Metabolomics Study of the Effect of Transcription Factor NOR-like1 on Flavonoids in Tomato at Different Stages of Maturity Using UPLC-MS/MS
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Materials and Sample Preparation
2.2. Ultra-Performance Liquid Chromatography (UPLC) Conditions
2.3. Electrospray Ionization (ESI)–Tandem Mass Spectrometry (MS/MS) (ESI-MS/MS) Conditions
2.4. Multivariate Statistical Analysis of Flavonoid Metabolites
3. Results and Discussion
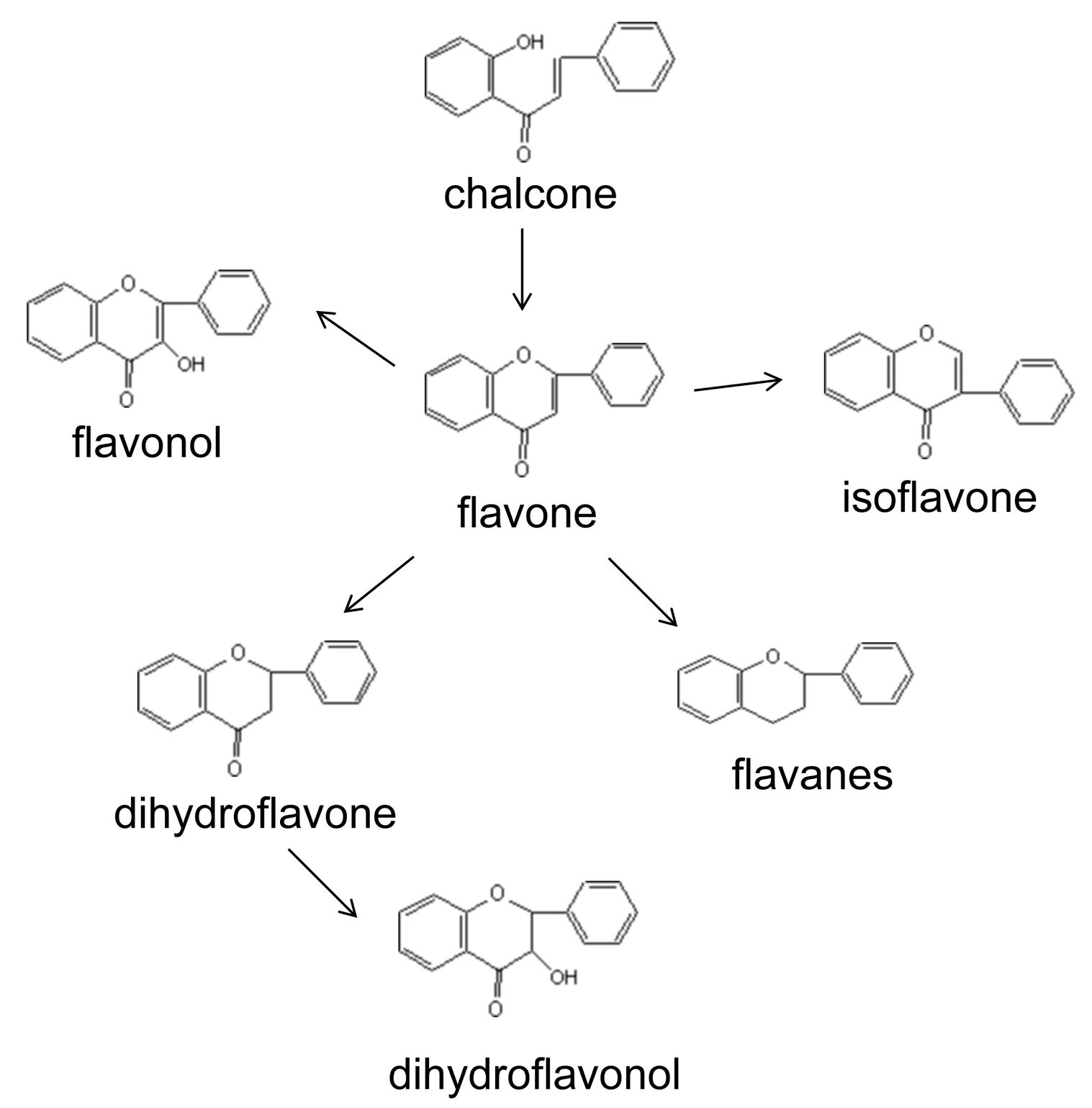
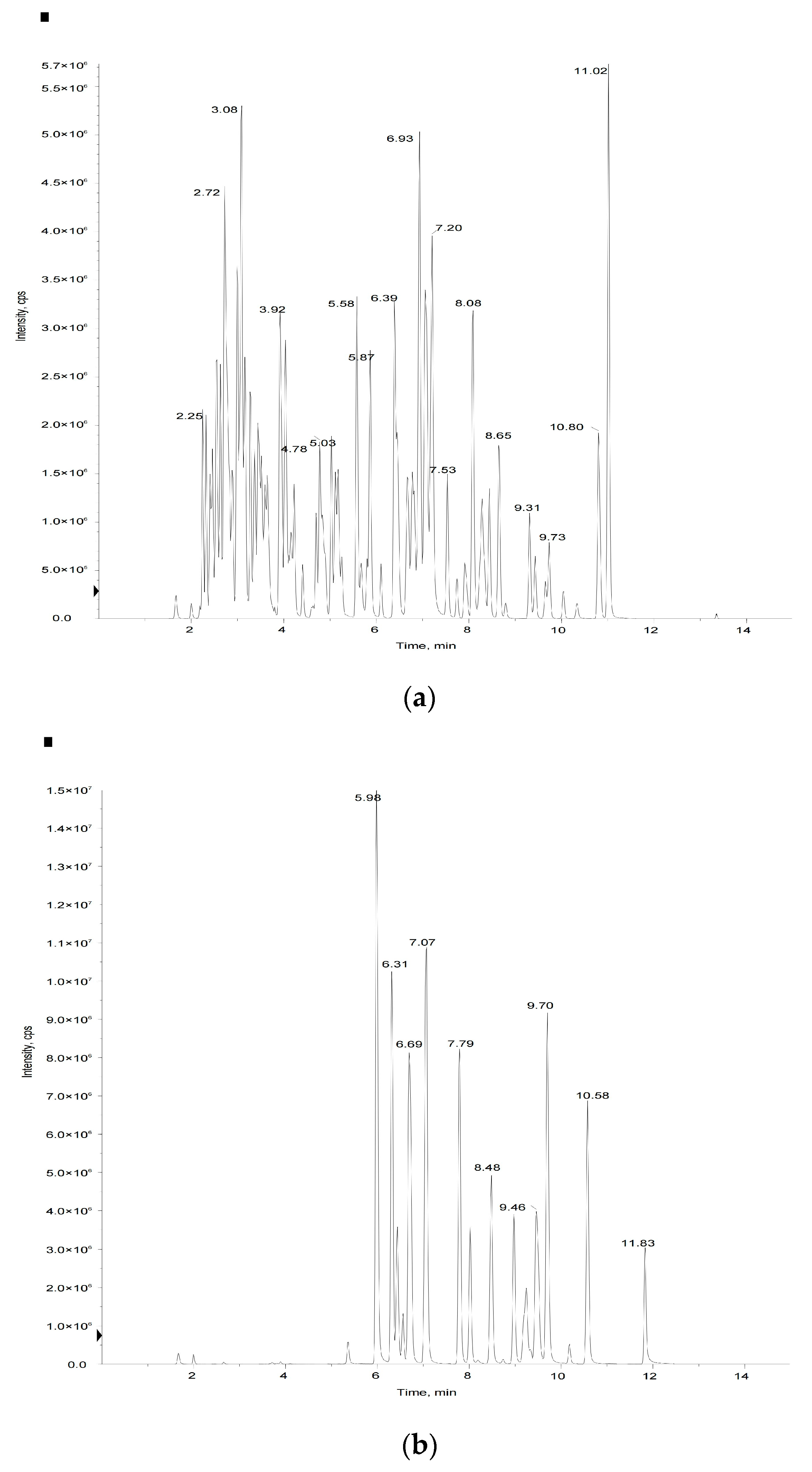
3.1. Comprehensive Analysis of Flavonoid Compounds
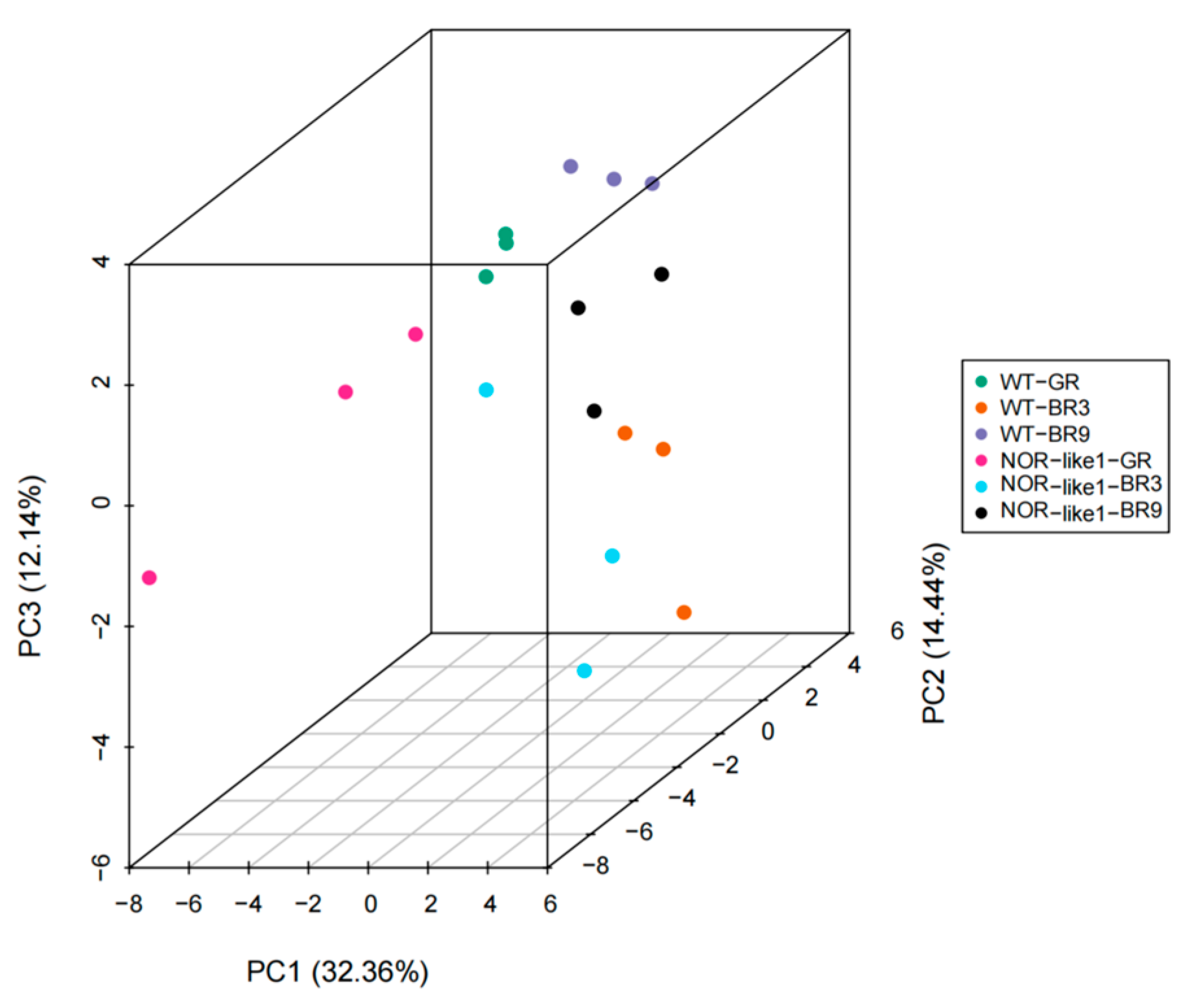
3.2. Differential Flavonoid Metabolites Based on Principal Component Analysis (PCA)
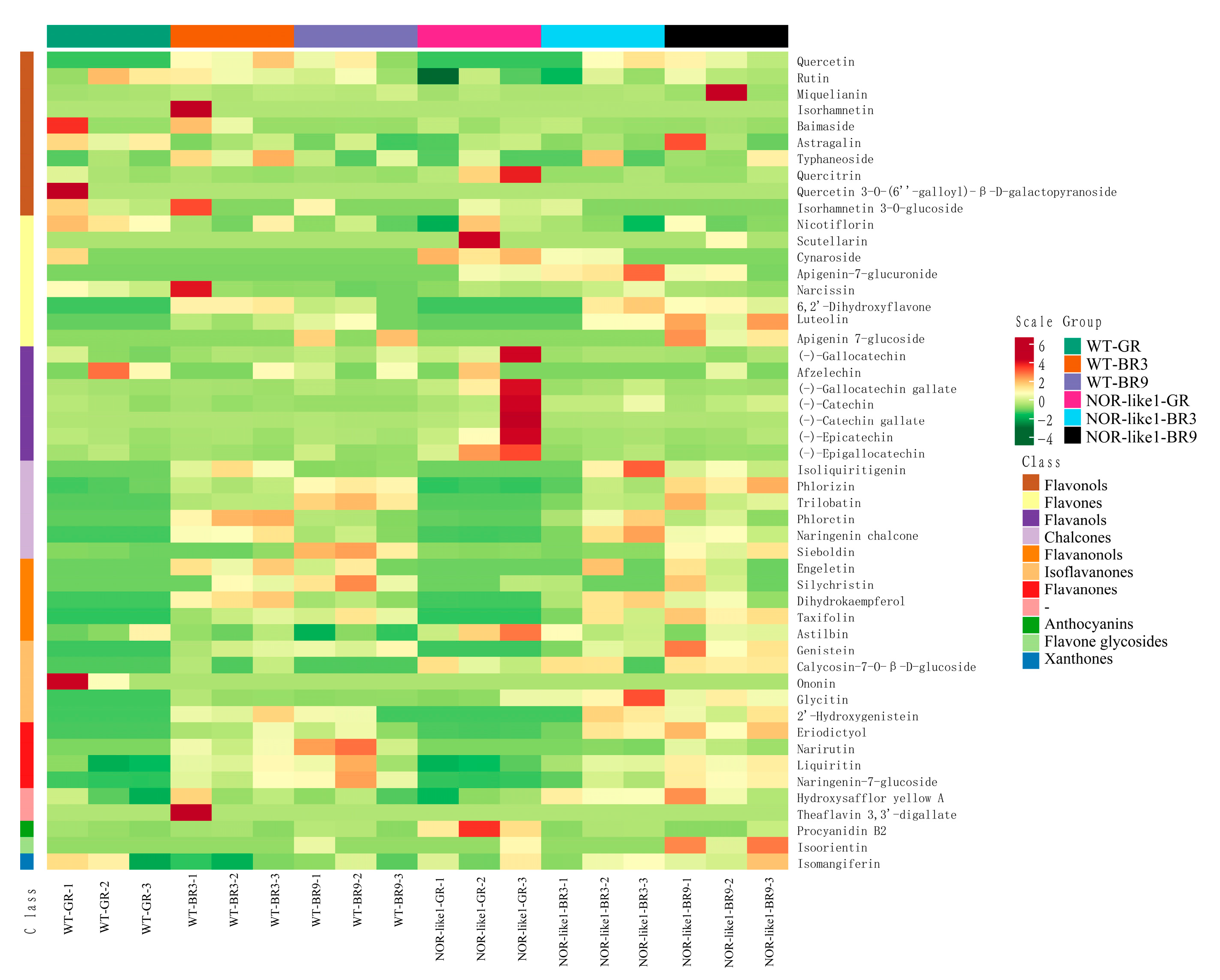
3.3. Screening and Analysis of Differential Flavonoid Metabolites
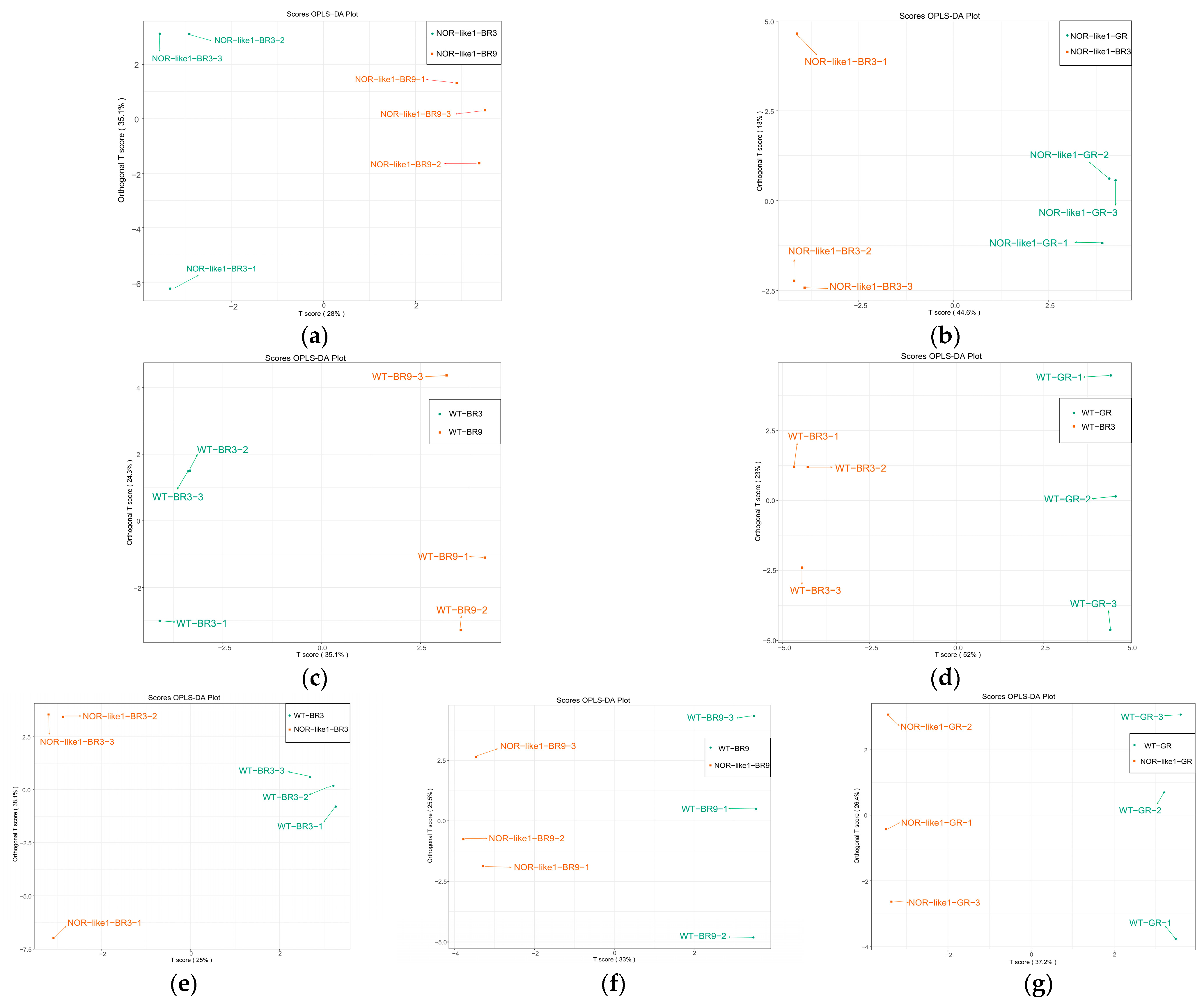
3.3.1. Orthogonal Projections to Latent Structures Discriminant Analysis (OPLS-DA) of Different Tomato Sample Groups
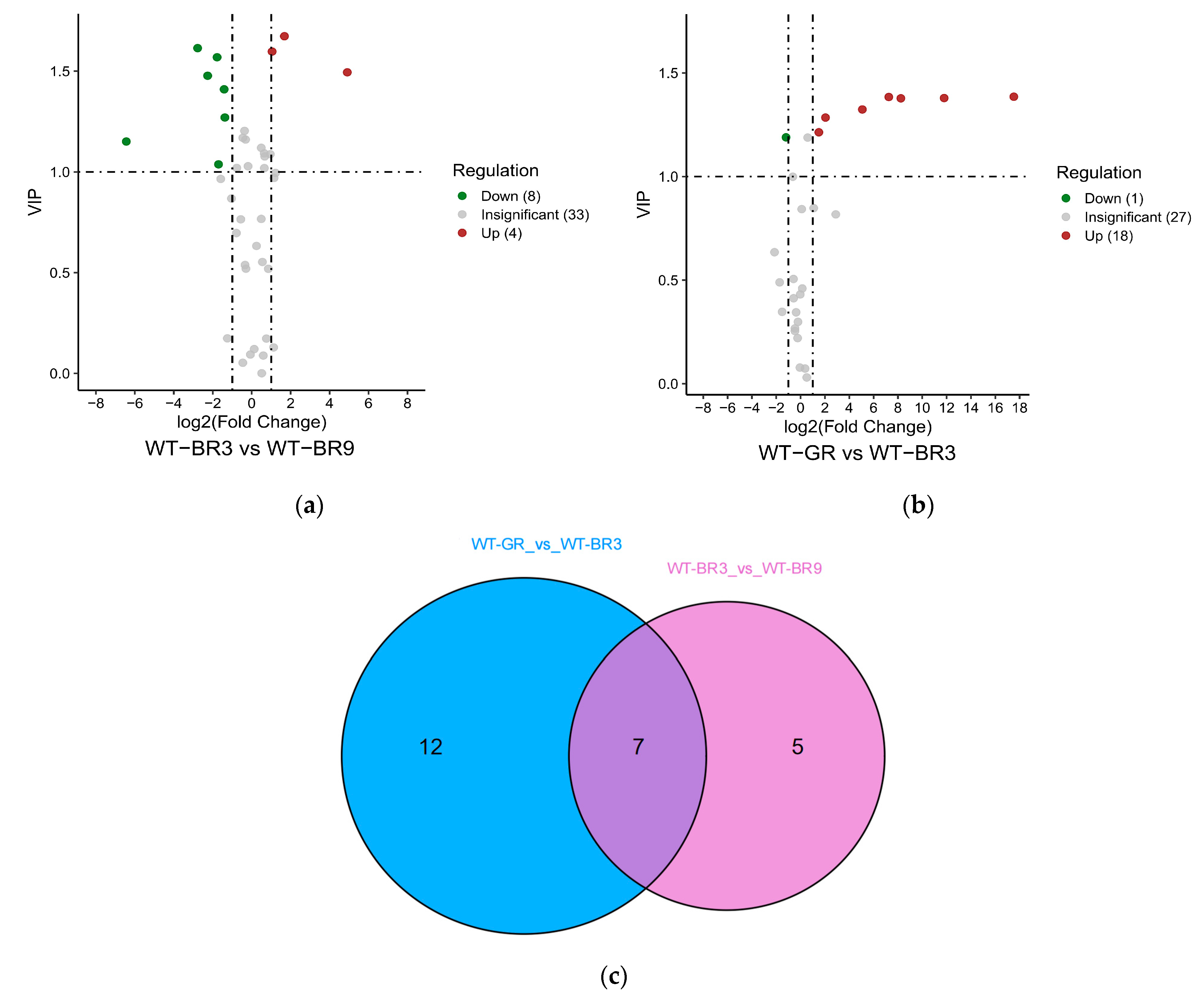
3.3.2. Changes in Flavonoid Metabolites in WT Tomato Fruits at Different Ripening Stages
