Foaming with Starch: Exploring Faba Bean Aquafaba as a Green Alternative
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Chemicals
2.2. Faba Bean Dehulling
2.3. Aqueous Extraction of Dehulled Faba Bean Cotyledons
2.4. Isolation of Starch and Protein from FBA through Ethanol Treatment
2.5. Foam Capacity and Stability
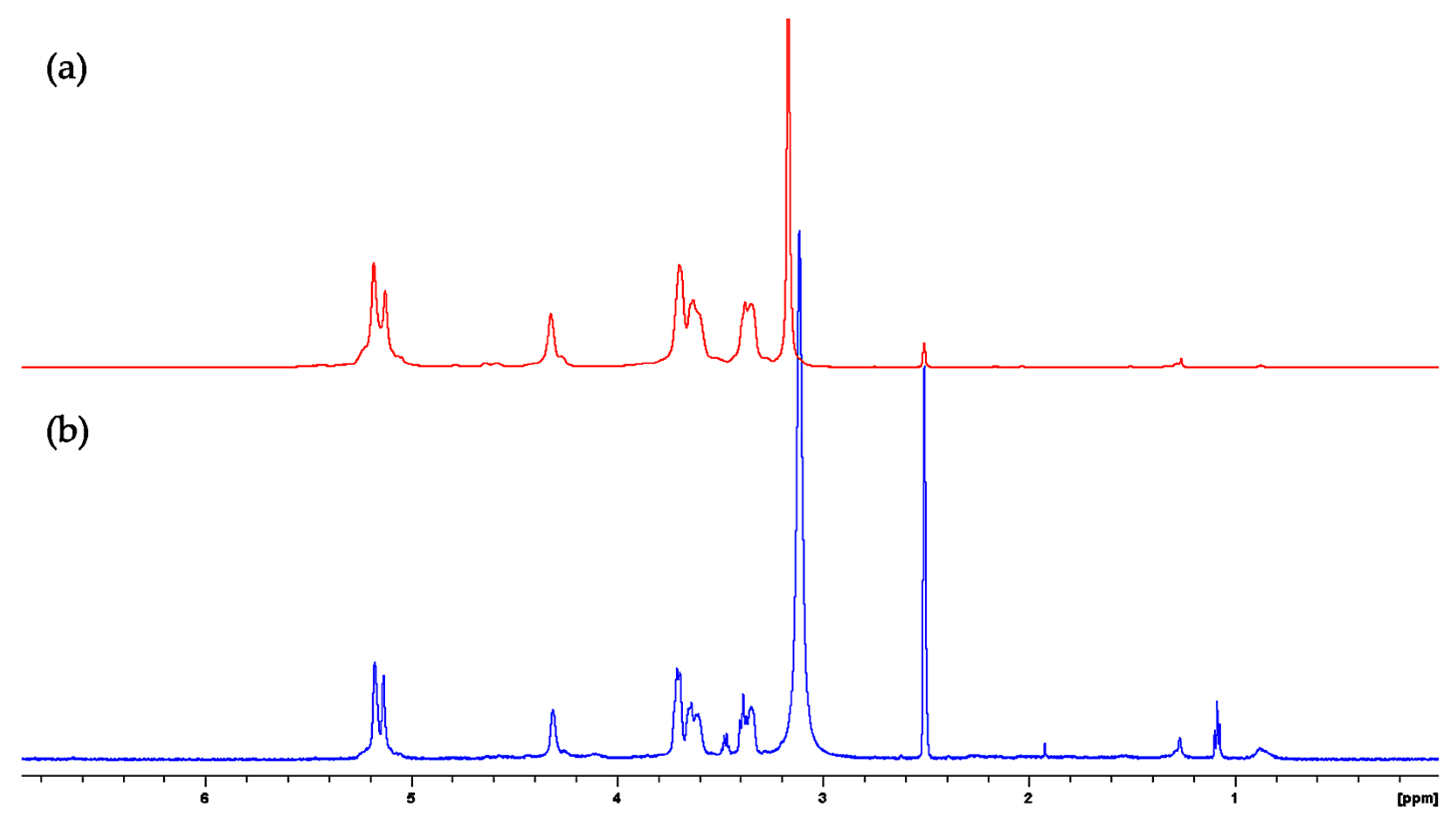
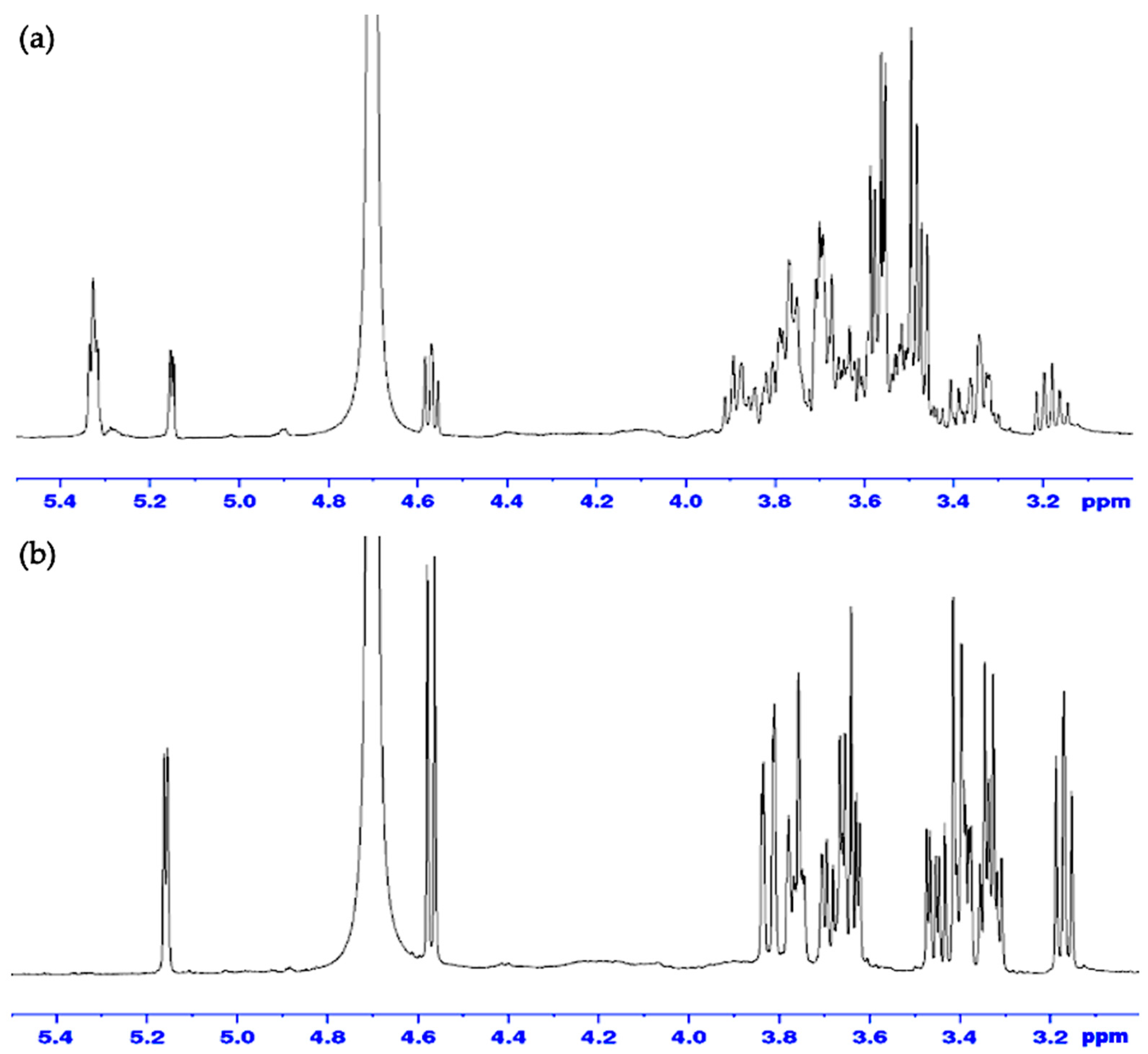
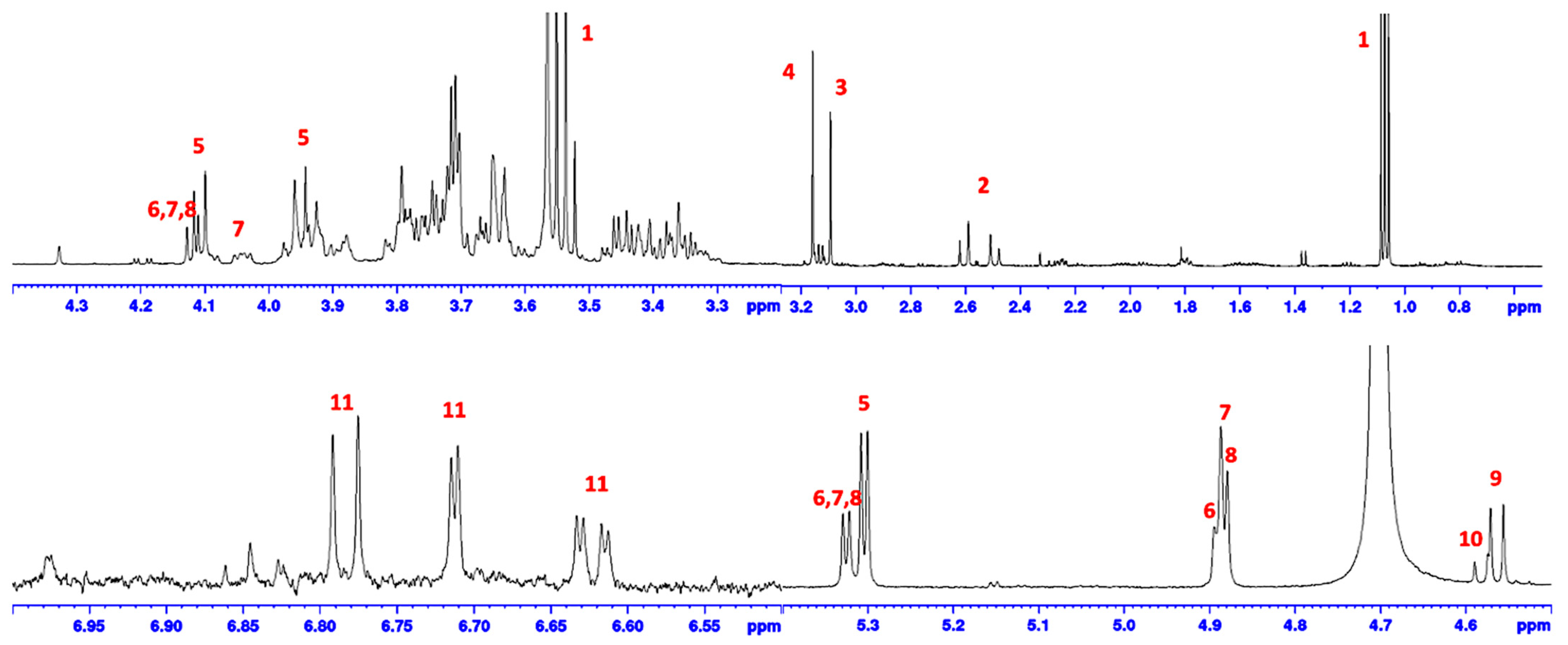
2.6. FBA-P and Starch Standard 1H-NMR Analysis
2.7. Analysis of Monosaccharide Content by α-Amylase Hydrolysis and 1H-NMR Analysis
2.8. Monosaccharide Content Determined by Gas Chromatography
2.9. GC-FID Analytical Methodology
2.10. Protein Content of FBA
2.10.1. Peptide Analysis via 1H-NMR Analysis
2.10.2. Determination of Total Nitrogen Content Using LECO Combustion Analyzer
2.10.3. Total Protein Content via Bradford Protein Assay
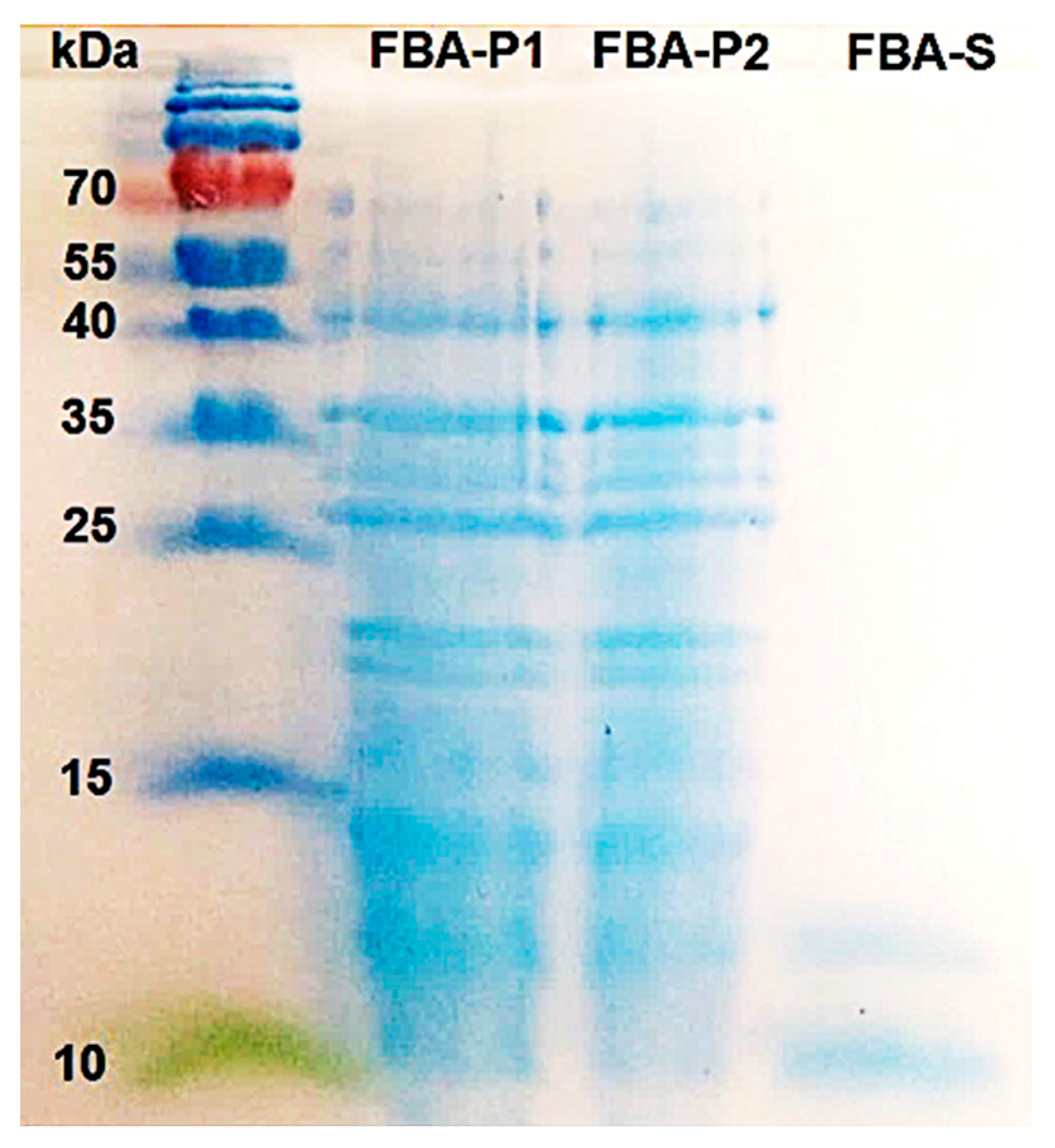
2.11. Separation of Protein via SDS-PAGE
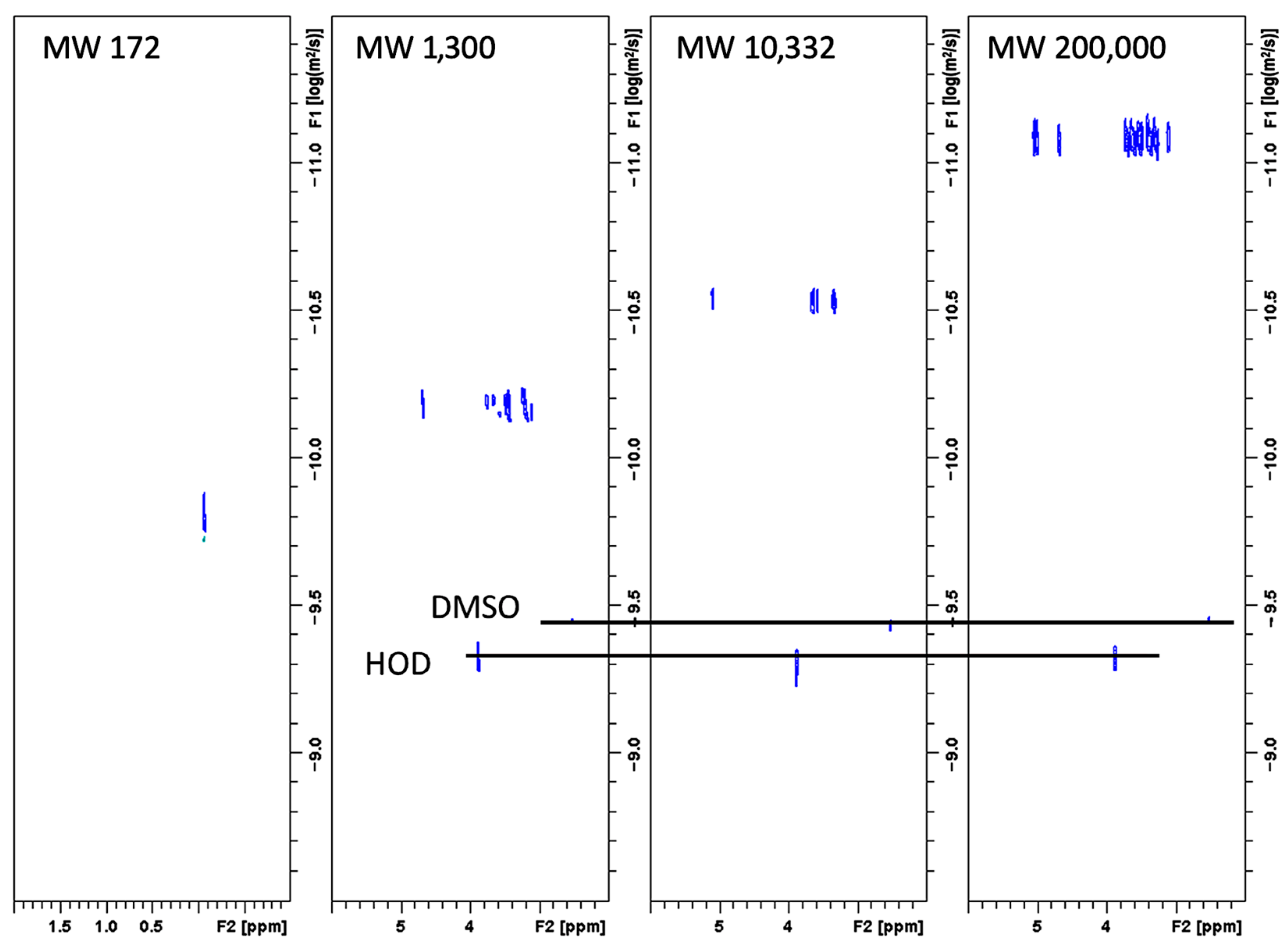
2.12. Molecular Weight Analysis of FBA-P via DOSY 1H-NMR
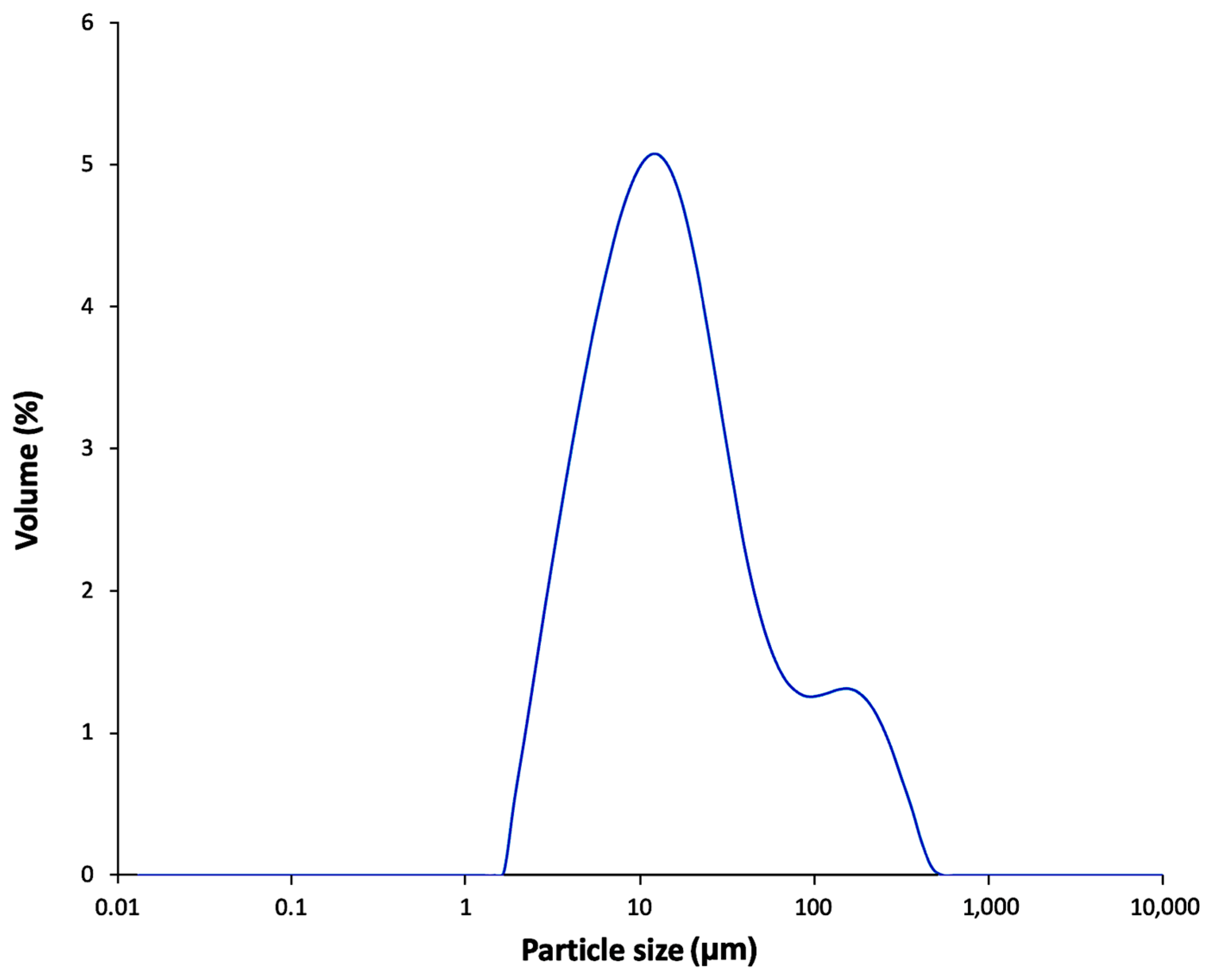
2.13. Particle Size Distribution of FBA-P
2.14. Protein–Starch Complex Interdependence for Foaming Ability
3. Results and Discussion
