Single Ultrasonic-Assisted Washing for Eco-Efficient Production of Mackerel (Auxis thazard) Surimi
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Surimi Preparation by UAW
2.3. Analyses of Surimi Characteristics
2.3.1. pH
2.3.2. Lipid Content
2.3.3. Heme Iron and Non-Heme Iron Contents
2.3.4. Absorption Spectra of Myoglobin, Myoglobin Content, and Myoglobin Derivatives
2.3.5. Trichloro Acetic Acid (TCA) Soluble Peptide
2.3.6. Ca2+-ATPase Activity
2.3.7. Reactive Sulfhydryl (SH) Content
2.3.8. Surface Hydrophobicity
2.3.9. Rheological Property
2.4. Determination of Surimi Gel Functionalities
2.4.1. Gel Formation
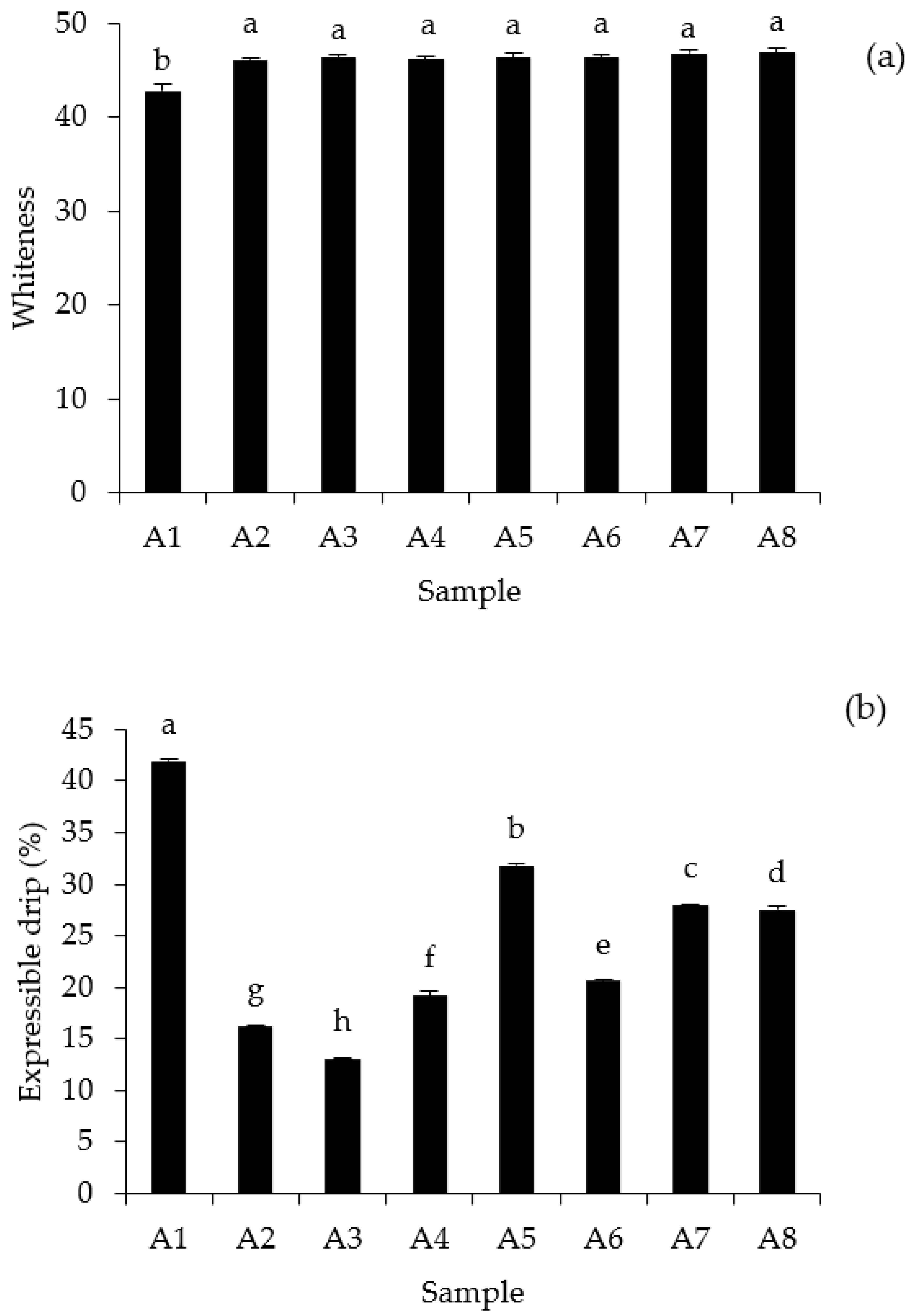
2.4.2. Determination of Whiteness
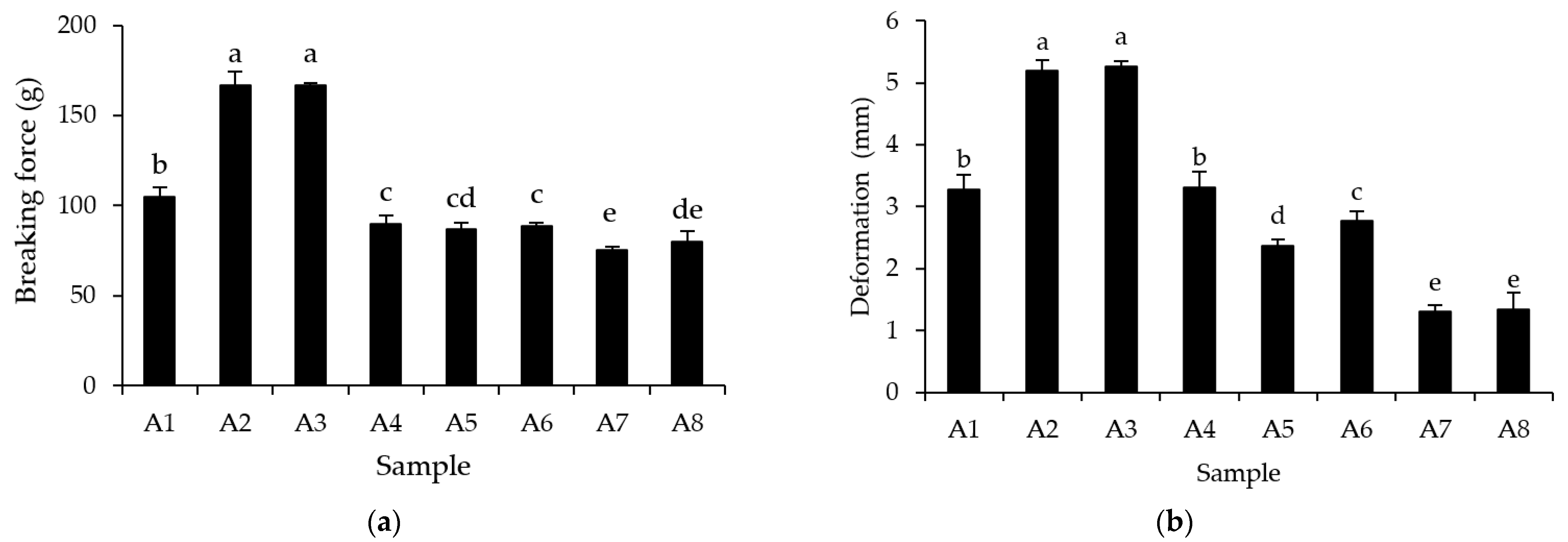
2.4.3. Texture Analysis
2.4.4. Determination of Expressible Moisture
2.4.5. Lipid Oxidation
2.4.6. Evaluation of Rancid and Fishy Odors
2.4.7. FTIR Spectra
2.4.8. Determination of Microstructure of Gel
2.5. Statistical Analysis
3. Results and Discussion
3.1. Yield and Chemical Characteristics
3.2. Myoglobin Content and Changes in Myoglobin Redox State
3.3. TCA-Soluble Peptides and Protein Denaturation
3.4. Lipid Oxidation and Off-Odor Development
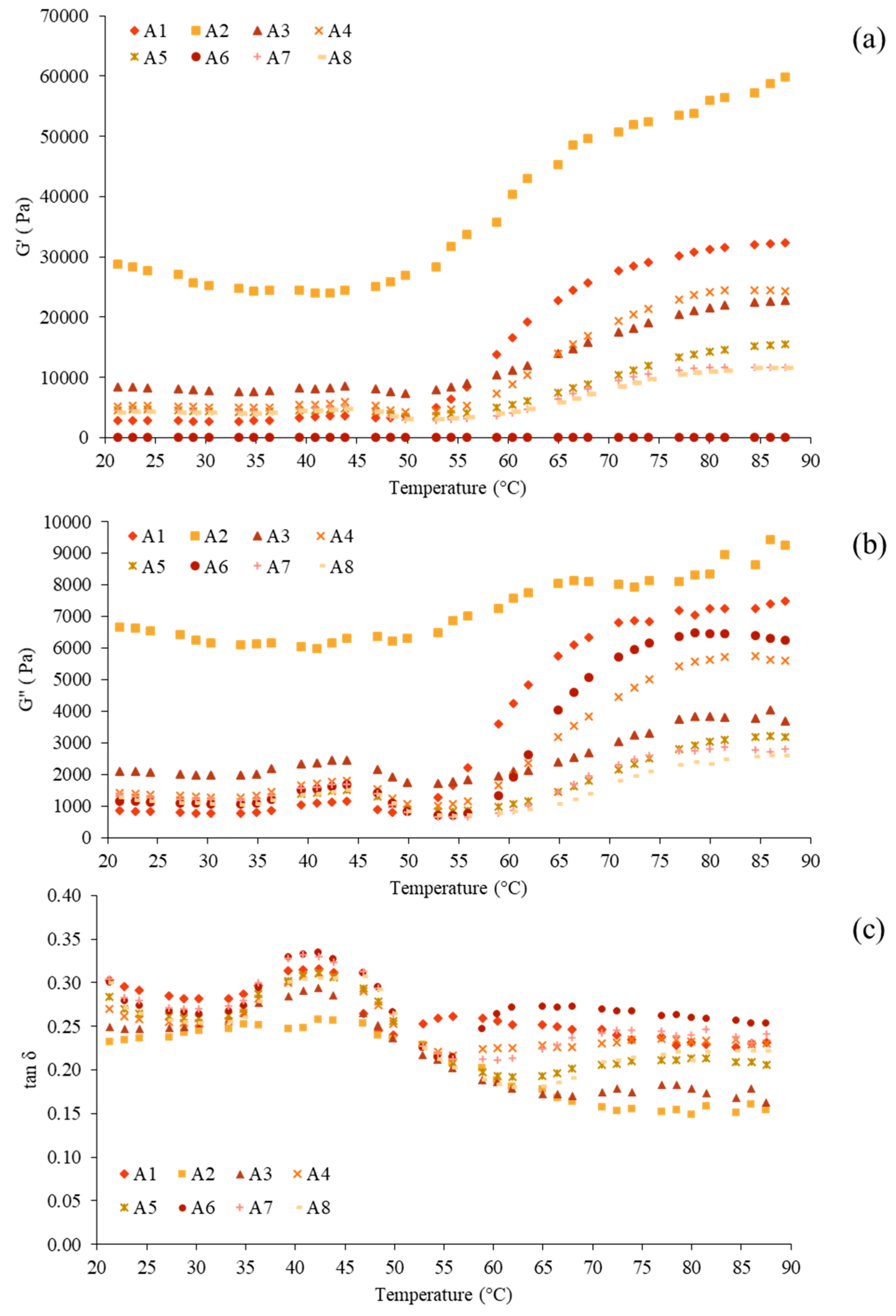
3.5. Rheological Properties
3.6. Gel Characteristics
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gao, W.; Huang, Y.; Zeng, X.A.; Brennan, M.A. Effect of soluble soybean polysaccharides on freeze-denaturation and structure of myofibrillar protein of bighead carp surimi with liquid nitrogen freezing. Int. J. Biol. Macromol. 2019, 135, 839–844. [Google Scholar] [CrossRef]
- Chaijan, M.; Panpipat, W.; Benjakul, S. Physicochemical properties and gel-forming ability of surimi from three species of mackerel caught in Southern Thailand. Food Chem. 2010, 121, 85–92. [Google Scholar] [CrossRef]
- Panpipat, W.; Chaijan, M.; Benjakul, S. Gel properties of croaker–mackerel surimi blend. Food Chem. 2010, 122, 1122–1128. [Google Scholar] [CrossRef]
- Chaijan, M. Lipid and myoglobin oxidations in muscle foods. SJST 2008, 30, 47–53. [Google Scholar]
