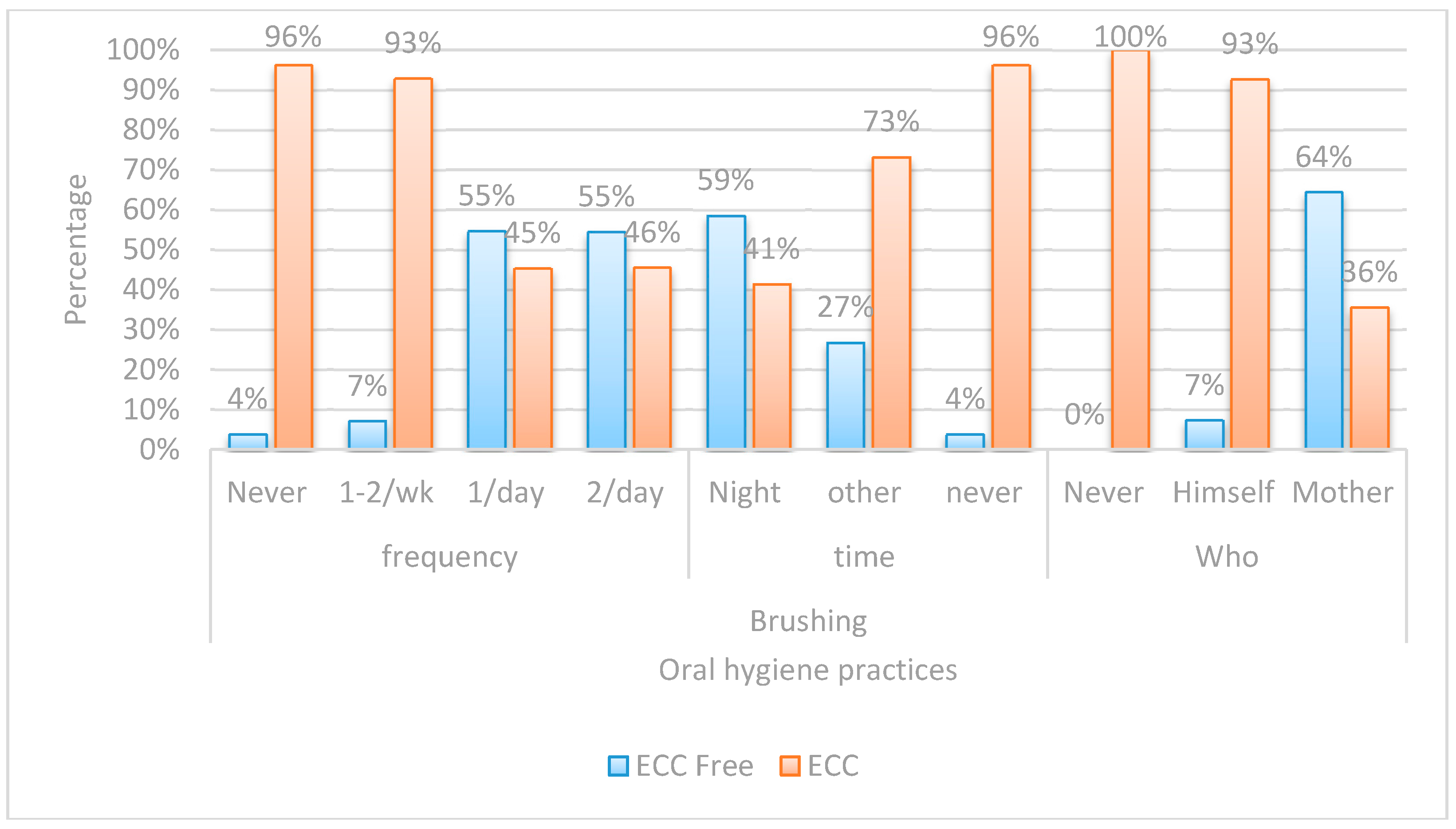
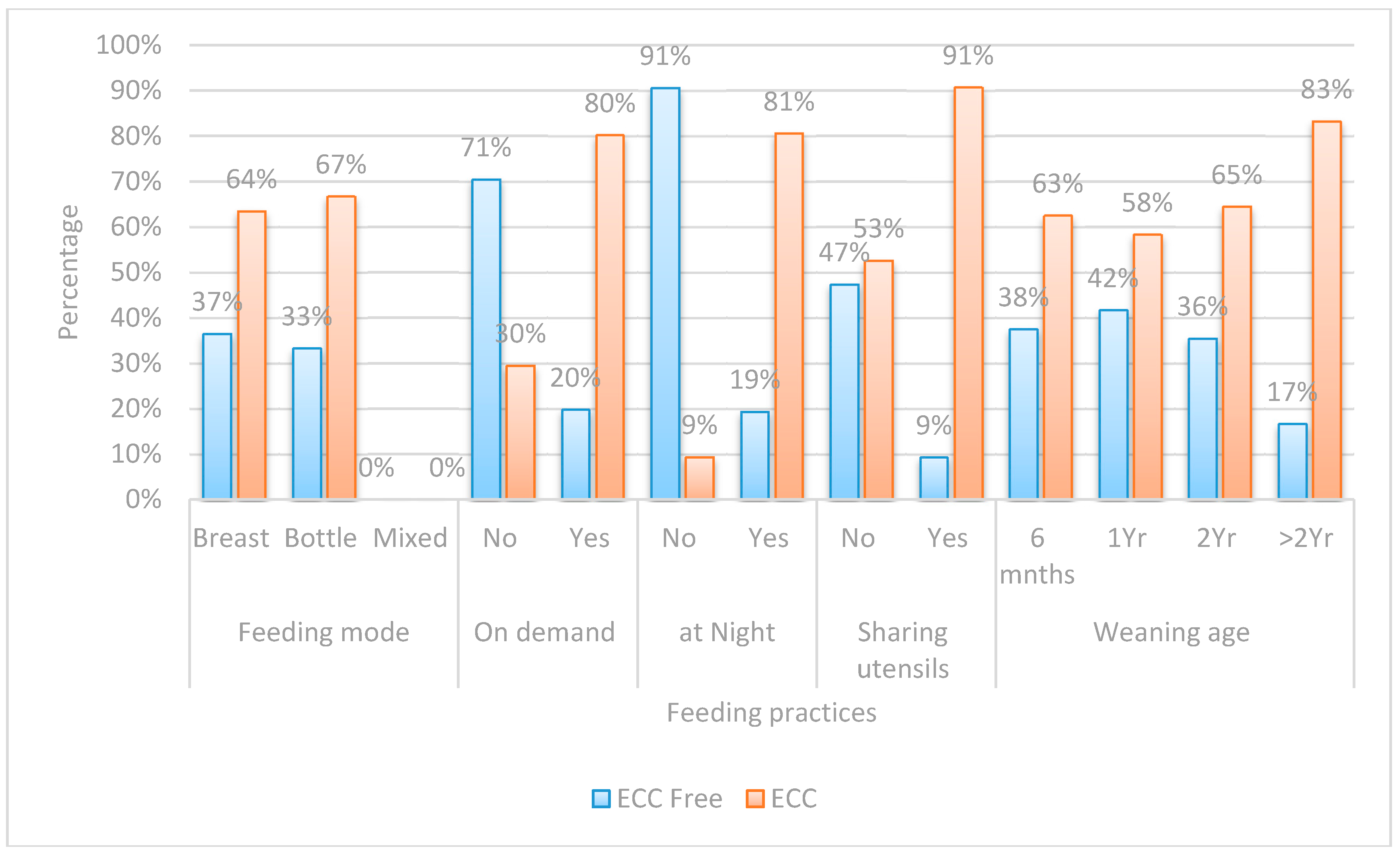
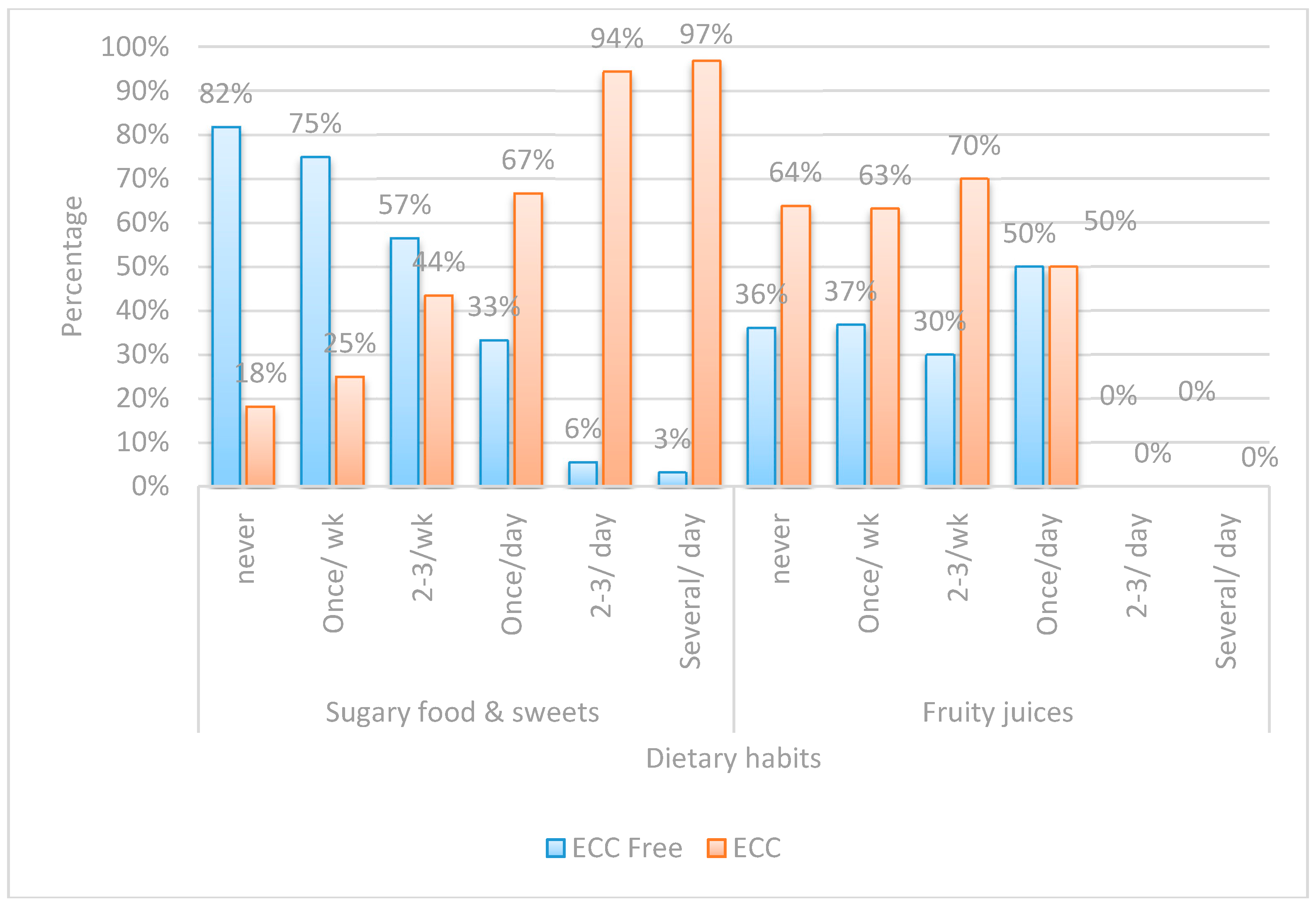
| Risk Factor | ECC Free | ECC | OR | 95% CI | p-Value | |
|---|---|---|---|---|---|---|
| (n = 50) | (n = 90) | |||||
| Frequency of Sugary Food Consumption | Never | 18(81.8%) | 4(18.2%) | Ref. | ||
| Several/Day | 1(3.2%) | 30(96.8%) | 135.0 | 13.98–1303.95 | <0.001 ** | |
| Two to Three Times/Day | 2(5.6%) | 34(94.4%) | 76.50 | 12.76–458.63 | <0.001 ** | |
| Three Siblings | Zero | 21(75.0%) | 7(25.0%) | Ref. | ||
| Three | 1(5.9%) | 16(94.1%) | 48.0 | 5.35–430.57 | 0.001 ** | |
| Night Feeding | No | 29(90.6%) | 3(9.4%) | Ref. | ||
| Yes | 21(19.4%) | 87(80.6%) | 40.05 | 11.13–144.13 | <0.001 ** | |
| Who Brushes | Mother | 47(64.4%) | 26(35.6%) | Ref. | ||
| Himself | 3(7.3%) | 38(92.7%) | 22.90 | 6.43–81.48 | <0.001 ** | |