3.1. Aquafaba Production, Functional Precipitates, and Starch Contain
3.2. Monosaccharide Content Determined by Gas Chromatography
3.3. Separation of Protein via SDS-PAGE
3.4. Molecular Weight Analysis of FBA-P via DOSY 1H-NMR
3.5. FBA-S Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kasprzak, M.M.; Macnaughtan, W.; Harding, S.; Wilde, P.; Wolf, B. Stabilisation of oil-in-water emulsions with non-chemical modified gelatinised starch. Food Hydrocoll. 2018, 81, 409–418. [Google Scholar] [CrossRef]
- Mu, M.; Karthik, P.; Chen, J.; Holmes, M.; Ettelaie, R. Effect of amylose and amylopectin content on the colloidal behaviour of emulsions stabilised by OSA-Modified starch. Food Hydrocoll. 2021, 111, 106363. [Google Scholar] [CrossRef]
- Bai, Y.; Kaufman, R.C.; Wilson, J.D.; Shi, Y.C. Position of modifying groups on starch chains of octenylsuccinic anhydride-modified waxy maize starch. Food Chem. 2014, 153, 193–199. [Google Scholar] [CrossRef] [PubMed]
- Asghari, A.K.; Norton, I.; Mills, T.; Sadd, P.; Spyropoulos, F. Interfacial and foaming characterisation of mixed protein-starch particle systems for food-foam applications. Food Hydrocoll. 2016, 53, 311–319. [Google Scholar] [CrossRef]
