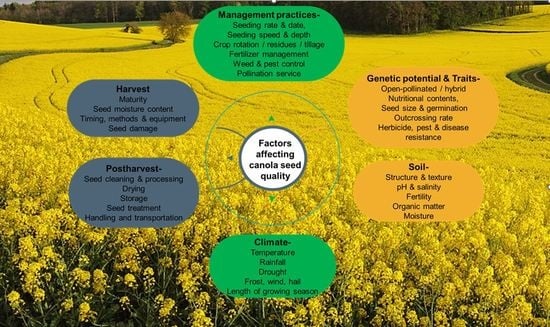
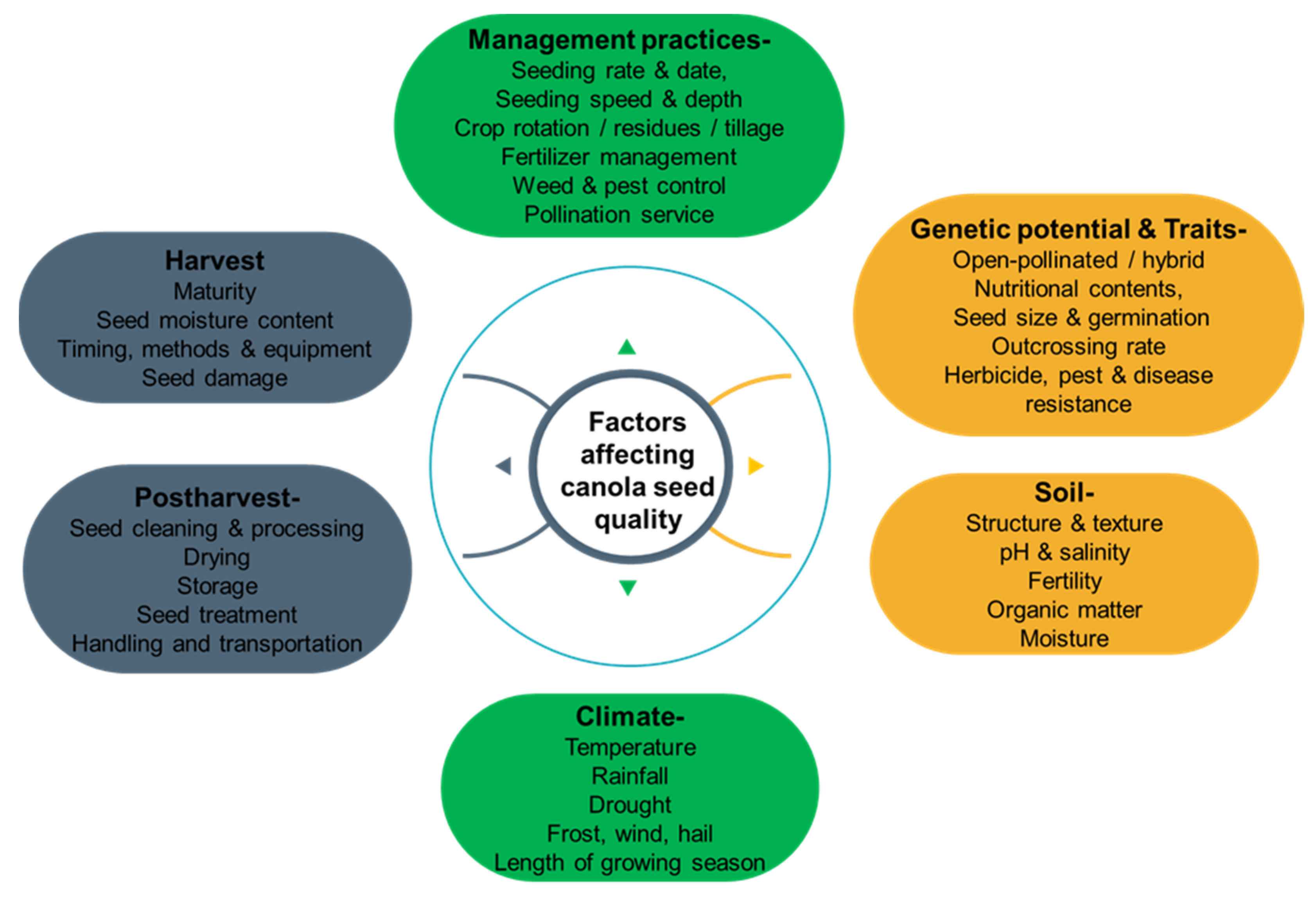
Factors Affecting the Quality of Canola Grains and Their Implications for Grain-Based Foods
Abstract
:1. Introduction
2. Factors Affecting the Quality of Canola Grains
2.1. Genetics and Breeding
- Genetic Diversity and Population Structure of Canola Germplasm
- Genetic Architecture and Molecular Mechanisms of Quality Traits in Canola
2.2. Environmental Factors
2.3. Agronomic Practices and Management Techniques
2.4. Harvest and Post-Harvest Management
- Harvesting, Drying, Storage and Transportation
- Quality Assessment Methods and Standards
3. Implications for Canola Grain-Based Foods
3.1. Canola Oil
- Factors Affecting Canola Oil Extraction
- Factors Affecting Canola Oil Refining
3.2. Canola Meal
3.3. Canola Flour and Bakery Products
- Factors Affecting Flour Production
- Factors Affecting Bakery Products
4. Challenges and Prospects
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Xiao, Z.; Pan, Y.; Wang, C.; Li, X.; Lu, Y.; Tian, Z.; Kuang, L.; Wang, X.; Dun, X.; Wang, H. Multi-Functional Development and Utilization of Rapeseed: Comprehensive Analysis of the Nutritional Value of Rapeseed Sprouts. Foods 2022, 11, 778. [Google Scholar] [CrossRef] [PubMed]
- Mag, T.K. Canola Oil Processing in Canada. J. Am. Oil Chem. Soc. 1983, 60, 380–384. [Google Scholar] [CrossRef]
- Assefa, Y.; Prasad, P.V.V.; Foster, C.; Wright, Y.; Young, S.; Bradley, P.; Stamm, M.; Ciampitti, I.A. Major Management Factors Determining Spring and Winter Canola Yield in North America. Crop Sci. 2018, 58, 2875–2880. [Google Scholar] [CrossRef]
- Secchi, M.A.; Fernandez, J.A.; Stamm, M.J.; Durrett, T.; Prasad, P.V.V.; Messina, C.D.; Ciampitti, I.A. Effects of Heat and Drought on Canola (Brassica napus L.) Yield, Oil, and Protein: A Meta-Analysis. Field Crops Res. 2023, 293, 108848. [Google Scholar] [CrossRef]
- Anwar, M.M.; Ali, S.E.; Nasr, E.H. Improving the Nutritional Value of Canola Seed by Gamma Irradiation. J. Radiat. Res. Appl. Sci. 2015, 8, 328–333. [Google Scholar] [CrossRef]
- Barthet, V.J.; Daun, J.K. 5—Seed Morphology, Composition, and Quality. In Canola; Daun, J.K., Eskin, N.A.M., Hickling, D., Eds.; AOCS Press: Hoboken, NJ, USA, 2011; pp. 119–162. ISBN 978-0-9818936-5-5. [Google Scholar]
- Guirrou, I.; El Harrak, A.; El Antari, A.; Hssaini, L.; Hanine, H.; El Fechtali, M.; Nabloussi, A. Bioactive Compounds Assessment in Six Moroccan Rapeseed (Brassica napus L.) Varieties Grown in Two Contrasting Environments. Agronomy 2023, 13, 460. [Google Scholar] [CrossRef]
- Petrie, J.R.; Zhou, X.-R.; Leonforte, A.; McAllister, J.; Shrestha, P.; Kennedy, Y.; Belide, S.; Buzza, G.; Gororo, N.; Gao, W.; et al. Development of a Brassica napus (Canola) Crop Containing Fish Oil-Like Levels of DHA in the Seed Oil. Front. Plant Sci. 2020, 11, 727. [Google Scholar] [CrossRef]
- Mohtashami, R.; Movahhedi Dehnavi, M.; Balouchi, H.; Faraji, H. Improving Yield, Oil Content and Water Productivity of Dryland Canola by Supplementary Irrigation and Selenium Spraying. Agric. Water Manag. 2020, 232, 106046. [Google Scholar] [CrossRef]
- Ghazani, S.M.; Marangoni, A.G. Minor Components in Canola Oil and Effects of Refining on These Constituents: A Review. J. Am. Oil Chem. Soc. 2013, 90, 923–932. [Google Scholar] [CrossRef]
- Johnson, G.H.; Keast, D.R.; Kris-Etherton, P.M. Dietary Modeling Shows That the Substitution of Canola Oil for Fats Commonly Used in the United States Would Increase Compliance with Dietary Recommendations for Fatty Acids. J. Am. Diet. Assoc. 2007, 107, 1726–1734. [Google Scholar] [CrossRef]
- Sey, A.A.; Pham, T.H.; Kavanagh, V.; Kaur, S.; Cheema, M.; Galagedara, L.; Thomas, R. Canola Produced under Boreal Climatic Conditions in Newfoundland and Labrador Have a Unique Lipid Composition and Expeller Press Extraction Retained the Composition for Commercial Use. J. Adv. Res. 2020, 24, 423–434. [Google Scholar] [CrossRef] [PubMed]
- Chew, S.C. Cold-Pressed Rapeseed (Brassica napus) Oil: Chemistry and Functionality. Food Res. Int. 2020, 131, 108997. [Google Scholar] [CrossRef] [PubMed]
- Goyal, A.; Tanwar, B.; Sihag, M.K.; Kumar, V.; Sharma, V.; Soni, S. Rapeseed/Canola (Brassica napus) Seed. In Oilseeds: Health Attributes and Food Applications; Tanwar, B., Goyal, A., Eds.; Springer: Singapore, 2021; pp. 47–71. ISBN 9789811541940. [Google Scholar]
- Aachary, A.A.; Thiyam-Hollander, U.; Eskin, M.N.A. Canola/Rapeseed Proteins and Peptides. In Applied Food Protein Chemistry; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2014; pp. 193–218. ISBN 978-1-118-86058-8. [Google Scholar]
- Wickramasuriya, S.S.; Yi, Y.-J.; Yoo, J.; Kang, N.K.; Heo, J.M. A Review of Canola Meal as an Alternative Feed Ingredient for Ducks. J. Anim. Sci. Technol. 2015, 57, 29. [Google Scholar] [CrossRef]
- Sarwar Gilani, G.; Wu Xiao, C.; Cockell, K.A. Impact of Antinutritional Factors in Food Proteins on the Digestibility of Protein and the Bioavailability of Amino Acids and on Protein Quality. Br. J. Nutr. 2012, 108 (Suppl. 2), S315–S332. [Google Scholar] [CrossRef] [PubMed]
- Khajali, F.; Slominski, B.A. Factors That Affect the Nutritive Value of Canola Meal for Poultry. Poult. Sci. 2012, 91, 2564–2575. [Google Scholar] [CrossRef] [PubMed]
- Bell, J.M. Factors Affecting the Nutritional Value of Canola Meal: A Review. Can. J. Anim. Sci. 1993, 73, 689–697. [Google Scholar] [CrossRef]
- Shahidi, F.; Naczk, M.; Hall, D.; Synowiecki, J. Insensitivity of the Amino Acids of Canola and Rapeseed to Methanol-Ammonia Extraction and Commercial Processing. Food Chem. 1992, 44, 283–285. [Google Scholar] [CrossRef]
