Hypotensive and Endothelium-Dependent Vasorelaxant Effects of Grayblue Spicebush Ethanol Extract in Rats
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Identification and Preparation of Plant Extract
2.2. Animals
2.3. Measurement of Blood Pressure in SHR
2.4. Chemicals and Solution Preparation
2.5. Measurement of Vascular Relaxation in Rat Aortic Rings
2.5.1. General Experimental Procedures
2.5.2. Vasorelaxant Effect of GSE on the Rat Aortic Rings
2.5.3. Vasorelaxant Effect of GSE on the Endothelium-Intact or -Denuded Rat Aortic Rings
2.5.4. Effects of NO Synthase Inhibitor or Cyclooxygenase (COX) Inhibitor Pretreatment on Vasorelaxation Effect of GSE
2.5.5. Effects of Soluble Guanylate Cyclase (sGC) Inhibitor or Cyclic Guanosine Monophosphate (cGMP) Inhibitor Pretreatment on Vasorelaxation of GSE
2.5.6. Effects of K+ Channel Blocker Pretreatment on Vasorelaxation of GSE
2.5.7. Inhibitory Effect of GSE on Aortic Rings Contracted with Extracellular Ca2+
2.5.8. Inhibitory Effect of GSE on Aortic Rings Contracted with Ang II
2.6. Statistical Analysis
3. Results
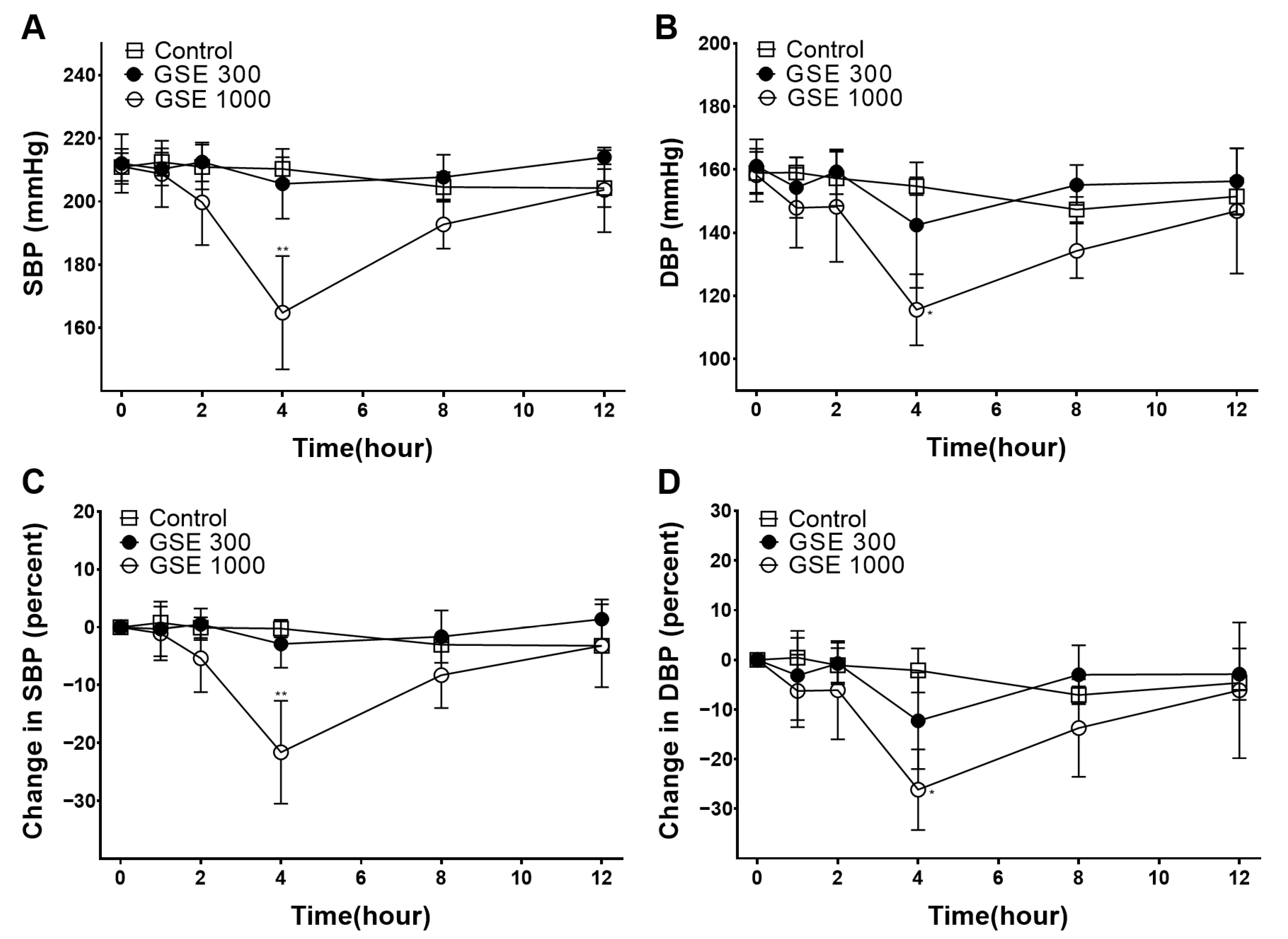
3.1. Hypotensive Effect of GSE
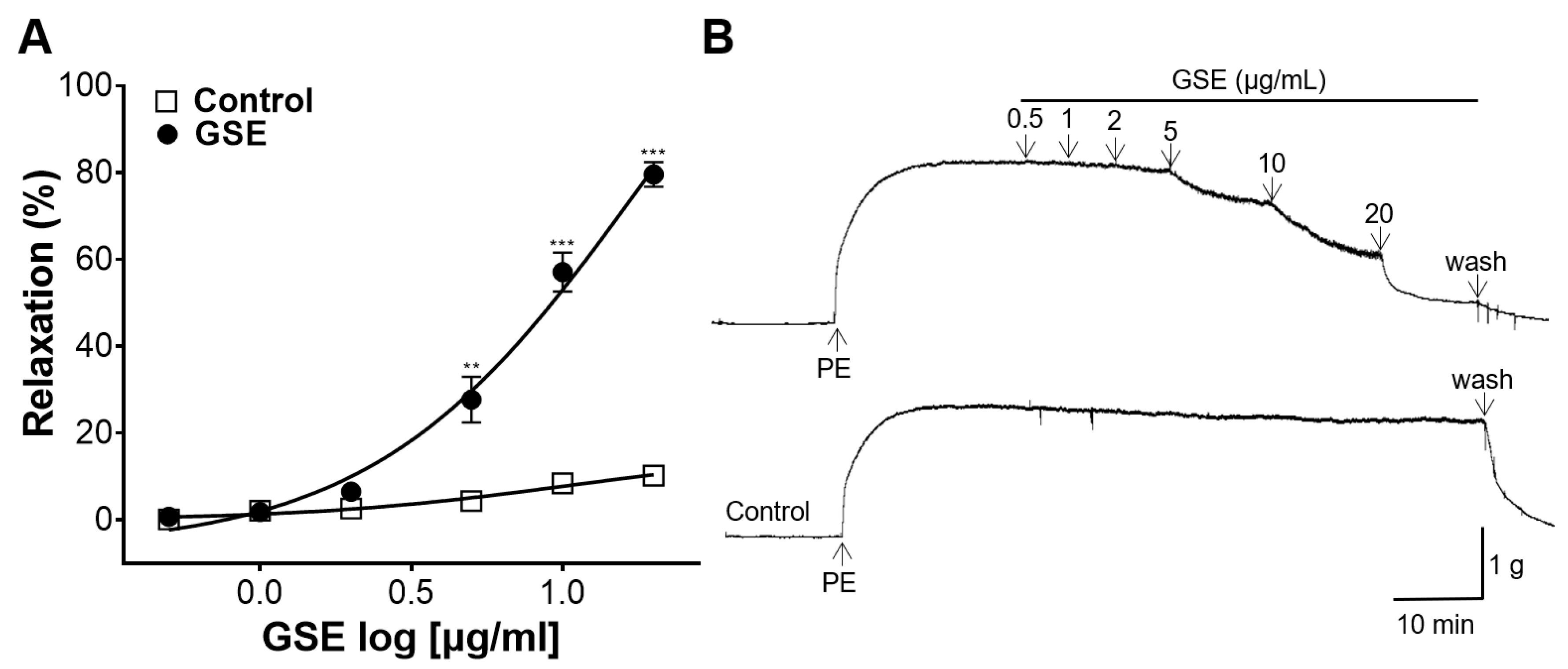
3.2. The Vasorelaxant Effect of GSE on the Rat Aortic Rings
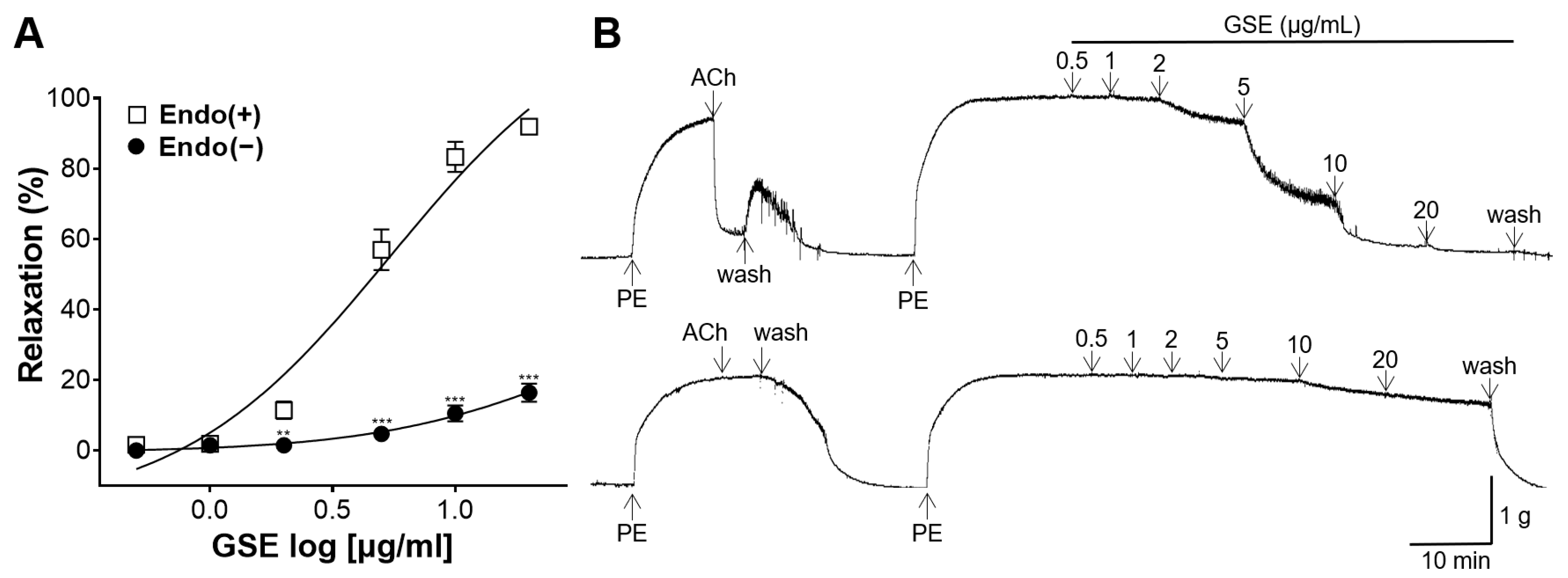
3.3. Vasorelaxant Effect of GSE on the Endothelium-Intact or -Denuded Rat Aortic Rings
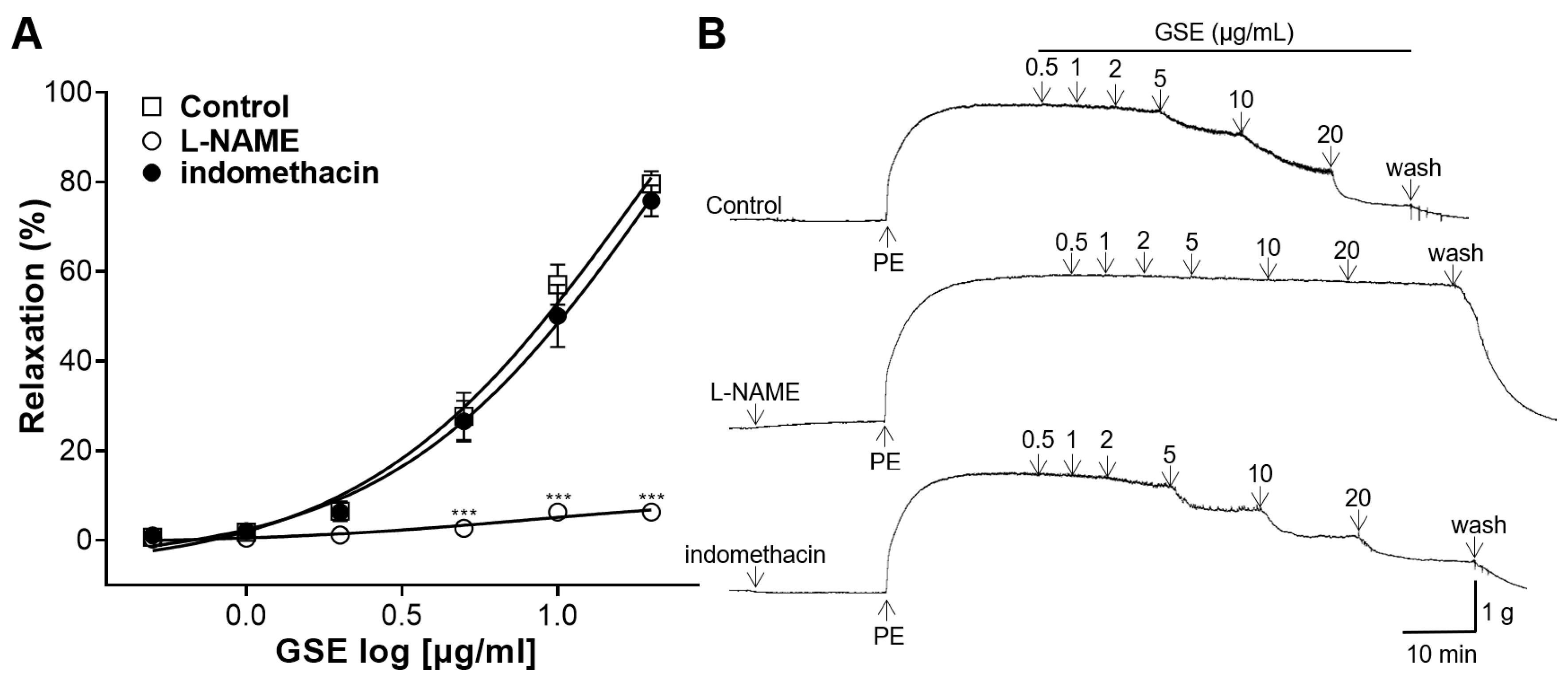
3.4. Effects of NO Synthase Inhibitor or COX Inhibitor Pretreatment on Vasorelaxation of GSE