- Buhl, T.F.; Christensen, C.H.; Hammershøj, M. Aquafaba as an egg white substitute in food foams and emulsions: Protein composition and functional behavior. Food Hydrocoll. 2019, 96, 354–364. [Google Scholar] [CrossRef]
- Gao, Y.; Chen, J.; Huang, Y.; Liu, Y. A comparative study on the emulsifying properties of plant proteins and animal proteins. Food Hydrocoll. 2019, 95, 273–279. [Google Scholar]
- He, Y.; Meda, V.; Reaney, M.J.T.; Mustafa, R. Aquafaba, a new plant-based rheological additive for food applications. Trends Food Sci. Technol. 2021, 111, 27–42. [Google Scholar]
- He, Y.; Purdy, S.K.; Tse, T.J.; Tar’an, B.; Meda, V.; Reaney, M.J.T.; Mustafa, R. Standardization of aquafaba production and application in vegan mayonnaise analogs. Foods 2021, 10, 1978. [Google Scholar] [CrossRef] [PubMed]
- Punia, S.; Dhull, S.B.; Sandhu, K.S.; Kaur, M. Faba bean (Vicia faba) starch: Structure, properties, and in vitro digestibility—A review. Legume Sci. 2019, 1, e18. [Google Scholar] [CrossRef]
- Mínguez, M.I.; Rubiales, D. Chapter 15—Faba bean. In Crop Physiology Case Histories for Major Crops; Sadras, V.O., Calderini, D.F., Eds.; Academic Press: London, UK, 2021; pp. 452–481. [Google Scholar]