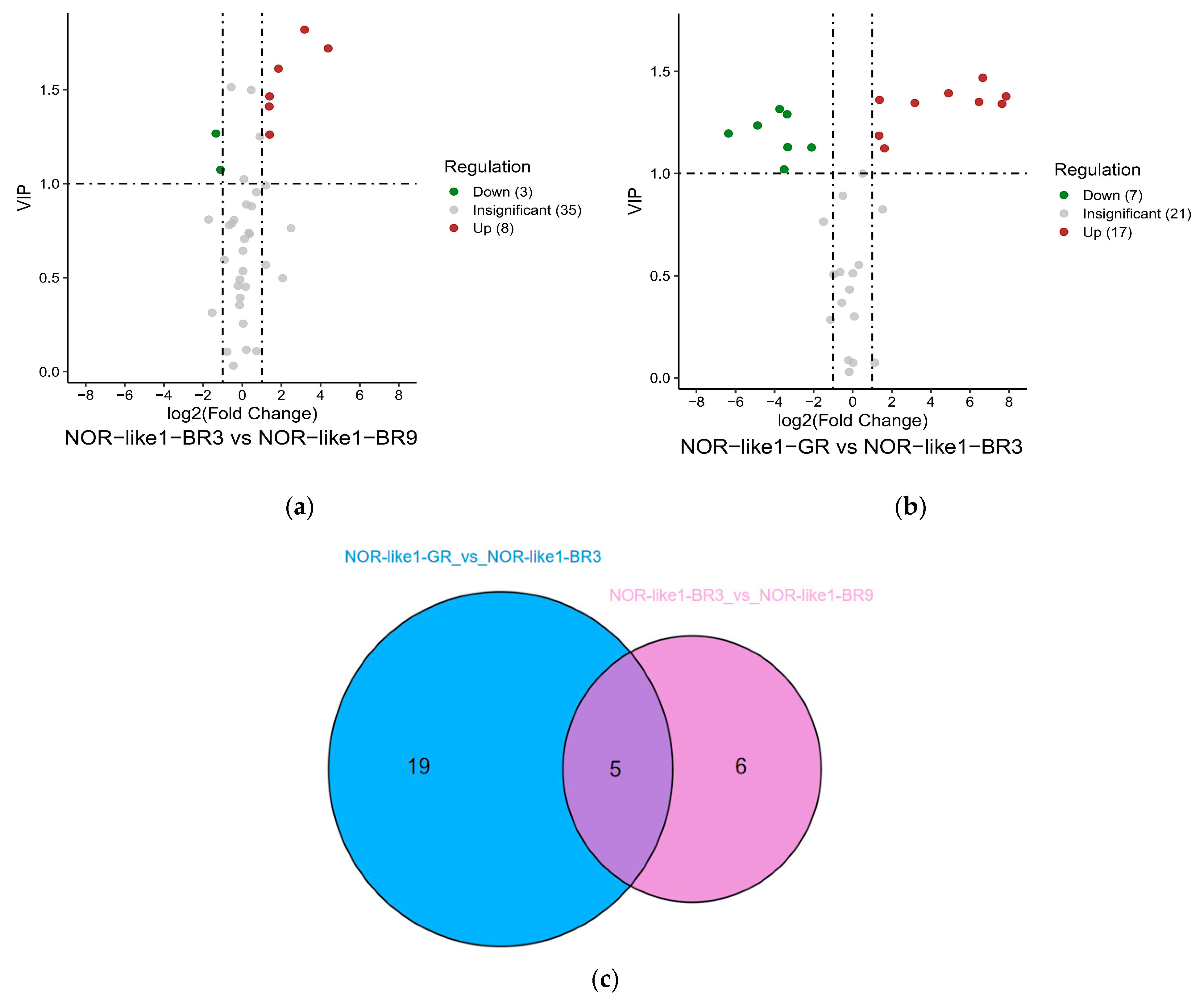
3.3.3. Change in Flavonoid Metabolites in NOR-like1 Tomato Fruits at Different Ripening Stages
3.3.4. Differential Flavonoid Metabolites between WT and NOR-like1 Tomato Fruits
3.4. Enrichment Analysis of Differential Flavonoid Metabolites
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gao, J.; Song, Y.; Mao, J. The determination of flavonoids in some fruit and vegetables. China Food Addit. 2007, 18, 211–213. [Google Scholar] [CrossRef]
- Treutter, D. Significance of flavonoids in plant resistance: A review. Environ. Chem. Lett. 2006, 4, 147–157. [Google Scholar] [CrossRef]
- Bui, T.T.; Nguyen, T.H. Natural product for the treatment of Alzheimer’s disease. J. Basic Clin. Physiol. Pharmacol. 2017, 28, 413–423. [Google Scholar] [CrossRef] [PubMed]
- Tan, T.; Li, Y.; Tang, B.; Chen, Y.; Chen, X.; Xie, Q.; Hu, Z.; Chen, G. Knockout of SlALKBH2 weakens the DNA damage repair ability of tomato. Plant Sci. 2022, 319, 111266. [Google Scholar] [CrossRef] [PubMed]