3.5. Effects of the sGC Inhibitor or the cGMP Inhibitor Pretreatment on Vasorelaxation of GSE
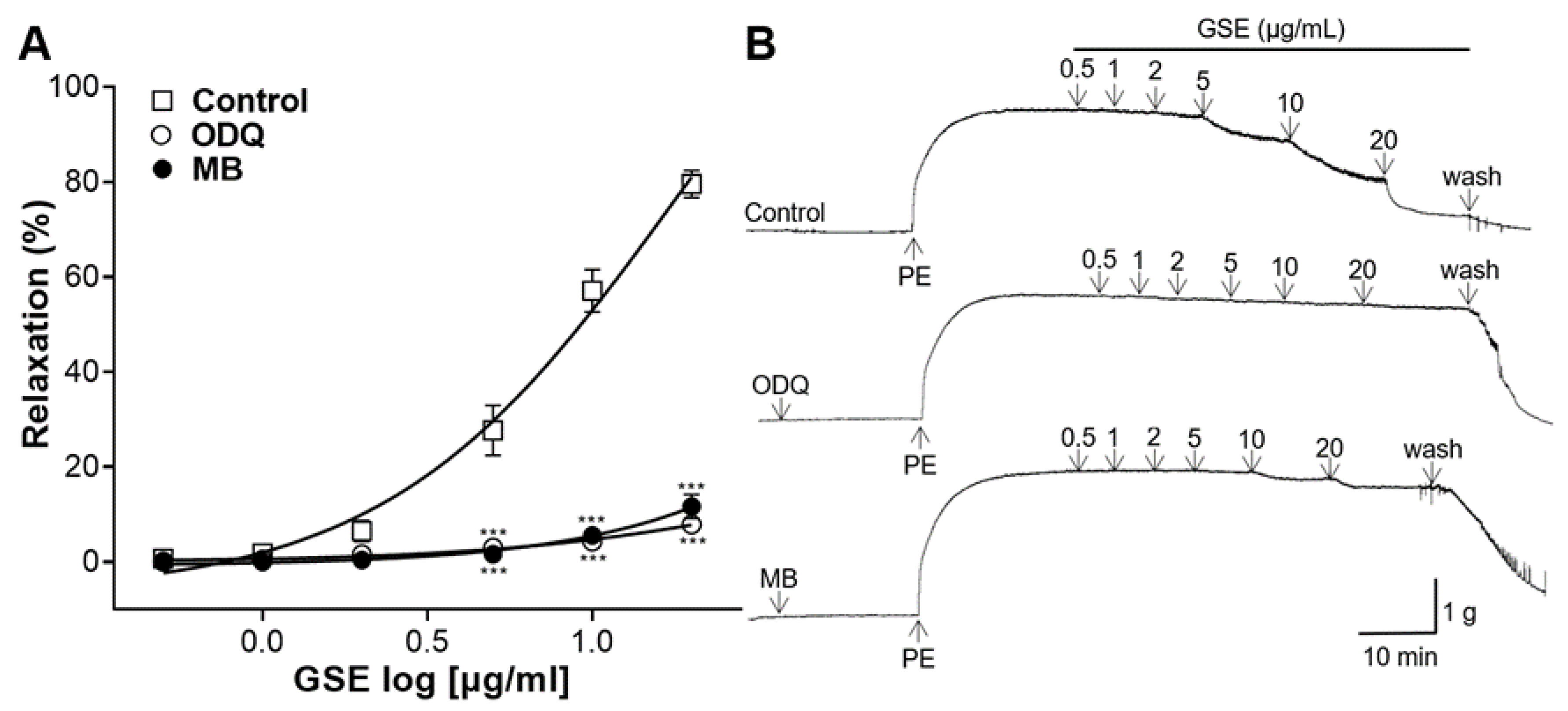
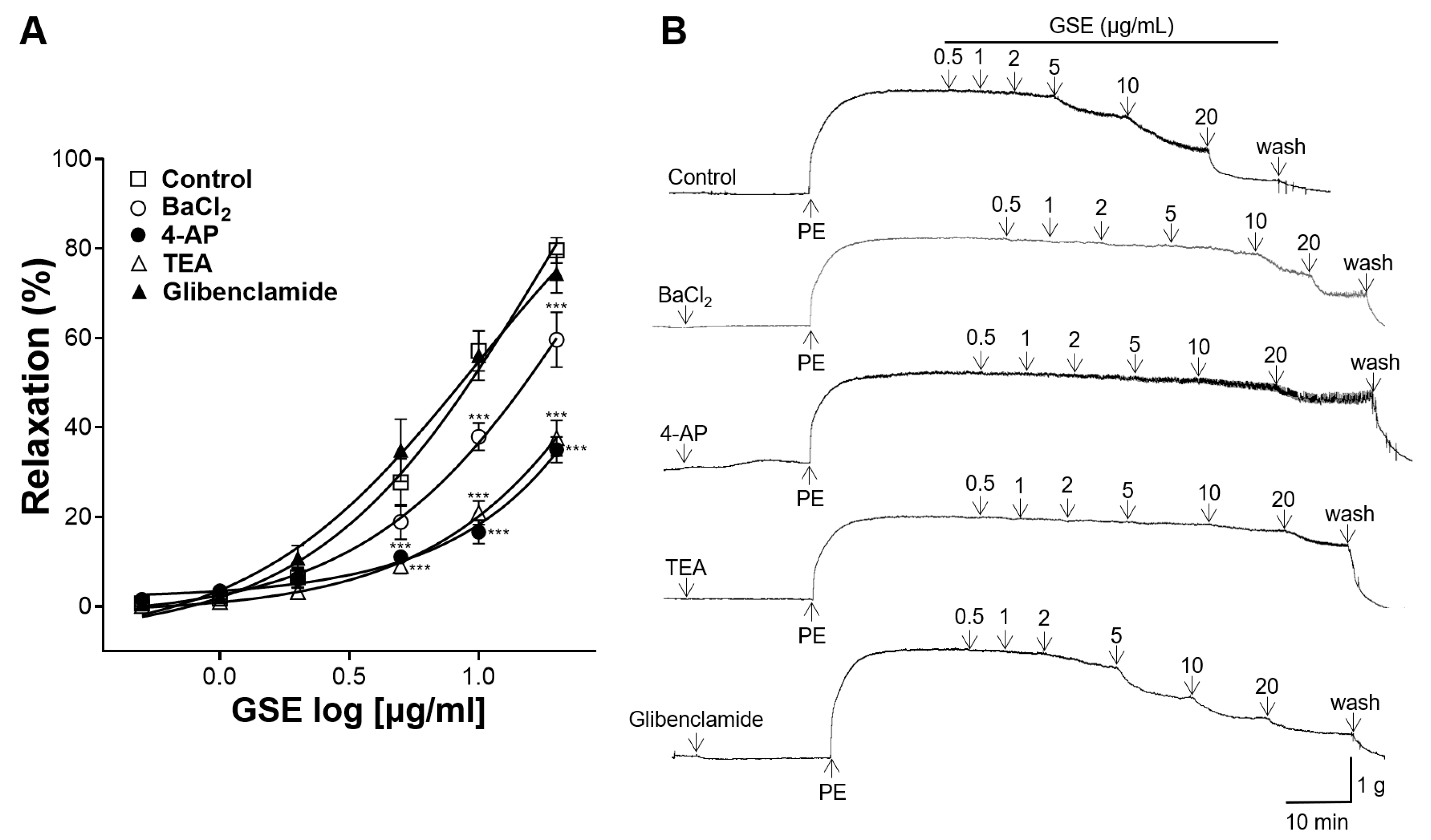
3.6. Effects of K+ Channel Blocker Pretreatment on Vasorelaxation of GSE
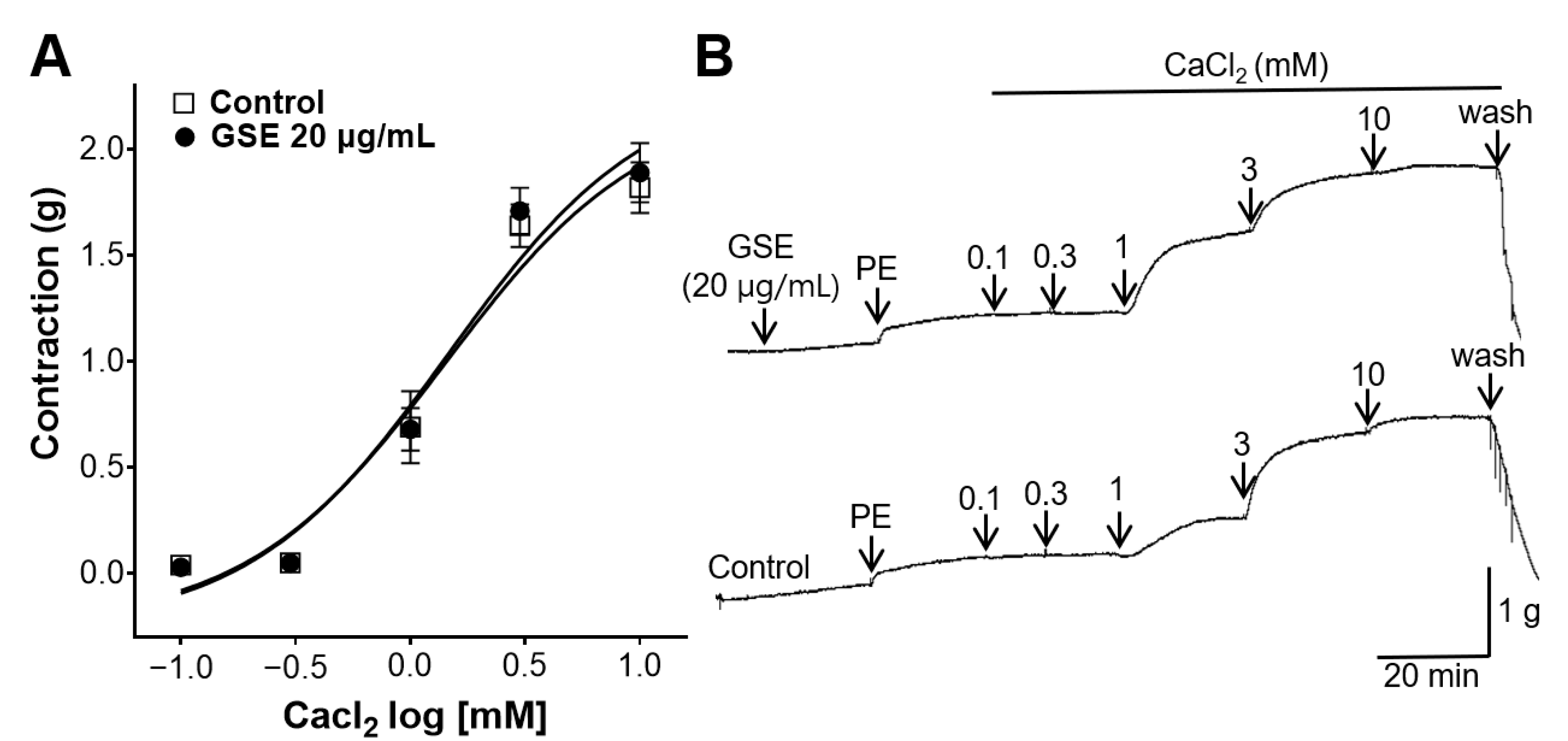
3.7. Inhibitory Effect of GSE on Rat Aortic Rings Constricted with Extracellular Ca2+
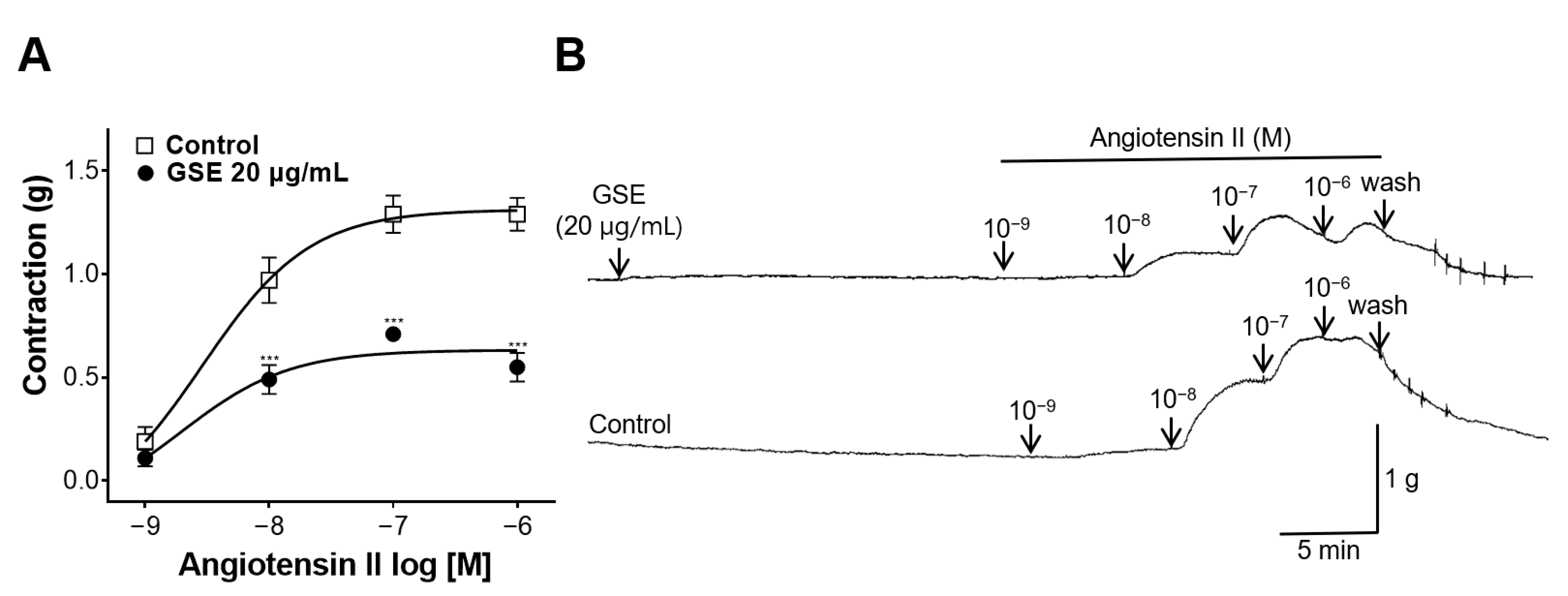
3.8. Inhibitory Effect of GSE on Rat Aortic Rings Constricted with Ang II
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Forouzanfar, M.H.; Liu, P.; Roth, G.A.; Ng, M.; Biryukov, S.; Marczak, L.; Alexander, L.; Estep, K.; Abate, K.H.; Akinyemiju, T.F.; et al. Global Burden of Hypertension and Systolic Blood Pressure of at Least 110 to 115 mm Hg, 1990–2015. JAMA 2017, 317, 165–182. [Google Scholar] [CrossRef] [PubMed]
- Brouwers, S.; Sudano, I.; Kokubo, Y.; Sulaica, E.M. Arterial hypertension. Lancet 2021, 398, 249–261. [Google Scholar] [CrossRef] [PubMed]
- Whelton, P.K.; Carey, R.M.; Aronow, W.S.; Casey, D.E., Jr.; Collins, K.J.; Himmelfarb, C.D.; DePalma, S.M.; Gidding, S.; Jamerson, K.A.; Jones, D.W.; et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA Guideline for the Prevention, Detection, Evaluation, and Management of High Blood Pressure in Adults: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J. Am. Coll. Cardiol. 2018, 71, 2275–2279. [Google Scholar]
