Correlation Analysis of Microbial Community Changes and Physicochemical Characteristics in Aged Vinegar Brewing
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents and Materials
2.2. Instruments and Equipment
2.3. Experimental Methods
2.3.1. Sample
2.3.2. Method for Determining Physicochemical Characteristics
2.3.3. Methods for Determining Microbial Community Dynamics
2.4. Data Analysis
3. Results and Analyses
3.1. Analysis of Physicochemical Characteristics
3.1.1. Analysis of the Physicochemical Characteristics of Cuqu
3.1.2. Analysis of Changes in Physicochemical Characteristics during the Fermentation of Aged Vinegar
3.2. Analysis of Microbial Community Changes during the Fermentation of Aged Vinegar
3.2.1. Dilution Curve Analysis
3.2.2. Alpha Diversity Analysis
3.2.3. Venn Plot Analysis of Different Fermentation Stages
3.2.4. Analysis of the Succession of Bacterial Community Structure
3.2.5. Analysis of Fungal Community Structure Succession
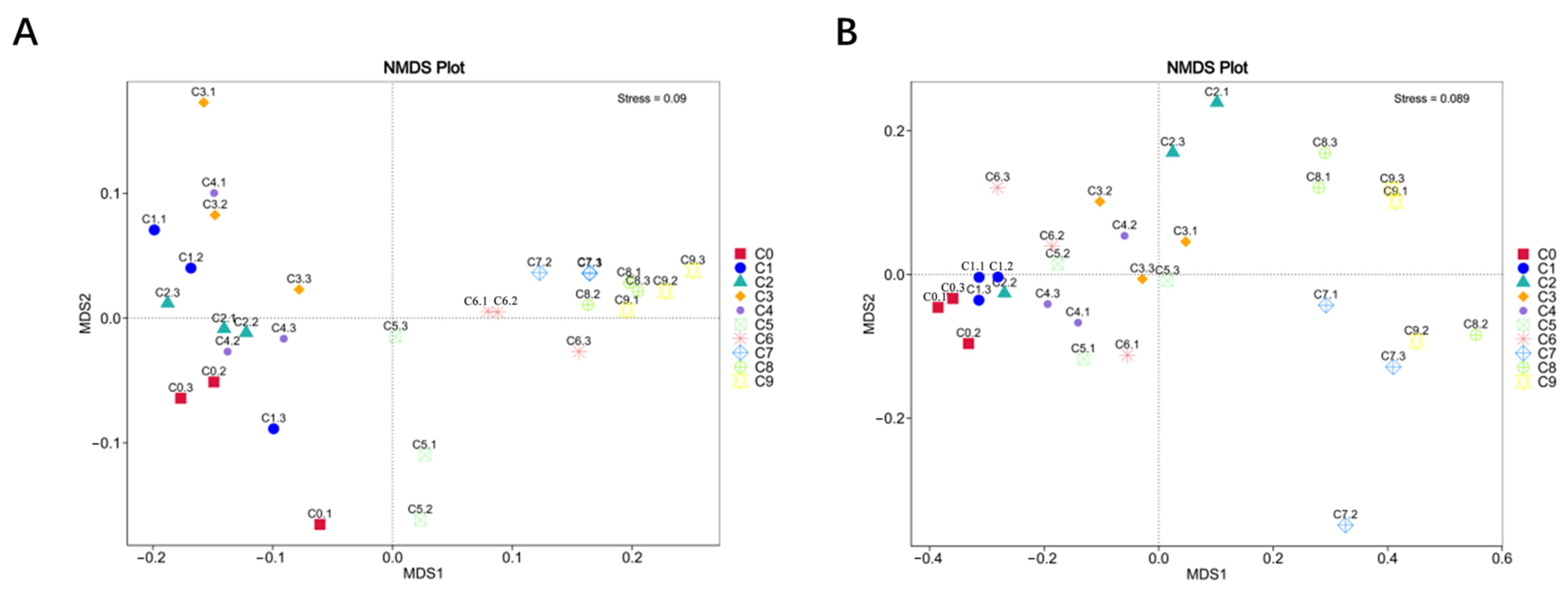
3.2.6. Beta Diversity Analysis
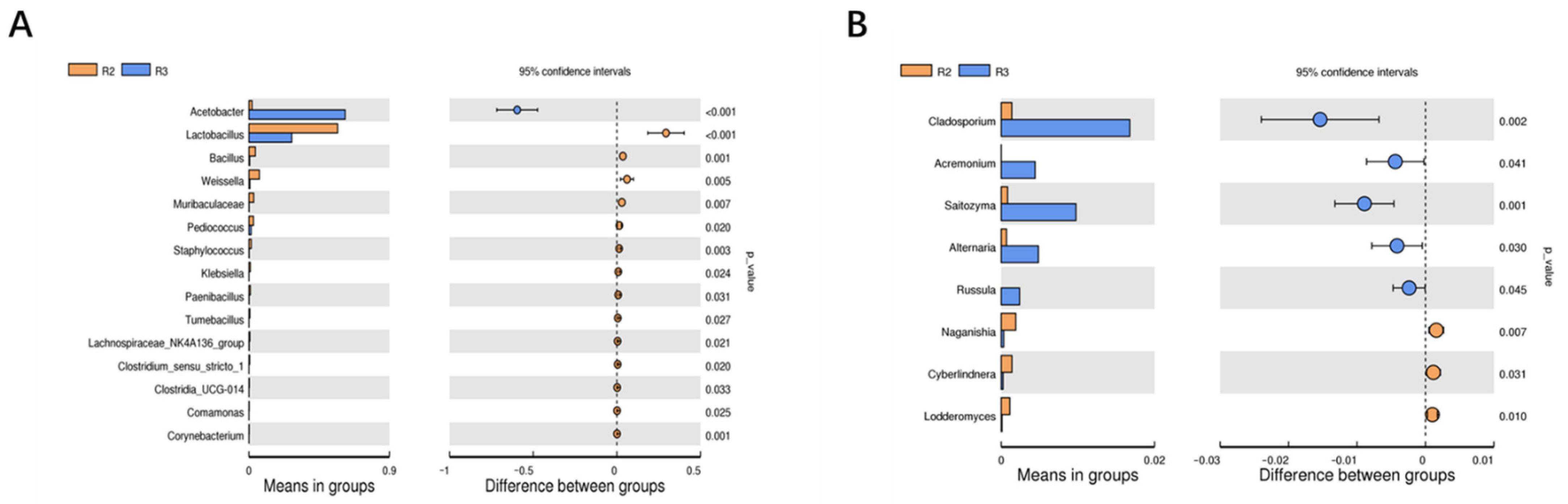
3.2.7. Screening of Differential Bacterial Genera in Two Fermentation Stages
3.3. Correlation Analysis
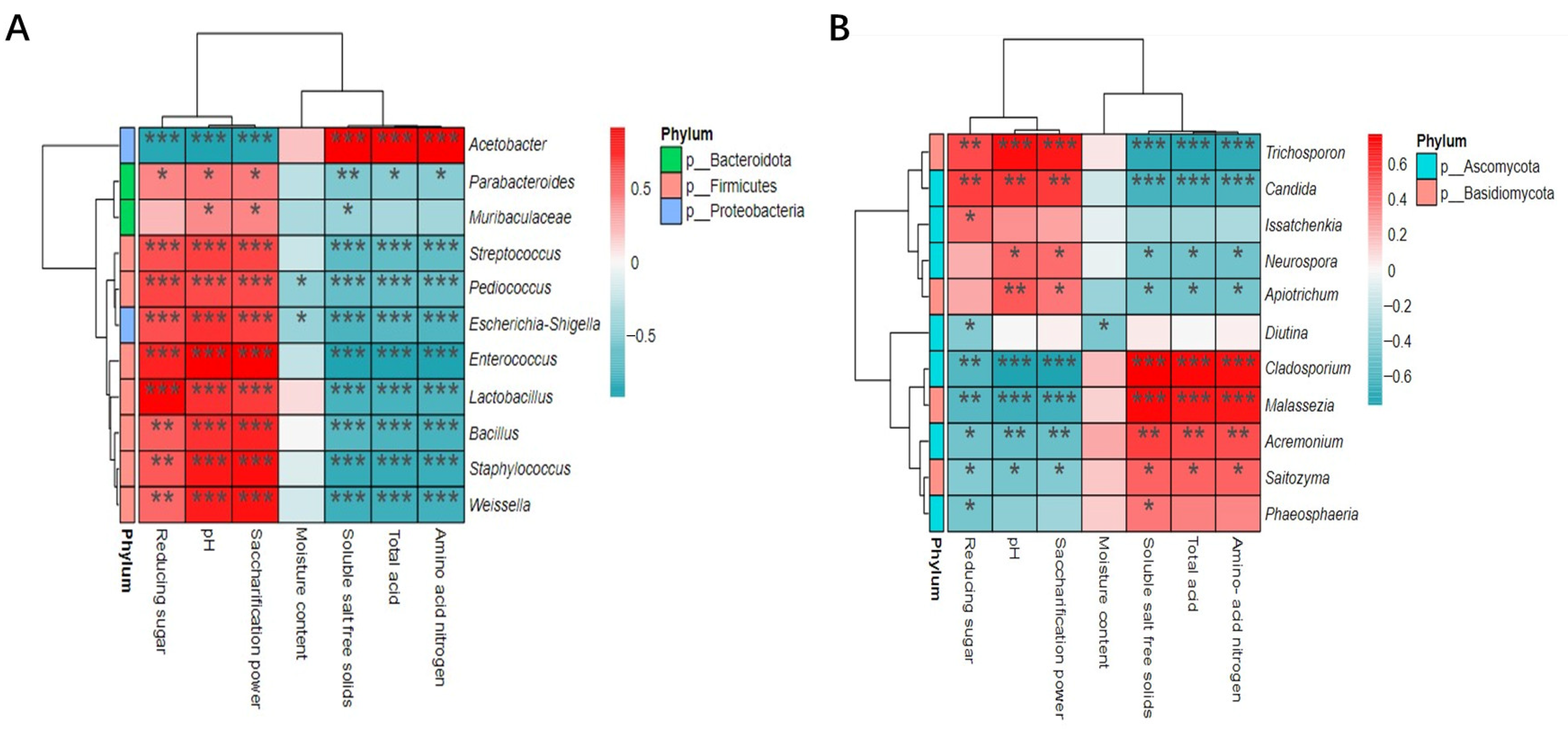
3.3.1. Correlation Analysis between the Physicochemical Characteristics of Cuqu and Microorganisms
3.3.2. Correlation Analysis between Physicochemical Characteristics of the Fermentation Process and Microorganisms
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Li, P.; Li, S.; Cheng, L.; Luo, L. Analyzing the relation between the microbial diversity of DaQu and the turbidity spoilage of traditional Chinese vinegar. Appl. Microbiol. Biotechnol. 2014, 98, 6073–6084. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.M.; Lu, Z.M.; Shi, J.S.; Xu, Z.H. Exploring flavour-producing core microbiota in multispecies solid-state fermentation of traditional Chinese vinegar. Sci. Rep. 2016, 6, 26818. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.Y.; Fan, B.Q.; Zheng, Y.; Song, J. Isolation, identification of acid-producing bacteria from Shanxi aged vinegar cupei and their alcohol tolerance ablity analysis. China Brew. 2021, 40, 128–132. [Google Scholar]