- Tao, X.; Wu, Q.; Huang, S.; Zhu, B.; Chen, F.; Liu, B.; Cai, L.; Mao, L.; Luo, Z.; Li, L.; et al. Exogenous abscisic acid regulates primary metabolism in postharvest cherry tomato fruit during ripening. Sci. Hortic. 2022, 299, 111008. [Google Scholar] [CrossRef]
- Monribot-Villanueva, J.L.; Altúzar-Molina, A.; Aluja, M.; Zamora-Briseño, J.A.; Elizalde-Contreras, J.M.; Bautista-Valle, M.V.; Arellano de Los Santos, J.; Sánchez-Martínez, D.E.; Rivera-Reséndiz, F.J.; Vázquez-Rosas-Landa, M.; et al. Integrating proteomics and metabolomics approaches to elucidate the ripening process in white Psidium guajava. Food Chem. 2022, 367, 130656. [Google Scholar] [CrossRef] [PubMed]
- Yonekura-Sakakibara, K.; Higashi, Y.; Nakabayashi, R. The Origin and Evolution of Plant Flavonoid Metabolism. Front. Plant Sci. 2019, 10, 943. [Google Scholar] [CrossRef] [PubMed]
- Xiao, J.; Muzashvili, T.S.; Georgiev, M.I. Advances in the biotechnological glycosylation of valuable flavonoids. Biotechnol. Adv. 2014, 32, 1145–1156. [Google Scholar] [CrossRef]
- Liu, Z.-Q. What about the progress in the synthesis of flavonoid from 2020? Eur. J. Med. Chem. 2022, 243, 114671. [Google Scholar] [CrossRef]
