Effect of the Pulsed Electric Field Treatment on Physical, Chemical and Structural Changes of Vacuum Impregnated Apple Tissue in Aloe Vera Juices
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Technological Processing
2.2.1. Pulsed Electric Field Treatment
2.2.2. Vacuum Impregnation Process (VI)
2.3. Analytical Methods
2.3.1. Water Content and Water Activity
2.3.2. Color Measurements
2.3.3. Texture Measurements
2.3.4. Structure Analysis
2.3.5. Assessment of Cell Viability
2.3.6. Assessment of Bioactive Compounds
Extract Preparation
Determination of Total Polyphenol Content (TPC)
Determination of Vitamin C
Determination of Antioxidant Activity (AA)
2.3.7. Fourier Infrared Spectroscopy (FTIR)
2.3.8. Thermogravimetric Analysis (TGA)
2.4. Statistical Methods
3. Results and Discussion
3.1. Vacuum Impregnation Process Combined with PEF Treatment of Apples
3.2. The Effect of Vacuum Impregnation of Apples in Aloe Vera Juice on the Change of Physical Properties
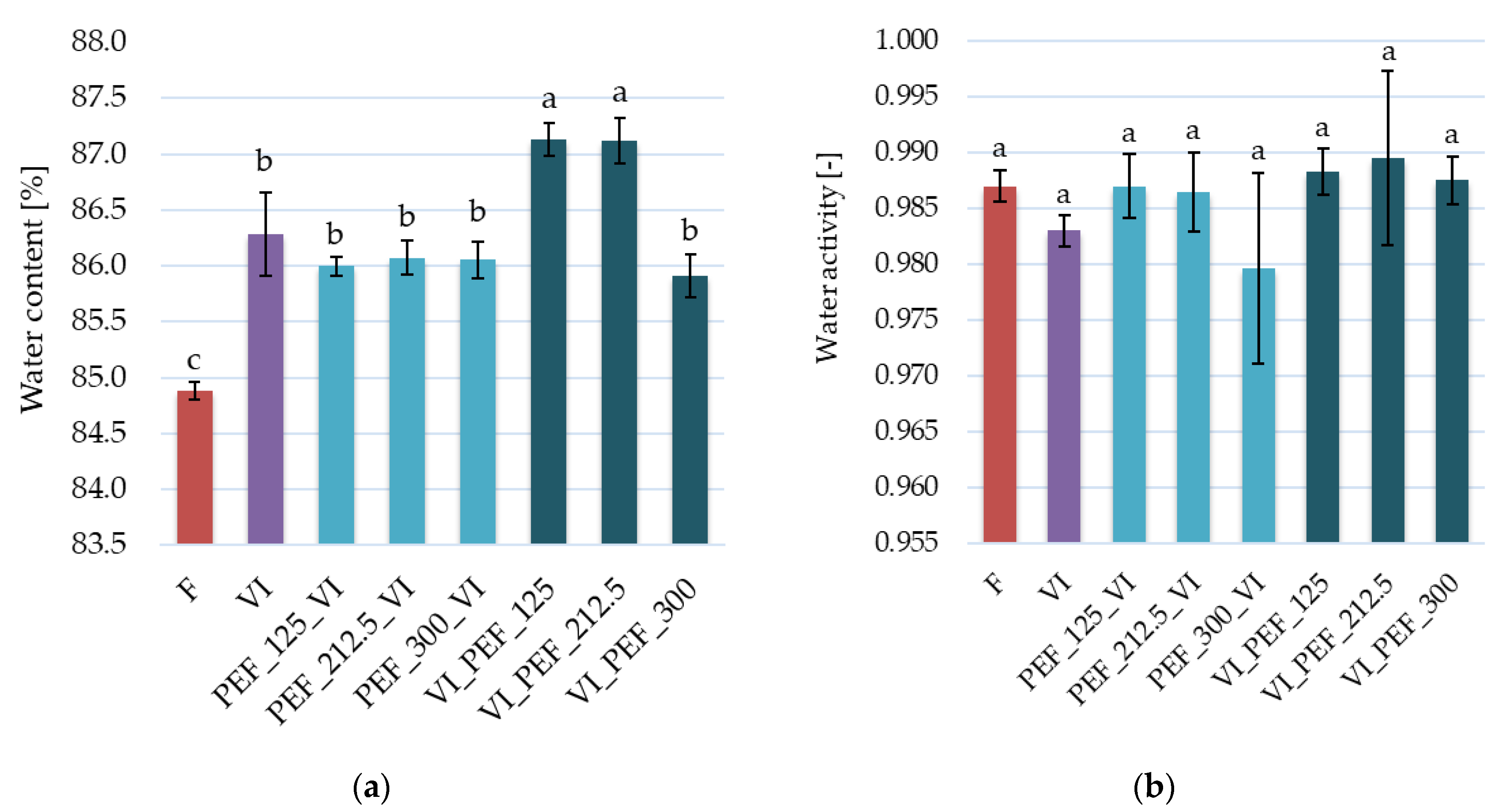
3.2.1. Changes in Water Content and Water Activity in Apples
3.2.2. Changes in Apple Color
3.2.3. Changes in Apples Texture and Structure
3.3. The Effect of Vacuum Impregnation of Apples in Aloe Vera Juice on the Change of Chemical Properties
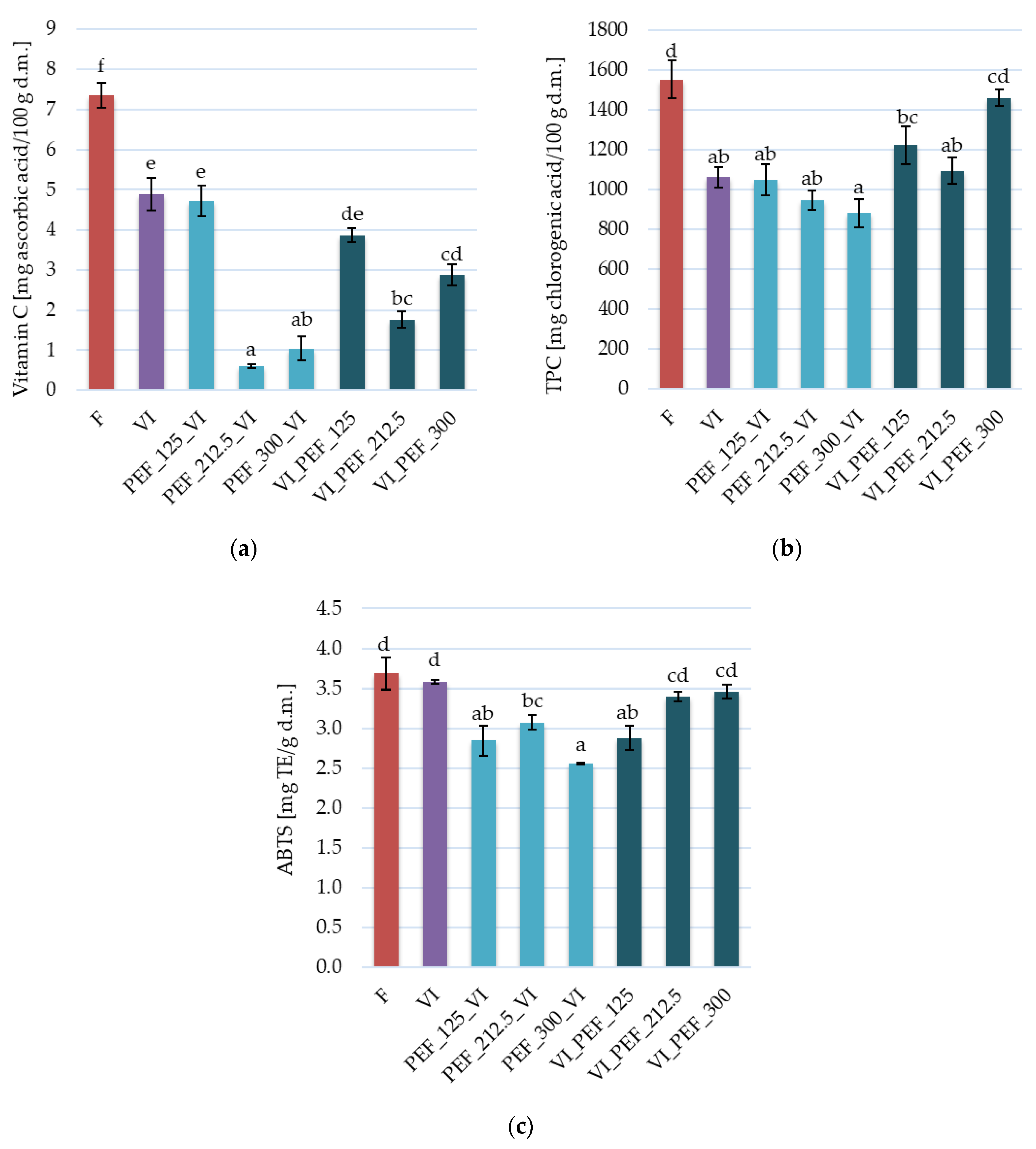
3.3.1. Bioactive Compounds and Antioxidant Activity in Apple Tissue
3.3.2. Fourier Infrared Spectroscopy (FTIR) of Apple Tissue
3.3.3. Thermogravimetric (TGA) Changes in Apple Tissue
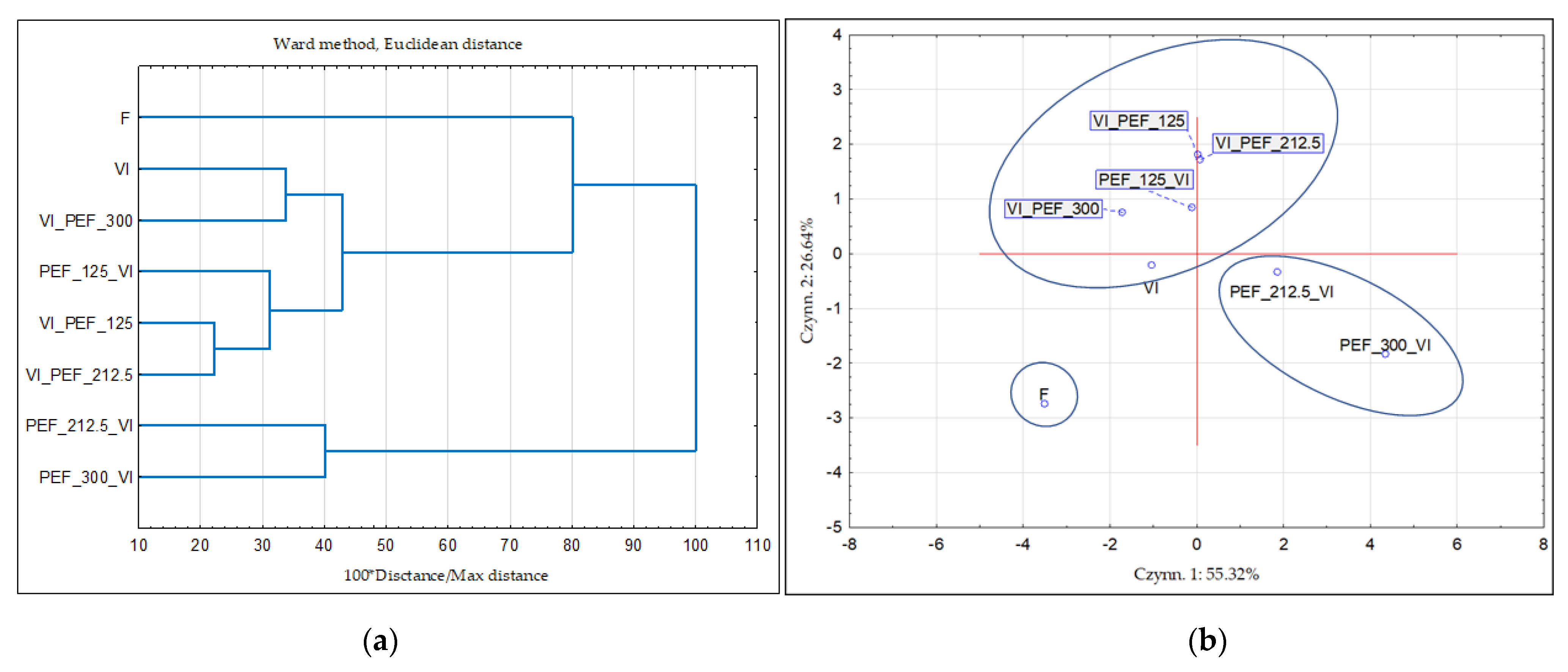
3.4. Cluster Analysis and Principal Components Analysis Results
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Saleena, P.; Jayashree, E.; Anees, K. A Comprehensive Review on Vacuum Impregnation: Mechanism, Applications and Prospects. Food Bioprocess Technol. 2023, 1–14. [Google Scholar] [CrossRef]
- Fito, P.; Andrés, A.; Chiralt, A.; Pardo, P. Coupling of Hydrodynamic Mechanism and Deformation-Relaxation Phenomena during Vacuum Treatments in Solid Porous Food-Liquid Systems. J. Food Eng. 1996, 27, 229–240. [Google Scholar] [CrossRef]
- Fito, P.; Chiralt, A.; Betoret, N.; Gras, M.; Cháfer, M.; Martínez-Monzó, J.; Andrés, A.; Vidal, D. Vacuum impregnation and osmotic dehydration in matrix engineering: Application in functional fresh food development. J. Food Eng. 2001, 49, 175–183. [Google Scholar] [CrossRef]
- Mújica-Paz, H.; Valdez-Fragoso, A.; López-Malo, A.; Palou, E.; Welti-Chanes, J. Impregnation and osmotic dehydration of some fruits: Effect of the vacuum pressure and syrup concentration. J. Food Eng. 2003, 57, 305–314. [Google Scholar] [CrossRef]
- Gras, M.; Vidal-Brotóns, N.; Betoret, A.; Chiralt; Fito, P. The response of some vegetables to vacuum impregnation. Innov. Food Sci. Emerg. Technol. 2002, 3, 263–269. [Google Scholar] [CrossRef]
- Tappi, S.; Tylewicz, U.; Romani, S.; Dalla Rosa, M.; Rizzi, F.; Rocculi, P. Study on the quality and stability of minimally processed apples impregnated with green tea polyphenols during storage. Innov. Food Sci. Emerg. Technol. 2017, 39, 148–155. [Google Scholar] [CrossRef]