- Liu, C. Analysis of Microbial Diversity in Vinegar Grains of Shanxi Aged Vinegar and Effects of Sorghum Tannin on Fungal Growth. Master’s Thesis, Shanxi University, Taiyuan, China, 2019. [Google Scholar]
- Xiong, X.; Hu, Y.; Yan, N.; Huang, Y.; Peng, N.; Liang, Y.; Zhao, S. PCR-DGGE analysis of the microbial communities in three different Chinese “Baiyunbian” liquor fermentation starters. J. Microbiol. Biotechnol. 2014, 24, 1088–1095. [Google Scholar] [CrossRef] [PubMed]
- Chiara, D.; Simona, B.; Giuditta, P. Antibiotic resistance determinants in the interplay between food and gut microbiota. Genes Nutr. 2011, 6, 275–284. [Google Scholar]
- Zhu, Y.; Zhang, F.; Zhang, C.; Yang, L.; Fan, G.; Xu, Y.; Sun, B.; Li, X. Dynamic microbial succession of Shanxi aged vinegar and its correlation with flavor metabolites during different stages of acetic acid fermentation. Sci. Rep. 2018, 8, 8612. [Google Scholar] [CrossRef] [PubMed]
- Nie, Z.; Zheng, Y.; Xie, S.; Zhang, X.; Song, J.; Xia, M.; Wang, M. Unraveling the correlation between microbiota succession and metabolite changes in traditional Shanxi aged vinegar. Sci. Rep. 2017, 7, 9240. [Google Scholar] [CrossRef]
- Gao, Y.; Jo, Y.; Chung, N.; Gu, S.-Y.; Jeong, Y.-J.; Kwon, J.-H. Physicochemical Qualities and Flavor Patterns of Traditional Chinese Vinegars Manufactured by Different Fermentation Methods and Aging Periods. Prev. Nutr. Food Sci. 2017, 22, 30–36. [Google Scholar] [CrossRef]
- Luo, A.; Yang, N.; Yang, J.; Hao, J.; Zhao, J.; Shi, S.; Hu, B. Effects of microbial interspecies relationships and physicochemical parameters on volatile flavors in sorghum-based fermented grains during the fermentation of Shanxi light-flavored liquor. Food Sci. Nutr. 2022, 11, 1452–1462. [Google Scholar] [CrossRef]