- Abegaz, T.M.; Shehab, A.; Gebreyohannes, E.A.; Bhagavathula, A.S.; Elnour, A.A. Nonadherence to antihypertensive drugs: A systematic review and meta-analysis. Medicine 2017, 96, e5641. [Google Scholar] [CrossRef] [PubMed]
- Kretchy, I.A.; Owusu-Daaku, F.T.; Danquah, S.A.; Asampong, E. A psychosocial perspective of medication side effects, experiences, coping approaches and implications for adherence in hypertension management. Clin. Hypertens. 2015, 21, 19. [Google Scholar] [CrossRef] [PubMed]
- Kim, B.; Jo, C.; Choi, H.-Y.; Lee, K. Prunetin Relaxed Isolated Rat Aortic Rings by Blocking Calcium Channels. Molecules 2018, 23, 2372. [Google Scholar] [CrossRef] [PubMed]
- Recinella, L.; Libero, M.L.; Citi, V.; Chiavaroli, A.; Martelli, A.; Foligni, R.; Mannozzi, C.; Acquaviva, A.; Simone, S.D.; Calderone, V.; et al. Anti-Inflammatory and Vasorelaxant Effects Induced by an Aqueous Aged Black Garlic Extract Supplemented with Vitamins D, C, and B12 on Cardiovascular System. Foods 2023, 12, 1558. [Google Scholar] [CrossRef] [PubMed]
- On-Nom, N.; Khaengamkham, K.; Kettawan, A.; Rungruang, T.; Suttisansanee, U.; Temviriyanukul, P.; Prangthip, P.; Chupeerach, C. Parboiled Germinated Brown Rice Improves Cardiac Structure and Gene Expression in Hypertensive Rats. Foods 2023, 12, 9. [Google Scholar] [CrossRef]
- Dixit, V.; Kamal, S.W.J.; Chole, P.B.; Dayal, D.; Chaubey, K.K.; Pal, A.K.; Xavier, J.; Manjunath, B.T.; Bachheti, R.K. Functional Foods: Exploring the Health Benefits of Bioactive Compounds from Plant and Animal Sources. J. Food Qual. 2023, 2023, 5546753. [Google Scholar] [CrossRef]