- Joint FAO/WHO/UNU Expert Consultation; United Nations University. Protein and Amino Acid Requirements in Human Nutrition: Report of a Joint FAO/WHO/UNU Expert Consultation; World Health Organization: Geneva, Switzerland, 2007. [Google Scholar]
- Renzyaeva, T.V.; Renzyaev, A.O.; Reznichenko, I.U.; Popov, A.M.; Kravchenko, S.N.; Miller, E.S. Rapeseed Processing Products as a Component of Flour-Based Food for Gerontological Purpose. IOP Conf. Ser. Earth Environ. Sci. 2021, 640, 042004. [Google Scholar] [CrossRef]
- Bermejo-Cruz, M.; Osorio-Ruiz, A.; Rodríguez-Canto, W.; Betancur-Ancona, D.; Martínez-Ayala, A.; Chel-Guerrero, L. Antioxidant Potential of Protein Hydrolysates from Canola (Brassica napus L.) Seeds. Biocatal. Agric. Biotechnol. 2023, 50, 102687. [Google Scholar] [CrossRef]
- Liu, Q.; Wu, L.; Pu, H.; Li, C.; Hu, Q. Profile and Distribution of Soluble and Insoluble Phenolics in Chinese Rapeseed (Brassica napus). Food Chem. 2012, 135, 616–622. [Google Scholar] [CrossRef]
- Liersch, A.; Bocianowski, J.; Nowosad, K.; Mikołajczyk, K.; Spasibionek, S.; Wielebski, F.; Matuszczak, M.; Szała, L.; Cegielska-Taras, T.; Sosnowska, K.; et al. Effect of Genotype × Environment Interaction for Seed Traits in Winter Oilseed Rape (Brassica napus L.). Agriculture 2020, 10, 607. [Google Scholar] [CrossRef]
- Cowling, W.A. Genetic Diversity in Australian Canola and Implications for Crop Breeding for Changing Future Environments. Field Crops Res. 2007, 104, 103–111. [Google Scholar] [CrossRef]
- Barthet, V.J. Comparison between Canadian Canola Harvest and Export Surveys. Plants 2016, 5, 30. [Google Scholar] [CrossRef] [PubMed]
- Niemann, J.; Bocianowski, J.; Wojciechowski, A. Effects of Genotype and Environment on Seed Quality Traits Variability in Interspecific Cross-Derived Brassica Lines. Euphytica 2018, 214, 193. [Google Scholar] [CrossRef]
- Gyawali, S.; Hegedus, D.D.; Parkin, I.A.P.; Poon, J.; Higgins, E.; Horner, K.; Bekkaoui, D.; Coutu, C.; Buchwaldt, L. Genetic Diversity and Population Structure in a World Collection of Brassica napus Accessions with Emphasis on South Korea, Japan, and Pakistan. Crop Sci. 2013, 53, 1537–1545. [Google Scholar] [CrossRef]
- Chen, S.; Nelson, M.N.; Ghamkhar, K.; Fu, T.; Cowling, W.A. Divergent Patterns of Allelic Diversity from Similar Origins: The Case of Oilseed Rape (Brassica napus L.) in China and Australia. Genome 2008, 51, 1–10. [Google Scholar] [CrossRef]
- Chen, R.; Shimono, A.; Aono, M.; Nakajima, N.; Ohsawa, R.; Yoshioka, Y. Genetic Diversity and Population Structure of Feral Rapeseed (Brassica napus L.) in Japan. PLoS ONE 2020, 15, e0227990. [Google Scholar] [CrossRef]
- Sharafi, Y.; Majidi, M.M.; Goli, S.A.H.; Rashidi, F. Oil Content and Fatty Acids Composition in Brassica Species. Int. J. Food Prop. 2015, 18, 2145–2154. [Google Scholar] [CrossRef]
- Fikere, M.; Barbulescu, D.M.; Malmberg, M.M.; Maharjan, P.; Salisbury, P.A.; Kant, S.; Panozzo, J.; Norton, S.; Spangenberg, G.C.; Cogan, N.O.I.; et al. Genomic Prediction and Genetic Correlation of Agronomic, Blackleg Disease, and Seed Quality Traits in Canola (Brassica napus L.). Plants 2020, 9, 719. [Google Scholar] [CrossRef]
- Hua, S.; Chen, Z.-H.; Zhang, Y.; Yu, H.; Lin, B.; Zhang, D. Chlorophyll and Carbohydrate Metabolism in Developing Silique and Seed Are Prerequisite to Seed Oil Content of Brassica napus L. Bot. Stud. 2014, 55, 34. [Google Scholar] [CrossRef]
- Lu, K.; Wei, L.; Li, X.; Wang, Y.; Wu, J.; Liu, M.; Zhang, C.; Chen, Z.; Xiao, Z.; Jian, H.; et al. Whole-Genome Resequencing Reveals Brassica napus Origin and Genetic Loci Involved in Its Improvement. Nat. Commun. 2019, 10, 1154. [Google Scholar] [CrossRef] [PubMed]
- Nesi, N.; Delourme, R.; Brégeon, M.; Falentin, C.; Renard, M. Genetic and Molecular Approaches to Improve Nutritional Value of Brassica napus L. Seed. Comptes Rendus Biol. 2008, 331, 763–771. [Google Scholar] [CrossRef] [PubMed]
- So, K.K.Y.; Duncan, R.W. Breeding Canola (Brassica napus L.) for Protein in Feed and Food. Plants 2021, 10, 2220. [Google Scholar] [CrossRef]
- Zum Felde, T.; Baumert, A.; Strack, D.; Becker, H.C.; Möllers, C. Genetic Variation for Sinapate Ester Content in Winter Rapeseed (Brassica napus L.) and Development of NIRS Calibration Equations. Plant Breed. 2007, 126, 291–296. [Google Scholar] [CrossRef]
- Hu, J.; Chen, B.; Zhao, J.; Zhang, F.; Xie, T.; Xu, K.; Gao, G.; Yan, G.; Li, H.; Li, L.; et al. Genomic Selection and Genetic Architecture of Agronomic Traits during Modern Rapeseed Breeding. Nat. Genet. 2022, 54, 694–704. [Google Scholar] [CrossRef]
- Behnke, N.; Suprianto, E.; Möllers, C. A Major QTL on Chromosome C05 Significantly Reduces Acid Detergent Lignin (ADL) Content and Increases Seed Oil and Protein Content in Oilseed Rape (Brassica napus L.). Appl. Genet. 2018, 131, 2477–2492. [Google Scholar] [CrossRef]
- Teh, L.; Möllers, C. Genetic Variation and Inheritance of Phytosterol and Oil Content in a Doubled Haploid Population Derived from the Winter Oilseed Rape Sansibar × Oase Cross. Appl. Genet. 2016, 129, 181–199. [Google Scholar] [CrossRef]
- Zhao, J.; Becker, H.C.; Zhang, D.; Zhang, Y.; Ecke, W. Conditional QTL Mapping of Oil Content in Rapeseed with Respect to Protein Content and Traits Related to Plant Development and Grain Yield. Appl. Genet. 2006, 113, 33–38. [Google Scholar] [CrossRef]
- Xiao, Z.; Zhang, C.; Qu, C.; Wei, L.; Zhang, L.; Yang, B.; Lu, K.; Li, J. Identification of Candidate Genes Regulating Seed Oil Content by QTL Mapping and Transcriptome Sequencing in Brassica napus. Front. Plant Sci. 2022, 13, 1067121. [Google Scholar] [CrossRef]
