Chaenomeles sinensis Extract Ameliorates Ovalbumin-Induced Allergic Rhinitis by Inhibiting the IL-33/ST2 Axis and Regulating Epithelial Cell Dysfunction
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of CSE
2.2. Analysis of CSE Using UPLC-Q-TOF-MS
2.3. Animal Studies
2.4. Group Size, Randomization, and Blinding
2.5. Experimental Design
2.6. Collection of Serum and Nasal Lavage Fluid (NLF)
2.7. Histological Analysis
2.8. Immunohistochemistry
2.9. Determination of Cytokines and Immunoglobulins
2.10. Immunoblotting
2.11. Statistical Analysis
3. Results
3.1. Active Components of CSE
3.2. CSE Inhibits Nasal Symptoms of AR and Suppresses Immunoglobulin Levels in AR Mice
3.3. CSE Reduces Infiltration of Inflammatory Cells in the NLF of AR Mice
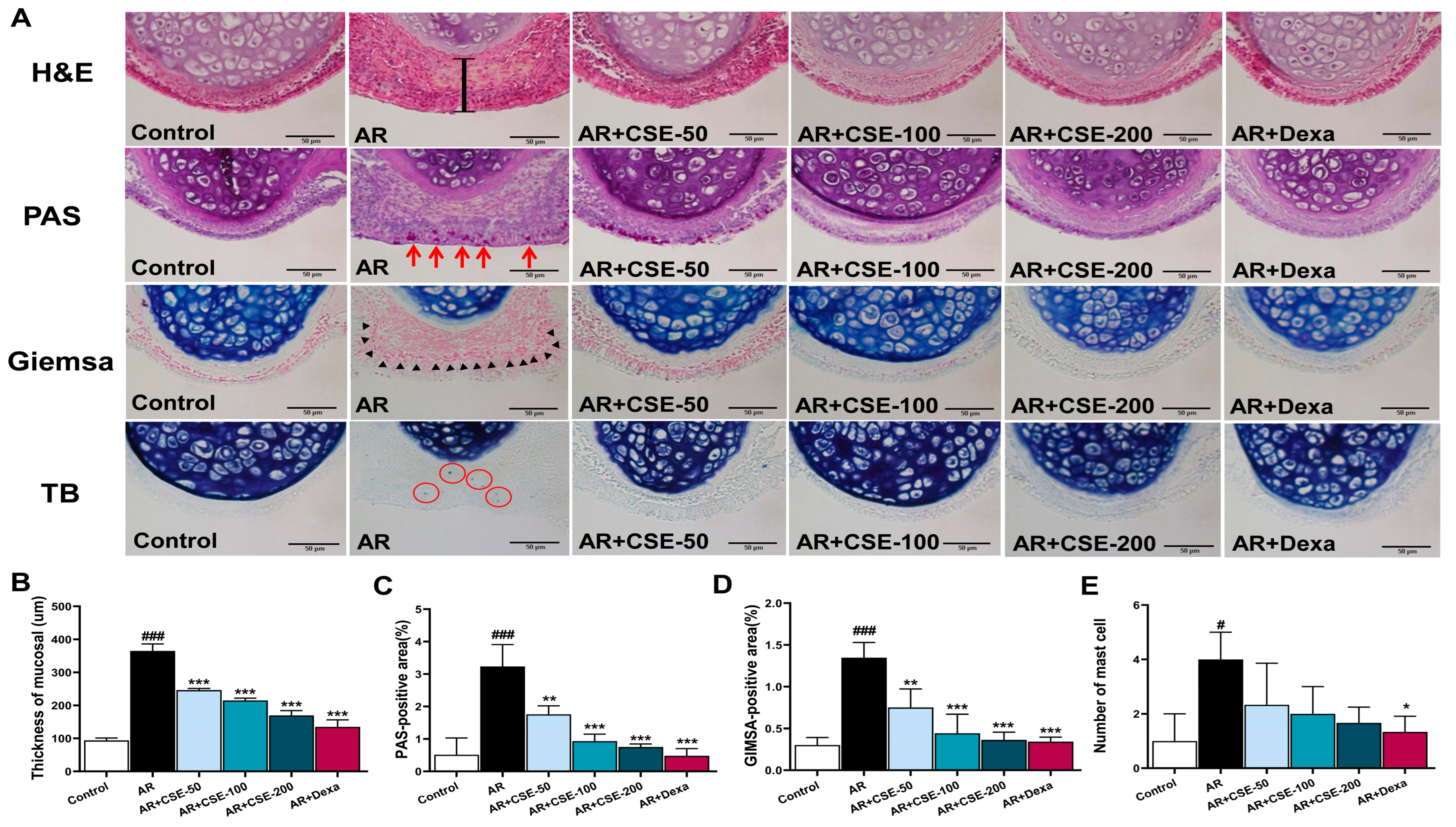
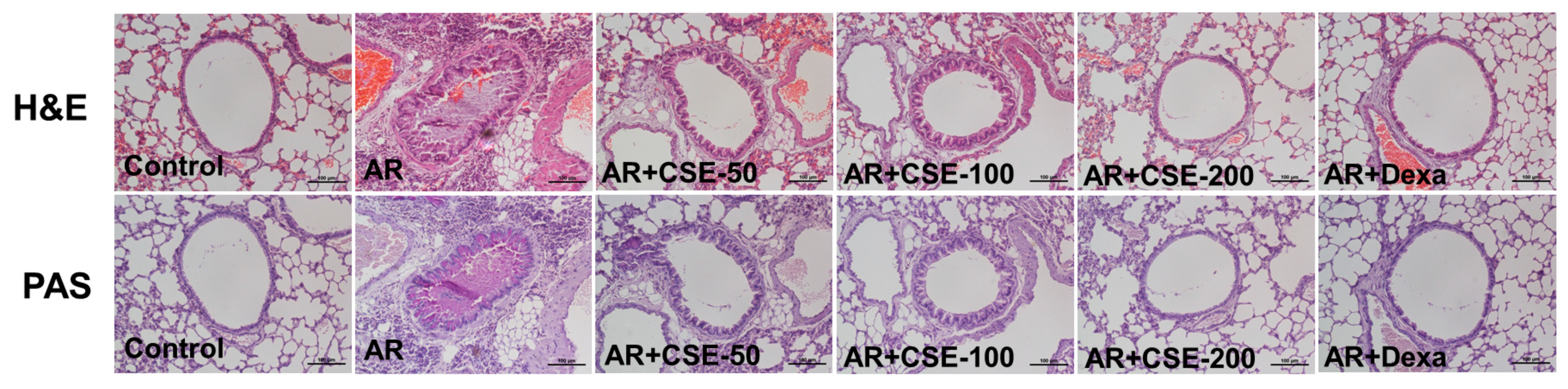
3.4. CSE Prevents Pathological Alterations in Nasal Mucosa and Lung Tissues of AR Mice
3.5. Influence of CSE on Inflammation in Lung Tissues of OVA-Induced AR Mice
3.6. CSE Inhibits IL-33 and ST2 in OVA-Induced AR Mice
3.7. CSE Modulates E-Cadherin, Occludin, and Zonula Occludens (ZO)-1 Expression in AR Mice
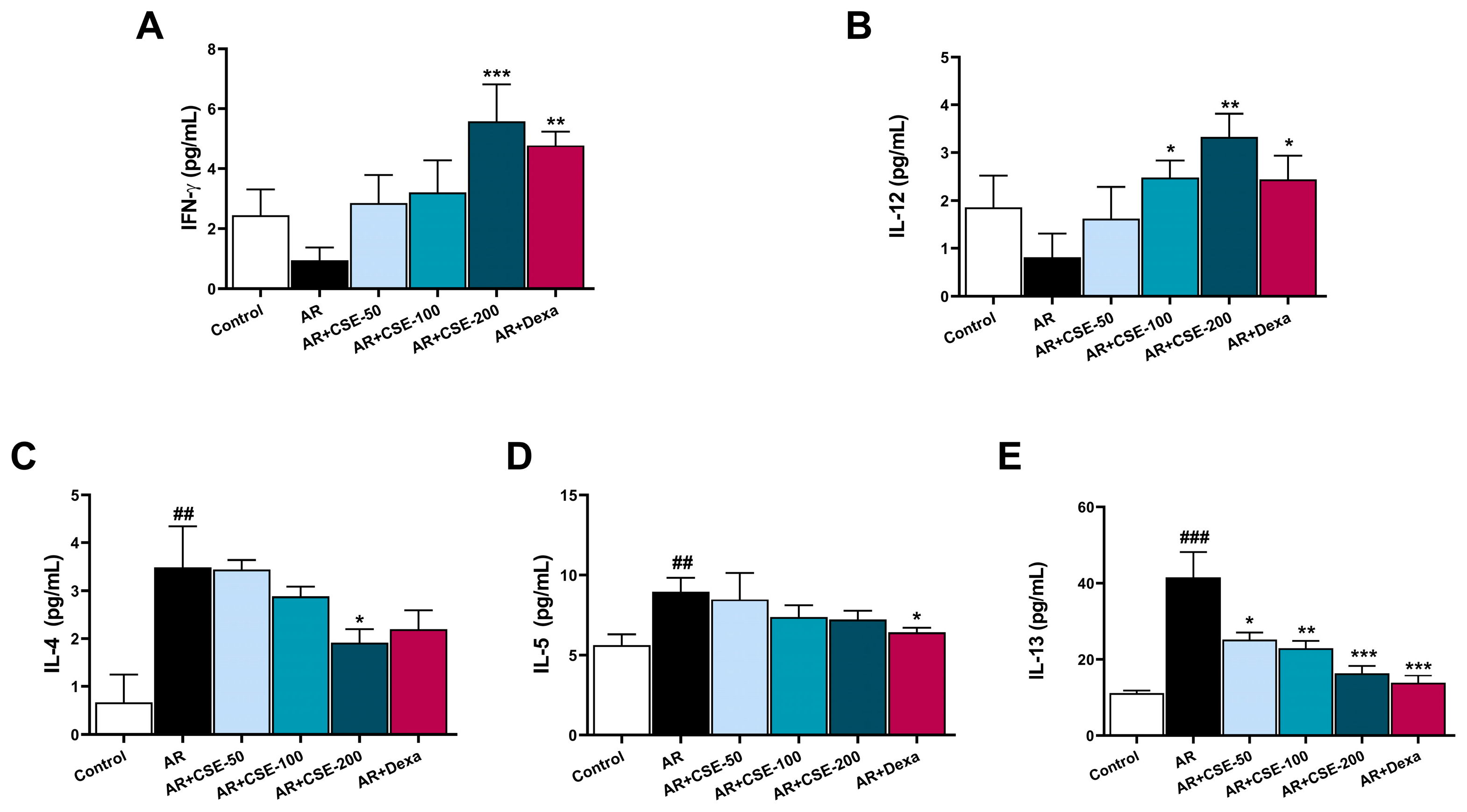
3.8. CSE Regulates T Helper 2 (Th2) and Th2 Associated Cytokine Production in NLF of AR Mice
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sacre Hazouri, J.A. Allergic rhinitis. Coexistent diseases and complications. A review and analysis. Rev. Alerg. Mex. 2006, 53, 9–29. [Google Scholar] [PubMed]
- Bousquet, J.; Khaltaev, N.; Cruz, A.A.; Denburg, J.; Fokkens, W.J.; Togias, A.; Zuberbier, T.; Baena-Cagnani, C.E.; Canonica, G.W.; van Weel, C.; et al. Allergic Rhinitis and its Impact on Asthma (ARIA) 2008 update (in collaboration with the World Health Organization, GA(2)LEN and AllerGen). Allergy 2008, 63 (Suppl. S86), 8–160. [Google Scholar] [CrossRef]
- Brozek, J.L.; Bousquet, J.; Agache, I.; Agarwal, A.; Bachert, C.; Bosnic-Anticevich, S.; Brignardello-Petersen, R.; Canonica, G.W.; Casale, T.; Chavannes, N.H.; et al. Allergic Rhinitis and its Impact on Asthma (ARIA) guidelines-2016 revision. J. Allergy Clin. Immunol. 2017, 140, 950–958. [Google Scholar] [CrossRef]
- Pawankar, R. Allergic diseases and asthma: A global public health concern and a call to action. World Allergy Organ. J. 2014, 7, 12. [Google Scholar] [CrossRef]
- Wheatley, L.M.; Togias, A. Clinical practice. Allergic rhinitis. N. Engl. J. Med. 2015, 372, 456–463. [Google Scholar] [CrossRef]
- Sin, B.; Togias, A. Pathophysiology of Allergic and Nonallergic Rhinitis. Proc. Am. Thorac. Soc. 2011, 8, 106–114. [Google Scholar] [CrossRef]
- Nakonechna, A.; Umanetz, T.; Lapshin, V.; van Bergen, A.R.D. The role of Th1 and Th2 response in allergic rhinitis children with or without sensitization to food allergens. J. Allergy Clin. Immunol. 2007, 119, S43. [Google Scholar] [CrossRef]