- Tappi, S.; Tylewicz, U.; Romani, S.; Siroli, L.; Patrignani, F.; Dalla Rosa, M.; Rocculi, P. Optimization of Vacuum Impregnation with Calcium Lactate of Minimally Processed Melon and Shelf-Life Study in Real Storage Conditions. J. Food Sci. 2016, 81, E2734–E2742. [Google Scholar] [CrossRef]
- Erihemu; Hironaka, K.; Oda, Y.; Koaze, H. Iron enrichment of whole potato tuber by vacuum impregnation. LWT 2014, 59, 504–509. [Google Scholar] [CrossRef]
- Hironaka, K.; Kikuchi, M.; Koaze, H.; Sato, T.; Kojima, M.; Yamamoto, K.; Yasuda, K.; Mori, M.; Tsuda, S. Ascorbic acid enrichment of whole potato tuber by vacuum-impregnation. Food Chem. 2011, 127, 1114–1118. [Google Scholar] [CrossRef]
- Park, S.I.; Kodihalli, I.; Zhao, Y. Nutritional, sensory, and physicochemical properties of vitamin E- and mineral-fortified fresh-cut apples by use of vacuum impregnation. J. Food Sci. 2005, 70, S593–S599. [Google Scholar] [CrossRef]
- de Oliveira, P.M.; Ramos, A.M.; Martins, E.M.F.; Vieira, É.N.R.; de Souza Soares, A.; de Noronha, M.C. Comparison of vacuum impregnation and soaking techniques for addition of the probiotic Lactobacillus acidophilus to minimally processed melon. Int. J. Food Sci. Technol. 2017, 52, 2547–2554. [Google Scholar] [CrossRef]
- Castagnini, J.M.; Tappi, S.; Tylewicz, U.; Laghi, L.; Rocculi, P. Study of Water Distribution, Textural and Colour Properties of Cold Formulated and Air-Dried Apple Snacks. Foods 2022, 11, 731. [Google Scholar] [CrossRef] [PubMed]
- González-Pérez, J.E.; Jiménez-González, O.; Ramírez-Corona, N.; Guerrero-Beltrán, J.A.; López-Malo, A. Vacuum impregnation on apples with grape juice concentrate: Effects of pressure, processing time, and juice concentration. Innov. Food Sci. Emerg. Technol. 2022, 7, 102981. [Google Scholar] [CrossRef]
- Betoret, N.; Puente, L.; Díaz, M.J.; Pagán, M.J.; García, M.J.; Gras, M.L.; Martínez-Monzó, J.; Fito, P. Development of probiotic-enriched dried fruits by vacuum impregnation. J. Food Eng. 2003, 56, 273–277. [Google Scholar] [CrossRef]
- Betoret, E.; Betoret, N.; Arilla, A.; Bennár, M.; Barrera, C.; Codoñer, P.; Fito, P. No invasive methodology to produce a probiotic low humid apple snack with potential effect against Helicobacter pylori. J. Food Eng. 2012, 110, 289–293. [Google Scholar] [CrossRef]
- Derossi, A.; Ricci, I.; Fiore Ricci, A.G.; Severini, C. Apple slices enriched with aloe vera by vacuum impregnation. Ital. J. Food Sci. 2018, 30, 256–267. [Google Scholar]
- Mitra, A.; Singh, M.; Banga, A.; Pandey, J.; Tripathi, S.S.; Singh, D. Bioactive compounds and therapeutic properties of Aloe vera—A review. Plant Sci. Today 2023, 10, 1–7. [Google Scholar] [CrossRef]
- Deep, A.; Kumar, D.; Bansal, N.; Narasimhan, B.; Marwaha, R.K.; Sharma, P.C. Understanding mechanistic aspects and therapeutic potential of natural substances as anticancer agents. Phytomed. Plus 2023, 3, 100418. [Google Scholar] [CrossRef]