- Zhao, C.; Xie, M.; Liang, L.; Yang, L.; Han, H.; Qin, X.; Zhao, J.; Hou, Y.; Dai, W.; Du, C.; et al. Genome-Wide Association Analysis Combined With Quantitative Trait Loci Mapping and Dynamic Transcriptome Unveil the Genetic Control of Seed Oil Content in Brassica napus L. Front. Plant Sci. 2022, 13, 929197. [Google Scholar] [CrossRef]
- Orsavova, J.; Misurcova, L.; Vavra Ambrozova, J.; Vicha, R.; Mlcek, J. Fatty Acids Composition of Vegetable Oils and Its Contribution to Dietary Energy Intake and Dependence of Cardiovascular Mortality on Dietary Intake of Fatty Acids. Int. J. Mol. Sci. 2015, 16, 12871–12890. [Google Scholar] [CrossRef]
- McVetty, P.B.E.; Duncan, R.W. Canola, Rapeseed, and Mustard: For Biofuels and Bioproducts. In Industrial Crops: Breeding for BioEnergy and Bioproducts; Cruz, V.M.V., Dierig, D.A., Eds.; Handbook of Plant Breeding; Springer: New York, NY, USA, 2015; pp. 133–156. ISBN 978-1-4939-1447-0. [Google Scholar]
- Spasibionek, S.; Mikołajczyk, K.; Ćwiek–Kupczyńska, H.; Piętka, T.; Krótka, K.; Matuszczak, M.; Nowakowska, J.; Michalski, K.; Bartkowiak-Broda, I. Marker Assisted Selection of New High Oleic and Low Linolenic Winter Oilseed Rape (Brassica napus L.) Inbred Lines Revealing Good Agricultural Value. PLoS ONE 2020, 15, e0233959. [Google Scholar] [CrossRef]
- Qu, C.; Jia, L.; Fu, F.; Zhao, H.; Lu, K.; Wei, L.; Xu, X.; Liang, Y.; Li, S.; Wang, R.; et al. Genome-Wide Association Mapping and Identification of Candidate Genes for Fatty Acid Composition in Brassica napus L. Using SNP Markers. BMC Genom. 2017, 18, 232. [Google Scholar] [CrossRef]
- Yusuf, A.O.; Richter, J.-C.; Möllers, C. Genetic Variation and QTL Analysis of Saturated Fatty Acids in Two Doubled Haploid Populations of Oilseed Rape (Brassica napus L.). Euphytica 2022, 218, 88. [Google Scholar] [CrossRef]
- Zhu, Q.; King, G.J.; Liu, X.; Shan, N.; Borpatragohain, P.; Baten, A.; Wang, P.; Luo, S.; Zhou, Q. Identification of SNP Loci and Candidate Genes Related to Four Important Fatty Acid Composition in Brassica napus Using Genome Wide Association Study. PLoS ONE 2019, 14, e0221578. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; Qi, G.; Sun, X.S.; Stamm, M.J.; Wang, D. Physicochemical Properties and Adhesion Performance of Canola Protein Modified with Sodium Bisulfite. J. Am. Oil Chem. Soc. 2012, 89, 897–908. [Google Scholar] [CrossRef]
- Knoch, D.; Abbadi, A.; Grandke, F.; Meyer, R.C.; Samans, B.; Werner, C.R.; Snowdon, R.J.; Altmann, T. Strong Temporal Dynamics of QTL Action on Plant Growth Progression Revealed through High-Throughput Phenotyping in Canola. Plant Biotechnol. J. 2020, 18, 68–82. [Google Scholar] [CrossRef] [PubMed]
- Gazave, E.; Tassone, E.E.; Baseggio, M.; Cryder, M.; Byriel, K.; Oblath, E.; Lueschow, S.; Poss, D.; Hardy, C.; Wingerson, M.; et al. Genome-Wide Association Study Identifies Acyl-Lipid Metabolism Candidate Genes Involved in the Genetic Control of Natural Variation for Seed Fatty Acid Traits in Brassica napus L. Ind. Crops Prod. 2020, 145, 112080. [Google Scholar] [CrossRef]
- Felker, P.; Bunch, R.; Leung, A.M. Concentrations of Thiocyanate and Goitrin in Human Plasma, Their Precursor Concentrations in Brassica Vegetables, and Associated Potential Risk for Hypothyroidism. Nutr. Rev. 2016, 74, 248–258. [Google Scholar] [CrossRef] [PubMed]
- Jhingan, S.; Harloff, H.-J.; Abbadi, A.; Welsch, C.; Blümel, M.; Tasdemir, D.; Jung, C. Reduced Glucosinolate Content in Oilseed Rape (Brassica napus L.) by Random Mutagenesis of BnMYB28 and BnCYP79F1 Genes. Sci. Rep. 2023, 13, 2344. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Huang, H.; Yi, X.; Zhang, Y.; Yang, Q.; Zhang, C.; Fan, C.; Zhou, Y. Dissection of Genetic Architecture for Glucosinolate Accumulations in Leaves and Seeds of Brassica napus by Genome-Wide Association Study. Plant Biotechnol. J. 2020, 18, 1472–1484. [Google Scholar] [CrossRef]
- Sontowski, R.; Gorringe, N.J.; Pencs, S.; Schedl, A.; Touw, A.J.; van Dam, N.M. Same Difference? Low and High Glucosinolate Brassica Rapa Varieties Show Similar Responses Upon Feeding by Two Specialist Root Herbivores. Front. Plant Sci. 2019, 10, 1451. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Yang, Z.; Tang, M.; Yang, Q.-Y.; Zhang, Y.; Liu, S. Enhancing Canola Breeding by Editing a Glucosinolate Transporter Gene Lacking Natural Variation. Plant Physiol. 2022, 188, 1848–1851. [Google Scholar] [CrossRef]
- Tang, Y.; Zhang, G.; Jiang, X.; Shen, S.; Guan, M.; Tang, Y.; Sun, F.; Hu, R.; Chen, S.; Zhao, H.; et al. Genome-Wide Association Study of Glucosinolate Metabolites (MGWAS) in Brassica napus L. Plants 2023, 12, 639. [Google Scholar] [CrossRef] [PubMed]
- Raman, H.; Shamaya, N.; Pirathiban, R.; McVittie, B.; Raman, R.; Cullis, B.; Easton, A. Quantitative Trait Loci for Genotype and Genotype by Environment Interaction Effects for Seed Yield Plasticity to Terminal Water-Deficit Conditions in Canola (Brassica napus L.). Plants 2023, 12, 720. [Google Scholar] [CrossRef]
- Menendez, Y.C.; Sanchez, D.H.; Snowdon, R.J.; Rondanini, D.P.; Botto, J.F. Unraveling the Impact on Agronomic Traits of the Genetic Architecture Underlying Plant-Density Responses in Canola. J. Exp. Bot. 2021, 72, 5426–5441. [Google Scholar] [CrossRef]
- Kirkegaard, J.A.; Lilley, J.M.; Berry, P.M.; Rondanini, D.P. Chapter 17—Canola. In Crop Physiology Case Histories for Major Crops; Sadras, V.O., Calderini, D.F., Eds.; Academic Press: Cambridge, MA, USA, 2021; pp. 518–549. ISBN 978-0-12-819194-1. [Google Scholar]