- Zhang, X.; Qi, X.; Chen, Y.; Yao, H.; Zheng, Y. Research Advances of Flavonoids in Strawberry. North. Hortic. 2020, 44, 128–133. [Google Scholar] [CrossRef]
- Tejada, S.; Pinya, S.; Martorell, M.; Capó, X.; Tur, J.A.; Pons, A.; Sureda, A. Potential Anti-inflammatory Effects of Hesperidin from the Genus Citrus. Curr. Med. Chem. 2018, 25, 4929–4945. [Google Scholar] [CrossRef]
- Shen, N.; Wang, T.; Gan, Q.; Liu, S.; Wang, L.; Jin, B. Plant flavonoids: Classification, distribution, biosynthesis, and antioxidant activity. Food Chem. 2022, 383, 132531. [Google Scholar] [CrossRef] [PubMed]
- Staszowska-Karkut, M.; Materska, M. Phenolic Composition, Mineral Content, and Beneficial Bioactivities of Leaf Extracts from Black Currant (Ribes nigrum L.), Raspberry (Rubus idaeus), and Aronia (Aronia melanocarpa). Nutrients 2020, 12, 463. [Google Scholar] [CrossRef] [PubMed]
- Tohge, T.; de Souza, L.P.; Fernie, A.R. Current understanding of the pathways of flavonoid biosynthesis in model and crop plants. J. Exp. Bot. 2017, 68, 4013–4028. [Google Scholar] [CrossRef] [PubMed]
- Song, X.; Wei, J.; Di, S.; Pang, Y. Recent Advances in the Regulation Mechanism of Transcription Factors and Metabolic Engineering of Anthocyanins. Chin. Bull. Bot. 2019, 54, 133–156. [Google Scholar] [CrossRef]