- Peng, W.-X.; Marchal, J.L.M.; Poel, A.F.B.v.d. Strategies to prevent and reduce mycotoxins for compound feed manufacturing. Anim. Feed Sci. Technol. 2018, 237, 129–153. [Google Scholar] [CrossRef]
- Tesfaye, W.; Morales, M.L.; Callejón, R.M.; Cerezo, A.B.; González, A.G.; García-Parrilla, M.C.; Troncoso, A.M. Descriptive Sensory Analysis of Wine Vinegar: Tasting Procedure and Reliability of New Attributes. J. Sens. Stud. 2010, 25, 216–230. [Google Scholar] [CrossRef]
- Katarína, V.; Viera, H. Starch degradation by glucoamylase Glm from Saccharomycopsis fibuligera IFO 0111 in the presence and absence of a commercial pullulanase. Chem. Biodivers. 2007, 4, 216–230. [Google Scholar]
- Kou, R.; Li, M.; Xing, J.; He, Y.; Wang, H.; Fan, X. Exploring of seasonal dynamics of microbial community in multispecies fermentation of Shanxi mature vinegar. J. Biosci. Bioeng. 2022, 133, 375–381. [Google Scholar] [CrossRef]
- Yang, Y.; Xia, Y.; Wang, G.; Tao, L.; Yu, J.; Ai, L. Effects of boiling, ultra-high temperature and high hydrostatic pressure on free amino acids, flavor characteristics and sensory profiles in Chinese rice wine. Food Chem. 2018, 275, 407–416. [Google Scholar] [CrossRef]
- Yu, H.; Zhao, J.; Li, F.; Tian, H.; Ma, X. Characterization of Chinese rice wine taste attributes using liquid chromatographic analysis, sensory evaluation, and an electronic tongue. J. Chromatogr. B 2015, 997, 129–135. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Gong, X.; Zhao, G.; Soe Htet, M.N.; Jia, Z.; Yan, Z.; Liu, L.; Zhai, Q.; Huang, T.; Feng, B.; et al. Liquor flavour is associated with the physicochemical property and microbial diversity of fermented grains in waxy and non-waxy sorghum (Sorghum bicolor) during fermentation. Front. Microbiol. 2021, 12, 618458. [Google Scholar] [CrossRef] [PubMed]