- Taukoorah, U.; Mahomoodally, M.F. Crude Aloe vera Gel Shows Antioxidant Propensities and Inhibits Pancreatic Lipase and Glucose Movement in Vitro. Adv. Pharmacol. Sci. 2016, 2016, 3720850. [Google Scholar] [CrossRef]
- Kumar, S.V.; Kumar, S.P.; Rupesh, D.; Nitin, K. Aloe vera: A Potential Herb and its Medicinal Importance. J. Chem. Pharm. Res. 2010, 2, 21–29. [Google Scholar]
- He, Q.; Changhong, L.; Kojo, E.; Tian, Z. Quality and safety assurance in the processing of aloe vera gel juice. Food Control 2005, 16, 95–104. [Google Scholar] [CrossRef]
- Kumar, S.; Kalita, S.; Das, A.; Kumar, P.; Singh, S.; Katiyar, V.; Mukherjee, A. Aloe vera: A contemporary overview on scope and prospects in food preservation and packaging. Prog. Org. Coat. 2022, 166, 106799. [Google Scholar] [CrossRef]
- Donsì, F.; Ferrari, G.; Pataro, G. Applications of pulsed electric field treatments for the enhancement of mass transfer from vegetable tissue. Food Eng. Rev. 2010, 2, 109–130. [Google Scholar] [CrossRef]
- Puértolas, E.; Luengo, E.; Álvarez, I.; Raso, J. Improving mass transfer to soften tissues by pulsed electric fields: Fundamentals and applications. Annu. Rev. Food Sci. Technol. 2012, 3, 263–282. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Dadmohammadi, Y.; Li, P.; Madarshahian, S.; Abbaspourrad, A. Generation of garlic flavor after frying by infusing alliin into potato strips using pulsed electric field and assisted infusion methods. Food Chem. 2022, 396, 133643. [Google Scholar] [CrossRef]
- Velickova, E.; Tylewicz, U.; Dalla Rosa, M.; Winkelhausen, E.; Kuzmanova, S.; Romani, S. Effect of pulsed electric field coupled with vacuum infusion on quality parameters of frozen/thawed strawberries. J. Food Eng. 2018, 233, 57–64. [Google Scholar] [CrossRef]
- Shiekh, K.A.; Benjakul, S.; Gulzar, S. Impact of pulsed electric field and vacuum impregnation with Chamuang leaf extract on quality changes in Pacific white shrimp packaged under modified atmosphere. LWT 2021, 149, 111899. [Google Scholar] [CrossRef]
- Tylewicz, U.; Lundin, P.; Cocola, L.; Dymek, K.; Rocculi, P.; Svanberg, S.; Dejmek, P.; Gόmez Galindo, F. Gas in scattering media absorption spectroscopy (GASMAS) detected persistent vacuum in apple tissue after vacuum impregnation. Food Biophys. 2012, 7, 28–34. [Google Scholar]
- Association of Official Analytical Collaboration International. Official Methods of Analysis of AOAC International, 17th ed.; The Association of Official Analytical Chemists: Gaithersburg, MD, USA, 2002. [Google Scholar]
- Genovese, J.; Tylewicz, U.; Mannozzi, C.; Castagnini, J.M.; Romani, S.; Rocculi, P.; Dalla Rosa, M. Kiwifruit waste valorisation through innovative snack development. Acta Hortic. 2022, 1332, 407–414. [Google Scholar] [CrossRef]
- Cerezal-Mezquita, P.; Bugueño-Muñoz, W. Drying of Carrot Strips in Indirect Solar Dehydrator with Photovoltaic Cell and Thermal Energy Storage. Sustainability 2022, 14, 2147. [Google Scholar] [CrossRef]