- Iqbal, I.; Wilairatana, P.; Saqib, F.; Nasir, B.; Wahid, M.; Latif, M.F.; Iqbal, A.; Naz, R.; Mubarak, M.S. Plant Polyphenols and Their Potential Benefits on Cardiovascular Health: A Review. Molecules 2023, 28, 6403. [Google Scholar] [CrossRef]
- Ma, M.; Feng, Y.; Miao, Y.; Shen, Q.; Tang, S.; Dong, J.; Zhang, J.Z.H.; Zhang, L. Revealing the Sequence Characteristics and Molecular Mechanisms of ACE Inhibitory Peptides by Comprehensive Characterization of 160,000 Tetrapeptides. Foods 2023, 12, 1573. [Google Scholar] [CrossRef] [PubMed]
- Jeong, E.-W.; Park, S.-Y.; Yang, Y.-S.; Baek, Y.-J.; Yun, D.-M.; Kim, H.-J.; Go, G.-W.; Lee, H.-G. Black Soybean and Adzuki Bean Extracts Lower Blood Pressure by Modulating the Renin-Angiotensin System in Spontaneously Hypertensive Rats. Foods 2021, 10, 1571. [Google Scholar] [CrossRef]
- Chupeerach, C.; Aursalung, A.; Watcharachaisoponsiri, T.; Whanmek, K.; Thiyajai, P.; Yosphan, K.; Sritalahareuthai, V.; Sahasakul, Y.; Santivarangkna, C.; Suttisansanee, U. The Effect of Steaming and Fermentation on Nutritive Values, Antioxidant Activities, and Inhibitory Properties of Tea Leaves. Foods 2021, 10, 117. [Google Scholar] [CrossRef] [PubMed]
- Marino, M.; Bo’, C.D.; Martini, D.; Porrini, M.; Riso, P. A Review of Registered Clinical Trials on Dietary (Poly)Phenols: Past Efforts and Possible Future Directions. Foods 2020, 9, 1606. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.; Xuan, B.; Peng, B.; Li, C.; Chai, X.; Tu, P. The genus Lindera: A source of structurally diverse molecules having pharmacological significance. Phytochem. Rev. 2015, 15, 869–906. [Google Scholar] [CrossRef]