- Rivelli, G.M.; Gomez, N.V.; Mantese, A.I.; Miralles, D.J.; Abeledo, L.G.; Rondanini, D.P. Photothermal Quotient Describes the Combined Effects of Heat and Shade Stresses on Canola Seed Productivity. Seeds 2023, 2, 149–164. [Google Scholar] [CrossRef]
- Mi, C.; Sun, C.; Yuan, Y.; Li, F.; Wang, Q.; Zhu, H.; Hua, S.; Lin, L. Effects of Low Nighttime Temperature on Fatty Acid Content in Developing Seeds from Brassica napus L. Based on RNA-Seq and Metabolome. Plants 2023, 12, 325. [Google Scholar] [CrossRef]
- Aksouh-Harradj, N.M.; Campbell, L.C.; Mailer, R.J. Canola Response to High and Moderately High Temperature Stresses during Seed Maturation. Can. J. Plant Sci. 2006, 86, 967–980. [Google Scholar] [CrossRef]
- McGeary, K.D.; de Koff, J.; Pokharel, B.; Link, R.; Saini, P.; Gill, T. Effect of Winter Canola Cultivar on Seed Yield, Oil, and Protein Content. Agrosyst. Geosci. Environ. 2022, 5, e20254. [Google Scholar] [CrossRef]
- Wang, S.X.; Oomah, B.D.; McGregor, D.I.; Downey, R.K. Genetic and Seasonal Variation in the Sinapine Content of Seed from Brassica and Sinapis Species. Can. J. Plant Sci. 1998, 78, 395–400. [Google Scholar] [CrossRef]
- Kirkegaard, J.A.; Lilley, J.M.; Brill, R.D.; Ware, A.H.; Walela, C.K. The Critical Period for Yield and Quality Determination in Canola (Brassica napus L.). Field Crops Res. 2018, 222, 180–188. [Google Scholar] [CrossRef]
- Zeleke, K.T.; Luckett, D.J.; Cowley, R.B. The Influence of Soil Water Conditions on Canola Yields and Production in Southern Australia. Agric. Water Manag. 2014, 144, 20–32. [Google Scholar] [CrossRef]
- Attia, Z.; Pogoda, C.S.; Reinert, S.; Kane, N.C.; Hulke, B.S. Breeding for Sustainable Oilseed Crop Yield and Quality in a Changing Climate. Appl. Genet. 2021, 134, 1817–1827. [Google Scholar] [CrossRef] [PubMed]
- Sinaki, J.M.; Heravan, E.M.; Rad, A.H.S.; Noormohammadi, G.; Zarei, G. The Effects of Water Deficit during Growth Stages of Canola (Brassica napus L.). Am. Eurasian J. Agric. Environ. Sci. 2007, 2, 417–422. [Google Scholar]
- Bilibio, C.; Carvalho, J.d.A.; Hensel, O.; Richter, U. Effect of Different Levels of Water Deficit on Rapeseed (Brassica napus L.) Crop. Ciênc. Agrotec. 2011, 35, 672–684. [Google Scholar] [CrossRef]
- Cadot, S.; Bélanger, G.; Ziadi, N.; Morel, C.; Sinaj, S. Critical Plant and Soil Phosphorus for Wheat, Maize, and Rapeseed after 44 Years of P Fertilization. Nutr. Cycl. Agroecosyst. 2018, 112, 417–433. [Google Scholar] [CrossRef]
- Grant, C.A.; Bailey, L.D. Fertility Management in Canola Production. Can. J. Plant Sci. 1993, 73, 651–670. [Google Scholar] [CrossRef]
- Ahmad, G.; Jan, A.; Arif, M.; Jan, M.T.; Khattak, R.A. Influence of Nitrogen and Sulfur Fertilization on Quality of Canola (Brassica napus L.) under Rainfed Conditions. J. Zhejiang Univ. Sci. B 2007, 8, 731–737. [Google Scholar] [CrossRef]
- Cheema, M.A.; Malik, M.A.; Hussain, A.; Shah, S.H.; Basra, S.M.A. Effects of Time and Rate of Nitrogen and Phosphorus Application on the Growth and the Seed and Oil Yields of Canola (Brassica napus L.). J. Agron. Crop Sci. 2001, 186, 103–110. [Google Scholar] [CrossRef]
- Ma, B.L.; Herath, A.W.; Ma, B.L.; Herath, A.W. Timing and Rates of Nitrogen Fertiliser Application on Seed Yield, Quality and Nitrogen-Use Efficiency of Canola. Crop Pasture Sci. 2016, 67, 167–180. [Google Scholar] [CrossRef]
- Aslam, M.M.; Farhat, F.; Siddiqui, M.A.; Yasmeen, S.; Khan, M.T.; Sial, M.A.; Khan, I.A. Exploration of Physiological and Biochemical Processes of Canola with Exogenously Applied Fertilizers and Plant Growth Regulators under Drought Stress. PLoS ONE 2021, 16, e0260960. [Google Scholar] [CrossRef]
- Wang, Z.-H.; Li, S.-X.; Malhi, S. Effects of Fertilization and Other Agronomic Measures on Nutritional Quality of Crops. J. Sci. Food Agric. 2008, 88, 7–23. [Google Scholar] [CrossRef]
- Van de Wouw, A.P.; Howlett, B.J. Advances in Understanding the Leptosphaeria Maculans—Brassica Pathosystem and Their Impact on Disease Management. Can. J. Plant Pathol. 2020, 42, 149–163. [Google Scholar] [CrossRef]
- Dolatabadian, A.; Cornelsen, J.; Huang, S.; Zou, Z.; Fernando, W.G.D. Sustainability on the Farm: Breeding for Resistance and Management of Major Canola Diseases in Canada Contributing towards an IPM Approach. Can. J. Plant Pathol. 2022, 44, 157–190. [Google Scholar] [CrossRef]
- Edde, P.A. 3—Arthropod Pests of Rapeseed (Canola) (Brassica napus L.). In Field Crop Arthropod Pests of Economic Importance; Edde, P.A., Ed.; Academic Press: Cambridge, MA, USA, 2022; pp. 140–207. ISBN 978-0-12-818621-3. [Google Scholar]
- Liu, Z.; Wang, H.; Xie, J.; Lv, J.; Zhang, G.; Hu, L.; Luo, S.; Li, L.; Yu, J. The Roles of Cruciferae Glucosinolates in Disease and Pest Resistance. Plants 2021, 10, 1097. [Google Scholar] [CrossRef]
- Nelson, M.N.; Nesi, N.; Barrero, J.M.; Fletcher, A.L.; Greaves, I.K.; Hughes, T.; Laperche, A.; Snowdon, R.; Rebetzke, G.J.; Kirkegaard, J.A. Chapter Two—Strategies to Improve Field Establishment of Canola: A Review. In Advances in Agronomy; Sparks, D.L., Ed.; Academic Press: Cambridge, MA, USA, 2022; Volume 175, pp. 133–177. [Google Scholar]
- Page, E.R.; Meloche, S.; Moran, M.; Caldbeck, B.; Barthet, V. Effect of Seeding Date on Winter Canola (Brassica napus L.) Yield and Oil Quality in Southern Ontario. Can. J. Plant Sci. 2021, 101, 490–499. [Google Scholar] [CrossRef]
- Pan, X.; Caldwell, C.D.; Falk, K.C.; Lada, R. The Effect of Cultivar, Seeding Rate and Applied Nitrogen on Brassica Carinata Seed Yield and Quality in Contrasting Environments. Can. J. Plant Sci. 2012, 92, 961–971. [Google Scholar] [CrossRef]