- Entani, E.; Ohmori, S.; Masai, H.; Suzuki, K.-I. Acetobacter polyoxogenes sp. Nov., a new species of an acetic acid bacterium useful for producing vinegar with high acidity. J. Gen. Appl. Microbiol. 1985, 31, 475–490. [Google Scholar] [CrossRef]
- Joyeux, A.; Lafon-Lafourcade, S.; Ribéreau-Gayon, P. Evolution of acetic Acid bacteria during fermentation and storage of wine. Appl. Environ. Microbiol. 1984, 48, 153–156. [Google Scholar] [CrossRef]
- Ruan, W.; Liu, J.; Li, P.; Zhao, W.; Zhang, A.; Liu, S.; Liu, J. Dynamics of Microbial Communities, Flavor, and Physicochemical Properties during Ziziphus jujube Vinegar Fermentation: Correlation between Microorganisms and Metabolites. Foods 2022, 11, 3334. [Google Scholar] [CrossRef]
- Babayan, T.L.; Bezrukov, M.G.; Latov, V.K.; Belikov, V.M.; Belavtseva, E.M.; Titova, E.F. Induced autolysis ofSaccharomyces cerevisiae: Morphological effects, rheological effects, and dynamics of accumulation of extracellular hydrolysis products. Curr. Microbiol. 1981, 5, 163–168. [Google Scholar] [CrossRef]
- Haruta, S.; Ueno, S.; Egawa, I.; Hashiguchi, K.; Fujii, A.; Nagano, M.; Ishii, M.; Igarashi, Y. Succession of bacterial and fungal communities during a traditional pot fermentation of rice vinegar assessed by PCR-mediated denaturing gradient gel electrophoresis. Int. J. Food Microbiol. 2006, 109, 79–87. [Google Scholar] [CrossRef] [PubMed]
- Comelli, R.N.; Seluy, L.G.; Isla, M.A. Performance of several Saccharomyces strains for the alcoholic fermentation of sugar-sweetened high-strength wastewaters: Comparative analysis and kinetic modelling. New Biotechnol. 2016, 33, 874–882. [Google Scholar] [CrossRef] [PubMed]
- Min, Z.; Zhuo, C.; Bo, L.H.; Xiang, M.; Ying, Y.; Hua, T.W.; Dan, H. Study of the Phase Characteristics of Sichuan Bran Vinegar Fermentation Based on Flavor Compounds and Core Bacteria. J. Am. Soc. Brew. Chem. 2021, 79, 201–211. [Google Scholar]