- Chaijan, M.; Panpipat, W.; Benjakul, S. Physicochemical and gelling properties of short-bodied mackerel (Rastrelliger brachysoma) protein isolate prepared using alkaline-aided process. Food Bioprod. Process. 2010, 88, 174–180. [Google Scholar] [CrossRef]
- Granata, L.A.; Flick, G.J., Jr.; Martin, R.E. (Eds.) The Seafood Industry: Species, Products, Processing, and Safety; John Wiley & Sons: Hoboken, NJ, USA, 2012. [Google Scholar]
- Priyadarshini, B.; Majumdar, R.K.; Parhi, J.; Maurya, P.K.; Roy, D.; Saha, A. Gel properties of sutchi catfish (Pangasius hypophthalmus) surimi as affected by selected washing process and number of washing cycles. Food Sci. Technol. Int. 2016, 22, 266–274. [Google Scholar] [CrossRef]
- Zhou, X.; Chong, Y.; Ding, Y.; Gu, S.; Liu, L. Determination of the effects of different washing processes on aroma characteristics in silver carp mince by MMSE–GC–MS, e-nose and sensory evaluation. Food Chem. 2016, 207, 205–213. [Google Scholar] [CrossRef]
- Somjid, P.; Panpipat, W.; Chaijan, M. Carbonated water as a novel washing medium for mackerel (Auxis thazard) surimi production. J. Food Sci. Technol. 2017, 54, 3979–3988. [Google Scholar] [CrossRef]
- Thongkam, P.; Chaijan, M.; Cheong, L.-Z.; Panpipat, W. Impact of washing with antioxidant-infused soda–saline solution on gel functionality of mackerel (Auxis thazard) surimi. Foods 2023, 12, 3178. [Google Scholar] [CrossRef]
- Gâmbuteanu, C.; Alexe, P. Principles and effects of acoustic cavitation. Food Technol. 2013, 37, 9–17. [Google Scholar]
- Benjakul, S.; Morrissey, M.T. Protein hydrolysates from Pacific whiting solid wastes. J. Agric. Food Chem. 1997, 45, 3423–3430. [Google Scholar] [CrossRef]
- Bligh, E.G.; Dyer, W.J. A rapid method of total lipid extraction and purification. Can. J. Biochem. Physiol. 1959, 37, 911–917. [Google Scholar] [CrossRef] [PubMed]
- Benjakul, S.; Bauer, F. Biochemical and physicochemical changes in catfish (Silurus glanis Linne) muscle as influenced by different freeze–thaw cycles. Food Chem. 2001, 72, 207–217. [Google Scholar] [CrossRef]
- Gomez-Basauri, J.V.; Regenstein, J.M. Vacuum packaging, ascorbic acid and frozen storage effects on heme and nonheme iron content of mackerel. J. Food Sci. 1992, 57, 1337–1339. [Google Scholar] [CrossRef]