- Harker, K.N.; O’Donovan, J.T.; Blackshaw, R.E.; Hall, L.M.; Willenborg, C.J.; Kutcher, H.R.; Gan, Y.; Lafond, G.P.; May, W.E.; Grant, C.A.; et al. Effect of Agronomic Inputs and Crop Rotation on Biodiesel Quality and Fatty Acid Profiles of Direct-Seeded Canola. Can. J. Plant Sci. 2013, 93, 577–588. [Google Scholar] [CrossRef]
- May, W.E.; Hume, D.J.; Hale, B.A. Effects of Agronomic Practices on Free Fatty Acid Levels in the Oil of Ontario-Grown Spring Canola. Can. J. Plant Sci. 1994, 74, 267–274. [Google Scholar] [CrossRef]
- Morrison, I.N.; Khan, R.; Rashid, A. Effects of Seeding Methods and Soil Crusting on Establishment of Rapeseed (Brassica napus) and Mustard (B. Juncea). Field Crops Res. 1988, 19, 27–39. [Google Scholar] [CrossRef]
- Mocniak, L.E.; Elkin, K.R.; Dillard, S.L.; Bryant, R.B.; Soder, K.J. Building Comprehensive Glucosinolate Profiles for Brassica Varieties. Talanta 2023, 251, 123814. [Google Scholar] [CrossRef] [PubMed]
- Ratajczak, K.; Sulewska, H.; Szymańska, G. New Winter Oilseed Rape Varieties—Seed Quality and Morphological Traits Depending on Sowing Date and Rate. Plant Prod. Sci. 2017, 20, 262–272. [Google Scholar] [CrossRef]
- Harker, K.N.; Clayton, G.W.; Blackshaw, R.E.; O’Donovan, J.T.; Stevenson, F.C. Seeding Rate, Herbicide Timing and Competitive Hybrids Contribute to Integrated Weed Management in Canola (Brassica napus). Can. J. Plant Sci. 2003, 83, 433–440. [Google Scholar] [CrossRef]
- Ma, B.L.; Zhao, H.; Zheng, Z.; Caldwell, C.; Mills, A.; Vanasse, A.; Earl, H.; Scott, P.; Smith, D.L. Optimizing Seeding Dates and Rates for Canola Production in the Humid Eastern Canadian Agroecosystems. Agron. J. 2016, 108, 1869–1879. [Google Scholar] [CrossRef]
- Nazeri, P.; Shirani Rad, A.H.; ValadAbadi, S.A.; Mirakhori, M.; Hadidi Masoule, E. Effect of Sowing Dates and Late Season Water Deficit Stress on Quantitative and Qualitative Traits of Canola Cultivars. Outlook Agric. 2018, 47, 291–297. [Google Scholar] [CrossRef]
- Righi, E.Z.; Vazata, S.; Dalmago, G.A.; Muller, A.L.; Carvalho, L.Z.G.d.; Daga, J. Sowing Times Influence Canola Grain Yield and Oil Quality. Agrometeoros 2018, 25, 415–425. [Google Scholar] [CrossRef]
- Pavlista, A.D.; Hergert, G.W.; Margheim, J.M.; Isbell, T.A. Growth of Spring Canola (Brassica napus) under Deficit Irrigation in Western Nebraska. Ind. Crops Prod. 2016, 83, 635–640. [Google Scholar] [CrossRef]
- Seleiman, M.F.; Al-Suhaibani, N.; Ali, N.; Akmal, M.; Alotaibi, M.; Refay, Y.; Dindaroglu, T.; Abdul-Wajid, H.H.; Battaglia, M.L. Drought Stress Impacts on Plants and Different Approaches to Alleviate Its Adverse Effects. Plants 2021, 10, 259. [Google Scholar] [CrossRef]
- Dogan, E.; Copur, O.; Kahraman, A.; Kirnak, H.; Guldur, M.E. Supplemental Irrigation Effect on Canola Yield Components under Semiarid Climatic Conditions. Agric. Water Manag. 2011, 98, 1403–1408. [Google Scholar] [CrossRef]
- Rahman, N.; Schoenau, J. Response of Wheat, Pea, and Canola to Micronutrient Fertilization on Five Contrasting Prairie Soils. Sci. Rep. 2020, 10, 18818. [Google Scholar] [CrossRef]
- Malhi, S.S.; Gill, K.S. Placement, Rate and Source of N, Seedrow Opener and Seeding Depth Effects on Canola Production. Can. J. Plant Sci. 2004, 84, 719–729. [Google Scholar] [CrossRef]
- Gill, K.S. Crop Rotations Compared with Continuous Canola and Wheat for Crop Production and Fertilizer Use over 6 Yr. Can. J. Plant Sci. 2018, 98, 1139–1149. [Google Scholar] [CrossRef]
- Mohammadi, K.; Rokhzadi, A. An Integrated Fertilization System of Canola (Brassica napus L.) Production under Different Crop Rotations. Ind. Crops Prod. 2012, 37, 264–269. [Google Scholar] [CrossRef]
- Peterson, M.A.; Collavo, A.; Ovejero, R.; Shivrain, V.; Walsh, M.J. The Challenge of Herbicide Resistance around the World: A Current Summary. Pest Manag. Sci. 2018, 74, 2246–2259. [Google Scholar] [CrossRef] [PubMed]
- Asaduzzaman, M.; Pratley, J.E.; Luckett, D.; Lemerle, D.; Wu, H. Weed Management in Canola (Brassica napus L): A Review of Current Constraints and Future Strategies for Australia. Arch. Agron. Soil Sci. 2020, 66, 427–444. [Google Scholar] [CrossRef]
- Asaduzzaman, M.; Pratley, J.E.; An, M.; Luckett, D.J.; Lemerle, D. Canola Interference for Weed Control. Springer Sci. Rev. 2014, 2, 63–74. [Google Scholar] [CrossRef]
- Capinera, J.L. Integrated Management of Insect Pests on Canola and Other Brassica Oilseed Crops. Fla. Entomol. 2017, 100, 833. [Google Scholar] [CrossRef]
- Wallenhammar, A.-C.; Vilvert, E.; Bergqvist, S.; Olson, Å.; Berlin, A. Scientific Evidence of Sustainable Plant Disease Protection Strategies for Oilseed Rape (Brassica napus) in Sweden: A Systematic Map. Environ. Evid. 2022, 11, 22. [Google Scholar] [CrossRef]
- Amro, A.M. Pollinators and Pollination Effects on Three Canola (Brassica napus L.) Cultivars: A Case Study in Upper Egypt. J. King Saud Univ. Sci. 2021, 33, 101240. [Google Scholar] [CrossRef]
- Sabbahi, R.; DeOliveira, D.; Marceau, J. Influence of Honey Bee (Hymenoptera: Apidae) Density on the Production of Canola (Crucifera: Brassicacae). J. Econ. Entomol. 2005, 98, 367–372. [Google Scholar] [CrossRef]
- Sabbahi, R.; de Oliveira, D.; Marceau, J. Does the Honeybee (Hymenoptera: Apidae) Reduce the Blooming Period of Canola? J Agron. Crop Sci. 2006, 192, 233–237. [Google Scholar] [CrossRef]
- Kumar, D.; Kalita, P. Reducing Postharvest Losses during Storage of Grain Crops to Strengthen Food Security in Developing Countries. Foods 2017, 6, 8. [Google Scholar] [CrossRef] [PubMed]
- Stamm, M.; Roozeboom, K.; Holman, J. Harvest Management of Canola. MF3092. Kansas State University Research and Extension. Available online: https://Www.Bookstore.Ksre.Ksu.Edu/ (accessed on 22 May 2023).