- Wu, J.J.; Ma, Y.K.; Zhang, F.F.; Chen, F.S. Biodiversity of yeasts, lactic acid bacteria and acetic acid bacteria in the fermentation of “Shanxi aged vinegar”, a traditional Chinese vinegar. Food Microbiol. 2012, 30, 289–297. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.Y.; Oh, C.G.; Lee, Y.J.; Choi, K.H.; Shin, D.S.; Lee, S.K.; Park, K.J.; Shin, S.; Park, M.S.; Lee, J.H. Sequence analysis of a cryptic plasmid pKW2124 from Weissella cibaria KLC140 and construction of a surface display vector. J. Microbiol. Biotechnol. 2013, 23, 545–554. [Google Scholar] [CrossRef] [PubMed]
- Zheng, X.-W.; Yan, Z.; Nout, M.J.R.; Smid, E.J.; Zwietering, M.H.; Boekhout, T.; Han, J.-S.; Han, B.-Z. Microbiota dynamics related to environmental conditions during the fermentative production of Fen-Daqu, a Chinese industrial fermentation starter. Int. J. Food Microbiol. 2014, 182–183, 57–62. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Mou, J.; Niu, J.; Yang, S.; Chen, L.; Xia, M.; Wang, M. Succession sequence of lactic acid bacteria driven by environmental factors and substrates throughout the brewing process of Shanxi aged vinegar. Appl. Microbiol. Biotechnol. 2018, 102, 2645–2658. [Google Scholar] [CrossRef]
- Signore, A.D. Chemometric analysis and volatile compounds of traditional balsamic vinegars from Modena. J. Food Eng. 2001, 50, 77–90. [Google Scholar] [CrossRef]
- Haghshenas, B.; Nami, Y.; Abdullah, N.; Radiah, D.; Rosli, R.; Khosroushahi, A.Y. Anticancer impacts of potentially probiotic acetic acid bacteria isolated from traditional dairy microbiota. LWT-Food Sci. Technol. 2015, 60, 690–697. [Google Scholar] [CrossRef]
- Hu, M.; Yu, J.; Yu, J.; Pan, Y.; Ou, Y. Isolation and Screening of Staphylococcus Xylosus P2 from Chinese Bacon: A Novel Starter Culture in Fermented Meat Products. Int. J. Food Eng. 2018, 15, 20180021. [Google Scholar] [CrossRef]