- Eccles, R. Substitution of phenylephrine for pseudoephedrine as a nasal decongeststant. An illogical way to control methamphetamine abuse. Br. J. Clin. Pharmacol. 2007, 63, 10–14. [Google Scholar] [CrossRef] [PubMed]
- Meltzer, E.O.; Bukstein, D.A. The economic impact of allergic rhinitis and current guidelines for treatment. Ann. Allergy Asthma Immunol. 2011, 106, S12–S16. [Google Scholar] [CrossRef]
- Small, P.; Keith, P.K.; Kim, H. Allergic rhinitis. Allergy Asthma Clin. Immunol. 2018, 14, 51. [Google Scholar] [CrossRef]
- Iwamura, C.; Shinoda, K.; Yoshimura, M.; Watanabe, Y.; Obata, A.; Nakayama, T. Naringenin chalcone suppresses allergic asthma by inhibiting the type-2 function of CD4 T cells. Allergol. Int. 2010, 59, 67–73. [Google Scholar] [CrossRef] [PubMed]
- Jung, C.H.; Lee, J.Y.; Cho, C.H.; Kim, C.J. Anti-asthmatic action of quercetin and rutin in conscious guinea-pigs challenged with aerosolized ovalbumin. Arch. Pharm. Res. 2007, 30, 1599–1607. [Google Scholar] [CrossRef]
- Chojnacka, K.; Lewandowska, U. Inhibition of Pro-Inflammatory Cytokine Secretion by Polyphenol-Rich Extracts in Macrophages via NF-κB Pathway. Food Rev. Int. 2023, 39, 5459–5478. [Google Scholar] [CrossRef]
- Du, L.; Li, J.; Zhang, X.; Wang, L.; Zhang, W.; Yang, M.; Hou, C. Pomegranate peel polyphenols inhibits inflammation in LPS-induced RAW264.7 macrophages via the suppression of TLR4/NF-kappaB pathway activation. Food Nutr. Res. 2019, 63, 3392. [Google Scholar] [CrossRef]
- Vugic, L.; Colson, N.; Nikbakht, E.; Gaiz, A.; Holland, O.J.; Kundur, A.R.; Singh, I. Anthocyanin supplementation inhibits secretion of pro-inflammatory cytokines in overweight and obese individuals. J. Funct. Foods 2020, 64, 103596. [Google Scholar] [CrossRef]
- Yahfoufi, N.; Alsadi, N.; Jambi, M.; Matar, C. The Immunomodulatory and Anti-Inflammatory Role of Polyphenols. Nutrients 2018, 10, 1618. [Google Scholar] [CrossRef]
- Dharmananda, S. CHAENOMELES—A Relaxing and Strengthening Fruit; Institute for Traditional Medicine: Portland, OR, USA, 2005. [Google Scholar]
- Chen, J.-C.; Chang, Y.-S.; Wu, S.-L.; Chao, D.-C.; Chang, C.-S.; Li, C.-C.; Ho, T.-Y.; Hsiang, C.-Y. Inhibition of Escherichia coli heat-labile enterotoxin-induced diarrhea by Chaenomeles speciosa. J. Ethnopharmacol. 2007, 113, 233–239. [Google Scholar] [CrossRef]
- Hamauzu, Y.; Yasui, H.; Inno, T.; Kume, C.; Omanyuda, M. Phenolic profile, antioxidant property, and anti-influenza viral activity of Chinese quince (Pseudocydonia sinensis Schneid.), quince (Cydonia oblonga Mill.), and apple (Malus domestica Mill.) fruits. J. Agric. Food Chem. 2005, 53, 928–934. [Google Scholar] [CrossRef]
