Huangshan Maofeng Green Tea Extracts Prevent Obesity-Associated Metabolic Disorders by Maintaining Homeostasis of Gut Microbiota and Hepatic Lipid Classes in Leptin Receptor Knockout Rats
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemical Analysis of Huangshan Maofeng Green Tea Water Extracts
2.2. Animals Experiment
2.3. Serum Biochemical Analysis
2.4. Histopathological Analysis
2.5. RNA Isolation and Real-Time PCR Analysis
2.6. Analysis of Liver Lipidomics
2.7. Analysis of Gut Microbiota
2.8. Quantification of Fecal Short–Chain Fatty Acids
2.9. Statistical Analysis
3. Results
3.1. Main Chemical Compounds in Huangshan Maofeng Green Tea Water Extract
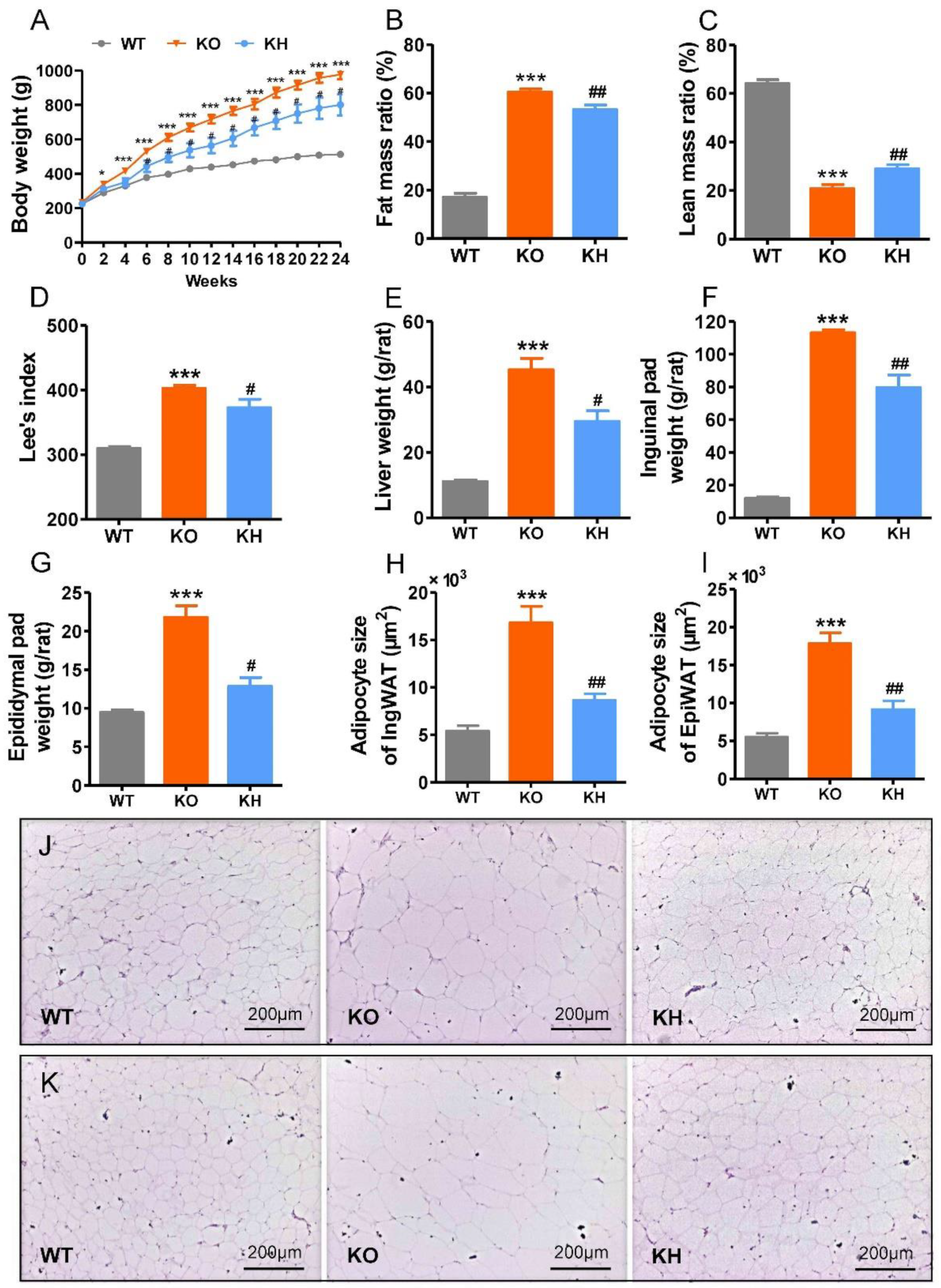