- Tucker, A.O.; Maciarello, M.J.; Burbage, P.W.; Sturtz, G. Spicebush [Lindera benzoin (L.) Blume var. benzoin, Lauraceae]: A tea, spice, and medicine. Econ. Bot. 1994, 48, 333–336. [Google Scholar]
- Yu, J.S.; Baek, J.; Park, H.B.; Moon, E.; Kim, S.Y.; Choi, S.U.; Kim, K.H. A new rearranged eudesmane sesquiterpene and bioactive sesquiterpenes from the twigs of Lindera glauca (Sieb. et Zucc.) Blume. Arch. Pharm. Res. 2016, 39, 1628–1634. [Google Scholar] [CrossRef]
- Chen, F.; Miao, X.; Lin, Z.; Xiu, Y.; Shi, L.; Zhang, Q.; Liang, D.; Lin, S.; He, B. Disruption of metabolic function and redox homeostasis as antibacterial mechanism of Lindera glauca fruit essential oil against Shigella flexneri. Food Control 2021, 130, 108282. [Google Scholar] [CrossRef]
- Kim, Y.-S.; Kim, E.-K.; Dong, X.; Park, J.-S.; Shin, W.-B.; Kim, S.-J.; Go, E.-A.; Park, P.-J.; Lim, B.-O. Lindera glauca (Siebold et Zucc.) Blume Stem Extracts Protect Against tert-Butyl Hydroperoxide-Induced Oxidative Stress. J. Med. Food 2019, 22, 508–520. [Google Scholar] [CrossRef]
- Kim, Y.-U.; Yun, J.-M. Antioxidative and antiproliferative effects of Lindera glauca Blume on human colorectal cancer cells. J. Korean Soc. Food Sci. Nutr. 2015, 44, 635–640. [Google Scholar] [CrossRef]