- Huang, Y.; Cui, L.; Chen, W.; Liu, Z.; Yuan, W.; Zhu, F.; Jiao, Z.; Zhang, Z.; Deng, X.; Wang, L.; et al. Comprehensive analysis of NAC transcription factors and their expressions during taproot coloration in radish (Raphanus sativus L.). Sci. Hortic. 2022, 299, 111047. [Google Scholar] [CrossRef]
- Sun, Q.; Jiang, S.; Zhang, T.; Xu, H.; Fang, H.; Zhang, J.; Su, M.; Wang, Y.; Zhang, Z.; Wang, N.; et al. Apple NAC transcription factor MdNAC52 regulates biosynthesis of anthocyanin and proanthocyanidin through MdMYB9 and MdMYB11. Plant Sci. 2019, 289, 110286. [Google Scholar] [CrossRef] [PubMed]
- Duan, A.-Q.; Yang, X.-L.; Feng, K.; Liu, J.-X.; Xu, Z.-S.; Xiong, A.-S. Genome-wide analysis of NAC transcription factors and their response to abiotic stress in celery (Apium graveolens L.). Comput. Biol. Chem. 2020, 84, 107186. [Google Scholar] [CrossRef]
- Hu, H.; Dai, M.; Yao, J.; Xiao, B.; Li, X.; Zhang, Q.; Xiong, L. Overexpressing a NAM, ATAF, and CUC (NAC) transcription factor enhances drought resistance and salt tolerance in rice. Proc. Natl. Acad. Sci. USA 2006, 103, 12987–12992. [Google Scholar] [CrossRef]