3.2. HTE Attenuated Phenotypes of Obesity in Lepr−/− Rats
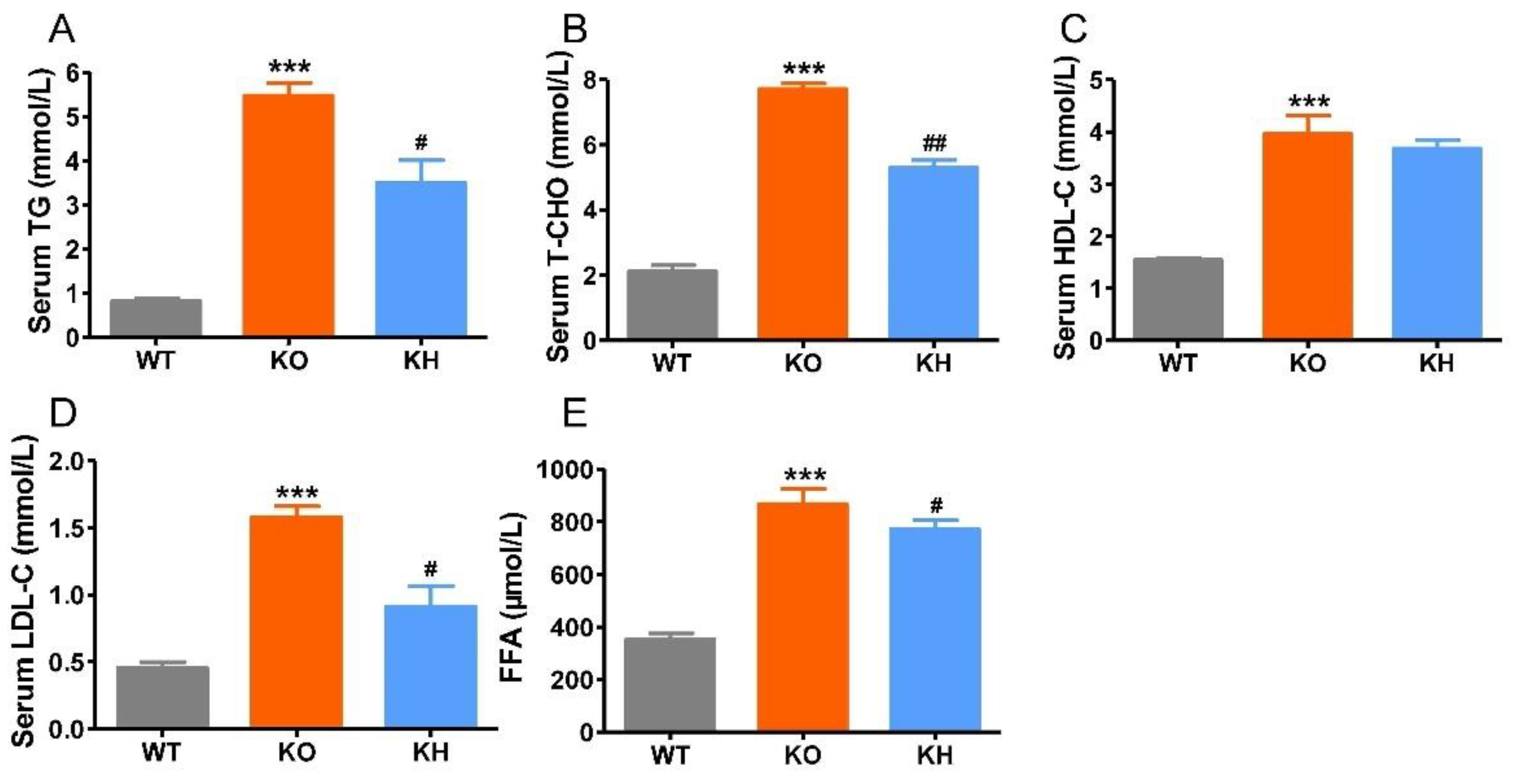
3.3. HTE Improved Dyslipidemia in Lepr−/− Rats
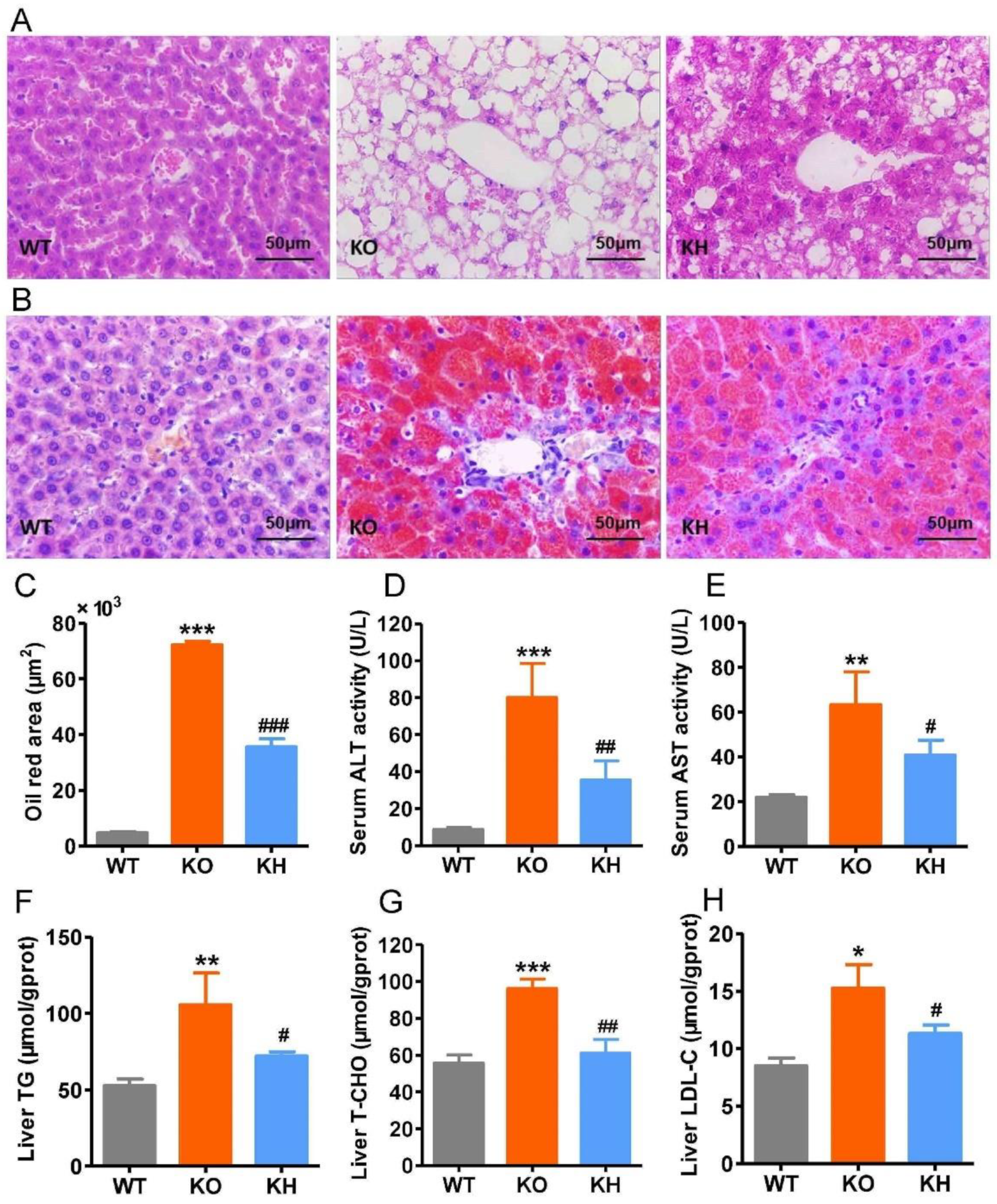
3.4. HTE Ameliorated Hepatic Steatosis in Lepr−/− Rats
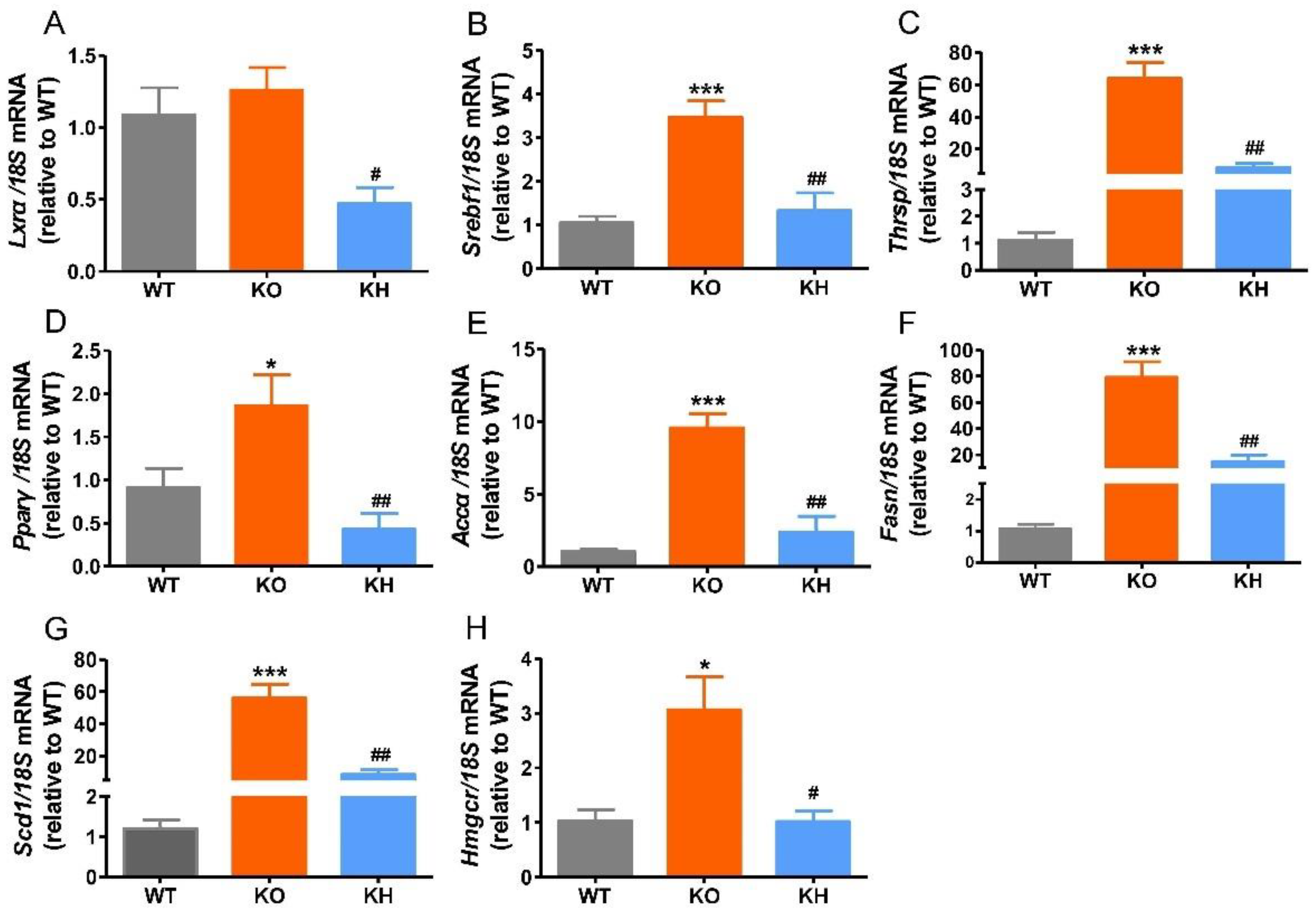
3.5. HTE Reduced the Expressions of Lipogenesis Related Genes in Liver of Lepr−/− Rats
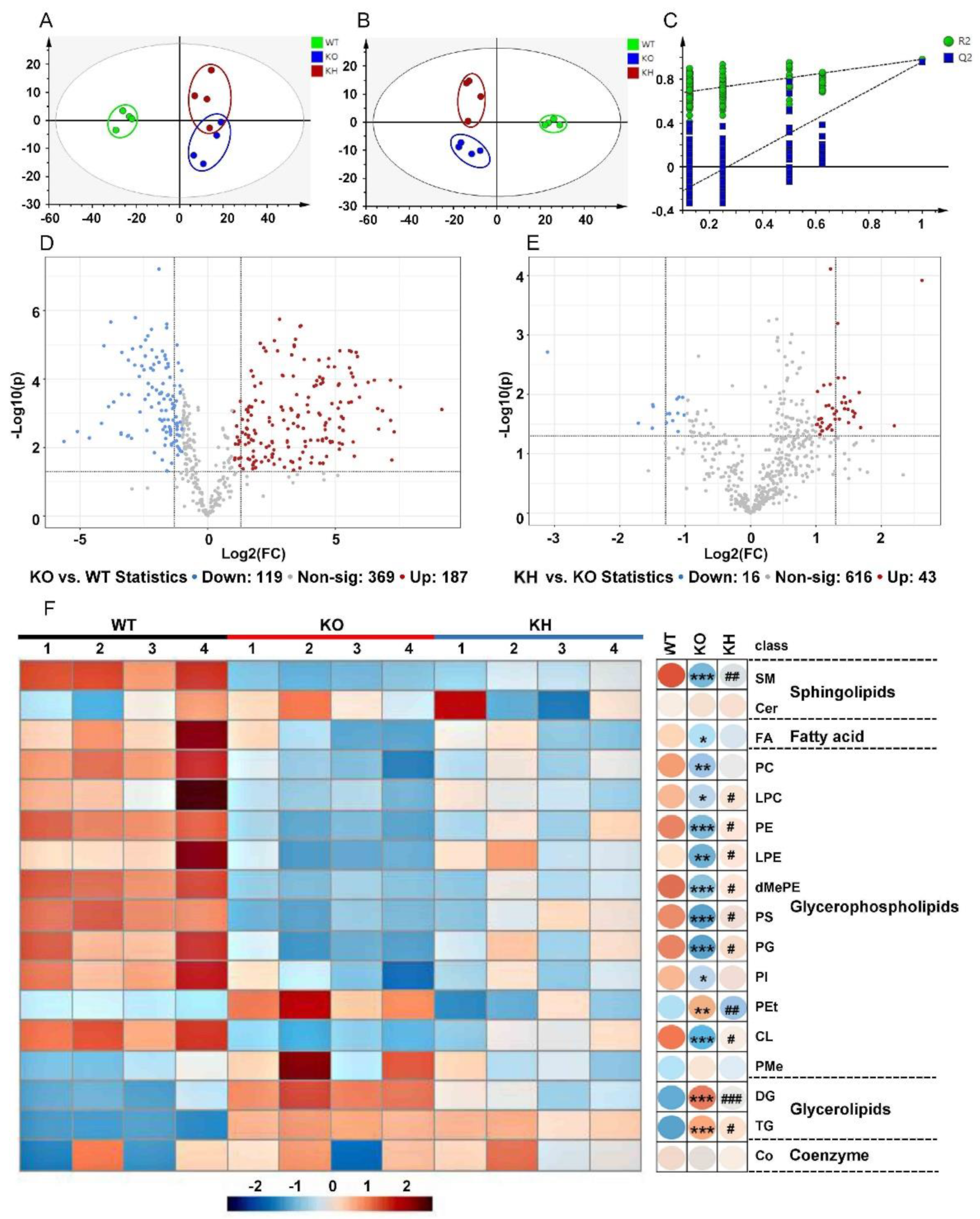
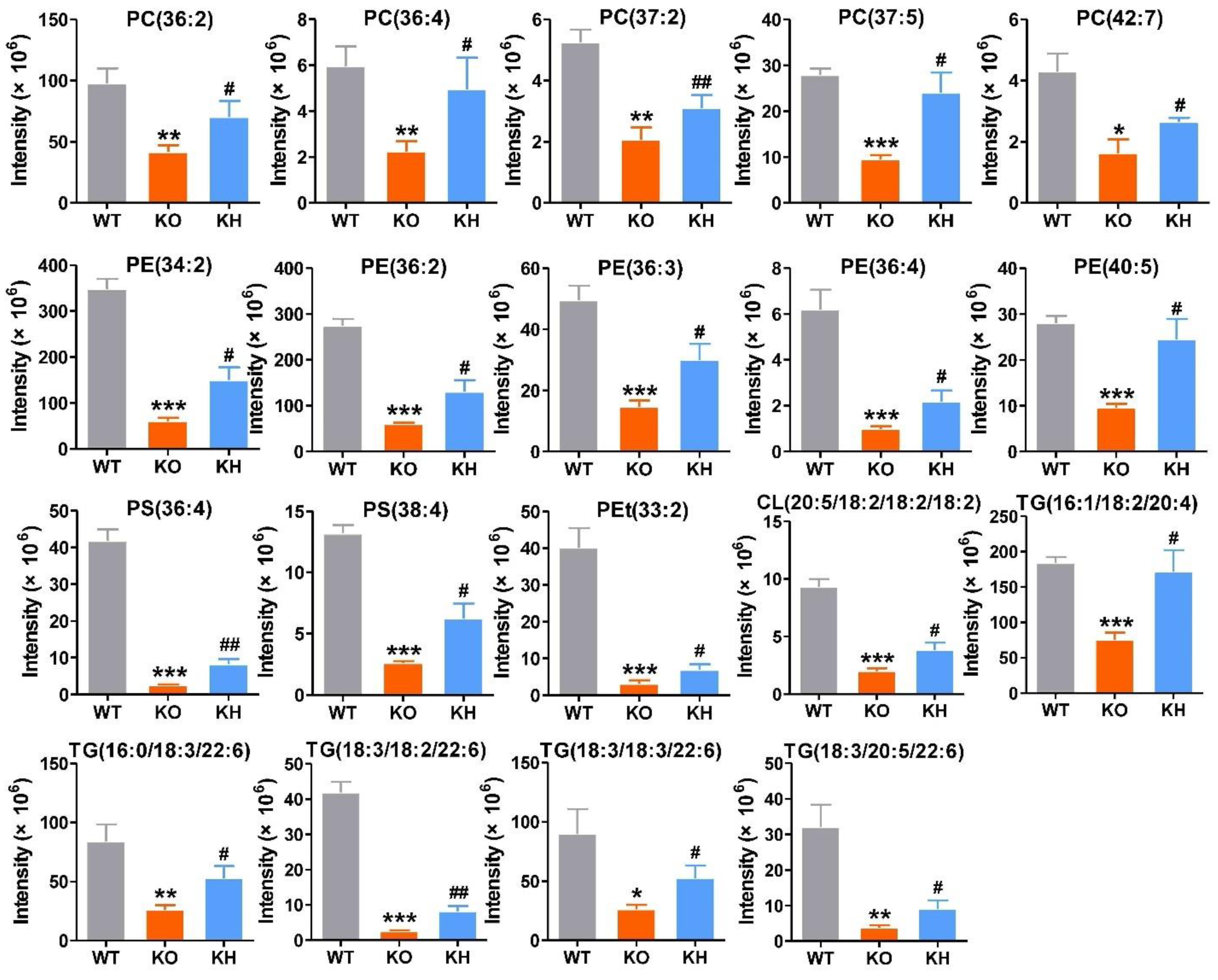
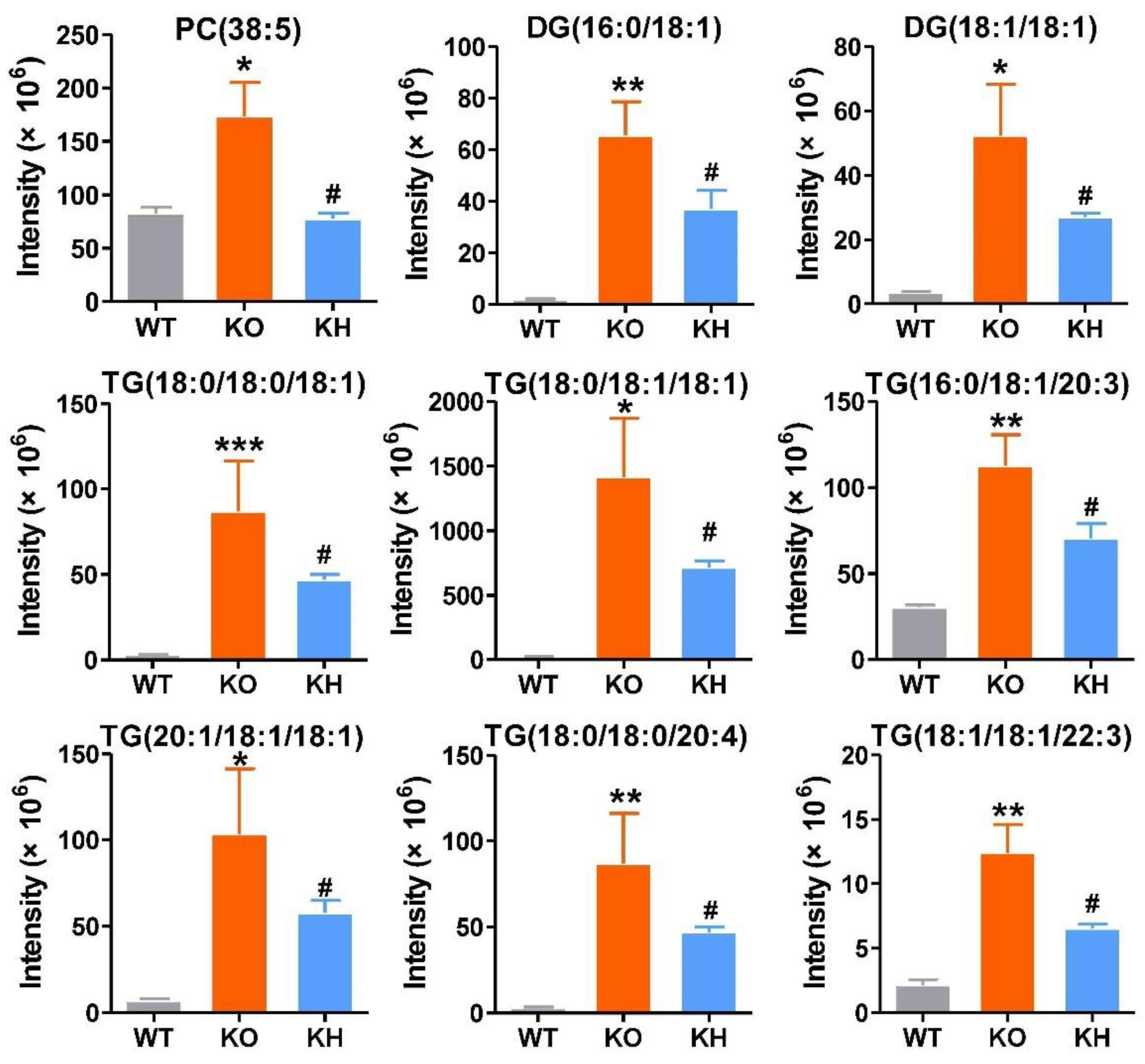
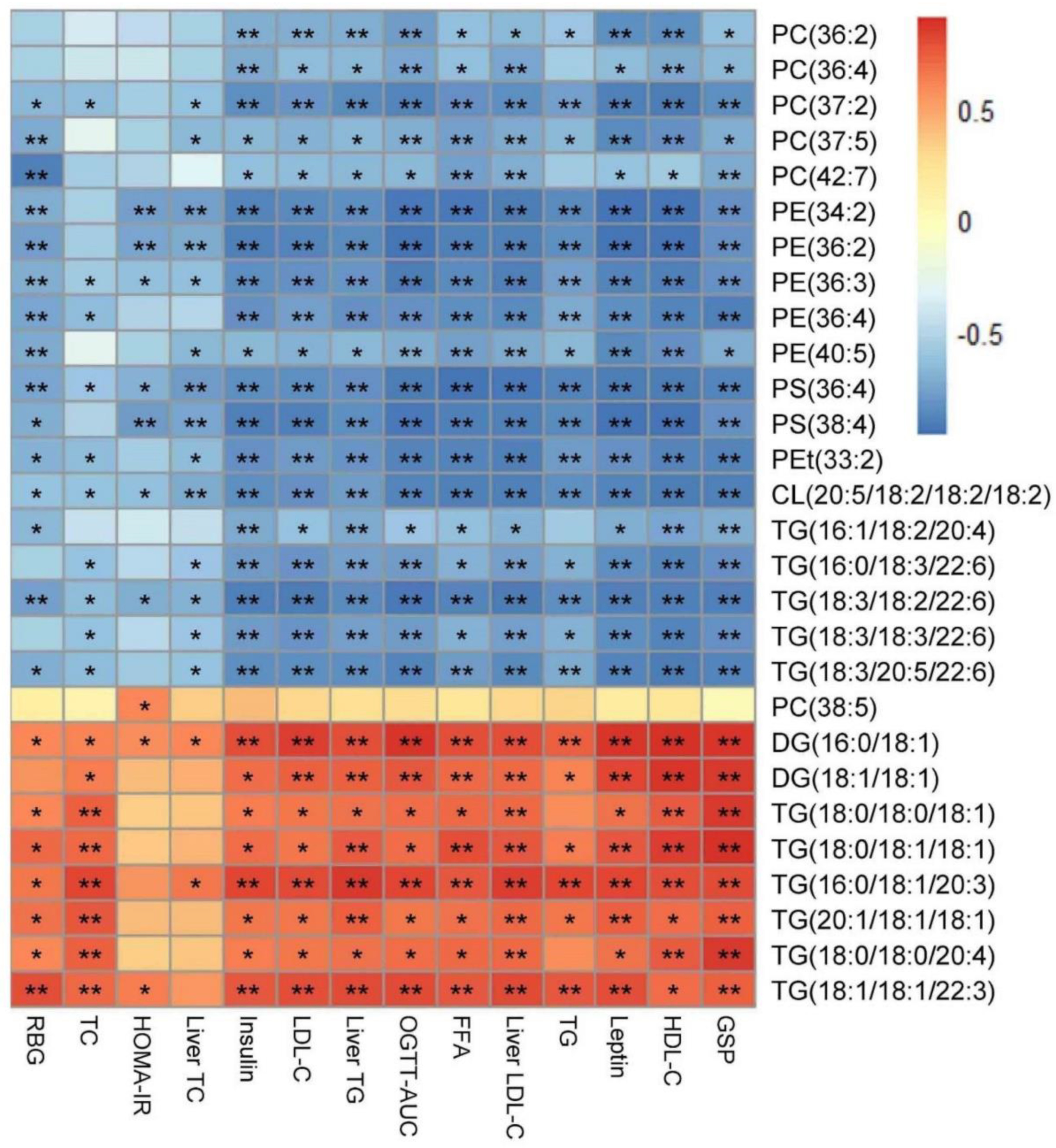
3.6. HTE Improved Hepatic Lipid Profiles by Lipidomics Analysis
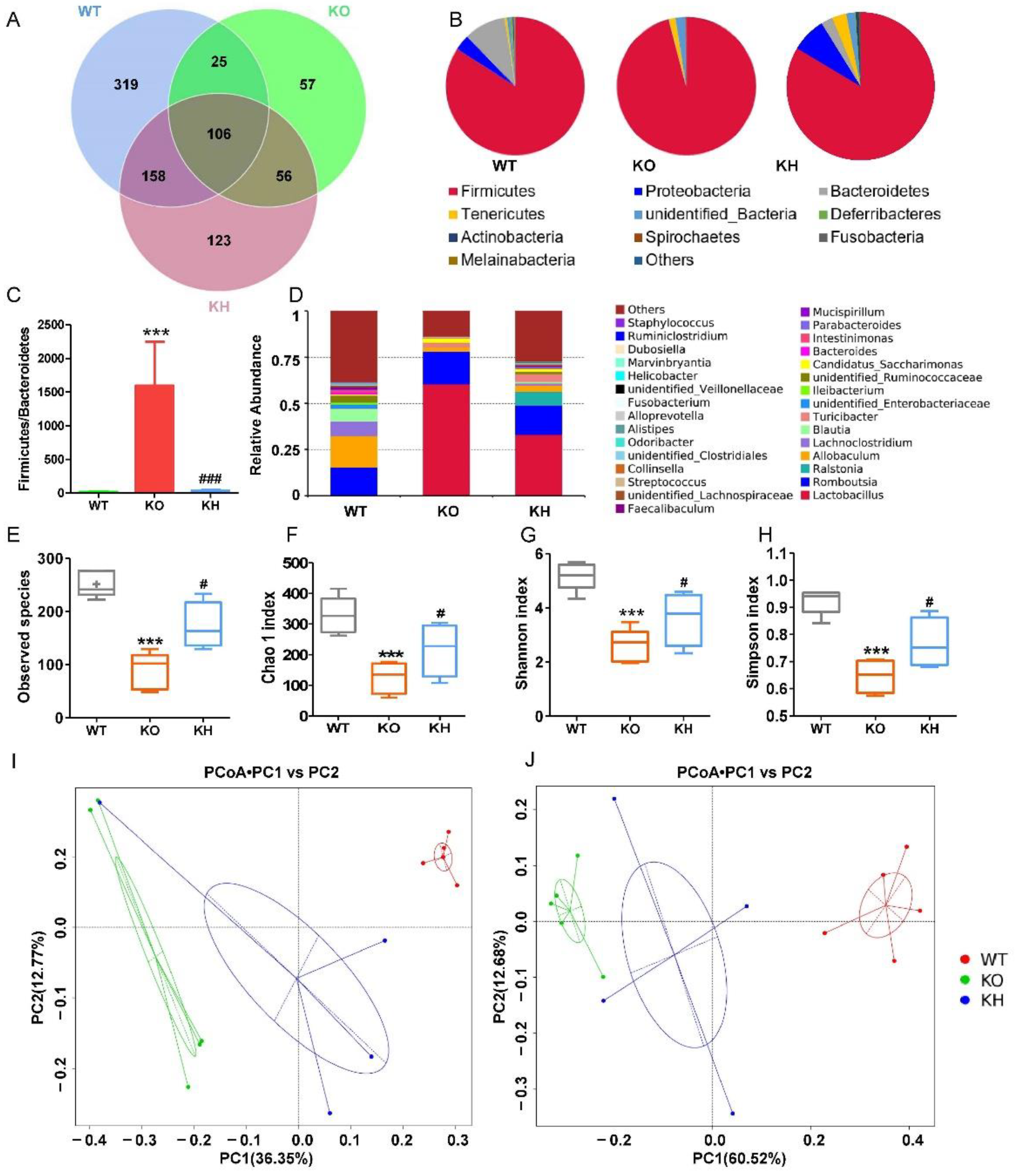
3.7. HTE Increased Gut Microbiota Diversity in Lepr−/− Rats