- Paula, A.M.; Conti-Silva, A.C. Texture profile and correlation between sensory and instrumental analyses on extruded snacks. J. Food Eng. 2014, 121, 9–14. [Google Scholar] [CrossRef]
- Biswas, M.S.; Terada, R.; Mano, J. Inactivation of Carbonyl-Detoxifying Enzymes by H2O2 Is a Trigger to Increase Carbonyl Load for Initiating Programmed Cell Death in Plants. Antioxidants 2020, 9, 141. [Google Scholar] [CrossRef] [PubMed]
- Bochnak-Niedźwiecka, J.; Szymanowska, U.; Świeca, M. The Protein-Rich Powdered Beverages Stabilized with Flax Seeds Gum—Antioxidant and Antiproliferative Properties of the Potentially Bioaccessible Fraction. Appl. Sci. 2022, 12, 7159. [Google Scholar] [CrossRef]
- Beya, M.M.; Netzel, M.E.; Sultanbawa, Y.; Smyth, H.; Hoffman, L.C. Kakadu plum (Terminalia ferdinandiana) bioactivity against spoilage microorganisms and oxidative reactions in refrigerated raw beef patties under modified atmosphere packaging. Meat Sci. 2023, 204, 109268. [Google Scholar] [CrossRef]
- Matys, A.; Dadan, M.; Witrowa-Rajchert, D.; Parniakov, O.; Wiktor, A. Response Surface Methodology as a Tool for Optimization of Pulsed Electric Field Pretreatment and Microwave-Convective Drying of Apple. Appl. Sci. 2022, 12, 3392. [Google Scholar] [CrossRef]
- Rivero-Ramos, P.; Unthank, M.G.; Sanz, T.; Rodrigo, M.D.; Benlloch-Tinoco, M. Synergistic depolymerisation of alginate and chitosan by high hydrostatic pressure (HHP) and pulsed electric fields (PEF) treatment in the presence of H2O2. Carbohydr. Polym. 2023, 316, 120999. [Google Scholar] [CrossRef]
- Mikus, M.; Galus, S.; Ciurzyńska, A.; Janowicz, M. Development and characterization of novel composite films based on soy protein isolate and oilseed flours. Molecules 2021, 26, 3738. [Google Scholar] [CrossRef]
- Panayampadan, A.S.; Alam, M.S.; Aslam, R.; Kaur, J. Vacuum Impregnation Process and Its Potential in Modifying Sensory, Physicochemical and Nutritive Characteristics of Food Products. Food Eng. Rev. 2022, 14, 229–256. [Google Scholar] [CrossRef]
- Martínez-Monzó, J.; Barat, J.M.; González-Martínez, C.; Chiralt, A.; Fito, P. Changes in thermal properties of apple due to vacuum impregnation. J. Food Eng. 2000, 43, 213–218. [Google Scholar] [CrossRef]
- Yılmaz, F.M.; Ersus Bilek, S. Ultrasound-assisted vacuum impregnation on the fortification of fresh-cut apple with calcium and black carrot phenolics. Ultrason. Sonochem. 2018, 48, 509–516. [Google Scholar] [CrossRef]
- Paes, S.S.; Stringari, G.B.; Laurindo, J.B. Effect of vacuum and relaxation periods and solution concentration on the osmotic dehydration of apples. Int. J. Food Sci. Technol. 2007, 42, 441–447. [Google Scholar] [CrossRef]
- Derossi, A.; Francavilla, M.; Monteleone, M.; Caporizzi, R.; Severini, C. From biorefinery of microalgal biomass to vacuum impregnation of fruit. A multidisciplinary strategy to develop innovative food with increased nutritional properties. Innov. Food Sci. Emerg. Technol. 2021, 70, 102677. [Google Scholar] [CrossRef]