| Mother’s Caries Experience | Free | 37(77.1%) | 11(22.9%) | Ref. | ||
| Carious | 13(14.1%) | 79(85.9%) | 20.44 | 8.37–49.92 | <0.001 ** | |
| Two Siblings | Zero | 21(75.0%) | 7(25.0%) | Ref. | ||
| Two | 9(19.1%) | 38(80.9%) | 12.67 | 4.12–38.91 | <0.001 ** | |
| On Demand Feeding | No | 31(70.5%) | 13(29.5%) | Ref. | ||
| Yes | 19(19.8%) | 77(80.2%) | 9.66 | 4.26–21.93 | <0.001 ** | |
| Sugary Food Once/Day | Never | 18(81.8%) | 4(18.2%) | Ref. | ||
| Once/Day | 4(33.3%) | 8(66.7%) | 9.0 | 1.79–45.34 | 0.008 ** | |
| Sharing Utensils | No | 46(47.4%) | 51(52.6%) | Ref. | ||
| Yes | 4(9.3%) | 39(90.7%) | 8.79 | 2.92–26.51 | <0.001 ** | |
| One Sibling | Zero | 21(75.0%) | 7(25.0%) | Ref. | ||
| One | 19(47.5%) | 21(52.5%) | 3.32 | 1.15–9.54 | 0.026 * | |
| Gender | Female | 33(47.8%) | 36(52.2%) | Ref. | ||