- Rhee, K.S.; Ziprin, Y.A. Modification of the Schricker nonheme iron method to minimize pigment effects for red meats. J. Food Sci. 1987, 52, 1174–1176. [Google Scholar] [CrossRef]
- Viriyarattanasak, C.; Hamada-Sato, N.; Watanabe, M.; Kajiwara, K.; Suzuki, T. Equations for spectrophotometric determination of relative concentrations of myoglobin derivatives in aqueous tuna meat extracts. Food Chem. 2011, 127, 656–661. [Google Scholar] [CrossRef]
- Lowry, C.O.; Rosebrough, N.; Farr, A.; Randall, R. Protein measurement with the Folin phenol reagent. J. Biol. Chem. 1951, 193, 265–275. [Google Scholar] [CrossRef]
- Morrissey, M.T.; Wu, J.W.; Lin, D.; An, H. Protease inhibitor effects on torsion measurements and autolysis of Pacific whiting surimi. J. Food Sci. 1993, 58, 1050–1054. [Google Scholar] [CrossRef]
- Ellman, G.L. Tissue sulfhydryl groups. Arch. Biochem. Biophys. 1959, 82, 70–77. [Google Scholar] [CrossRef]
- Chelh, I.; Gatellier, P.; Santé-Lhoutellier, V. A simplified procedure for myofibril hydrophobicity determination. Meat Sci. 2006, 74, 681–683. [Google Scholar] [CrossRef]
- Somjid, P.; Panpipat, W.; Cheong, L.Z.; Chaijan, M. Reduced washing cycle for sustainable mackerel (Rastrelliger kanagurta) surimi production: Evaluation of bio-physico-chemical, rheological, and gel-forming properties. Foods 2021, 10, 2717. [Google Scholar] [CrossRef]
- Somjid, P.; Panpipat, W.; Cheong, L.Z.; Chaijan, M. Comparative effect of cricket protein powder and soy protein isolate on gel properties of Indian mackerel surimi. Foods 2022, 11, 3445. [Google Scholar] [CrossRef]
- Panpipat, W.; Chaijan, M. Biochemical and physicochemical characteristics of protein isolates from bigeye snapper (Priacanthus tayenus) head by-product using pH shift method. Turkish J. Fish. Aquat. Sci. 2016, 16, 41–50. [Google Scholar]
- Botsoglou, N.A.; Fletouris, D.J.; Papageorgiou, G.E.; Vassilopoulos, V.N.; Mantis, A.J.; Trakatellis, A.G. Rapid, sensitive, and specific thiobarbituric acid method for measuring lipid peroxidation in animal tissue, food, and feedstuff samples. J. Agric. Food Chem. 1994, 42, 1931–1937. [Google Scholar] [CrossRef]