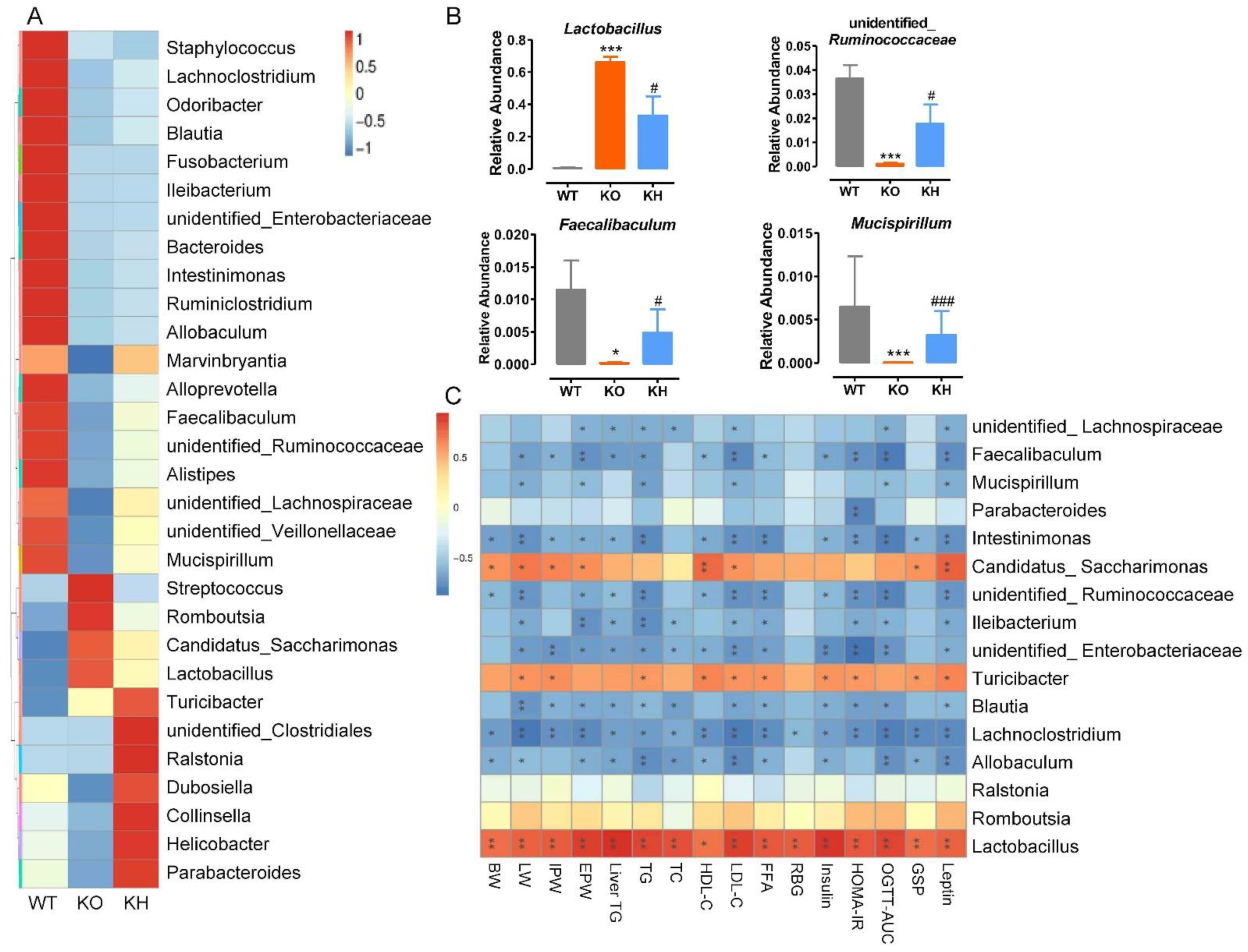
3.8. HTE Altered the Gut Microbiota Composition in Lepr−/− Rats
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Marie-Eve, P.; André, T.; Jean-Pierre, D. Obesity Phenotypes, Diabetes, and Cardiovascular Diseases. Circ. Res. 2020, 126, 1477–1500. [Google Scholar]
- Damsgaard, C.T.; Michaelsen, K.F.; Molbo, D.; Mortensen, E.L.; Sørensen, T. Trends in adult body-mass index in 200 countries from 1975 to 2014: A pooled analysis of 1698 population-based measurement studies with 19.2 million participants. Lancet 2016, 387, 1377. [Google Scholar]
- Afshin, A.; Reitsma, M.B.; Murray, C.J.L. Health Effects of Overweight and Obesity in 195 Countries. N. Engl. J. Med. 2017, 377, 1495–1497. [Google Scholar]
- Gammone, M.A.; D’Orazio, N. COVID-19 and Obesity: Overlapping of Two Pandemics. Obes. Facts. 2021, 14, 579–585. [Google Scholar] [CrossRef]
- Landecho, M.F.; Marin-Oto, M.; Recalde-Zamacona, B.; Bilbao, I.; Frühbeck, G. Obesity as an Adipose tissue dysfunction disease and A Risk Factor for Infections—COVID-19 as a case study. Eur. J. Intern. Med. 2021, 91, 3–9. [Google Scholar] [CrossRef]
- World Health Organization. World Health Statistics 2021: Monitoring Health for the SDGs, Sustainable Development Goals. 2021. Available online: www.who.int/data/gho/publications/world-health-statistics (accessed on 5 March 2022).