- Akbar, R. Nuskha Dar Fanni-Falahat: The Art of Agriculture (Persion Manuscript Compiled in the 17th Century by the Mughal Prince Dara Shikoh); Agri-History Bulletin No. 3; Munshiram Manoharlal Publishers Pvt. Ltd.: New Delhi, India, 2000; p. 98. [Google Scholar]
- Naqvi, H.K. Cultivation under the Sultans of Delhi c. 1206–1555. Indian J. History Sci. 1984, 19, 329–340. [Google Scholar]
- Singh, A.K.; Bhatt, B.P. Faba Bean (Vicia faba L.): A Potential Leguminous Crop of India; Indian Council for Agricultural Research Research Complex for Eastern Region: Patna, India, 2012; p. 518. ISBN 978-93-5067-773-5. [Google Scholar]
- Sinclair, T.R.; Marrou, H.; Ghanem, M.E.; Kharrat, M.; Amri, M. Review of quantitative sensitivity of faba bean physiology to temperature and soil-water deficit. Crop Pasture Sci. 2022, 74, 344–352. [Google Scholar] [CrossRef]
- Mohseni, M.S.M.; Golshani, B. Simultaneous determination of levodopa and cardidopa from faba bean, green peas and green beans by high performance liquid gas chromatography. J. Clin. Diagn. Res. Doctors 2013, 7, 1004–1007. [Google Scholar]
- Etemadi, F.; Hashemi, M.; Randhir, R.; Zandvakili, O.; Ebadi, A. Accumulation of L-Dopa in various organs of faba bean and influence of drought, nitrogen stress, and processing methods on L-Dopa yield. Crop J. 2018, 6, 426–434. [Google Scholar] [CrossRef]