- José, L.N.-B.; Jiménez-Islas, H.; Botello-Alvarez, E.; Rico-Martínez, R.; Paredes-López, O. An optimization study of solid-state fermentation: Xanthophylls extraction from marigold flowers. Appl. Microbiol. Biotechnol. 2004, 65, 383–390. [Google Scholar]
- Yusuf, A.; Ahmet, S.; Fehim, K.E.; Metin, E. Postbiotic metabolites, antioxidant and anticancer activities of probiotic Leuconostoc pseudomesenteroides strains in natural pickles. Arch. Microbiol. 2022, 204, 571. [Google Scholar]
- Dhaliwal, S.S.; Oberoi, H.S.; Sandhu, S.K.; Nanda, D.; Kumar, D.; Uppal, S.K. Enhanced ethanol production from sugarcane juice by galactose adaptation of a newly isolated thermotolerant strain of Pichia kudriavzevii. Bioresour. Technol. 2011, 102, 5968–5975. [Google Scholar] [CrossRef] [PubMed]
- Stenuit, B.; Agathos, S.N. Deciphering microbial community robustness through synthetic ecology and molecular systems synecology. Curr. Opin. Biotechnol. 2015, 33, 305–317. [Google Scholar] [CrossRef] [PubMed]
- Hirokazu, T.; Abe, M.S.; Chiharu, I.; Yoshie, H.; Hiroaki, F.; Shinji, F. Scoring Species for Synthetic Community Design: Network Analyses of Functional Core Microbiomes. Front. Microbiol. 2020, 11, 1361. [Google Scholar]
- Li, P.; Lin, W.; Liu, X.; Wang, X.; Gan, X.; Luo, L.; Lin, W.-T. Effect of bioaugmented inoculation on microbiota dynamics during solid-state fermentation of Daqu starter using autochthonous of Bacillus, Pediococcus, Wickerhamomyces and Saccharomycopsis. Food Microbiol. 2017, 61, 83–92. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Huang, Y.; Huang, Y.; Yi, Z.; Yi, Z.; Jin, Y.; Jin, Y.; Huang, M.; Huang, M.; He, K.; et al. Metatranscriptomics Reveals the Functions and Enzyme Profiles of the Microbial Community in Chinese Nong-Flavor Liquor Starter. Front. Microbiol. 2017, 8, 1747. [Google Scholar] [CrossRef]