- Caballero, B. Humans against Obesity: Who Will Win. Adv. Nutr. 2019, 10, S4–S9. [Google Scholar] [CrossRef]
- Siavash, M.; Tabbakhian, M.; Sabzghabaee, A.M.; Razavi, N. Severity of gastrointestinal side effects of metformin tablet compared to metformin capsule in type 2 diabetes mellitus patients. J. Res. Pharm. Pract. 2017, 6, 73–76. [Google Scholar]
- Turner, R.M.; Pirmohamed, M. Statin-Related Myotoxicity: A Comprehensive Review of Pharmacokinetic, Pharmacogenomic and Muscle Components. J. Clin. Med. 2019, 9, 22. [Google Scholar] [CrossRef]
- Mammen, A.L.; Tiniakou, E. Intravenous Immune Globulin for Statin-Triggered Autoimmune Myopathy. N. Engl. J. Med. 2015, 373, 1680–1682. [Google Scholar] [CrossRef]
- Chen, H.; Nie, Q.; Hu, J.; Huang, X.; Zhang, K.; Pan, S.; Nie, S. Hypoglycemic and Hypolipidemic Effects of Glucomannan Extracted from Konjac on Type 2 Diabetic Rats. J. Agric. Food Chem. 2019, 67, 5278–5288. [Google Scholar] [CrossRef]
- Teng, Y.; Li, D.; Guruvaiah, P.; Xu, N.; Xie, Z. Dietary Supplement of Large Yellow Tea Ameliorates Metabolic Syndrome and Attenuates Hepatic Steatosis in db/db Mice. Nutrients 2018, 10, 75. [Google Scholar] [CrossRef]
- Wang, H.N.; Xiang, J.Z.; Qi, Z.; Du, M. Plant extracts in prevention of obesity. Crit. Rev. Food Sci. Nutr. 2022, 62, 2221–2234. [Google Scholar] [CrossRef]
- Busetto, L.; Sbraccia, P.; Vettor, R. Obesity management: At the forefront against disease stigma and therapeutic inertia. Eat. Weight Disord. 2022, 27, 761–768. [Google Scholar] [CrossRef]
- Aron-Wisnewsky, J.; Warmbrunn, M.V.; Nieuwdorp, M.; Clément, K. Metabolism and Metabolic Disorders and the Microbiome: The Intestinal Microbiota Associated with Obesity, Lipid Metabolism, and Metabolic Health-Pathophysiology and Therapeutic Strategies. Gastroenterology 2021, 160, 573–599. [Google Scholar] [CrossRef]
- Górowska-Kowolik, K.; Chobot, A. The role of gut micorbiome in obesity and diabetes. World J. Pediatr. 2019, 15, 332–340. [Google Scholar] [CrossRef]
- Chen, T.; Yang, C.S. Biological fates of tea polyphenols and their interactions with microbiota in the gastrointestinal tract: Implications on health effects. Crit. Rev. Food Sci. Nutr. 2020, 60, 2691–2709. [Google Scholar] [CrossRef]
- Nie, Q.; Chen, H.; Hu, J.; Fan, S.; Nie, S. Dietary compounds and traditional Chinese medicine ameliorate type 2 diabetes by modulating gut microbiota. Crit. Rev. Food Sci. Nutr. 2019, 59, 848–863. [Google Scholar] [CrossRef]
- Fang, Q.; Hu, J.; Nie, Q.; Nie, S. Effects of polysaccharides on glycometabolism based on gut microbiota alteration. Trends Food Sci. Technol. 2019, 92, 65–70. [Google Scholar] [CrossRef]
- Sang, S.; Lambert, J.D.; Ho, C.T.; Yang, C.S. The chemistry and biotransformation of tea constituents. Pharmacol. Res. 2011, 64, 87–99. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.S.; Zhang, J.; Zhang, L.; Huang, J.; Wang, Y. Mechanisms of body weight reduction and metabolic syndrome alleviation by tea. Mol. Nutr. Food Res. 2016, 60, 160–174. [Google Scholar] [CrossRef] [PubMed]
- Khan, N.; Mukhtar, H. Tea Polyphenols in Promotion of Human Health. Nutrients 2018, 11, 39. [Google Scholar] [CrossRef]
- Li, D.; Wang, R.; Huang, J.; Cai, Q.; Yang, C.S.; Wan, X.; Xie, Z. Effects and Mechanisms of Tea Regulating Blood Pressure: Evidences and Promises. Nutrients 2019, 11, 1115. [Google Scholar] [CrossRef]
- Liu, Z.; Chen, Z.; Guo, H.; He, D.; Zhao, H.; Wang, Z.; Zhang, W.; Liao, L.; Zhang, C.; Ni, L. The modulatory effect of infusions of green tea, oolong tea, and black tea on gut microbiota in high-fat-induced obese mice. Food Funct. 2016, 7, 4869–4879. [Google Scholar] [CrossRef]
- Li, H.; Fang, Q.; Nie, Q.; Hu, J.; Yang, C.; Huang, T.; Li, H.; Nie, S. Hypoglycemic and Hypolipidemic Mechanism of Tea Polysaccharides on Type 2 Diabetic Rats via Gut Microbiota and Metabolism Alteration. J. Agric. Food Chem. 2020, 68, 10015–10028. [Google Scholar] [CrossRef]
- Liu, D.; Huang, J.; Luo, Y.; Wen, B.; Wu, W.; Zeng, H.; Zhonghua, L. Fuzhuan Brick Tea Attenuates High-Fat Diet-Induced Obesity and Associated Metabolic Disorders by Shaping Gut Microbiota. J. Agric. Food Chem. 2019, 67, 13589–13604. [Google Scholar] [CrossRef]
- Chen, T.; Liu, A.B.; Sun, S.; Ajami, N.J.; Ross, M.C.; Wang, H.; Zhang, L.; Reuhl, K.; Kobayashi, K.; Onishi, J.C. Green Tea Polyphenols Modify the Gut Microbiome in db/db Mice as Co-Abundance Groups Correlating with the Blood Glucose Lowering Effect. Mol. Nutr. Food Res. 2019, 63, e1801064. [Google Scholar] [CrossRef]
- Wu, G.; Sun, X.; Cheng, H.; Xu, S.; Li, D.; Xie, Z. Large Yellow Tea Extract Ameliorates Metabolic Syndrome by Suppressing Lipogenesis through SIRT6/SREBP1 Pathway and Modulating Microbiota in Leptin Receptor Knockout Rats. Foods 2022, 11, 1638. [Google Scholar] [CrossRef]
- Han, Z.; Wen, M.; Zhang, H.; Zhang, L.; Wan, X.; Ho, C.T. LC-MS based metabolomics and sensory evaluation reveal the critical compounds of different grades of Huangshan Maofeng green tea. Food Chem. 2022, 374, 131796. [Google Scholar] [CrossRef]
- Zeng, X.; Tian, J.; Cai, K.; Wu, X.; Wang, Y.; Zheng, Y.; Su, Y.; Cui, L. Promoting osteoblast differentiation by the flavanes from Huangshan Maofeng tea is linked to a reduction of oxidative stress. Phytomedicine 2014, 21, 217–224. [Google Scholar] [CrossRef]
- Cui, Y.; Yang, X.; Lu, X.; Chen, J.; Zhao, Y. Protective effects of polyphenols-enriched extract from Huangshan Maofeng green tea against CCl4-induced liver injury in mice. Chem. Biol. Interact. 2014, 220, 75–83. [Google Scholar] [CrossRef]
- Liu, Z.; Xiao, M.; Du, Z.; Li, M.; Guo, H.; Yao, M.; Wan, X.; Xie, Z. Dietary supplementation of Huangshan Maofeng green tea preventing hypertension of older C57BL/6 mice induced by desoxycorticosterone acetate and salt. J. Nutr. Biochem. 2021, 88, 108530. [Google Scholar] [CrossRef]
- Bechmann, L.P.; Hannivoort, R.A.; Gerken, G.; Hotamisligil, G.S.; Trauner, M.; Canbay, A. The interaction of hepatic lipid and glucose metabolism in liver diseases. J. Hepatol. 2012, 56, 952–964. [Google Scholar] [CrossRef]
- Preuss, C.; Jelenik, T.; Bódis, K.; Müssig, K.; Burkart, V.; Szendroedi, J.; Roden, M.; Markgraf, D.F. A New Targeted Lipidomics Approach Reveals Lipid Droplets in Liver, Muscle and Heart as a Repository for Diacylglycerol and Ceramide Species in Non-Alcoholic Fatty Liver. Cells 2019, 8, 277. [Google Scholar] [CrossRef]
- Marchesini, G.; Petta, S.; Dalle Grave, R. Diet, Weight Loss, and Liver Health in NAFLD: Pathophysiology, Evidence and Practice. Hepatology 2016, 63, 2032–2043. [Google Scholar] [CrossRef]
- Yang, K.; Han, X. Lipidomics: Techniques, Applications, and Outcomes Related to Biomedical Sciences. Trends Biochem. Sci. 2016, 41, 954–969. [Google Scholar] [CrossRef]