- Etemadi, F.; Hashemi, M.; Barker, A.V.; Zandvakili, O.R.; Liu, X. Agronomy, nutritional value, and medicinal application of faba bean (Vicia faba L.). Hortic. Plant J. 2019, 5, 170–182. [Google Scholar] [CrossRef]
- Landry, E.J.; Fuchs, S.J.; Hu, J. Carbohydrate composition of mature and immature faba bean seeds. J. Food Compost. Anal. 2016, 50, 55–60. [Google Scholar] [CrossRef]
- Alghamdi, S.S. Chemical composition of faba bean (Vicia faba L.) genotypes under various water regimes. Pak. J. Nutr. 2009, 8, 477–482. [Google Scholar] [CrossRef]
- Kumar, Y.; Basu, S.; Goswami, D.; Devi, M.; Shivhare, U.S.; Vishwakarma, R.K. Anti-nutritional compounds in pulses: Implications and alleviation methods. Legume Sci. 2022, 4, e111. [Google Scholar] [CrossRef]
- Liu, C.; Pei, R.; Heinonen, M. Faba bean protein: A promising plant-based emulsifier for improving physical and oxidative stabilities of oil-in-water emulsions. Food Chem. 2022, 369, 130879. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Chiu, H.T.; Feng, Z.; Maes, E.; Serventi, L. Effect of spray-drying and freeze-drying on the composition, physical properties, and sensory quality of pea processing water (Liluva). Foods 2021, 10, 1401. [Google Scholar] [CrossRef]