- Frankel, E.N. Antioxidants in lipid foods and their impact on food quality. Food Chem. 1996, 57, 51–55. [Google Scholar] [CrossRef]
- Buege, J.A.; Aust, S.D. Microsomal lipid peroxidation. In Methods in Enzymology; Academic Press: Cambridge, MA, USA, 1978; Volume 52, pp. 302–310. [Google Scholar]
- Phetsang, H.; Panpipat, W.; Undeland, I.; Panya, A.; Phonsatta, N.; Chaijan, M. Comparative quality and volatilomic characterisation of unwashed mince, surimi, and pH-shift-processed protein isolates from farm-raised hybrid catfish (Clarias macrocephalus × Clarias gariepinus). Food Chem. 2021, 364, 130365. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.H. Decoloration and gel-forming ability of horse mackerel mince by air-flotation washing. J. Food Sci. 2002, 67, 2970–2975. [Google Scholar] [CrossRef]
- Chaijan, M.; Panpipat, W. Mechanism of oxidation in foods of animal origin. In Natural Antioxidants, 1st ed.; Banerjee, R., Verma, A.K., Siddiqui, M.W., Eds.; Apple Academic Press: Palm Bay, FL, USA, 2017; pp. 1–37. [Google Scholar]
- Singh, A.; Prabowo, F.F.; Benjakul, S.; Pranoto, Y.; Chantakun, K. Combined effect of microbial transglutaminase and ethanolic coconut husk extract on the gel properties and in-vitro digestibility of spotted golden goatfish (Parupeneus heptacanthus) surimi gel. Food Hydrocoll. 2020, 109, 106107. [Google Scholar] [CrossRef]
- Zhang, L.; Li, Q.; Hong, H.; Luo, Y. Prevention of protein oxidation and enhancement of gel properties of silver carp (Hypophthalmichthys molitrix) surimi by addition of protein hydrolysates derived from surimi processing by-products. Food Chem. 2020, 316, 126343. [Google Scholar] [CrossRef]
- Tang, L.; Yongsawatdigul, J. Physicochemical properties of tilapia (Oreochromis niloticus) acto-myosin subjected to high intensity ultrasound in low NaCl concentrations. Ultrason. Sonochem. 2020, 63, 104922. [Google Scholar] [CrossRef]
- Gülseren, İ.; Güzey, D.; Bruce, B.D.; Weiss, J. Structural and functional changes in ultrasonicated bovine serum albumin solutions. Ultrason. Sonochem. 2007, 14, 173–183. [Google Scholar] [CrossRef] [PubMed]