- Shon, J.C.; Kim, W.C.; Ryu, R.; Wu, Z.; Seo, J.S.; Choi, M.S.; Liu, K.H. Plasma Lipidomics Reveals Insights into Anti-Obesity Effect of Chrysanthemum morifolium Ramat Leaves and Its Constituent Luteolin in High-Fat Diet-Induced Dyslipidemic Mice. Nutrients 2020, 12, 2973. [Google Scholar] [CrossRef]
- Nam, M.; Choi, M.S.; Choi, J.Y.; Kim, N.; Kim, M.S.; Jung, S.; Kim, J.; Ryu, D.H.; Hwang, G.S. Effect of green tea on hepatic lipid metabolism in mice fed a high-fat diet. J. Nutr. Biochem. 2018, 51, 1–7. [Google Scholar] [CrossRef]
- Bao, D.; Ma, Y.; Zhang, X.; Guan, F.; Chen, W.; Gao, K.; Qin, C.; Zhang, L. Preliminary Characterization of a Leptin Receptor Knockout Rat Created by CRISPR/Cas9 System. Sci. Rep. 2015, 5, 15942. [Google Scholar] [CrossRef]
- Yao, F.; Jiang, D.D.; Guo, W.H.; Guo, L.S.; Gao, M.M.; Bai, Y.; Wang, X.; Zhang, L.S. FABP4 inhibitor attenuates inflammation and endoplasmic reticulum stress of islet in leptin receptor knockout rats. Eur. Rev. Med. Pharmacol. Sci. 2020, 24, 12808–12820. [Google Scholar]
- Li, R.; Peng, S.; Ye, H.; Li, D.; Granato, D.; Guo, H.; Xie, Z. Metabolite differentiation and antiobesity effects between different grades of Yuexi Cuilan green tea. J. Funct. Foods 2021, 87, 104794. [Google Scholar] [CrossRef]
- Wen, J.J.; Li, M.Z.; Gao, H.; Hu, J.L.; Nie, Q.X.; Chen, H.H.; Zhang, Y.L.; Xie, M.Y.; Nie, S.P. Polysaccharides from fermented Momordica charantia L. with Lactobacillus plantarum NCU116 ameliorate metabolic disorders and gut microbiota change in obese rats. Food Funct. 2021, 12, 2617–2630. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Gu, M.; Wang, R.; Li, M.; Li, D.; Xie, Z. Dietary supplement of Yunkang 10 green tea and treadmill exercise ameliorate high fat diet induced metabolic syndrome of C57BL/6J mice. Nutr. Metab. 2020, 17, 14. [Google Scholar] [CrossRef] [PubMed]
- Xie, Z.; Su, W.; Liu, S.; Zhao, G.; Esser, K.; Schroder, E.A.; Lefta, M.; Stauss, H.M.; Guo, Z.; Gong, M.C. Smooth-muscle BMAL1 participates in blood pressure circadian rhythm regulation. J. Clin. Investig. 2015, 125, 324–336. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Shen, Y.; Ling, T.; Ho, C.T.; Li, D.; Guo, H.; Xie, Z. Analysis of Differentiated Chemical Components between Zijuan Purple Tea and Yunkang Green Tea by UHPLC-Orbitrap-MS/MS Combined with Chemometrics. Foods 2021, 10, 1070. [Google Scholar] [CrossRef]
- Pourabedin, M.; Guan, L.; Zhao, X. Xylo-oligosaccharides and virginiamycin differentially modulate gut microbial composition in chickens. Microbiome 2015, 3, 15. [Google Scholar] [CrossRef] [Green Version]
- Besten, G.D.; Bleeker, A.; Gerding, A.; Eunen, K.V.; Havinga, R.; Dijk, T.V.; Oosterveer, M.H.; Jonker, J.W.; Groen, A.K.; Reijngoud, D.J. Short-Chain Fatty Acids Protect Against High-Fat Diet-Induced Obesity via a PPARγ-Dependent Switch from Lipogenesis to Fat Oxidation. Diabetes 2015, 64, 2398–2408. [Google Scholar] [CrossRef]
- Turnbaugh, P.J.; Hamady, M.; Yatsunenko, T.; Cantarel, B.L.; Duncan, A.; Ley, R.E.; Sogin, M.L.; Jones, W.J.; Roe, B.A.; Affourtit, J.P.; et al. A core gut microbiome in obese and lean twins. Nature 2009, 457, 480–484. [Google Scholar] [CrossRef]
- Huang, J.; Wang, Y.; Xie, Z.; Zhou, Y.; Zhang, Y.; Wan, X. The anti-obesity effects of green tea in human intervention and basic molecular studies. Eur. J. Clin. Nutr. 2014, 68, 1075–1087. [Google Scholar] [CrossRef]
- Dinh, T.C.; Thi Phuong, T.N.; Minh, L.B.; Minh Thuc, V.T.; Bac, N.D.; Van Tien, N.; Pham, V.H.; Show, P.L.; Tao, Y.; Nhu Ngoc, V.T.; et al. The effects of green tea on lipid metabolism and its potential applications for obesity and related metabolic disorders—An existing update. Diabetes Metab. Syndr. 2019, 13, 1667–1673. [Google Scholar] [CrossRef]
- Huang, J.; Feng, S.; Liu, A.; Dai, Z.; Wang, H.; Reuhl, K.; Lu, W.; Yang, C.S. Green Tea Polyphenol EGCG Alleviates Metabolic Abnormality and Fatty Liver by Decreasing Bile Acid and Lipid Absorption in Mice. Mol. Nutr. Food Res. 2018, 62, 101002. [Google Scholar] [CrossRef]
- Choi, C.; Song, H.D.; Son, Y.; Cho, Y.K.; Ahn, S.Y.; Jung, Y.S.; Yoon, Y.C.; Kwon, S.W.; Lee, Y.H. Epigallocatechin-3-Gallate Reduces Visceral Adiposity Partly through the Regulation of Beclin1-Dependent Autophagy in White Adipose Tissues. Nutrients 2020, 12, 3072. [Google Scholar] [CrossRef]
- Chen, G.; Chen, R.; Chen, D.; Ye, H.; Hu, B.; Zeng, X.; Liu, Z. Tea Polysaccharides as Potential Therapeutic Options for Metabolic Diseases. J. Agric. Food Chem. 2019, 67, 5350–5360. [Google Scholar] [CrossRef]
- Wu, L.; Meng, J.; Shen, Q.; Zhang, Y.; Pan, S.; Chen, Z.; Zhu, L.Q.; Lu, Y.; Huang, Y.; Zhang, G. Caffeine inhibits hypothalamic A1R to excite oxytocin neuron and ameliorate dietary obesity in mice. Nat. Commun. 2017, 8, 15904. [Google Scholar] [CrossRef]
- Zhu, M.Z.; Zhou, F.; Ouyang, J.; Wang, Q.Y.; Li, Y.L.; Wu, J.L.; Huang, J.A.; Liu, Z.H. Combined use of epigallocatechin-3-gallate (EGCG) and caffeine in low doses exhibits marked anti-obesity synergy through regulation of gut microbiota and bile acid metabolism. Food Funct. 2021, 12, 4105–4116. [Google Scholar] [CrossRef]
- Wu, G.; Liu, A.B.; Xu, Y.; Wang, Y.; Zhao, L.; Hara, Y.; Lam, Y.Y.; Yang, C.S. The Effects of Green Tea on Diabetes and Gut Microbiome in db/db Mice: Studies with Tea Extracts vs. Tea Powder. Nutrients 2021, 13, 3135. [Google Scholar] [CrossRef]
- Yousaf, S.; Butt, M.S.; Suleria, H.A.R.; Iqbal, M.J. The role of green tea extract and powder in mitigating metabolic syndromes with special reference to hyperglycemia and hypercholesterolemia. Food Funct. 2014, 5, 545–556. [Google Scholar] [CrossRef]
- Rocha, A.; Bolin, A.P.; Cardoso, C.A.; Otton, R. Green tea extract activates AMPK and ameliorates white adipose tissue metabolic dysfunction induced by obesity. Eur. J. Nutr. 2016, 55, 2231–2244. [Google Scholar] [CrossRef]
- Xia, H.M.; Wang, J.; Xie, X.J.; Xu, L.J.; Tang, S.Q. Green tea polyphenols attenuate hepatic steatosis, and reduce insulin resistance and inflammation in high-fat diet-induced rats. Int. J. Mol. Med. 2019, 44, 1523–1530. [Google Scholar] [CrossRef]
- Han, M.; Zhao, G.; Wang, Y.; Wang, D.; Sun, F.; Ning, J.; Wan, X.; Zhang, J. Safety and anti-hyperglycemic efficacy of various tea types in mice. Sci. Rep. 2016, 6, 31703. [Google Scholar] [CrossRef]
- Hawkins, M.; Tonelli, J.; Kishore, P.; Stein, D.; Ragucci, E.; Gitig, A.; Reddy, K. Contribution of Elevated Free Fatty Acid Levels to the Lack of Glucose Effectiveness in Type 2 Diabetes. Diabetes 2003, 52, 2748–2758. [Google Scholar] [CrossRef]
- Bogdanski, P.; Suliburska, J.; Szulinska, M.; Stepien, M.; Pupek-Musialik, D.; Jablecka, A. Green tea extract reduces blood pressure, inflammatory biomarkers, and oxidative stress and improves parameters associated with insulin resistance in obese, hypertensive patients. Nutr. Res. 2012, 32, 421–427. [Google Scholar] [CrossRef] [PubMed]