| Male | 17(23.9%) | 54(76.1%) | 2.91 | 1.42–5.99 | 0.004 ** | |
| Father’s Education | School | 8(21.1%) | 30(78.9%) | Ref. | ||
| University | 42(41.2%) | 60(58.8%) | 0.381 | 0.16–0.91 | 0.030 * | |
| Late First Child Dental Visit | None | 4(8.3%) | 44(91.7%) | Ref. | ||
| Late | 11(26.8%) | 30(73.2%) | 0.248 | 0.072–0.852 | <0.027 * | |
| Brushing Time | Never | 34(58.6%) | 24(41.4%) | Ref. | ||
| Others | 1(3.8%) | 25(96.2%) | 0.109 | 0.014–0.879 | 0.037 * | |
| Mother’s Education | School | 6(9.7%) | 56(90.3%) | Ref. | ||
| University | 44(56.4%) | 34(43.6%) | 0.083 | 0.03–0.21 | <0.001 ** | |
| Early First Child Dental Visit | None | 4(8.3%) | 44(91.7%) | Ref. | ||
| Early | 35(68.6%) | 16(31.4%) | 0.042 | 0.013–0.136 | <0.001 ** | |
| Brushing Frequency | Never | 1(3.8%) | 25(96.2%) | Ref. | ||
| Once/Day | 29(54.7%) | 24(45.3%) | 0.033 | 0.004–0.263 | 0.001 ** | |
| Twice/Day | 18(54.5%) | 15(45.5%) | 0.033 | 0.004–0.276 | 0.002 ** | |
| Brushing Time | Never | 34(58.6%) | 24(41.4%) | Ref. | ||
| Night | 15(26.8%) | 41(73.2%) | 0.028 | 0.004–0.223 | 0.001 ** |
© 2017 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kabil, N.S.; Eltawil, S. Prioritizing the Risk Factors of Severe Early Childhood Caries. Dent. J. 2017, 5, 4. https://doi.org/10.3390/dj5010004
Kabil NS, Eltawil S. Prioritizing the Risk Factors of Severe Early Childhood Caries. Dentistry Journal. 2017; 5(1):4. https://doi.org/10.3390/dj5010004
Chicago/Turabian StyleKabil, Noha Samir, and Sherif Eltawil. 2017. "Prioritizing the Risk Factors of Severe Early Childhood Caries" Dentistry Journal 5, no. 1: 4. https://doi.org/10.3390/dj5010004