- Nguyen, T.M.N.; Nguyen, T.P.; Tran, G.B.; Le, P.T.Q. Effect of processing methods on foam properties and application of lima bean (Phaseolus lunatus L.) aquafaba in eggless cupcakes. J. Food Process. Preserv. 2020, 44, e14886. [Google Scholar] [CrossRef]
- Shim, Y.Y.; Mustafa, R.; Shen, J.; Ratanapariyanuch, R.; Reaney, M.J.T. Composition and properties of aquafaba: Water recovered from commercially canned chickpeas. J. Vis. Exp. 2018, 132, e56305. [Google Scholar]
- Ratnayake, W.S.; Hoover, R.; Warkentin, T. Pea Starch: Composition, Structure and Properties—A Review. Starch-Stärke 2002, 54, 217–234. [Google Scholar] [CrossRef]
- Mustafa, R.; He, Y.; Shim, Y.Y.; Reaney, M.J.T. Aquafaba, wastewater from chickpea canning, functions as an egg replacer in sponge cake. Int. J. Food Sci. Tech. 2018, 53, 2247–2255. [Google Scholar] [CrossRef]
- Sosulski, F.W.; Imafidon, G.I. Amino acid composition and nitrogen-to-protein conversion factors for animal and plant foods. J. Agric. Food Chem. 1990, 38, 1351–1356. [Google Scholar] [CrossRef]
- Kleks, G.; Holland, D.C.; Porter, J.; Carroll, A.R. Natural products dereplication by diffusion ordered NMR spectroscopy (DOSY). Chem. Sci. 2021, 12, 10930–10943. [Google Scholar] [CrossRef]