- Kim, Y.; Cho, S.-H. Lindera glauca Blume ameliorates amyloid-β1-42-induced memory impairment in mice with neuroprotection and activation of the CREB-BDNF pathway. Neurochem. Int. 2021, 147, 105071. [Google Scholar] [CrossRef] [PubMed]
- Doggrell, S.A.; Brown, L. Rat models of hypertension, cardiac hypertrophy and failure. Cardiovasc. Res. 1998, 39, 89–105. [Google Scholar] [CrossRef] [PubMed]
- Jung, J.; Shin, S.; Park, J.; Lee, K.; Choi, H.-Y. Hypotensive and Vasorelaxant Effects of Sanguisorbae Radix Ethanol Extract in Spontaneously Hypertensive and Sprague Dawley Rats. Nutrients 2023, 15, 4510. [Google Scholar] [CrossRef] [PubMed]
- Nair, A.B.; Jacob, S. A simple practice guide for dose conversion between animals and human. J. Basic Clin. Pharm. 2016, 7, 27–31. [Google Scholar] [CrossRef] [PubMed]
- Zhonghua Bencao Edit Committee. Zhonghua Bencao; Shanghai Science and Technology Publications: Shanghai, China, 1999. [Google Scholar]
- Kim, J.S.; Kang, B.H.; Park, S.J.; Yang, W.I.; Kim, M.S.; Lee, B.S.; Cha, D.S.; Lee, S.Y.; Kwon, J.; Jeon, H. Anti-inflammatory and Anti-nociceptive Effects of Ethyl Acetate Fraction of Lindera glauca. Korean J. Pharmacogn. 2022, 53, 49–56. [Google Scholar]
- Zelis, R. Mechanisms of vasodilation. Am. J. Med. 1983, 74, 3–12. [Google Scholar] [CrossRef] [PubMed]
- Brozovich, F.V.; Nicholson, C.J.; Degen, C.V.; Gao, Y.Z.; Aggarwal, M.; Morgan, K.G. Mechanisms of Vascular Smooth Muscle Contraction and the Basis for Pharmacologic Treatment of Smooth Muscle Disorders. Pharmacol. Rev. 2016, 68, 476–532. [Google Scholar] [CrossRef]