- Qin, S.; He, Z.; Wu, Y.; Zeng, C.; Zheng, Z.; Zhang, H.; Lv, C.; Yuan, Y.; Wu, H.; Ye, J.; et al. Instant Dark Tea Alleviates Hyperlipidaemia in High-Fat Diet-Fed Rat: From Molecular Evidence to Redox Balance and Beyond. Front. Nutr. 2022, 9, 819980. [Google Scholar] [CrossRef] [PubMed]
- Sanders, F.W.; Griffin, J.L. De novo lipogenesis in the liver in health and disease: More than just a shunting yard for glucose. Biol. Rev. Camb. Philos. Soc. 2016, 91, 452–468. [Google Scholar] [CrossRef] [PubMed]
- Shrestha, S.; Ehlers, S.J.; Lee, J.Y.; Fernandez, M.L.; Koo, S.I. Dietary green tea extract lowers plasma and hepatic triglycerides and decreases the expression of sterol regulatory element-binding protein-1c mRNA and its responsive genes in fructose-fed, ovariectomized rats. J. Nutr. 2009, 139, 640–645. [Google Scholar] [CrossRef]
- Oppi-Williams, C.; Suagee, J.K.; Corl, B.A. Regulation of lipid synthesis by liver X receptor α and sterol regulatory element-binding protein 1 in mammary epithelial cells. J. Dairy. Sci. 2013, 96, 112–121. [Google Scholar] [CrossRef] [Green Version]
- Joseph, S.B.; Laffitte, B.A.; Patel, P.H.; Watson, M.A.; Matsukuma, K.E.; Walczak, R.; Collins, J.L.; Osborne, T.F.; Tontonoz, P. Direct and indirect mechanisms for regulation of fatty acid synthase gene expression by liver X receptors. J. Biol. Chem. 2002, 277, 11019–11025. [Google Scholar] [CrossRef]
- Jing, W.; Wang, C.; Li, S.; Sha, L.; Wang, W.; Jing, L.; Chi, Y.; Hang, Y.; Kong, X. Thrsp promotes hepatic lipogenesis, and its expression is regulated by LXRα through an SREBP1c-dependent mechanism. Hepatology 2013, 58, 617–628. [Google Scholar]
- Zhu, Q.; Anderson, G.W.; Mucha, G.T.; Parks, E.J.; Metkowski, J.K.; Mariash, C.N. The Spot 14 protein is required for de novo lipid synthesis in the lactating mammary gland. Endocrinology 2005, 146, 3343. [Google Scholar] [CrossRef]
- Evans, R.M.; Barish, G.D.; Wang, Y.X. PPARs and the complex journey to obesity. Nat. Med. 2004, 10, 355–361. [Google Scholar] [CrossRef]
- Matsusue, K.; Haluzik, M.; Lambert, G.; Yim, S.H.; Gavrilova, O.; Ward, J.M.; Brewer, B., Jr.; Reitman, M.L.; Gonzalez, F.J. Liver-specific disruption of PPARγ in leptin-deficient mice improves fatty liver but aggravates diabetic phenotypes. J. Clin. Investig. 2003, 111, 737. [Google Scholar] [CrossRef]
- Li, Z.Z.; Berk, M.; McIntyre, T.M.; Feldstein, A.E. Hepatic lipid partitioning and liver damage in nonalcoholic fatty liver disease: Role of stearoyl-CoA desaturase. J. Biol. Chem. 2009, 284, 5637–5644. [Google Scholar] [CrossRef]
- Chirala, S.S.; Chang, H.; Matzuk, M.; Abu-Elheiga, L.; Mao, J.; Mahon, K.; Finegold, M.; Wakil, S.J. Fatty acid synthesis is essential in embryonic development: Fatty acid synthase null mutants and most of the heterozygotes die in utero. Proc. Natl. Acad. Sci. USA 2003, 100, 6358–6363. [Google Scholar] [CrossRef]
- Zhong, S.; Li, L.; Liang, N.; Zhang, L.; Xu, X.; Chen, S.; Yin, H. Acetaldehyde Dehydrogenase 2 regulates HMG-CoA reductase stability and cholesterol synthesis in the liver. Redox Biol. 2021, 41, 101919. [Google Scholar] [CrossRef]
- Feng, S.; Dai, Z.; Liu, A.B.; Huang, J.; Narsipur, N.; Guo, G.; Kong, B.; Reuhl, K.; Lu, W.; Luo, Z.; et al. Intake of stigmasterol and β-sitosterol alters lipid metabolism and alleviates NAFLD in mice fed a high-fat western-style diet. Biochim. Biophys. Acta Mol. Cell Biol. Lipids 2018, 1863, 1274–1284. [Google Scholar] [CrossRef]
- Gorden, D.L.; Ivanova, P.T.; Myers, D.S.; McIntyre, J.O.; VanSaun, M.N.; Wright, J.K.; Matrisian, L.M.; Brown, H.A. Increased diacylglycerols characterize hepatic lipid changes in progression of human nonalcoholic fatty liver disease; comparison to a murine model. PLoS ONE 2011, 6, e22775. [Google Scholar] [CrossRef]
- Ruby, M.A.; Massart, J.; Hunerdosse, D.M.; Schönke, M.; Correia, J.C.; Louie, S.M.; Ruas, J.L.; Näslund, E.; Nomura, D.K.; Zierath, J.R. Human Carboxylesterase 2 Reverses Obesity-Induced Diacylglycerol Accumulation and Glucose Intolerance. Cell Rep. 2017, 18, 636–646. [Google Scholar] [CrossRef] [Green Version]
- Feng, K.; Lan, Y.; Zhu, X.; Li, J.; Chen, T.; Huang, Q.; Ho, C.T.; Chen, Y.; Cao, Y. Hepatic Lipidomics Analysis Reveals the Antiobesity and Cholesterol-Lowering Effects of Tangeretin in High-Fat Diet-Fed Rats. J. Agric. Food Chem. 2020, 68, 6142–6153. [Google Scholar] [CrossRef]
- Rhee, E.P.; Cheng, S.; Larson, M.G.; Walford, G.A.; Lewis, G.D.; McCabe, E.; Yang, E.; Farrell, L.; Fox, C.S.; O’Donnell, C.J.; et al. Lipid profiling identifies a triacylglycerol signature of insulin resistance and improves diabetes prediction in humans. J. Clin. Investig. 2011, 121, 1402–1411. [Google Scholar] [CrossRef]
- Stegemann, C.; Pechlaner, R.; Willeit, P.; Langley, S.R.; Mangino, M.; Mayr, U.; Menni, C.; Moayyeri, A.; Santer, P.; Rungger, G.; et al. Lipidomics profiling and risk of cardiovascular disease in the prospective population-based Bruneck study. Circulation 2014, 129, 1821–1831. [Google Scholar] [CrossRef]
- Kihara, A. Very long-chain fatty acids: Elongation, physiology and related disorders. J. Biochem. 2012, 152, 387–395. [Google Scholar] [CrossRef]
- Niu, Z.; Wu, Q.; Sun, L.; Qi, Q.; Zheng, H.; Li, H.; Zeng, R.; Lin, X.; Zong, G. Circulating Glycerolipids, Fatty Liver Index, and Incidence of Type 2 Diabetes: A Prospective Study Among Chinese. J. Clin. Endocrinol. Metab. 2021, 106, 2010–2020. [Google Scholar] [CrossRef] [PubMed]
- Tabe, S.; Hikiji, H.; Ariyoshi, W.; Hashidate-Yoshida, T.; Shindou, H.; Shimizu, T.; Okinaga, T.; Seta, Y.; Tominaga, K.; Nishihara, T. Lysophosphatidylcholine acyltransferase 4 is involved in chondrogenic differentiation of ATDC5 cells. Sci. Rep. 2017, 7, 16701. [Google Scholar] [CrossRef] [PubMed]
- Hishikawa, D.; Hashidate, T.; Shimizu, T.; Shindou, H. Diversity and function of membrane glycerophospholipids generated by the remodeling pathway in mammalian cells. J. Lipid Res. 2014, 55, 799–807. [Google Scholar] [CrossRef] [PubMed]
- Feng, S.; Gan, L.; Yang, C.S.; Liu, A.B.; Lu, W.; Shao, P.; Dai, Z.; Sun, P.; Luo, Z. Effects of Stigmasterol and beta-Sitosterol on Nonalcoholic Fatty Liver Disease in a Mouse Model: A Lipidomic Analysis. J. Agric. Food Chem. 2018, 66, 3417–3425. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Chen, T.; Xie, X.; Wang, X.; Luo, Y.; Xu, N.; Sa, Z.; Zhang, M.; Chen, Z.; Hu, X.; et al. Hepatic Lipidomics Analysis Reveals the Ameliorative Effects of Highland Barley β-Glucan on Western Diet-Induced Nonalcoholic Fatty Liver Disease Mice. J. Agric. Food Chem. 2021, 69, 9287–9298. [Google Scholar] [CrossRef] [PubMed]
- Calzada, E.; Onguka, O.; Claypool, S.M. Phosphatidylethanolamine Metabolism in Health and Disease. Int. Rev. Cell Mol. Bio. 2016, 321, 29–88. [Google Scholar]