- Vogelsang-O’Dwyer, M.; Petersen, I.L.; Joehnke, M.S.; Sørensen, J.C.; Bez, J.; Detzel, A.; Busch, M.; Krueger, M.; O’Mahony, J.A.; Arendt, E.K.; et al. Comparison of faba bean protein ingredients produced using dry fractionation and isoelectric precipitation: Techno-functional, nutritional and environmental performance. Foods 2020, 9, 322. [Google Scholar] [CrossRef] [PubMed]
- Adebowale, K.O.; Lawal, O.S. Foaming, gelation and electrophoretic characteristics of mucuna bean (Mucuna pruriens) protein concentrates. Food Chem. 2003, 83, 237–246. [Google Scholar] [CrossRef]
- Bergthaller, W.; Hollmann, J. Starch. In Reference Module in Chemistry, Molecular Sciences and Chemical Engineering; Elsevier Science: Amsterdam, The Netherlands, 2014; p. 579612. [Google Scholar] [CrossRef]
- Sun, X.S. Plastics derived from starch and poly (lactic acids). In Bio-Based Polymers and Composites; Academic Press: New York, NY, USA, 2005; pp. 369–410. [Google Scholar] [CrossRef]
- Etemadi, F.; Hashemi, M.; Mangan, F.; Weis, S. Faba Beans; Growers Guide in New England. 2015. Available online: https://ag.umass.edu/sites/ag.umass.edu/files/research-reports/fava_bean_guide_2.pdf (accessed on 9 September 2023).