- Endo, A.; Okada, S. Monitoring the lactic acid bacterial diversity during shochu fermentation by PCR-denaturing gradient gel electrophoresis. J. Biosci. Bioeng. 2005, 99, 216–221. [Google Scholar] [CrossRef]
- Lipke, P.N.; Ovalle, R. Cell wall architecture in yeast: New structure and new challenges. J. Bacteriol. 1998, 180, 3735–3740. [Google Scholar] [CrossRef]
- Abrha, B.; Gashe, B.A. Cellulase production and activity in a species ofCladosporium. World J. Microbiol. Biotechnol. 1992, 8, 164–166. [Google Scholar] [CrossRef]
- Hu, L.; Wang, J.; Ji, X.; Liu, R.; Chen, F.; Zhang, X. Selection of non- Saccharomyces yeasts for orange wine fermentation based on their enological traits and volatile compounds formation. J. Food Sci. Technol. 2018, 55, 4001–4012. [Google Scholar] [CrossRef]
- Zhao, S.; Zhao, Y.; Li, S.; Zhao, J.; Zhang, G.; Wang, H.; Ng, T.B. A novel lectin with highly potent antiproliferative and HIV-1 reverse transcriptase inhibitory activities from the edible wild mushroom Russula delica. Glycoconj. J. 2010, 27, 259–265. [Google Scholar] [CrossRef]
- Yang, Y.; Na, W.Y.; Erdenebat, O.; Rong, W.Y.; Zhuang, G.; Quan, S. Correlation analysis between microbial diversity and physicochemical indices of Koumiss. Food Biosci. 2022, 49, 101922. [Google Scholar] [CrossRef]
- Yang, Y.; Fan, Y.; Li, T.; Yang, Y.; Zeng, F.; Wang, H.; Zhang, Y. Microbial composition and correlation between microbiota and quality-related physiochemical characteristics in chongqing radish paocai. Food Chem. 2022, 369, 130897. [Google Scholar] [CrossRef]
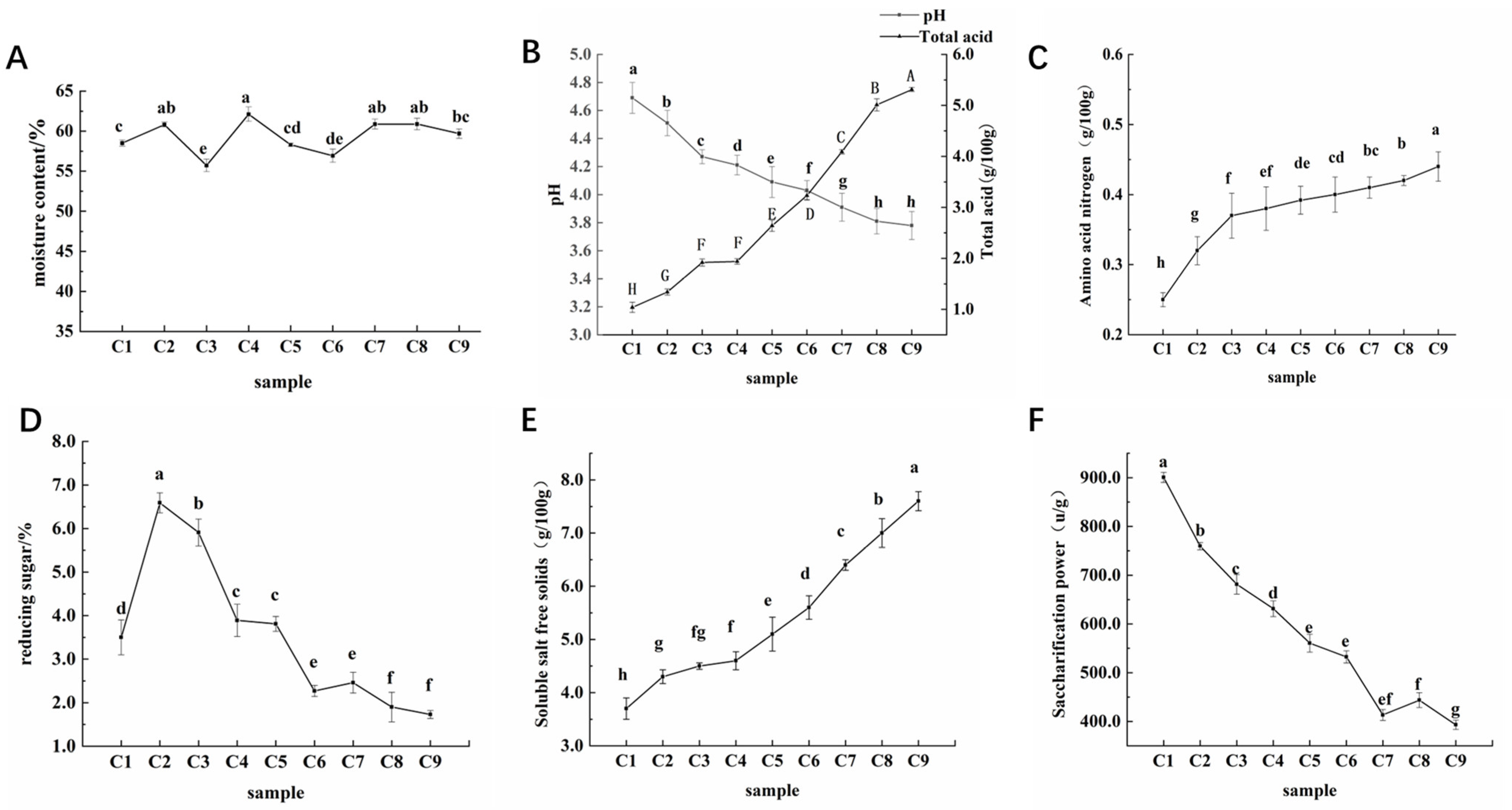
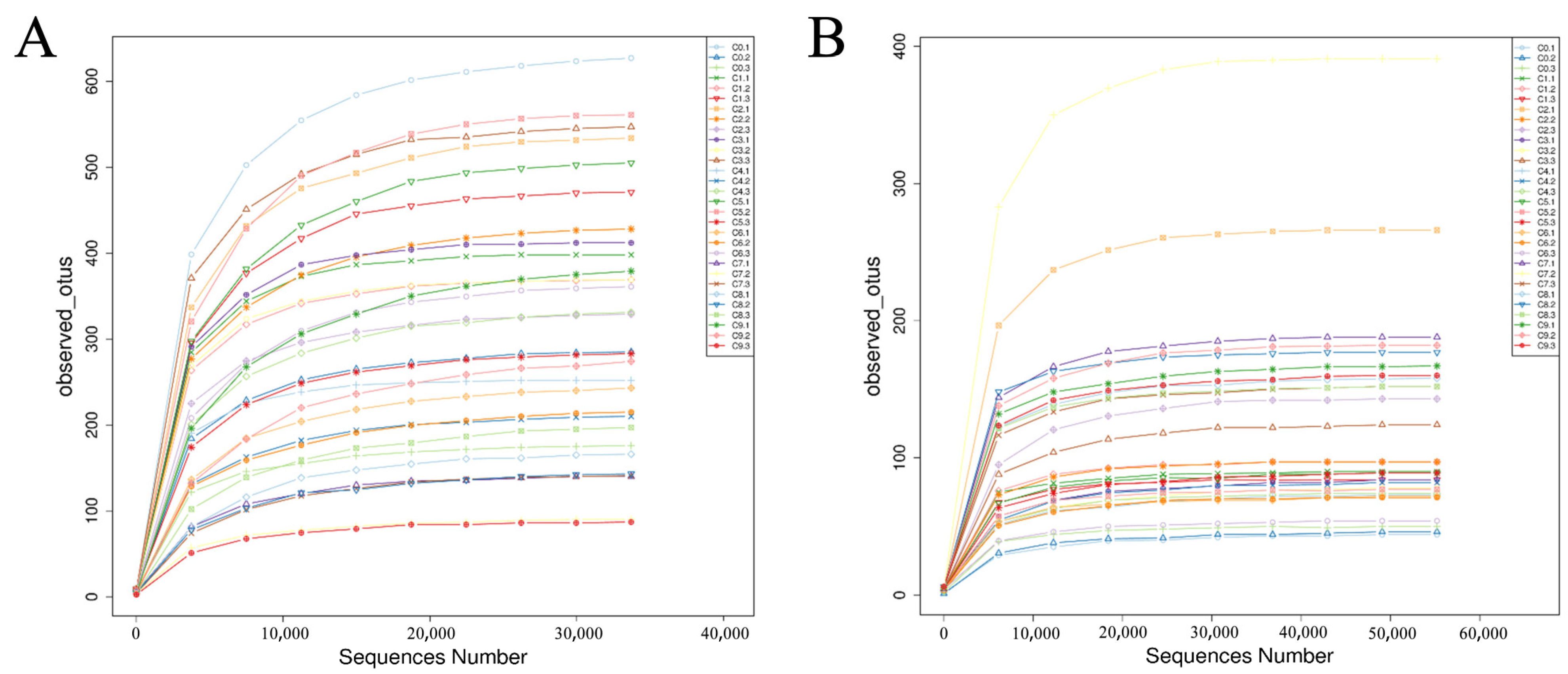
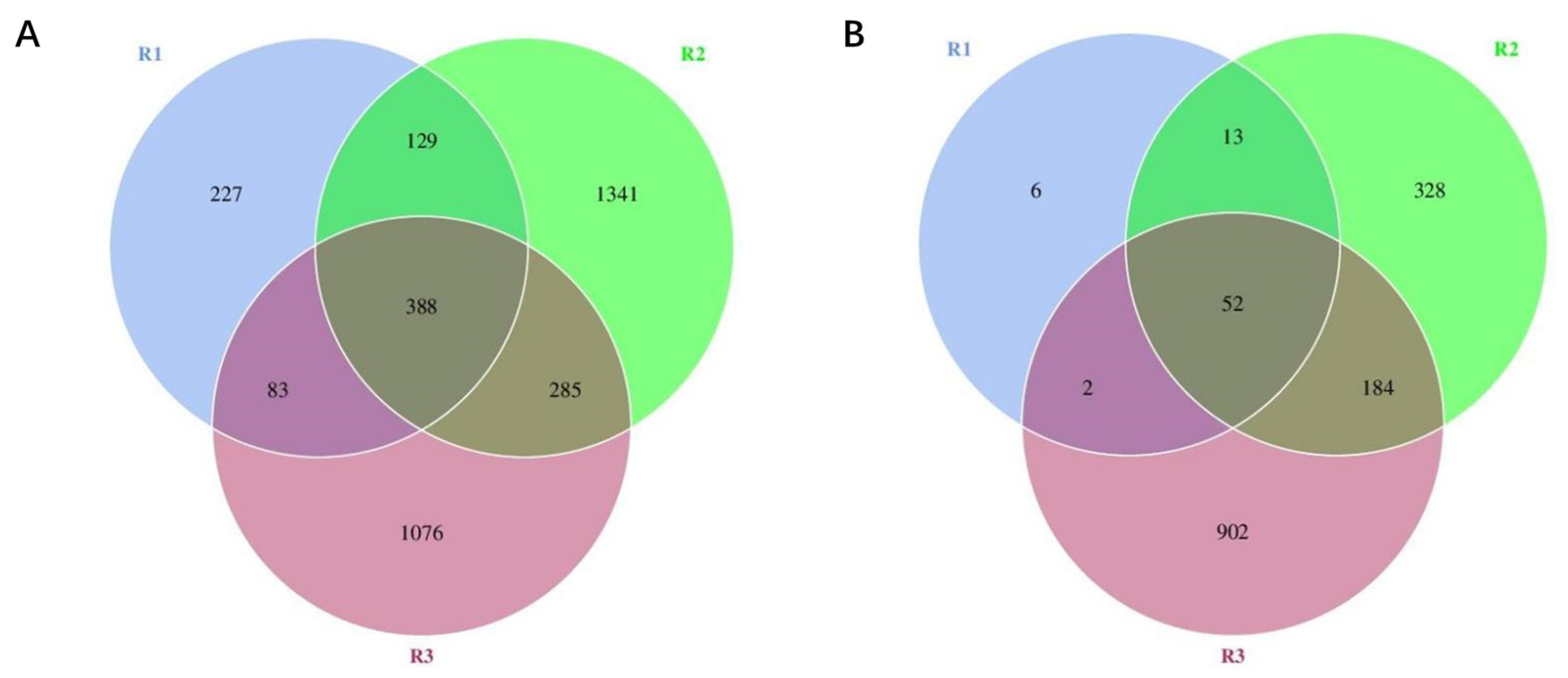
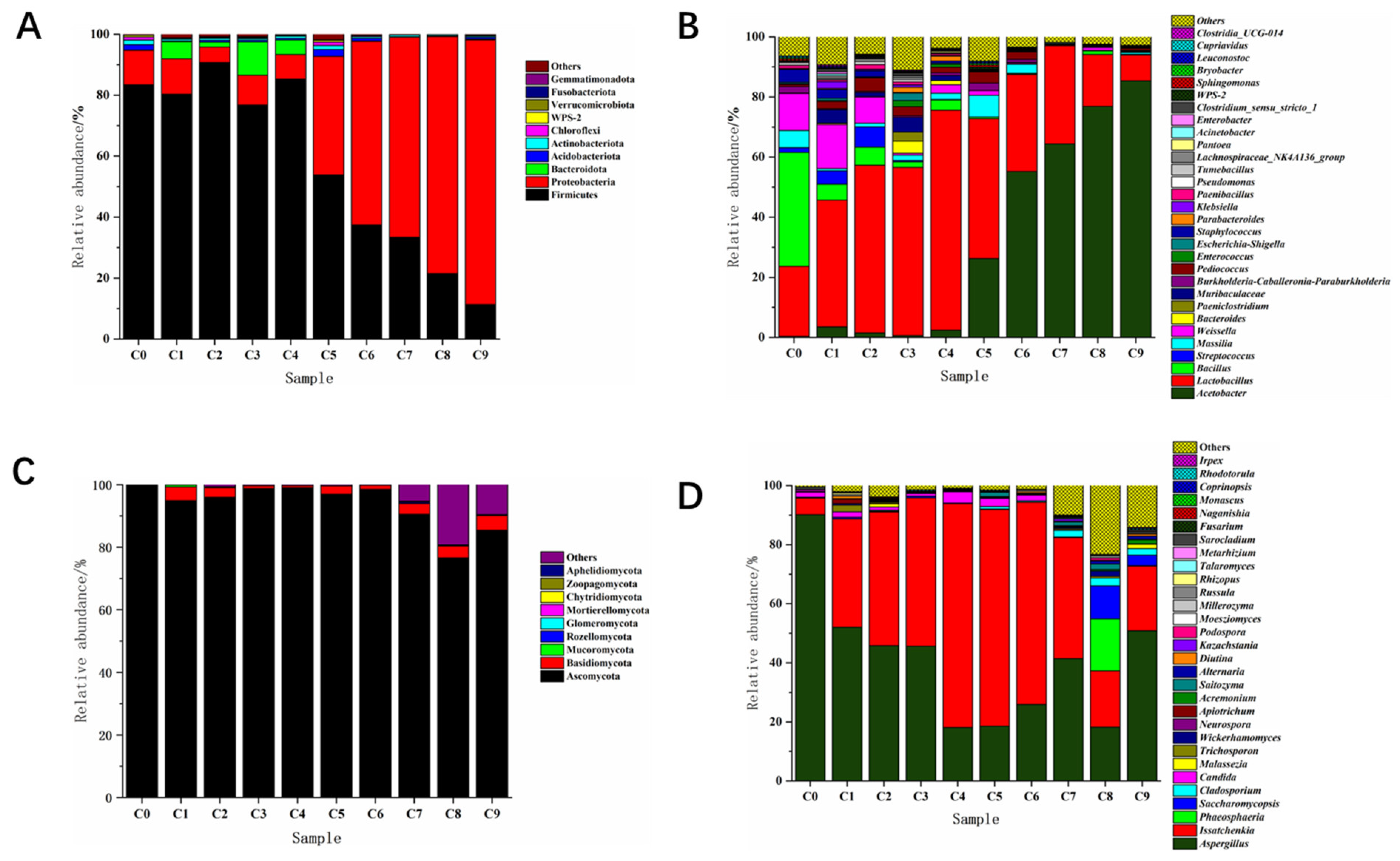

| Sample | Moisture Content (%) | pH | Total Acid (g/100 g) | Amino Acid Nitrogen (g/100 g) | Reducing Sugar (%) | Soluble Salt-Free Solids (g/100 g) | Saccharifying Power (U/g) |
|---|---|---|---|---|---|---|---|