- Tappi, S.; Velickova, E.; Mannozzi, C.; Tylewicz, U.; Laghi, L.; Rocculi, P. Multi-Analytical Approach to Study Fresh-Cut Apples Vacuum Impregnated with Different Solutions. Foods 2022, 11, 488. [Google Scholar] [CrossRef] [PubMed]
- Assis, F.R.; Rodrigues, L.G.G.; Tribuzi, G.; de Souza, P.G.; Carciofi, B.A.M.; Laurindo, J.B. Fortified apple (Malus spp., var. Fuji) snacks by vacuum impregnation of calcium lactate and convective drying. LWT—Food Sci. Technol. 2019, 113, 108298. [Google Scholar]
- Mashkour, M.; Maghsoudlou, Y.; Kashaninejad, M.; Aalami, M. Iron Fortification of Whole Potato Using Vacuum Impregnation Technique with a Pulsed Electric Field Pretreatment. Potato Res. 2018, 61, 375–389. [Google Scholar] [CrossRef]
- Lahiri, R.; Adams, L.B. Cultivation and Viability Determination of Mycobacterium leprae. In International Textbook of Leprosy; Scollard, D.M., Gillis, T.P., Eds.; American Leprosy Missions: Greenville, SC, USA, 2016. [Google Scholar]
- Panarese, V.; Dejmek, P.; Rocculi, P.; Galindo, F.G. Microscopic studies providing insight into the mechanisms of mass transfer in vacuum impregnation. Innov. Food Sci. Emerg. Technol. 2013, 18, 169–176. [Google Scholar]
- Tylewicz, U.; Romani, S.; Widell, S.; Gόmez Galindo, F. Induction of Vesicle Formation by Exposing Apple Tissue to Vacuum Impregnation. Food Bioprocess Technol. 2013, 6, 1099–1104. [Google Scholar] [CrossRef]
- Velickova, E.; Tylewicz, U.; Dalla Rosa, M.; Winkelhausen, E.; Kuzmanova, S.; Gómez Galindo, F. Effect of vacuum infused cryoprotectants on the freezing tolerance of strawberry tissues. LWT 2013, 52, 146–150. [Google Scholar] [CrossRef]
- Mauro, M.A.; Dellarosa, N.; Tylewicz, U.; Tappi, S.; Laghi, L.; Rocculi, P.; Rosa, M.D. Calcium and ascorbic acid affect cellular structure and water mobility in apple tissue during osmotic dehydration in sucrose solutions. Food Chem. 2016, 195, 19–28. [Google Scholar] [CrossRef]
- Tylewicz, U.; Tappi, S.; Genovese, J.; Mozzon, M.; Rocculi, P. Metabolic response of organic strawberries and kiwifruit subjected to PEF assisted-osmotic dehydration. Innov. Food Sci. Emerg. Technol. 2019, 56, 102190. [Google Scholar] [CrossRef]
- Tylewicz, U.; Tappi, S.; Mannozzi, C.; Romani, S.; Dellarosa, N.; Laghi, L.; Ragni, L.; Rocculi, P.; Dalla Rosa, M. Effect of pulsed electric field (PEF) pre-treatment coupled with osmotic dehydration on physico-chemical characteristics of organic strawberries. J. Food Eng. 2017, 213, 2–9. [Google Scholar] [CrossRef]
- de Lima, M.M.; Tribuzi, G.; de Souza, J.A.R.; de Souza, I.G.; Laurindo, J.B.; Carciofi, B.A.M. Vacuum impregnation and drying of calcium-fortified pineapple snacks. LWT 2016, 72, 501–509. [Google Scholar] [CrossRef]
- Nawirska-Olszańska, A.; Pasławska, M.; Stępień, B.; Oziembłowski, M.; Sala, K.; Smorowska, A. Effect of vacuum impregnation with apple-pear juice on content of bioactive compounds and antioxidant activity of dried chokeberry fruit. Foods 2020, 9, 108. [Google Scholar] [CrossRef] [PubMed]