- Osawa, K.; Yasuda, H.; Morita, H.; Takeya, K.; Itokawa, H. Antibacterial and Antihemolytic Activity of Triterpenes and -Sitosterol Isolated from Chinese Quince (Chaenomeles sinensis). Nat. Med. 1997, 51, 365–367. [Google Scholar]
- Zhang, R.; Zhan, S.; Li, S.; Zhu, Z.; He, J.; Lorenzo, J.M.; Barba, F.J. Anti-hyperuricemic and nephroprotective effects of extracts from Chaenomeles sinensis (Thouin) Koehne in hyperuricemic mice. Food Funct. 2018, 9, 5778–5790. [Google Scholar] [CrossRef]
- Han, Y.-K.; Kim, Y.-S.; Natarajan, S.B.; Kim, W.-S.; Hwang, J.-W.; Jeon, N.-J.; Jeong, J.-H.; Moon, S.-H.; Jeon, B.-T.; Park, P.-J.J.M. Antioxidant and anti-inflammatory effects of Chaenomeles sinensis leaf extracts on LPS-stimulated RAW 264.7 cells. Molecules 2016, 21, 422. [Google Scholar] [CrossRef] [PubMed]
- Yang, E.J.; Lee, S.H. Anti-Inflammatory Effects of Chaenomeles sinensis Extract in an ALS Animal Model. Front. Biosci.-Landmark 2023, 28, 326. [Google Scholar] [CrossRef] [PubMed]
- Xu, R.; Kuang, M.; Li, N. Phytochemistry and pharmacology of plants in the genus Chaenomeles. Arch. Pharmacal Res. 2023, 46, 825–854. [Google Scholar] [CrossRef] [PubMed]
- Escobar, A.; Pérez, M.; Romanelli, G.; Blustein, G. Thymol bioactivity: A review focusing on practical applications. Arab. J. Chem. 2020, 13, 9243–9269. [Google Scholar] [CrossRef]
- Kim, T.; Song, B.; Cho, K.S.; Lee, I.S. Therapeutic Potential of Volatile Terpenes and Terpenoids from Forests for Inflammatory Diseases. Int. J. Mol. Sci. 2020, 21, 2187. [Google Scholar] [CrossRef] [PubMed]
- de las Heras, B.; Hortelano, S. Molecular basis of the anti-inflammatory effects of terpenoids. Inflamm. Allergy Drug Targets 2009, 8, 28–39. [Google Scholar] [CrossRef]
- Del Prado-Audelo, M.L.; Cortes, H.; Caballero-Floran, I.H.; Gonzalez-Torres, M.; Escutia-Guadarrama, L.; Bernal-Chavez, S.A.; Giraldo-Gomez, D.M.; Magana, J.J.; Leyva-Gomez, G. Therapeutic Applications of Terpenes on Inflammatory Diseases. Front. Pharmacol. 2021, 12, 704197. [Google Scholar] [CrossRef]
- Ge, J.; Liu, Z.; Zhong, Z.; Wang, L.; Zhuo, X.; Li, J.; Jiang, X.; Ye, X.-Y.; Xie, T.; Bai, R. Natural terpenoids with anti-inflammatory activities: Potential leads for anti-inflammatory drug discovery. Bioorg. Chem. 2022, 124, 105817. [Google Scholar] [CrossRef]
- Bocsan, I.C.; Magureanu, D.C.; Pop, R.M.; Levai, A.M.; Macovei, S.O.; Patrasca, I.M.; Chedea, V.S.; Buzoianu, A.D. Antioxidant and Anti-Inflammatory Actions of Polyphenols from Red and White Grape Pomace in Ischemic Heart Diseases. Biomedicines 2022, 10, 2337. [Google Scholar] [CrossRef]
- Kruk, J.; Aboul-Enein, B.H.; Duchnik, E.; Marchlewicz, M. Antioxidative properties of phenolic compounds and their effect on oxidative stress induced by severe physical exercise. J. Physiol. Sci. 2022, 72, 19. [Google Scholar] [CrossRef]