| Cuqu (C0) | 13.71 ± 0.36 | 5.77 ± 0.14 | 1.49 ± 0.11 | 0.42 ± 0.05 | 12.48 ± 0.67 | 3.30 ± 0.24 | 1811.30 ± 2.31 |
| Sample | Bacteria | Fungi | ||
|---|---|---|---|---|
| Chao1 Index | Simpson Index | Chao1 Index | Simpson Index | |
| C0 | 230.904 ± 15.567 g | 0.885 ± 0.092 e | 47.083 ± 7.091 j | 0.654 ± 0.077 f |
| C1 | 441.878 ± 18.685 c | 0.960 ± 0.054 a | 90.333 ± 13.953 f | 0.752 ± 0.079 c |
| C2 | 413.833 ± 17.683 d | 0.943 ± 0.041 b | 168.667 ± 11.669 c | 0.710 ± 0.147 e |
| C3 | 444.700 ± 22.778 b | 0.939 ± 0.184 c | 96.306 ± 9.954 e | 0.614 ± 0.064 g |
| C4 | 258.899 ± 9.078 f | 0.840 ± 0.121 f | 76.569 ± 15.553 h | 0.539 ± 0.072 j |
| C5 | 546.000 ± 14.770 a | 0.914 ± 0.009 d | 85.778 ± 12.221 g | 0.602 ± 0.080 i |
| C6 | 230.177 ± 24.448 h | 0.803 ± 0.116 g | 66.333 ± 9.005 i | 0.607 ± 0.034 h |
| C7 | 137.011 ± 10.986 j | 0.748 ± 0.029 h | 170.375 ± 16.866 a | 0.713 ± 0.091 d |
| C8 | 165.389 ± 15.007 i | 0.676 ± 0.116 i | 163.287 ± 19.099 d | 0.868 ± 0.011 a |
| C9 | 335.181 ± 8.903 e | 0.536 ± 0.151 j | 170.017 ± 15.022 b | 0.801 ± 0.0663 b |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hou, Z.; Zhang, J.; Dang, L.; Xue, H.; Chen, M.; Bai, B.; Yang, Y.; Bo, T.; Fan, S. Correlation Analysis of Microbial Community Changes and Physicochemical Characteristics in Aged Vinegar Brewing. Foods 2023, 12, 3430. https://doi.org/10.3390/foods12183430
Hou Z, Zhang J, Dang L, Xue H, Chen M, Bai B, Yang Y, Bo T, Fan S. Correlation Analysis of Microbial Community Changes and Physicochemical Characteristics in Aged Vinegar Brewing. Foods. 2023; 12(18):3430. https://doi.org/10.3390/foods12183430
Chicago/Turabian StyleHou, Zhixing, Jinhua Zhang, Ling Dang, Hugui Xue, Min Chen, Baoqing Bai, Yukun Yang, Tao Bo, and Sanhong Fan. 2023. "Correlation Analysis of Microbial Community Changes and Physicochemical Characteristics in Aged Vinegar Brewing" Foods 12, no. 18: 3430. https://doi.org/10.3390/foods12183430