- Surwase, S.N.; Patil, S.A.; Jadhav, S.B.; Jadhav, J.P. Optimization of L-Dopa production by Brevundimonas sp. SGJ using response surface methodology. Microb. Biotechnol. 2012, 5, 731–737. [Google Scholar] [CrossRef]
- Inamdar, S.A.; Surwase, S.N.; Jadhav, S.B.; Bapat, V.A.; Jadhav, J.P. Statistically optimized biotransformation protocol for continuous production of L-Dopa using Mucuna monosperma callus culture. SpringerPlus 2013, 2, 570. [Google Scholar] [CrossRef]
- Hu, J.; Kwon, S.J.; Park, J.J.; Landry, E.; Mattinson, D.S.; Gang, D.R. LC-MS determination of L-Dopa concentration in the leaf and flower tissues of six faba bean (Vicia faba L.) lines with common and rare flower colors. Funct. Foods Health Dis. 2015, 5, 243–250. [Google Scholar] [CrossRef]
- Etemadi, F.; Hashemi, M.; Autio, W.; Mangan, F.; Zandvakili, O. Accumulation and distribution trend of L-Dopa in different parts of eight varieties of faba bean plant through its growth period. J. Crop Sci. 2018, 6, 426–434. [Google Scholar]
- Soares, A.R.; Marchiosi, R.; Siqueira-Soares, R.d.C.; de Lima, R.B.; dos Santos, W.D.; Ferrarese-Filho, O. The role of L-Dopa in plants. Plant Signal Behav. 2014, 9, e28275. [Google Scholar] [CrossRef] [PubMed]
- Ramakrishna, A.; Ravishankar, G.A. Influence of abiotic stress signals on secondary metabolites in plants. Plant Signal Behav. 2011, 1, 1720–1731. [Google Scholar]
- Echeverria, G.C.L.; Bressani, R. Effect of different cooking treatments of Mucuna beans on its L-Dopa content. Arch Latinoam Nutr. 2006, 56, 175–184. [Google Scholar]
- Vadivel, V.; Pugalenthi, M. Studies on the incorporation of velvet bean (Mucuna pruriens var. utilis) as an alternative protein source in poultry feed and its effect on growth performance of broiler chickens. Trop Anim. Health Prod. 2010, 42, 1367–1376. [Google Scholar] [CrossRef]
- Dahouda, M.; Toleba, S.S.; Youssao, A.K.I.; Hambuckers, A.; Dangou-Sapoho, R.; Martin, G.B.; Fillet, M.; Hornick, J.L. Nutrient digestibility of Mucuna (Mucuna pruriens var. utilis) bean in guineafowl (Numida meleagris L.): Effects of heat treatment and levels of incorporation in diets. Br. Poult. Sci. 2009, 50, 564–572. [Google Scholar] [CrossRef] [PubMed]
| Compound | Retention Time (min) |
|---|---|
| D-arabinose | 14.286 |
| D-fucose | 14.212 |
| D-galactose | 17.869 |
| D-glucose | 17.657 |
| D-mannose | 17.606 |
| L-rhamnose | 14.053 |
| D-xylose | 14.503 |
| TFA hydrolyzed FBA-P | 17.666 |
| Characteristics | Yield (%) | Foaming Capacity (%) | Foaming Stability (%) |
|---|---|---|---|
| FBA | 87 a | 600.01 ± 54.98 | 94.44 ± 8.10 |
| FBA-P | 0.62 b 0.95 c | 533.33 ± 43.32 | 93.75 ± 7.12 |
| FBA-S | 1.73 d 1.14 e | NA | NA |
| Compound | Profile (%) |
|---|---|
| Ethanol | 7.61 |
| Citric acid | 5.31 |
| Phosphocholine | 2.12 |
| Betaine phosphate | 3.39 |
| Sucrose | 24.41 |
| Raffinose | 7.63 |
| Stachyose | 9.98 |
| Verbascose | 24.82 |
| Vicine | 9.75 |
| Convicine | 3.70 |
| L-Dopa | 1.30 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ramos-Figueroa, J.S.; Tse, T.J.; Shen, J.; Purdy, S.K.; Kim, J.K.; Kim, Y.J.; Han, B.K.; Hong, J.Y.; Shim, Y.Y.; Reaney, M.J.T. Foaming with Starch: Exploring Faba Bean Aquafaba as a Green Alternative. Foods 2023, 12, 3391. https://doi.org/10.3390/foods12183391
Ramos-Figueroa JS, Tse TJ, Shen J, Purdy SK, Kim JK, Kim YJ, Han BK, Hong JY, Shim YY, Reaney MJT. Foaming with Starch: Exploring Faba Bean Aquafaba as a Green Alternative. Foods. 2023; 12(18):3391. https://doi.org/10.3390/foods12183391
Chicago/Turabian StyleRamos-Figueroa, Josseline S., Timothy J. Tse, Jianheng Shen, Sarah K. Purdy, Jae Kyeom Kim, Young Jun Kim, Bok Kyung Han, Ji Youn Hong, Youn Young Shim, and Martin J. T. Reaney. 2023. "Foaming with Starch: Exploring Faba Bean Aquafaba as a Green Alternative" Foods 12, no. 18: 3391. https://doi.org/10.3390/foods12183391