- Hinestroza-Córdoba, L.I.; Barrera, C.; Seguí, L.; Betoret, N. Potential use of vacuum impregnation and high-pressure homogenization to obtain functional products from lulo fruit (Solanum quitoense lam.). Foods 2021, 10, 817. [Google Scholar] [CrossRef]
- Oblitas Cruz, J.F.; Rojas Gutierrez, E.L. Optimizing the incorporation of aloe vera in yacon slices (Smallanthus sonchifolius Poepp. & Endl.) through vacuum impregnation using response surface methodology. Ing. Univ. 2018, 22, 117–136. [Google Scholar] [CrossRef]
- Nowacka, M.; Mannozzi, C.; Dalla Rosa, M.; Tylewicz, U. Sustainable Approach for Development Dried Snack Based on Actinidia deliciosa Kiwifruit. Appl. Sci. 2023, 13, 2189. [Google Scholar] [CrossRef]
- Mokrzycki, W.S.; Tatol, M. Colour difference ∆E—A survey Mokrzycki. Mach. Graph. Vis. 2011, 20, 383–411. [Google Scholar]
- Abalos, R.A.; Naef, E.F.; Aviles, M.V.; Gómez, M.B. Vacuum impregnation: A methodology for the preparation of a ready-to-eat sweet potato enriched in polyphenols. LWT—Food Sci. Technol. 2020, 131, 109773. [Google Scholar] [CrossRef]
- Jalaee, F.; Fazeli, A.; Fatemian, H.; Tavakolipour, H. Mass transfer coefficient and the characteristics of coated apples in osmotic dehydrating. Food Bioprod. Process. 2011, 89, 367–374. [Google Scholar] [CrossRef]
- Wiktor, A.; Witrowa-Rajchert, D. Zastosowanie pulsacyjnego pola elektrycznego do wspomagania procesów usuwania wody z tkanek roślinnych. Żywność Nauk. Technol. Jakość 2012, 2, 22–32. [Google Scholar]
- Toivonen, P.M.A.; Brummell, D.A. Biochemical bases of appearance and texture changes in fresh-cut fruit and vegetables. Postharvest Biol. Technol. 2008, 48, 1–14. [Google Scholar] [CrossRef]
- Moreno, J.; Simpson, R.; Sayas, M.; Segura, I.; Aldana, O.; Almonacid, S. Influence of ohmic heating and vacuum impregnation on the osmotic dehydration kinetics and microstructure of pears (cv. Packham’s Triumph). J. Food Eng. 2011, 104, 621–627. [Google Scholar] [CrossRef]
- Moreno, J.; Simpson, R.; Baeza, A.; Morales, J.; Muñoz, C.; Sastry, S.; Almonacid, S. Effect of ohmic heating and vacuum impregnation on the osmodehydration kinetics and microstructure of strawberries (cv. Camarosa). LWT 2012, 45, 148–154. [Google Scholar] [CrossRef]
- Ertek, G.; Taştan, Ö.; Baysal, T. Combined use of vacuum impregnation and encapsulation technologies for phenolic enrichment of strawberries. Food Chem. 2023, 398, 133853. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Dodds, E.; Leong, S.Y.; Eyres, G.T.; Burritt, D.J.; Oey, I. Effect of pulsed electric fields on the structure and frying quality of “kumara” sweet potato tubers. Innov. Food Sci. Emerg. Technol. 2017, 39, 197–208. [Google Scholar] [CrossRef]
- Rahaman, A.; Siddeeg, A.; Manzoor, M.F.; Zeng, X.A.; Ali, S.; Baloch, Z.; Li, J.; Wen, Q.H. Impact of pulsed electric field treatment on drying kinetics, mass transfer, colour parameters and microstructure of plum. J. Food Sci. Technol. 2019, 56, 2670–2678. [Google Scholar] [CrossRef]