- Cenkowski, S.; Sokhansanj, S.; Sosulski, F.W. Effect of Harvest Date and Swathing on Moisture Content and Chlorophyll Content of Canola Seed. Can. J. Plant Sci. 1989, 69, 925–928. [Google Scholar] [CrossRef]
- Haile, T.A.; Shirtliffe, S.J. Effect of Harvest Timing on Dormancy Induction in Canola Seeds. Weed Sci. 2014, 62, 548–554. [Google Scholar] [CrossRef]
- Gan, Y.; Angadi, S.V.; Cutforth, H.; Potts, D.; Angadi, V.V.; McDonald, C.L. Canola and Mustard Response to Short Periods of Temperature and Water Stress at Different Developmental Stages. Can. J. Plant Sci. Rev. Can. Phytotech. 2004, 84, 697–704. [Google Scholar] [CrossRef]
- Cenkowski, S.; Sokhansanj, S.; Sosulski, F.W. Effect of Drying Temperature on Green Color and Chlorophyll Content of Canola Seed. Can. Inst. Food Sci. Technol. J. 1989, 22, 383–386. [Google Scholar] [CrossRef]
- Sokhansanj, S.; Yang, W. Technical Notes: Revision of the ASAE Standard D245.4: Moisture Relationships of Grains. Trans. ASAE 1996, 39, 639–642. [Google Scholar] [CrossRef]
- Karunakaran, C.; Muir, W.E.; Jayas, D.S.; White, N.D.G.; Abramson, D. Safe Storage Time of High Moisture Wheat. J. Stored Prod. Res. 2001, 37, 303–312. [Google Scholar] [CrossRef]
- Canadian Grain Commission Canola and Rapeseed: Grading. Available online: https://grainscanada.gc.ca/en/grain-quality/official-grain-grading-guide/10-canola-rapeseed/grading.html (accessed on 23 April 2023).
- Vidal, N.P.; Rahimi, J.; Kroetsch, B.; Martinez, M.M. Quality and Chemical Stability of Long-Term Stored Soy, Canola, and Sunflower Cold-Pressed Cake Lipids before and after Thermomechanical Processing: A 1H NMR Study. LWT 2023, 173, 114409. [Google Scholar] [CrossRef]
- Cai, Z.; Li, K.; Lee, W.J.; Reaney, M.T.J.; Zhang, N.; Wang, Y. Recent Progress in the Thermal Treatment of Oilseeds and Oil Oxidative Stability: A Review. Fundam. Res. 2021, 1, 767–784. [Google Scholar] [CrossRef]
- Daou, R.; Joubrane, K.; Maroun, R.G.; Khabbaz, L.R.; Ismail, A.; Khoury, A.E.; Daou, R.; Joubrane, K.; Maroun, R.G.; Khabbaz, L.R.; et al. Mycotoxins: Factors Influencing Production and Control Strategies. AIMS Agric. Food 2021, 6, 416–447. [Google Scholar] [CrossRef]
- Radi, M.; Abbasi, S. Optimization of Novel Oil Extraction Technique From Canola Seeds: Lecithin-Based Microemulsion. Eur. J. Lipid Sci. Technol. 2018, 120, 1700267. [Google Scholar] [CrossRef]
- Gaber, M.A.F.M.; Tujillo, F.J.; Mansour, M.P.; Juliano, P. Improving Oil Extraction from Canola Seeds by Conventional and Advanced Methods. Food Eng. Rev. 2018, 10, 198–210. [Google Scholar] [CrossRef]
- Manjarrez Juárez, F.J.; Díaz Huacuz, R.; Carballo-Carballo, A.; Estrada-Gómez, A.; Vaquera-Huerta, H.; Acosta-Gallegos, J.A.; Ávila Perches, M.A.; Gámez Vázquez, A.J.; Manjarrez Juárez, F.J.; Díaz Huacuz, R.; et al. Effect of Storage Time on Canola Seed Quality. Rev. Mex. Cienc. Agrícolas 2017, 8, 933–948. [Google Scholar] [CrossRef]
- Barthet, V.J.; Petryk, M.W.P.; Siemens, B. Rapid Nondestructive Analysis of Intact Canola Seeds Using a Handheld Near-Infrared Spectrometer. J. Am. Oil Chem. Soc. 2020, 97, 577–589. [Google Scholar] [CrossRef]
- Jian, F.; Vellaichamy, C.; Jayas, D.; White, N.D.G. Three-Dimensional Transient Heat, Mass, and Momentum Transfer Model to Predict Conditions of Canola Stored inside Silo Bags under Canadian Prairie Conditions: Part II. Model of Canola Bulk Temperature and Moisture Content. Trans. ASABE 2015, 58, 1135–1144. [Google Scholar] [CrossRef]
- Canadian Grain Commission Oilseeds Methods and Tests Used to Measure Quality. Available online: https://grainscanada.gc.ca/en/grain-research/export-quality/oilseeds/methods-tests.html (accessed on 23 April 2023).
- GrainCorp Canola Standards 2022–2023. Available online: https://grains.graincorp.com.au/wp-content/uploads/2022/10/Canola-Standards-2022_23.pdf (accessed on 22 May 2023).
- Canadian Grain Comission Quality of Western Canadian Canola. Available online: https://grainscanada.gc.ca/en/grain-research/export-quality/oilseeds/canola/2019/pdf/canola-quality-report-19-en.pdf (accessed on 22 May 2023).
- Canadian Grain Commission Fatty Acid Composition: Quality of Western Canadian Canola 2021. Available online: https://grainscanada.gc.ca/en/grain-research/export-quality/oilseeds/canola/2021/10-fatty-acid-composition.html (accessed on 23 April 2023).