- Ruiz-Ruiz, J.C.; Matus-Basto, A.J.; Acereto-Escoffié, P.; Segura-Campos, M.R. Antioxidant and anti-inflammatory activities of phenolic compounds isolated from Melipona beecheii honey. Food Agric. Immunol. 2017, 28, 1424–1437. [Google Scholar] [CrossRef]
- Kelm, M.A.; Nair, M.G.; Strasburg, G.M.; DeWitt, D.L. Antioxidant and cyclooxygenase inhibitory phenolic compounds from Ocimum sanctum Linn. Phytomedicine 2000, 7, 7–13. [Google Scholar] [CrossRef] [PubMed]
- Rahman, M.M.; Rahaman, M.S.; Islam, M.R.; Rahman, F.; Mithi, F.M.; Alqahtani, T.; Almikhlafi, M.A.; Alghamdi, S.Q.; Alruwaili, A.S.; Hossain, M.S.; et al. Role of Phenolic Compounds in Human Disease: Current Knowledge and Future Prospects. Molecules 2022, 27, 233. [Google Scholar] [CrossRef] [PubMed]
- Sawai-Kuroda, R.; Kikuchi, S.; Shimizu, Y.K.; Sasaki, Y.; Kuroda, K.; Tanaka, T.; Yamamoto, T.; Sakurai, K.; Shimizu, K. A polyphenol-rich extract from Chaenomeles sinensis (Chinese quince) inhibits influenza A virus infection by preventing primary transcription in vitro. J. Ethnopharmacol. 2013, 146, 866–872. [Google Scholar] [CrossRef] [PubMed]
- Nagle, D.G.; Ferreira, D.; Zhou, Y.D. Epigallocatechin-3-gallate (EGCG): Chemical and biomedical perspectives. Phytochemistry 2006, 67, 1849–1855. [Google Scholar] [CrossRef] [PubMed]
- Bui, T.T.; Piao, C.H.; Hyeon, E.; Fan, Y.; Van Nguyen, T.; Jung, S.Y.; Choi, D.W.; Lee, S.-y.; Shin, H.S.; Song, C.H.; et al. The protective role of Piper nigrum fruit extract in an ovalbumin-induced allergic rhinitis by targeting of NFκBp65 and STAT3 signalings. Biomed. Pharmacother. 2019, 109, 1915–1923. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Shao, L.; Li, Y.; Dai, M.; Liu, H.; Xiang, N.; Chen, H. Tanshinone IIA alleviates ovalbumin-induced allergic rhinitis symptoms by inhibiting Th2 cytokine production and mast cell histamine release in mice. Pharm. Biol. 2022, 60, 326–333. [Google Scholar] [CrossRef]
- Piao, C.H.; Fan, Y.J.; Nguyen, T.V.; Song, C.H.; Chai, O.H. Mangiferin Alleviates Ovalbumin-Induced Allergic Rhinitis via Nrf2/HO-1/NF-κB Signaling Pathways. Int. J. Mol. Sci. 2020, 21, 3415. [Google Scholar] [CrossRef]
- Jin, J.; Fan, Y.J.; Nguyen, T.V.; Yu, Z.N.; Song, C.H.; Lee, S.-Y.; Shin, H.S.; Chai, O.H. Fallopia japonica Root Extract Ameliorates Ovalbumin-Induced Airway Inflammation in a CARAS Mouse Model by Modulating the IL-33/TSLP/NF-κB Signaling Pathway. Int. J. Mol. Sci. 2023, 24, 12514. [Google Scholar]
- Nguyen, T.V.; Vo, C.T.; Vo, V.M.; Nguyen, C.T.T.; Pham, T.M.; Piao, C.H.; Fan, Y.J.; Chai, O.H.; Bui, T.T. Phaeanthus vietnamensis Ban Ameliorates Lower Airway Inflammation in Experimental Asthmatic Mouse Model via Nrf2/HO-1 and MAPK Signaling Pathway. Antioxidants 2023, 12, 1301. [Google Scholar] [CrossRef]