- Glade, M.J.; Smith, K. Phosphatidylserine and the human brain. Nutrition 2015, 31, 781–786. [Google Scholar] [CrossRef] [PubMed]
- Osman, C.; Voelker, D.R.; Langer, T. Making heads or tails of phospholipids in mitochondria. J. Cell Biol. 2011, 192, 7–16. [Google Scholar] [CrossRef]
- Bouter, K.E.; Raalte, D.V.; Groen, A.K.; Nieuwdorp, M. Role of the Gut Microbiome in the Pathogenesis of Obesity and Obesity-Related Metabolic Dysfunction. Gastroenterology 2017, 152, 1671. [Google Scholar] [CrossRef]
- Goodrich, J.; Waters, J.; Poole, A.; Sutter, J.; Koren, O.; Blekhman, R.; Beaumont, M.; Van Treuren, W.; Knight, R.; Bell, J. Human genetics shape the gut microbiome. Cell 2014, 159, 789–799. [Google Scholar] [CrossRef]
- Wang, L.; Zeng, B.; Liu, Z.; Liao, Z.; Zhong, Q.; Gu, L.; Wei, H.; Fang, X. Green Tea Polyphenols Modulate Colonic Microbiota Diversity and Lipid Metabolism in High-Fat Diet Treated HFA Mice. J. Food Sci. 2018, 83, 864–873. [Google Scholar] [CrossRef]
- Santacruz, A.; Collado, M.C.; García-Valdés, L.; Segura, M.T.; Martín-Lagos, J.A.; Anjos, T.; Martí-Romero, M.; Lopez, R.M.; Florido, J.; Campoy, C.; et al. Gut microbiota composition is associated with body weight, weight gain and biochemical parameters in pregnant women. Br. J. Nutr. 2010, 104, 83–92. [Google Scholar] [CrossRef]
- Chen, G.; Xie, M.; Dai, Z.; Wan, P.; Ye, H.; Zeng, X.; Sun, Y. Kudingcha and Fuzhuan Brick Tea Prevent Obesity and Modulate Gut Microbiota in High-Fat Diet Fed Mice. Mol. Nutr. Food Res. 2018, 62, e1700485. [Google Scholar] [CrossRef]
- Darnaud, M.; Dos Santos, A.; Gonzalez, P.; Augui, S.; Lacoste, C.; Desterke, C.; De Hertogh, G.; Valentino, E.; Braun, E.; Zheng, J.; et al. Enteric Delivery of Regenerating Family Member 3 alpha Alters the Intestinal Microbiota and Controls Inflammation in Mice with Colitis. Gastroenterology 2018, 154, 1009–1023.e14. [Google Scholar] [CrossRef]
- Wang, L.; Liao, Y.; Yang, R.; Zhu, Z.; Zhang, L.; Wu, Z.; Sun, X. An engineered probiotic secreting Sj16 ameliorates colitis via Ruminococcaceae/butyrate/retinoic acid axis. Bioeng. Transl. Med. 2021, 6, e10219. [Google Scholar] [CrossRef]
- Coppola, S.; Avagliano, C.; Calignano, A.; Berni Canani, R. The Protective Role of Butyrate against Obesity and Obesity-Related Diseases. Molecules 2021, 26, 682. [Google Scholar] [CrossRef]
- Alsharairi, N.A. The Role of Short-Chain Fatty Acids in Mediating Very Low-Calorie Ketogenic Diet-Infant Gut Microbiota Relationships and Its Therapeutic Potential in Obesity. Nutrients 2021, 13, 3702. [Google Scholar] [CrossRef]
- Menni, C.; Lin, C.; Cecelja, M.; Mangino, M.; Matey-Hernandez, M.L.; Keehn, L.; Mohney, R.P.; Steves, C.J.; Spector, T.D.; Kuo, C.F.; et al. Gut microbial diversity is associated with lower arterial stiffness in women. Eur. Heart J. 2018, 39, 2390–2397. [Google Scholar] [CrossRef]
- Aroor, A.R.; Jia, G.; Sowers, J.R. Cellular mechanisms underlying obesity-induced arterial stiffness. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2018, 314, R387–R398. [Google Scholar] [CrossRef]
- Koenen, M.; Hill, M.A.; Cohen, P.; Sowers, J.R. Obesity, Adipose Tissue and Vascular Dysfunction. Circ. Res. 2021, 128, 951–968. [Google Scholar] [CrossRef]
- Mao, G.; Li, S.; Orfila, C.; Shen, X.; Zhou, S.; Linhardt, R.J.; Ye, X.; Chen, S. Depolymerized RG-I-enriched pectin from citrus segment membranes modulates gut microbiota, increases SCFA production, and promotes the growth of Bifidobacterium spp., Lactobacillus spp. and Faecalibaculum spp. Food Funct. 2019, 10, 7828–7843. [Google Scholar] [CrossRef]
- González-Bosch, C.; Boorman, E.; Zunszain, P.A.; Mann, G.E. Short-chain fatty acids as modulators of redox signaling in health and disease. Redox Biol. 2021, 47, 102165. [Google Scholar] [CrossRef]
- He, J.; Zhang, P.; Shen, L.; Niu, L.; Tan, Y.; Chen, L.; Zhao, Y.; Bai, L.; Hao, X.; Li, X.; et al. Short-Chain Fatty Acids and Their Association with Signalling Pathways in Inflammation, Glucose and Lipid Metabolism. Int. J. Mol. Sci. 2020, 21, 6356. [Google Scholar] [CrossRef]
- Hu, J.; Lin, S.; Zheng, B.; Cheung, P.C.K. Short-chain fatty acids in control of energy metabolism. Crit. Rev. Food Sci. Nutr. 2018, 58, 1243–1249. [Google Scholar] [CrossRef]
- Herp, S.; Brugiroux, S.; Garzetti, D.; Ring, D.; Jochum, L.M.; Beutler, M.; Eberl, C.; Hussain, S.; Walter, S.; Gerlach, R.G.; et al. Mucispirillum schaedleri Antagonizes Salmonella Virulence to Protect Mice against Colitis. Cell Host Microbe 2019, 25, 681–694.e8. [Google Scholar] [CrossRef]
- Wang, J.; Tang, H.; Zhang, C.; Zhao, Y.; Derrien, M.; Rocher, E.; van-Hylckama Vlieg, J.E.; Strissel, K.; Zhao, L.; Obin, M.; et al. Modulation of gut microbiota during probiotic-mediated attenuation of metabolic syndrome in high fat diet-fed mice. ISME J. 2015, 9, 1–15. [Google Scholar] [CrossRef]
- Nie, Q.; Hu, J.; Chen, H.; Geng, F.; Nie, S. Arabinoxylan ameliorates type 2 diabetes by regulating the gut microbiota and metabolites. Food Chem. 2022, 371, 131106. [Google Scholar] [CrossRef]
- Sato, J.; Kanazawa, A.; Ikeda, F.; Yoshihara, T.; Goto, H.; Abe, H.; Komiya, K.; Kawaguchi, M.; Shimizu, T.; Ogihara, T.; et al. Gut dysbiosis and detection of “live gut bacteria” in blood of Japanese patients with type 2 diabetes. Diabetes Care 2014, 37, 2343–2350. [Google Scholar] [CrossRef] [Green Version]
| Compound | LTE (mg/g) | HTE (mg/g) |
|---|---|---|
| EGCG | 127.77 ± 8.06 | 178.14 ± 2.37 ** |
| EC | 50.49 ± 5.60 | 63.54 ± 2.37 |
| GCG | 63.33 ± 0.93 | 54.52 ± 0.50 ** |
| GC | 44.53 ± 0.49 | 42.62 ± 0.36 * |
| C | 27.71 ± 0.46 | 43.02 ± 2.34 ** |
| Total catechins | 313.83 ± 14.45 | 381.84 ± 4.56 * |
| Polysaccharide | 188.02 ± 4.33 | 153.89 ± 1.36 *** |
| Caffeine | 111.25 ± 0.52 | 106.92 ± 0.93 * |
| Theanine | 20.74 ± 0.14 | 30.51 ± 1.43 *** |
| Total amino acids | 50.31 ± 0.42 | 74.63 ± 5.58 *** |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, G.; Gu, W.; Cheng, H.; Guo, H.; Li, D.; Xie, Z. Huangshan Maofeng Green Tea Extracts Prevent Obesity-Associated Metabolic Disorders by Maintaining Homeostasis of Gut Microbiota and Hepatic Lipid Classes in Leptin Receptor Knockout Rats. Foods 2022, 11, 2939. https://doi.org/10.3390/foods11192939
Wu G, Gu W, Cheng H, Guo H, Li D, Xie Z. Huangshan Maofeng Green Tea Extracts Prevent Obesity-Associated Metabolic Disorders by Maintaining Homeostasis of Gut Microbiota and Hepatic Lipid Classes in Leptin Receptor Knockout Rats. Foods. 2022; 11(19):2939. https://doi.org/10.3390/foods11192939
Chicago/Turabian StyleWu, Guohuo, Wei Gu, Huijun Cheng, Huimin Guo, Daxiang Li, and Zhongwen Xie. 2022. "Huangshan Maofeng Green Tea Extracts Prevent Obesity-Associated Metabolic Disorders by Maintaining Homeostasis of Gut Microbiota and Hepatic Lipid Classes in Leptin Receptor Knockout Rats" Foods 11, no. 19: 2939. https://doi.org/10.3390/foods11192939