- Thiyam-Holländer, U.; Eskin, N.A.M.; Matthäus, B. (Eds.) Canola and Rapeseed: Production, Processing, Food Quality, and Nutrition; CRC Press: Boca Raton, FL, USA, 2013; ISBN 978-0-429-08691-5. [Google Scholar]
- Chmielewska, A.; Kozłowska, M.; Rachwał, D.; Wnukowski, P.; Amarowicz, R.; Nebesny, E.; Rosicka-Kaczmarek, J. Canola/Rapeseed Protein—Nutritional Value, Functionality and Food Application: A Review. Crit. Rev. Food Sci. Nutr. 2021, 61, 3836–3856. [Google Scholar] [CrossRef]
- Carcea, M. Nutritional Value of Grain-Based Foods. Foods 2020, 9, 504. [Google Scholar] [CrossRef]
- Rondanini, D.P.; Borrás, L.; Savin, R. Grain Qualityoilgrain Qualityin Oiloiland Cereal Cropscerealcrops. In Encyclopedia of Sustainability Science and Technology; Meyers, R.A., Ed.; Springer: New York, NY, USA, 2012; pp. 4550–4563. ISBN 978-1-4419-0851-3. [Google Scholar]
- Li, X.; Shi, J.; Scanlon, M.; Xue, S.J.; Lu, J. Effects of Pretreatments on Physicochemical and Structural Properties of Proteins Isolated from Canola Seeds after Oil Extraction by Supercritical-CO2 Process. LWT 2021, 137, 110415. [Google Scholar] [CrossRef]
- Nde, D.B.; Foncha, A.C. Optimization Methods for the Extraction of Vegetable Oils: A Review. Processes 2020, 8, 209. [Google Scholar] [CrossRef]
- Dunford, N.T.; Temelli, F. Extraction Conditions and Moisture Content of Canola Flakes as Related to Lipid Composition of Supercritical CO2 Extracts. J. Food Sci. 1997, 62, 155–159. [Google Scholar] [CrossRef]
- Lavenburg, V.M.; Rosentrater, K.A.; Jung, S. Extraction Methods of Oils and Phytochemicals from Seeds and Their Environmental and Economic Impacts. Processes 2021, 9, 1839. [Google Scholar] [CrossRef]
- Vafaei, N.; Rempel, C.B.; Scanlon, M.G.; Jones, P.J.H.; Eskin, M.N.A. Application of Supercritical Fluid Extraction (SFE) of Tocopherols and Carotenoids (Hydrophobic Antioxidants) Compared to Non-SFE Methods. AppliedChem 2022, 2, 68–92. [Google Scholar] [CrossRef]
- Sampaio, K.A.; Ayala, J.V.; Van Hoed, V.; Monteiro, S.; Ceriani, R.; Verhé, R.; Meirelles, A.J.A. Impact of Crude Oil Quality on the Refining Conditions and Composition of Nutraceuticals in Refined Palm Oil. J. Food Sci. 2017, 82, 1842–1850. [Google Scholar] [CrossRef] [PubMed]
- Gaber, M.A.F.M.; Juliano, P.; Mansour, M.P.; Shrestha, P.; Taylor, C.; Smith, R.; Trujillo, F.J. Improvement of the Canola Oil Degumming Process by Applying a Megasonic Treatment. Ind. Crops Prod. 2020, 158, 112992. [Google Scholar] [CrossRef]
- Ma, Y.; Shi, L.; Liu, Y.; Lu, Q. Effects of Neutralization, Decoloration, and Deodorization on Polycyclic Aromatic Hydrocarbons during Laboratory-Scale Oil Refining Process. J. Chem. 2017, 2017, e7824761. [Google Scholar] [CrossRef]
- Gharby, S.; Harhar, H.; Mamouni, R.; Matthäus, B.; Addi, E.H.A.; Charrouf, Z. Chemical Characterization and Kinetic Parameter Determination under Rancimat Test Conditions of Four Monovarietal Virgin Olive Oils Grown in Morocco. OCL 2016, 23, A401. [Google Scholar] [CrossRef]
- Monte, M.L.; Monte, M.L.; Pohndorf, R.S.; Crexi, V.T.; Pinto, L.A.A. Bleaching with Blends of Bleaching Earth and Activated Carbon Reduces Color and Oxidation Products of Carp Oil. Eur. J. Lipid Sci. Technol. 2015, 117, 829–836. [Google Scholar] [CrossRef]
- Pohndorf, R.S.; Cadaval, T.R.S.; Pinto, L.A.A. Kinetics and Thermodynamics Adsorption of Carotenoids and Chlorophylls in Rice Bran Oil Bleaching. J. Food Eng. 2016, 185, 9–16. [Google Scholar] [CrossRef]
- Gharby, S. Refining Vegetable Oils: Chemical and Physical Refining. Sci. World J. 2022, 2022, e6627013. [Google Scholar] [CrossRef] [PubMed]
- Rhazi, L.; Depeint, F.; Ayerdi Gotor, A. Loss in the Intrinsic Quality and the Antioxidant Activity of Sunflower (Helianthus annuus L.) Oil during an Industrial Refining Process. Molecules 2022, 27, 916. [Google Scholar] [CrossRef] [PubMed]
- Gotor, A.A.; Rhazi, L. Effects of Refining Process on Sunflower Oil Minor Components: A Review. OCL 2016, 23, D207. [Google Scholar] [CrossRef]
- Daun, J.K.; Eskin, M.N.A.; Hickling, D. (Eds.) Canola: Chemistry, Production, Processing, and Utilization, 1st ed.; Academic Press and AOCS Press: Urbana, IL, USA, 2011; ISBN 978-0-9818936-5-5. [Google Scholar]
- Mawson, R.; Heaney, R.K.; Zdunczyk, Z.; Kozlowska, H. Rapeseed meal-glucosinolates and their antinutritional effects Part 3. Animal growth and performance. Nahrung 1994, 38, 167–177. [Google Scholar] [CrossRef]
- Gupta, R.K.; Gangoliya, S.S.; Singh, N.K. Reduction of Phytic Acid and Enhancement of Bioavailable Micronutrients in Food Grains. J. Food Sci. Technol. 2015, 52, 676–684. [Google Scholar] [CrossRef]
- Newkirk, R. Canada, C.C.O., Canola Meal: Feed Industry Guide, 4th ed.; Canola Council Council of Canada: Winnipeg, MB, Canada, 2009. [Google Scholar]
- Tian, Y.; Zhou, Y.; Kriisa, M.; Anderson, M.; Laaksonen, O.; Kütt, M.-L.; Föste, M.; Korzeniowska, M.; Yang, B. Effects of Fermentation and Enzymatic Treatment on Phenolic Compounds and Soluble Proteins in Oil Press Cakes of Canola (Brassica napus). Food Chem. 2023, 409, 135339. [Google Scholar] [CrossRef]
- Hansen, J.Ø.; Skrede, A.; Mydland, L.T.; Øverland, M. Fractionation of Rapeseed Meal by Milling, Sieving and Air Classification—Effect on Crude Protein, Amino Acids and Fiber Content and Digestibility. Anim. Feed Sci. Technol. 2017, 230, 143–153. [Google Scholar] [CrossRef]
- Liu, C.; Liu, L.; Li, L.; Hao, C.; Zheng, X.; Bian, K.; Zhang, J.; Wang, X. Effects of Different Milling Processes on Whole Wheat Flour Quality and Performance in Steamed Bread Making. LWT Food Sci. Technol. 2015, 62, 310–318. [Google Scholar] [CrossRef]
- Mejicanos, G.A.; Rogiewicz, A.; Nyachoti, C.M.; Slominski, B.A. Fractionation of Canola Meal Using Sieving Technology. Can. J. Anim. Sci. 2017, 97, 613–621. [Google Scholar] [CrossRef]
- Ratnayake, W.S.; Hoover, R.; Warkentin, T. Pea Starch: Composition, Structure and Properties—A Review. Starch-Stärke 2002, 54, 217–234. [Google Scholar] [CrossRef]
- Aider, M.; Barbana, C. Canola Proteins: Composition, Extraction, Functional Properties, Bioactivity, Applications as a Food Ingredient and Allergenicity—A Practical and Critical Review. Trends Food Sci. Technol. 2011, 22, 21–39. [Google Scholar] [CrossRef]
- Nicolosi, A.; Laganà, V.R.; Di Gregorio, D. Habits, Health and Environment in the Purchase of Bakery Products: Consumption Preferences and Sustainable Inclinations before and during COVID-19. Foods 2023, 12, 1661. [Google Scholar] [CrossRef] [PubMed]