- Sarmah, N.; Nauli, A.M.; Ally, A.; Nauli, S.M. Interactions among Endothelial Nitric Oxide Synthase, Cardiovascular System, and Nociception during Physiological and Pathophysiological States. Molecules 2022, 27, 2835. [Google Scholar] [CrossRef]
- Stankevicius, E.; Kevelaitis, E.; Vainorius, E.; Simonsen, U. Role of nitric oxide and other endothelium-derived factors. Medicina 2003, 39, 333–341. [Google Scholar]
- Sharma, V.; Berkelhamer, S.; Lakshminrusimha, S. Persistent pulmonary hypertension of the newborn. Matern. Health Neonatol. Perinatol. 2015, 1, 14. [Google Scholar] [CrossRef]
- Grześk, G.; Nowaczyk, A. Current Modulation of Guanylate Cyclase Pathway Activity-Mechanism and Clinical Implications. Molecules 2021, 26, 3418. [Google Scholar] [CrossRef] [PubMed]
- Michelakis, E.D.; Reeve, H.L.; Huang, J.M.; Tolarova, S.; Nelson, D.P.; Weir, E.K.; Archer, S.L. Potassium channel diversity in vascular smooth muscle cells. Can. J. Physiol. Pharmacol. 1997, 75, 889–897. [Google Scholar] [CrossRef] [PubMed]
- Sobey, C.G. Potassium channel function in vascular disease. Arterioscler. Thromb. Vasc. Biol. 2001, 21, 28–38. [Google Scholar] [CrossRef] [PubMed]
- Touyz, R.M.; Alves-Lopes, R.; Rios, F.J.; Camargo, L.L.; Anagnostopoulou, A.; Arner, A.; Montezano, A.C. Vascular smooth muscle contraction in hypertension. Cardiovasc. Res. 2018, 114, 529–539. [Google Scholar] [CrossRef] [PubMed]