- Curtis, M.J.; Bond, R.A.; Spina, D.; Ahluwalia, A.; Alexander, S.P.; Giembycz, M.A.; Gilchrist, A.; Hoyer, D.; Insel, P.A.; Izzo, A.A.; et al. Experimental design and analysis and their reporting: New guidance for publication in BJP. Br. J. Pharmacol. 2015, 172, 3461. [Google Scholar] [CrossRef] [PubMed]
- Zeng, Y.; Xue, M.; Zhang, T.; Sun, S.; Lin, R.; Li, N.; Zheng, P.; Zhen, Y.; Hu, H.; Zhang, X.D.; et al. Soluble form of suppression of tumorigenicity-2 predicts clinical stability of inpatients with community-acquired pneumonia. Exp. Biol. Med. (Maywood) 2021, 246, 2297–2306. [Google Scholar] [CrossRef]
- Ariel, A.C.; Colin, D.; David, F.C.; Rajita, P.; Michele, A.G.; Divya, M.; Kian Fan, C. Targeting interleukin-33 and thymic stromal lymphopoietin pathways for novel pulmonary therapeutics in asthma and COPD. Eur. Respir. Rev. 2023, 32, 220144. [Google Scholar] [CrossRef]
- Chen, W.-Y.; Tsai, T.-H.; Yang, J.-L.; Li, L.-C. Therapeutic Strategies for Targeting IL-33/ST2 Signalling for the Treatment of Inflammatory Diseases. Cell. Physiol. Biochem. 2018, 49, 349–358. [Google Scholar] [CrossRef]
- Takatori, H.; Makita, S.; Ito, T.; Matsuki, A.; Nakajima, H. Regulatory Mechanisms of IL-33-ST2-Mediated Allergic Inflammation. Front. Immunol. 2018, 9, 2004. [Google Scholar] [CrossRef]
- Divekar, R.; Kita, H. Recent advances in epithelium-derived cytokines (IL-33, IL-25, and thymic stromal lymphopoietin) and allergic inflammation. Curr. Opin. Allergy Clin. Immunol. 2015, 15, 98–103. [Google Scholar] [CrossRef]
- Bourke, C.D.; Prendergast, C.T.; Sanin, D.E.; Oulton, T.E.; Hall, R.J.; Mountford, A.P. Epidermal keratinocytes initiate wound healing and pro-inflammatory immune responses following percutaneous schistosome infection. Int. J. Parasitol. 2015, 45, 215–224. [Google Scholar] [CrossRef] [PubMed]
- Gu, C.; Liu, M.; Zhao, T.; Wang, D.; Wang, Y. Protective role of p120-catenin in maintaining the integrity of adherens and tight junctions in ventilator-induced lung injury. Respir. Res. 2015, 16, 1–15. [Google Scholar] [CrossRef]
- Naser, A.N.; Lu, Q.; Chen, Y.H. Trans-Compartmental Regulation of Tight Junction Barrier Function. Tissue Barriers 2023, 11, 2133880. [Google Scholar] [CrossRef]
- Kang, J.S.; Zhao, X.Y.; Lee, J.H.; Lee, J.-S.; Keum, Y.-S. Ethanol Extract of Chaenomeles sinensis Inhibits the Development of Benign Prostatic Hyperplasia by Exhibiting Anti-oxidant and Anti-inflammatory Effects. J. Cancer Prev. 2022, 27, 42. [Google Scholar] [CrossRef]
- Tian, C.; Shao, Y.; Jin, Z.; Liang, Y.; Li, C.; Qu, C.; Sun, S.; Cui, C.; Liu, M. The protective effect of rutin against lipopolysaccharide induced acute lung injury in mice based on the pharmacokinetic and pharmacodynamic combination model. J. Pharm. Biomed. Anal. 2022, 209, 114480. [Google Scholar] [CrossRef]