- Chandrapala, J.; Zisu, B.; Palmer, M.; Kentish, S.; Ashokkumar, M. Effects of ultrasound on the thermal and structural characteristics of proteins in reconstituted whey protein concentrate. Ultrason. Sonochem. 2011, 18, 951–957. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.D.; Holley, R.A. Factors influencing gel formation by myofibrillar proteins in muscle foods. Compr. Rev. Food Sci. Food Saf. 2011, 10, 33–51. [Google Scholar] [CrossRef]
- Eymard, S.; Carcouët, E.; Rochet, M.J.; Dumay, J.; Chopin, C.; Genot, C. Development of lipid oxidation during manufacturing of horse mackerel surimi. J. Sci. Food Agric. 2005, 85, 1750–1756. [Google Scholar] [CrossRef]
- Kang, D.; Zhang, W.; Lorenzo, J.M.; Chen, X. Structural and functional modification of food proteins by high power ultrasound and its application in meat processing. Crit. Rev. Food Sci. Nutr. 2021, 61, 1914–1933. [Google Scholar] [CrossRef] [PubMed]
- Lorenzo, J.M.; Cittadini, A.; Munekata, P.E.; Domínguez, R. Physicochemical properties of foal meat as affected by cooking methods. Meat Sci. 2015, 108, 50–54. [Google Scholar] [CrossRef]
- Mehta, N.K.; Balange, A.K.; Lekshmi, M.; Nayak, B.B. Changes in dynamic viscoelastic and functional properties of Indian squid mantle during ice storage. J. Food Process. Preserv. 2017, 41, e12891. [Google Scholar] [CrossRef]
- Campo-Deaño, L.; Tovar, C.A.; Borderías, J. Effect of several cryoprotectants on the physicochemical and rheological properties of suwari gels from frozen squid surimi made by two methods. J. Food Eng. 2010, 97, 457–464. [Google Scholar] [CrossRef]
- Egelandsdal, B.; Martinsen, B.; Autio, K. Rheological parameters as predictors of protein functionality: A model study using myofibrils of different fiber-type composition. Meat Sci. 1995, 39, 97–111. [Google Scholar] [CrossRef]
- Buamard, N.; Benjakul, S.; Konno, K. Improvement of gel quality of sardine surimi with low setting phenomenon by ethanolic coconut husk extract. J. Texture Stud. 2017, 48, 47–56. [Google Scholar] [CrossRef]
- Rawdkuen, S.; Benjakul, S.; Visessanguan, W.; Lanier, T.C. Rheological and textural properties of pacific whiting surimi gels as influenced by chicken plasma. Int. J. Food Prop. 2008, 11, 820–832. [Google Scholar] [CrossRef]