- Mehta, P.K.; Griendling, K.K. Angiotensin II cell signaling: Physiological and pathological effects in the cardiovascular system. Am. J. Physiol. Cell Physiol. 2007, 292, C82–C97. [Google Scholar] [CrossRef] [PubMed]
- Cat, A.N.D.; Touyz, R.M. Cell signaling of angiotensin II on vascular tone: Novel mechanisms. Curr. Hypertens. Rep. 2011, 13, 122–128. [Google Scholar]
- Câmara, J.S.; Albuquerque, B.R.; Aguiar, J.; Corrêa, R.C.G.; Gonçalves, J.L.; Granato, D.; Pereira, J.A.M.; Barros, L.; Ferreira, I.C.F.R. Food Bioactive Compounds and Emerging Techniques for Their Extraction: Polyphenols as a Case Study. Foods 2021, 10, 37. [Google Scholar] [CrossRef]
- Fredes, C.; Parada, A.; Salinas, J.; Robert, P. Phytochemicals and Traditional Use of Two Southernmost Chilean Berry Fruits: Murta (Ugni molinae Turcz) and Calafate (Berberis buxifolia Lam.). Foods 2020, 9, 54. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shin, S.; Park, J.; Choi, H.-Y.; Lee, K. Hypotensive and Endothelium-Dependent Vasorelaxant Effects of Grayblue Spicebush Ethanol Extract in Rats. Foods 2023, 12, 4282. https://doi.org/10.3390/foods12234282
Shin S, Park J, Choi H-Y, Lee K. Hypotensive and Endothelium-Dependent Vasorelaxant Effects of Grayblue Spicebush Ethanol Extract in Rats. Foods. 2023; 12(23):4282. https://doi.org/10.3390/foods12234282
Chicago/Turabian StyleShin, Sujin, Junkyu Park, Ho-Young Choi, and Kyungjin Lee. 2023. "Hypotensive and Endothelium-Dependent Vasorelaxant Effects of Grayblue Spicebush Ethanol Extract in Rats" Foods 12, no. 23: 4282. https://doi.org/10.3390/foods12234282