- Chen, W.-Y.; Huang, Y.-C.; Yang, M.-L.; Lee, C.-Y.; Chen, C.-J.; Yeh, C.-H.; Pan, P.-H.; Horng, C.-T.; Kuo, W.-H.; Kuan, Y.-H. Protective effect of rutin on LPS-induced acute lung injury via down-regulation of MIP-2 expression and MMP-9 activation through inhibition of Akt phosphorylation. Int. Immunopharmacol. 2014, 22, 409–413. [Google Scholar] [CrossRef] [PubMed]
- Coleman, S.; Hurst, R.; Sawyer, G.; Kruger, M. The in vitro evaluation of isolated procyanidins as modulators of cytokine-induced eotaxin production in human alveolar epithelial cells. J. Berry Res. 2016, 6, 115–124. [Google Scholar] [CrossRef]
- Tie, S.; Zhang, L.; Li, B.; Xing, S.; Wang, H.; Chen, Y.; Cui, W.; Gu, S.; Tan, M. Effect of dual targeting procyanidins nanoparticles on metabolomics of lipopolysaccharide-stimulated inflammatory macrophages. Food Sci. Hum. Wellness 2023, 12, 2252–2262. [Google Scholar] [CrossRef]
- Chen, F.; Wang, H.; Zhao, J.; Yan, J.; Meng, H.; Zhan, H.; Chen, L.; Yuan, L. Grape seed proanthocyanidin inhibits monocrotaline-induced pulmonary arterial hypertension via attenuating inflammation: In vivo and in vitro studies. J. Nutr. Biochem. 2019, 67, 72–77. [Google Scholar] [CrossRef]
- Enomoto, T.; Nagasako-Akazome, Y.; Kanda, T.; Ikeda, M.; Dake, Y. Clinical effects of apple polyphenols on persistent allergic rhinitis: A randomized double-blind placebo-controlled parallel arm study. J. Investig. Allergol. Clin. Immunol. 2006, 16, 283–289. [Google Scholar]
- Lv, C.; Zhang, Y.; Shen, L. Preliminary Clinical Effect Evaluation of Resveratrol in Adults with Allergic Rhinitis. Int. Arch. Allergy Immunol. 2018, 175, 231–236. [Google Scholar] [CrossRef]
- Kennedy-Feitosa, E.; Okuro, R.T.; Pinho Ribeiro, V.; Lanzetti, M.; Barroso, M.V.; Zin, W.A.; Porto, L.C.; Brito-Gitirana, L.; Valenca, S.S. Eucalyptol attenuates cigarette smoke-induced acute lung inflammation and oxidative stress in the mouse. Pulm. Pharmacol. Ther. 2016, 41, 11–18. [Google Scholar] [CrossRef]
- Qin, W.; Deng, T.; Cui, H.; Zhang, Q.; Liu, X.; Yang, X.; Chen, M. Exposure to diisodecyl phthalate exacerbated Th2 and Th17-mediated asthma through aggravating oxidative stress and the activation of p38 MAPK. Food Chem. Toxicol. 2018, 114, 78–87. [Google Scholar] [CrossRef]
- Melo, R.C.; Weller, P.F. Contemporary understanding of the secretory granules in human eosinophils. J. Leukoc. Biol. 2018, 104, 85–93. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, H.; Sedgwick, J.B.; Vrtis, R.F.; Busse, W.W. The Effect of Transendothelial Migration on Eosinophil Function. ATS J. 2000, 23, 379–388. [Google Scholar] [CrossRef]
- Chiu, S.; Bharat, A. Role of monocytes and macrophages in regulating immune response following lung transplantation. Curr. Opin. Organ. Transpl. 2016, 21, 239–245. [Google Scholar] [CrossRef]
- Li, D.; Wu, M. Pattern recognition receptors in health and diseases. Signal Transduct. Target. Ther. 2021, 6, 291. [Google Scholar] [CrossRef]