- Rybak, K.; Wiktor, A.; Witrowa-Rajchert, D.; Parniakov, O.; Nowacka, M. The effect of traditional and non-thermal treatments on the bioactive compounds and sugars content of red bell pepper. Molecules 2020, 25, 4287. [Google Scholar] [CrossRef]
- Gustavo, V.; Barbosa-Cánovas, M.; Góngora-Nieto, M.; Pothakamury, U.R.; Swanson, B.G. PEF inactivation of vegetative cells, spores, and enzymes in foods. In Preservation of Foods with Pulsed Electric Fields; Gustavo, V., Barbosa-Cánovas, M., Góngora-Nieto, M., Pothakamury, U.R., Swanson, B.G., Eds.; Academic Press: Cambridge, MA, USA, 1999. [Google Scholar]
- Castro, S.M.; Saraiva, J.A.; Lopes-da-Silva, J.A.; Delgadillo, I.; Van Loey, A.; Smout, C.; Hendrickx, M. Effect of thermal blanching and of high pressure treatments on sweet green and red bell pepper fruits (Capsicum annuum L.). Food Chem. 2008, 107, 1436–1449. [Google Scholar] [CrossRef]
- Ciurzynska, A.; Trusinska, M.; Rybak, K.; Wiktor, A.; Nowacka, M. The Influence of Pulsed Electric Field and Air Temperature on the Course of Hot-Air Drying and the Bioactive Compounds of Apple Tissue. Molecules 2023, 28, 2970. [Google Scholar] [CrossRef]
- Dinçer, C. Modeling of hibiscus anthocyanins transport to apple tissue during ultrasound-assisted vacuum impregnation. J. Food Process. Preserv. 2022, 46, e15886. [Google Scholar] [CrossRef]
- Galindo, F.G. Responses of plant cells and tissues to pulsed electric field treatments. In Handbook of Electroporation; Miklavčič, D., Ed.; Springer International Publishing: New York, NY, USA, 2017; pp. 2621–2635. [Google Scholar]
- Bureau, S.; Cozzolino, D.; Clark, C.J. Contributions of Fourier-transform mid infrared (FT-MIR) spectroscopy to the study of fruit and vegetables: A review. Postharvest Biol. Technol. 2019, 148, 1–14. [Google Scholar] [CrossRef]
- Jankovic, B.; Marinovic-Cincovic, M.; Jankovic, M. TG-DTA-FTIR analysis and isoconversional reaction profiles for thermal and thermo-oxidative degradation processes in black chokeberry (Aroniamelanocarpa). Chem. Pap. 2016, 70, 1094–1105. [Google Scholar] [CrossRef]
- Lam, S.S.; Liew, R.K.; Lim, X.Y.; Ani, F.N.; Jusoh, A. Fruit waste as feedstock for recovery by pyrolysis technique. Int. Biodeterior. Biodegrad. 2016, 113, 325–333. [Google Scholar] [CrossRef]
- Kowalska, H.; Trusinska, M.; Rybak, K.; Wiktor, A.; Witrowa-Rajchert, D.; Nowacka, M. Shaping the Properties of Osmo-Dehydrated Strawberries in Fruit Juice Concentrates. Appl. Sci. 2023, 13, 2728. [Google Scholar] [CrossRef]
- Przybył, K.; Koszela, K.; Adamski, F.; Samborska, K. Analysis to Detect Polysaccharide in Raspberry Powders. Sensors 2021, 21, 5823. [Google Scholar] [CrossRef]
- Zlatanović, S.; Ostojić, S.; Micić, D.; Rankov, S.; Dodevska, M.; Vukosavljević, P.; Gorjanović, S. Thermal behaviour and degradation kinetics of apple pomace flours. Thermochim. Acta 2019, 673, 17–25. [Google Scholar] [CrossRef]
- Cheng, X.C.; Cui, X.Y.; Qin, Z.; Liu, H.M.; Wang, X.D.; Liu, Y.L. Effect of drying pretreatment methods on structural features and antioxidant activities of Brauns native lignin extracted from Chinese quince fruit. Process Biochem. 2021, 106, 70–77. [Google Scholar] [CrossRef]