- Duan, S.; Shi, Q.; Hong, J.; Zhu, D.; Lin, Y.; Li, Y.; Wu, J. Water-modulated biomimetic hyper-attribute-gel electronic skin for robotics and skin-attachable wearables. ACS Nano 2023, 17, 1355–1371. [Google Scholar] [CrossRef]
- Zhang, Y.I.N.; Zeng, Q.X.; Zhu, Z.W.; Zhou, R.U.I. Effect of ultrasonic treatment on the gel strength of tilapia (Sarotherodon nilotica) surimi. J. Food Process Eng. 2011, 34, 533–548. [Google Scholar] [CrossRef]
- Cando, D.; Herranz, B.; Borderías, A.J.; Moreno, H.M. Effect of high pressure on reduced sodium chloride surimi gels. Food Hydrocoll. 2015, 51, 176–187. [Google Scholar] [CrossRef]
- Urbonaite, V.; De Jongh, H.H.J.; Van der Linden, E.; Pouvreau, L. Origin of water loss from soy protein gels. J. Agric. Food Chem. 2014, 62, 7550–7558. [Google Scholar] [CrossRef]
- Jiao, X.; Cao, H.; Fan, D.; Huang, J.; Zhao, J.; Yan, B.; Zhang, H. Effects of fish oil incorporation on the gelling properties of silver carp surimi gel subjected to microwave heating combined with conduction heating treatment. Food Hydrocoll. 2019, 94, 164–173. [Google Scholar] [CrossRef]
- Pelton, J.T.; McLean, L.R. Spectroscopic methods for analysis of protein secondary structure. Anal. Biochem. 2000, 277, 167–176. [Google Scholar] [CrossRef]
- Ge, L.; Zhu, M.; Li, X.; Xu, Y.; Ma, X.; Shi, R.; Mu, C. Development of active rosmarinic acid-gelatin biodegradable films with antioxidant and long-term antibacterial activities. Food Hydrocoll. 2018, 83, 308–316. [Google Scholar] [CrossRef]
- Wei, W.; Hu, W.; Zhang, X.Y.; Zhang, F.P.; Sun, S.Q.; Liu, Y.; Xu, C.H. Analysis of protein structure changes and quality regulation of surimi during gelation based on infrared spectroscopy and microscopic imaging. Sci. Rep. 2018, 8, 5566. [Google Scholar] [CrossRef]
- Fecko, C.J.; Eaves, J.D.; Loparo, J.J.; Tokmakoff, A.; Geissler, P.L. Ultrafast hydrogen-bond dynamics in the infrared spectroscopy of water. Science 2003, 301, 1698–1702. [Google Scholar] [CrossRef]
- Duan, W.; Qiu, H.; Htwe, K.K.; Sun, Q.; Han, Z.; Wang, Z.; Liu, S. Investigation of the relationship between gel strength and microstructure of surimi gel induced by dense phase carbon dioxide based on quantitative analysis. Food Hydrocoll. 2024, 146, 109209. [Google Scholar] [CrossRef]