- Lee, Y.G.; Jeong, J.J.; Nyenhuis, S.; Berdyshev, E.; Chung, S.; Ranjan, R.; Karpurapu, M.; Deng, J.; Qian, F.; Kelly, E.A.; et al. Recruited alveolar macrophages, in response to airway epithelial–derived monocyte chemoattractant protein 1/CCL2, regulate airway inflammation and remodeling in allergic asthma. Am. J. Respir. Cell Mol. Biol. 2015, 52, 772–784. [Google Scholar] [CrossRef]
- Lewkowich, I.P.; Rempel, J.D.; HayGlass, K.T. Antigen-specific versus total immunoglobulin synthesis: Total IgE and IgG1, but not IgG2a levels predict murine antigen-specific responses. Int. Arch. Allergy Immunol. 2004, 133, 145–153. [Google Scholar] [CrossRef] [PubMed]
- Mountford, A.P.; Fisher, A.; Wilson, R.A. The profile of IgG1 and IgG2a antibody responses in mice exposed to Schistosoma mansoni. Parasite Immunol. 1994, 16, 521–527. [Google Scholar] [CrossRef] [PubMed]
- Rostamian, M.; Sohrabi, S.; Kavosifard, H.; Niknam, H.M. Lower levels of IgG1 in comparison with IgG2a are associated with protective immunity against Leishmania tropica infection in BALB/c mice. J. Microbiol. Immunol. Infect. 2017, 50, 160–166. [Google Scholar] [CrossRef] [PubMed]
- Cicchese, J.M.; Evans, S.; Hult, C.; Joslyn, L.R.; Wessler, T.; Millar, J.A.; Marino, S.; Cilfone, N.A.; Mattila, J.T.; Linderman, J.J.; et al. Dynamic balance of pro- and anti-inflammatory signals controls disease and limits pathology. Immunol. Rev. 2018, 285, 147–167. [Google Scholar] [CrossRef]
- Grund, M.; Choi, S.J.; Powell, L.; Lukomski, S. Intranasal immunization with a Bucl8-based vaccine ameliorates bacterial burden and pathological inflammation, and promotes an IgG2a/b dominant response in an outbred mouse model of Burkholderia infection. Front. Immunol. 2023, 14, 1177650. [Google Scholar] [CrossRef]
- Kim, S.; Kim, C.-H.; Yoon, J.-H.; Kim, H.-J. Alternative method for primary nasal epithelial cell culture using intranasal brushing and feasibility for the study of epithelial functions in allergic rhinitis. Allergy Asthma Immunol. Res. 2016, 8, 69–78. [Google Scholar]
- Li, Y.Q.; Zhong, Y.; Xiao, X.P.; Li, D.D.; Zhou, Z.; Tian, Y.Y. IL-33/ST2 axis promotes the inflammatory response of nasal mucosal epithelial cells through inducing the ERK1/2 pathway. Innate Immun. 2020, 26, 505–513. [Google Scholar] [CrossRef]
- Zhao, W.; Hu, Z. The enigmatic processing and secretion of interleukin-33. Cell. Mol. Immunol. 2010, 7, 260–262. [Google Scholar] [CrossRef]
- Hu, J.; Gao, N.; Zhang, Y.; Chen, X.; Li, J.; Bian, F.; Chi, W.; Liu, Z.; de Paiva, C.S.; Pflugfelder, S.C.; et al. IL-33/ST2/IL-9/IL-9R signaling disrupts ocular surface barrier in allergic inflammation. Mucosal Immunol. 2020, 13, 919–930. [Google Scholar] [CrossRef]