- Martínez-Burgos, W.J.; Serra, J.L.; MarsigliaF, R.M.; Montoya, P.; Sarmiento-Vásquez, Z.; Marin, O.; Gallego-Cartagena, E.; Paternina-Arboleda, C.D. Aloe vera: From ancient knowledge to the patent and innovation landscape—A review. S. Afr. J. Bot. 2022, 147, 993–1006. [Google Scholar] [CrossRef]
- Nema, J. Physicochemical study of acemannan polysaccharide in Aloe species under the influence of soil reaction (pH) and moisture application. Afr. J. Pure Appl. Chem. 2012, 6, 132–136. [Google Scholar] [CrossRef]








| Treatments * | Electrical Capacitance [nF] | Resistance [kΩ] | ||
|---|---|---|---|---|
| Average | SD | Average | SD | |
| F | 3.89 | 0.47 | 5.14 | 0.47 |
| PEF_125 | 4.14 | 0.63 | 4.77 | 0.63 |
| PEF_212.5 | 4.96 | 0.50 | 4.90 | 0.36 |
| PEF_300 | 5.27 | 0.32 | 2.97 | 0.50 |
| Code | VI | PEF Intensities [V/cm] | |
|---|---|---|---|
| Pre-Processing | Post-Processing | ||
| F | − | ||
| VI | + | ||
| PEF_125_VI | + | 125 | |
| PEF_212.5_VI | + | 212.5 | |
| PEF_300_VI | + | 300 | |
| VI_PEF_125 | + | 125 | |
| VI_PEF_212.5 | + | 212.5 | |
| VI_PEF_300 | + | 300 | |
| Sample | L* | a* | b* | ΔE | BI |
|---|---|---|---|---|---|
| F | 78.63 ± 2.22 c* | 0.92 ± 0.23 a | 23.16 ± 1.10 abc | 33.56 ± 2.16 a | |
| VI | 50.35 ± 6.55 ab | 0.77 ± 1.10 a | 20.99 ± 6.09 abc | 28.93 ± 6.61 abc | 46.02 ± 7.14 bc |
| PEF_125_VI | 45.36 ± 2.73 a | 1.34 ± 1.18 a | 18.49 ± 3.02 a | 33.64 ± 2.85 bc | 55.32 ± 9.69 cd |
| PEF_212.5_VI | 53.09 ± 2.18 b | 4.31 ± 2.24 b | 23.54 ± 1.69 bc | 25.80 ± 2.33 a | 59.25 ± 7.25 d |
| PEF_300_VI | 52.25 ± 2.53 b | 7.75 ± 2.03 c | 25.87 ± 0.87 c | 27.44 ± 2.90 ab | 79.60 ± 6.97 e |
| VI_PEF_125 | 44.43 ± 5.43 a | 1.06 ± 0.51 a | 19.13 ± 4.29 ab | 34.56 ± 5.65 c | 52.05 ± 6.05 cd |
| VI_PEF_212.5 | 47.62 ± 5.41 ab | 1.00 ± 1.72 a | 20.43 ± 5.00 ab | 31.40 ± 5.70 abc | 48.49 ± 9.19 bc |
| VI_PEF_300 | 49.30 ± 4.37 ab | 0.10 ± 1.01 a | 18.41 ± 3.20 a | 29.76 ± 4.73 abc | 41.54 ± 5.35 ab |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Trusinska, M.; Drudi, F.; Rybak, K.; Tylewicz, U.; Nowacka, M. Effect of the Pulsed Electric Field Treatment on Physical, Chemical and Structural Changes of Vacuum Impregnated Apple Tissue in Aloe Vera Juices. Foods 2023, 12, 3957. https://doi.org/10.3390/foods12213957
Trusinska M, Drudi F, Rybak K, Tylewicz U, Nowacka M. Effect of the Pulsed Electric Field Treatment on Physical, Chemical and Structural Changes of Vacuum Impregnated Apple Tissue in Aloe Vera Juices. Foods. 2023; 12(21):3957. https://doi.org/10.3390/foods12213957
Chicago/Turabian StyleTrusinska, Magdalena, Federico Drudi, Katarzyna Rybak, Urszula Tylewicz, and Malgorzata Nowacka. 2023. "Effect of the Pulsed Electric Field Treatment on Physical, Chemical and Structural Changes of Vacuum Impregnated Apple Tissue in Aloe Vera Juices" Foods 12, no. 21: 3957. https://doi.org/10.3390/foods12213957