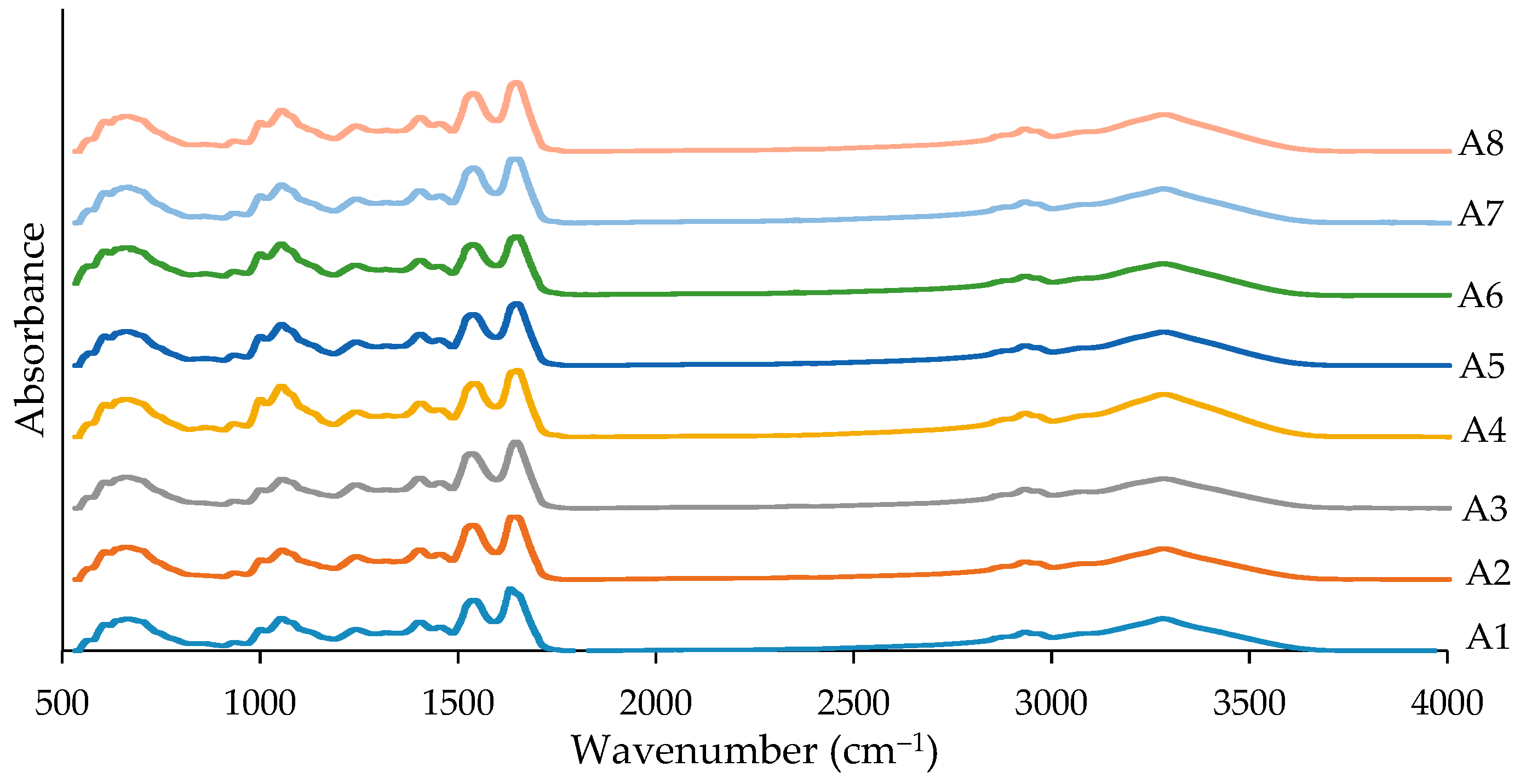

| Treatment | 1st Cycle | 2nd Cycle | 3rd Cycle |
|---|---|---|---|
| A1 (unwashed mince) | - | - | - |
| A2 | Water (10 min) | Water (10 min) | Water (10 min) |
| A3 | CSA + US (5 min) | - | - |
| A4 | CSA + US (10 min) | - | - |
| A5 | CSA + US (15 min) | - | - |
| A6 | CSA − US (5 min) | - | - |
| A7 | CSA − US (10 min) | - | - |
| A8 | CSA − US (15 min) | - | - |
| Treatment | Yield (%) | pH | Lipid (g/100 g) | Heme Iron (mg/100 g) | Non-Heme Iron (mg/100 g) |
|---|---|---|---|---|---|
| A1 | - | 5.52 ± 0.01g | 1.97 ± 0.03a | 35.92 ± 0.40a | 16.15 ± 0.33a |
| A2 | 71.56 ± 1.20c | 5.87 ± 0.01f | 0.62 ± 0.10de | 15.07 ± 0.07d | 15.19 ± 0.56b |
| A3 | 66.11 ± 1.53d | 5.90 ± 0.01e | 0.70 ± 0.03e | 10.46 ± 0.85f | 8.79 ± 0.18cd |
| A4 | 65.52 ± 1.60d | 5.93 ± 0.01d | 0.69 ± 0.03e | 15.80 ± 0.12cd | 8.38 ± 0.07d |
| A5 | 58.10 ± 1.01e | 6.00 ± 0.01b | 0.64 ± 0.02de | 10.43 ± 0.81f | 9.39 ± 0.85c |
| A6 | 80.26 ± 1.18a | 5.96 ± 0.01c | 1.50 ± 0.03c | 18.45 ± 0.11b | 16.85 ± 0.37a |
| A7 | 77.62 ± 1.16b | 6.02 ± 0.01a | 1.77 ± 0.05b | 13.38 ± 0.02e | 9.21 ± 0.02c |
| A8 | 78.14 ± 1.39b | 6.01 ± 0.01ab | 1.69 ± 0.03b | 16.05 ± 0.11c | 8.87 ± 0.44cd |
| Treatment | Myoglobin (g/100 g) | Myoglobin Redox State (%) | ||
|---|---|---|---|---|
| Deoxymyoglobin | Oxymyoglobin | Metmyoglobin | ||
| A1 | 0.73 ± 0.09a | 9.81 ± 1.18e | 10.44 ± 2.16b | 8.10 ± 1.34a |
| A2 | 0.30 ± 0.05cd | 25.12 ± 0.14a | 12.57 ± 0.89a | 2.45 ± 0.04e |
| A3 | 0.23 ± 0.02d | 17.39 ± 0.50b | 8.15 ± 0.69c | 4.31 ± 0.24d |
| A4 | 0.24 ± 0.00d | 12.53 ± 0.31cd | 6.39 ± 0.14cd | 6.40 ± 0.17bc |
| A5 | 0.23 ± 0.02d | 13.43 ± 0.52c | 8.22 ± 1.52c | 5.70 ± 0.23c |
| A6 | 0.42 ± 0.03b | 9.62 ± 0.84e | 5.78 ± 0.74d | 8.67 ± 0.89a |
| A7 | 0.28 ± 0.0.03cd | 11.73 ± 0.03d | 6.15 ± 0.95cd | 6.99 ± 0.03b |
| A8 | 0.31 ± 0.01c | 13.09 ± 0.66c | 7.28 ± 0.65cd | 6.07 ± 0.27bc |
| Treatment | TCA-Soluble Peptide (µmol Tyrosine/g Sample) | Ca2+-ATPase Activity (µmol/mg Protein/min) | Reactive Sulfhydryl Content (mol/108 g Protein) | Hydrophobicity (BPB Bound (µg)) |