- Landskron, G.; De la Fuente Lopez, M.; Dubois-Camacho, K.; Diaz-Jimenez, D.; Orellana-Serradell, O.; Romero, D.; Sepulveda, S.A.; Salazar, C.; Parada-Venegas, D.; Quera, R.; et al. Interleukin 33/ST2 Axis Components Are Associated to Desmoplasia, a Metastasis-Related Factor in Colorectal Cancer. Front. Immunol. 2019, 10, 1394. [Google Scholar] [CrossRef] [PubMed]
- Ryu, W.-I.; Lee, H.; Bae, H.; Jeon, J.; Ryu, H.J.; Kim, J.; Kim, J.; Son, J.; Kim, J.; Imai, Y.; et al. IL-33 down-regulates CLDN1 expression through the ERK/STAT3 pathway in keratinocytes. J. Dermatol. Sci. 2018, 90, 313–322. [Google Scholar] [CrossRef] [PubMed]
- Kidd, P. Th1/Th2 balance: The hypothesis, its limitations, and implications for health and disease. Altern. Med. Rev. 2003, 8, 223–246. [Google Scholar] [PubMed]
- Alspach, E.; Lussier, D.M.; Schreiber, R.D. Interferon gamma and Its Important Roles in Promoting and Inhibiting Spontaneous and Therapeutic Cancer Immunity. Cold Spring Harb. Perspect. Biol. 2019, 11, a028480. [Google Scholar] [CrossRef] [PubMed]
- Teixeira, L.K.; Fonseca, B.P.; Barboza, B.A.; Viola, J.P. The role of interferon-gamma on immune and allergic responses. Mem. Inst. Oswaldo Cruz 2005, 100 (Suppl. S1), 137–144. [Google Scholar] [CrossRef] [PubMed]
- Foster, P.S.; Hogan, S.P.; Matthaei, K.I.; Young, I.G. Interleukin-4 and interleukin-5 as targets for the inhibition of eosinophilic inflammation and allergic airways hyperreactivity. Mem. Inst. Oswaldo Cruz 1997, 92 (Suppl. S2), 55–61. [Google Scholar] [CrossRef] [PubMed]
- Kuperman, D.A.; Huang, X.; Koth, L.L.; Chang, G.H.; Dolganov, G.M.; Zhu, Z.; Elias, J.A.; Sheppard, D.; Erle, D.J. Direct effects of interleukin-13 on epithelial cells cause airway hyperreactivity and mucus overproduction in asthma. Nat. Med. 2002, 8, 885–889. [Google Scholar] [CrossRef] [PubMed]






Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jin, J.; Fan, Y.J.; Nguyen, T.V.; Yu, Z.N.; Song, C.H.; Lee, S.-Y.; Shin, H.S.; Chai, O.H. Chaenomeles sinensis Extract Ameliorates Ovalbumin-Induced Allergic Rhinitis by Inhibiting the IL-33/ST2 Axis and Regulating Epithelial Cell Dysfunction. Foods 2024, 13, 611. https://doi.org/10.3390/foods13040611
Jin J, Fan YJ, Nguyen TV, Yu ZN, Song CH, Lee S-Y, Shin HS, Chai OH. Chaenomeles sinensis Extract Ameliorates Ovalbumin-Induced Allergic Rhinitis by Inhibiting the IL-33/ST2 Axis and Regulating Epithelial Cell Dysfunction. Foods. 2024; 13(4):611. https://doi.org/10.3390/foods13040611
Chicago/Turabian StyleJin, Juan, Yan Jing Fan, Thi Van Nguyen, Zhen Nan Yu, Chang Ho Song, So-Young Lee, Hee Soon Shin, and Ok Hee Chai. 2024. "Chaenomeles sinensis Extract Ameliorates Ovalbumin-Induced Allergic Rhinitis by Inhibiting the IL-33/ST2 Axis and Regulating Epithelial Cell Dysfunction" Foods 13, no. 4: 611. https://doi.org/10.3390/foods13040611