- Barragán-Martínez, L.P.; Román-Guerrero, A.; Vernon-Carter, E.J.; Alvarez-Ramirez, J. Impact of Fat Replacement by a Hybrid Gel (Canola Oil/Candelilla Wax Oleogel and Gelatinized Corn Starch Hydrogel) on Dough Viscoelasticity, Color, Texture, Structure, and Starch Digestibility of Sugar-Snap Cookies. Int. J. Gastron. Food Sci. 2022, 29, 100563. [Google Scholar] [CrossRef]
- Salama, H.H.; Hashim, A.F. A Functional Spreadable Canola and Milk Proteins Oleogels as a Healthy System for Candy Gummies. Sci. Rep. 2022, 12, 12619. [Google Scholar] [CrossRef]
- Martins, Z.E.; Pinho, O.; Ferreira, I.M.P.L.V.O. Food Industry By-Products Used as Functional Ingredients of Bakery Products. Trends Food Sci. Technol. 2017, 67, 106–128. [Google Scholar] [CrossRef]
- Singh, R.; Langyan, S.; Sangwan, S.; Rohtagi, B.; Khandelwal, A.; Shrivastava, M. Protein for Human Consumption From Oilseed Cakes: A Review. Front. Sustain. Food Syst. 2022, 6, 856401. [Google Scholar] [CrossRef]
- Jang, A.; Bae, W.; Hwang, H.-S.; Lee, H.G.; Lee, S. Evaluation of Canola Oil Oleogels with Candelilla Wax as an Alternative to Shortening in Baked Goods. Food Chem. 2015, 187, 525–529. [Google Scholar] [CrossRef]
- Hesso, N.; Loisel, C.; Chevallier, S.; Le-Bail, A.; Queveau, D.; Pontoire, B.; Le-Bail, P. Monitoring Cake Baking by Studying Different Ingredient Interactions: From a Model System to a Real System. Food Hydrocoll. 2015, 51, 7–15. [Google Scholar] [CrossRef]
- Van Bockstaele, F.; Debonne, E.; De Leyn, I.; Wagemans, K.; Eeckhout, M. Impact of Temporary Frozen Storage on the Safety and Quality of Four Typical Belgian Bakery Products. LWT 2021, 137, 110454. [Google Scholar] [CrossRef]
- Qian, M.; Liu, D.; Zhang, X.; Yin, Z.; Ismail, B.B.; Ye, X.; Guo, M. A Review of Active Packaging in Bakery Products: Applications and Future Trends. Trends Food Sci. Technol. 2021, 114, 459–471. [Google Scholar] [CrossRef]
- Nhouchi, Z.; Botosoa, E.P.; Karoui, R. Critical Assessment of Formulation, Processing and Storage Conditions on the Quality of Alveolar Baked Products Determined by Different Analytical Techniques: A Review. Trends Food Sci. Technol. 2018, 81, 159–171. [Google Scholar] [CrossRef]
- Gagnon, Y.; Mhemdi, H.; Delbecq, F.; Van Hecke, E. Extended Surfactants and Their Tailored Applications for Vegetable Oils Extraction: An Overview. OCL 2021, 28, 7. [Google Scholar] [CrossRef]
- Carré, P. Reinventing the Oilseeds Processing to Extract Oil While Preserving the Protein. OCL 2021, 28, 13. [Google Scholar] [CrossRef]
| Quality Parameter | 2022 | 2021 | 2017 to 2021 Mean |
|---|---|---|---|
| Oil content (%, 8.5% moisture) | 42.1 | 41.3 | 43.8 |
| Protein content (%, 8.5% moisture) | 22.4 | 24.0 | 21.3 |
| Oil-free protein of the meal (%, 12% moisture) | 39.9 | 42.0 | 39.2 |
| Chlorophyll content (mg/kg in seed) | 9 | 10 | 11 |
| Total seed glucosinolates (µmol/g, 8.5% moisture) | 12 | 12 | 10 |
| Oil-free total glucosinolates of the meal (µmol/g, 8.5% moisture) | 21 | 20 | 19 |
| Free fatty acids (%) | 0.26 | 0.24 | 0.17 |
| Oleic acid (% in oil) | 64.6 | 64.2 | 63.5 |
| α-Linolenic acid (% in oil) | 8.2 | 8.6 | 9.2 |
| Total saturated fatty acids (% in oil) | 6.9 | 6.6 | 6.6 |
| Iodine value (unit of oil) | 109.5 | 110.9 | 112.0 |
| Total mono-unsaturated fatty acids (% in oil) | 66.1 | 65.8 | 65.1 |
| Total poly-unsaturated fatty acids (% in oil) | 26.3 | 27.0 | 27.7 |
| Amino Acid | Canola Meal | Soybean Meal | FAO/WHO Requirements for Adults |
|---|---|---|---|
| Histidine | 2.7 | 2.6 | 1.5 |
| Isoleucine | 4.0 | 4.0 | 3.0 |
| Leucine | 7.0 | 7.8 | 5.9 |
| Lysine | 5.8 | 6.4 | 4.5 |
| Methionine | 1.9 | 1.3 | 2.2 |
| Phenylalanine | 3.8 | 5.0 | 3.8 |
| Threonine | 4.5 | 4.0 | 2.3 |
| Tryptophan | 1.3 | 1.3 | 0.6 |
| Valine | 5.0 | 4.8 | 3.9 |
| Quality Parameter | Definition | Measurement Method | Limit or Range |
|---|---|---|---|
| Moisture | Amount of water present in canola grains | Oven drying or electronic moisture meters | Maximum limit of 8.0% |
| Test weight | Weight per unit volume of canola grains (i.e., reflect the density, size, shape, and uniformity of seeds) | Standardized test weight apparatus | Minimum limit of 62 kg/hl |
| Oil content | Amount of oil present in canola grains | Nuclear magnetic resonance (NMR) or near-infrared reflectance (NIR) spectroscopy | No minimum or maximum limit |
| Protein content | Amount of protein present in canola grains | Combustion analysis or NIR spectroscopy | No minimum or maximum limit |
| Fatty acid composition | Proportion of different fatty acids present in canola oil | Gas chromatography (GC) or NIR spectroscopy | No specific limits, market preferences for certain fatty acid profiles may exist |
| Glucosinolate content | Amount of glucosinolates present in canola meal | High-performance liquid chromatography (HPLC) or NIR spectroscopy | Maximum limit of 30 μmol/g |
| Impurities | Foreign materials present in canola grains | Sieving and visual inspection methods | Maximum limit of 3.0% by weight |
| Seed contaminants | Weed seeds or other seeds present in canola grains (Seed contaminants classified into five types, A to E, based on their potential harm to humans, animals, or plants) | Counting methods per half L or per 2.5 L of canola grains | Maximum limits vary by type of contaminant |
| Genetic purity | Degree of conformity of canola grains to a specific variety or trait affects the identity preservation and traceability of canola products. | Molecular markers or bioassays | Different standards for conventional, GM (herbicide-tolerant), or specialty (high-oleic) varieties |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sabbahi, R.; Azzaoui, K.; Rhazi, L.; Ayerdi-Gotor, A.; Aussenac, T.; Depeint, F.; Taleb, M.; Hammouti, B. Factors Affecting the Quality of Canola Grains and Their Implications for Grain-Based Foods. Foods 2023, 12, 2219. https://doi.org/10.3390/foods12112219
Sabbahi R, Azzaoui K, Rhazi L, Ayerdi-Gotor A, Aussenac T, Depeint F, Taleb M, Hammouti B. Factors Affecting the Quality of Canola Grains and Their Implications for Grain-Based Foods. Foods. 2023; 12(11):2219. https://doi.org/10.3390/foods12112219
Chicago/Turabian StyleSabbahi, Rachid, Khalil Azzaoui, Larbi Rhazi, Alicia Ayerdi-Gotor, Thierry Aussenac, Flore Depeint, Mustapha Taleb, and Belkheir Hammouti. 2023. "Factors Affecting the Quality of Canola Grains and Their Implications for Grain-Based Foods" Foods 12, no. 11: 2219. https://doi.org/10.3390/foods12112219