|---|---|---|---|---|
| A1 | 1.39 ± 0.12a | 1.89 ± 0.06a | 2.36 ± 0.01d | 52.95 ± 0.20b |
| A2 | 0.54 ± 0.01f | 0.29 ± 0.04e | 4.49 ± 0.17a | 18.97 ± 0.41g |
| A3 | 0.58 ± 0.05f | 1.16 ± 0.21c | 4.00 ± 0.17b | 38.43 ± 0.78d |
| A4 | 0.80 ± 0.04e | 0.98 ± 0.03d | 4.48 ± 0.06a | 50.60 ± 0.36c |
| A5 | 0.89 ± 0.04de | 0.89 ± 0.05d | 4.54 ± 0.13a | 13.44 ± 0.36h |
| A6 | 0.93 ± 0.05cd | 1.26 ± 0.03c | 3.54 ± 0.21c | 32.15 ± 1.26e |
| A7 | 0.97 ± 0.03cd | 1.63 ± 0.03b | 4.13 ± 0.10b | 59.12 ± 0.16a |
| A8 | 1.07 ± 0.04b | 1.63 ± 0.02b | 4.09 ± 0.06b | 26.08 ± 0.05f |
| Treatment | Peroxide Value (meq/kg Lipid) | Conjugated Diene (A234) | TBARS (mg MDA Equivalent/kg) | Rancid Odor * NS | Fishy Odor * |
|---|---|---|---|---|---|
| A1 | 1.97 ± 0.11ab | 21.22 ± 0.19d | 0.48 ± 0.01a | 0.60 ± 0.84 | 2.20 ± 0.92a |
| A2 | 0.92 ± 0.03c | 67.70 ± 0.79a | 0.34 ± 0.01d | 0.50 ± 0.71 | 1.90 ± 0.74ab |
| A3 | 0.66 ± 0.01d | 15.31 ± 0.36e | 0.38 ± 0.02cd | 0.70 ± 0.82 | 1.80 ± 0.79abc |
| A4 | 2.22 ± 0.05a | 22.01 ± 0.69d | 0.38 ± 0.03c | 0.30 ± 0.48 | 1.30 ± 0.67bc |
| A5 | 1.70 ± 0.47b | 31.32 ± 0.79b | 0.43 ± 0.00b | 0.40 ± 0.70 | 1.30 ± 0.82bc |
| A6 | 1.84 ± 0.38ab | 29.64 ± 0.30c | 0.39 ± 0.03bc | 0.30 ± 0.67 | 1.40 ± 0.70bc |
| A7 | 1.81 ± 0.16ab | 10.61 ± 0.51g | 0.48 ± 0.01a | 0.20 ± 0.42 | 1.30 ± 0.67bc |
| A8 | 0.95 ± 0.12c | 14.04 ± 0.76f | 0.48 ± 0.02a | 0.10 ± 0.32 | 1.10 ± 0.57c |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Panpipat, W.; Tongkam, P.; Çavdar, H.K.; Chaijan, M. Single Ultrasonic-Assisted Washing for Eco-Efficient Production of Mackerel (Auxis thazard) Surimi. Foods 2023, 12, 3817. https://doi.org/10.3390/foods12203817
Panpipat W, Tongkam P, Çavdar HK, Chaijan M. Single Ultrasonic-Assisted Washing for Eco-Efficient Production of Mackerel (Auxis thazard) Surimi. Foods. 2023; 12(20):3817. https://doi.org/10.3390/foods12203817
Chicago/Turabian StylePanpipat, Worawan, Pornthip Tongkam, Hasene Keskin Çavdar, and Manat Chaijan. 2023. "Single Ultrasonic-Assisted Washing for Eco-Efficient Production of Mackerel (Auxis thazard) Surimi" Foods 12, no. 20: 3817. https://doi.org/10.3390/foods12203817