- Jeong, J.S.; Kim, Y.S.; Baek, K.H.; Jung, H.; Ha, S.-H.; Do Choi, Y.; Kim, M.; Reuzeau, C.; Kim, J.-K. Root-Specific Expression of OsNAC10 Improves Drought Tolerance and Grain Yield in Rice under Field Drought Conditions. Plant Physiol. 2010, 153, 185–197. [Google Scholar] [CrossRef]
- Guo, F.; Liu, S.; Zhang, C.; Dong, T.; Meng, X.; Zhu, M. Genome-wide systematic survey and analysis of NAC transcription factor family and their response to abiotic stress in sweetpotato. Sci. Hortic. 2022, 299, 111048. [Google Scholar] [CrossRef]
- Li, M.; Chen, R.; Jiang, Q.; Sun, X.; Zhang, H.; Hu, Z. GmNAC06, a NAC domain transcription factor enhances salt stress tolerance in soybean. Plant Mol. Biol. 2021, 105, 333–345. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.; Wang, L.-Y.; Dai, J.-X.; Wang, Y.; Lin, D. The NAC-type transcription factor CaNAC46 regulates the salt and drought tolerance of transgenic Arabidopsis thaliana. BMC Plant Biol. 2021, 21, 11. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Hu, Z.; Zhu, M.; Zhu, Z.; Hu, J.; Qanmber, G.; Chen, G. The abiotic stress-responsive NAC transcription factor SlNAC11 is involved in drought and salt response in tomato (Solanum lycopersicum L.). Plant Cell Tissue Organ Cult. (PCTOC) 2017, 129, 161–174. [Google Scholar] [CrossRef]
- Zhu, M.; Chen, G.; Zhang, J.; Zhang, Y.; Xie, Q.; Zhao, Z.; Pan, Y.; Hu, Z. The abiotic stress-responsive NAC-type transcription factor SlNAC4 regulates salt and drought tolerance and stress-related genes in tomato (Solanum lycopersicum). Plant Cell Rep. 2014, 33, 1851–1863. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Wei, W.; Zhao, X.; Tan, X.; Fan, Z.; Zhang, Y.; Jing, Y.; Meng, L.; Zhu, B.; Zhu, H.; et al. A NAC transcription factor, NOR-like1, is a new positive regulator of tomato fruit ripening. Hortic. Res. 2018, 5, 75. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Wei, W.; Fan, Z.; Zhao, X.; Zhang, Y.; Jing, Y.; Zhu, B.; Zhu, H.; Shan, W.; Chen, J.; et al. Re-evaluation of the nor mutation and the role of the NAC-NOR transcription factor in tomato fruit ripening. J. Exp. Bot. 2020, 71, 3560–3574. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Zhao, X.; Fu, D.; Zhao, Y. Integrated Analysis of Widely Targeted Metabolomics and Transcriptomics Reveals the Effects of Transcription Factor NOR-like1 on Alkaloids, Phenolic Acids, and Flavonoids in Tomato at Different Ripening Stages. Metabolites 2022, 12, 1296. [Google Scholar] [CrossRef]
- Zhang, H.; Du, X.; Yu, J.; Jin, H.; Liu, N. Comparative Metabolomics study of flavonoids in the pericarp of different coloured bitter gourds (Momordica charantia L.). Physiol. Mol. Biol. Plants 2022, 28, 1347–1357. [Google Scholar] [CrossRef]
- Wang, Y.; Cheng, J.; Jiang, W.; Chen, S. Metabolomics study of flavonoids in Coreopsis tinctoria of different origins by UPLC-MS/MS. PeerJ 2022, 10, e14580. [Google Scholar] [CrossRef]
- Li, J.; Hossain, M.S.; Ma, H.; Yang, Q.; Gong, X.; Yang, P.; Feng, B. Comparative metabolomics reveals differences in flavonoid metabolites among different coloured buckwheat flowers. J. Food Compos. Anal. 2020, 85, 103335. [Google Scholar] [CrossRef]
- Hunt, G.M.; Baker, E.A. Phenolic constituents of tomato fruit cuticles. Phytochemistry 1980, 19, 1415–1419. [Google Scholar] [CrossRef]
- Iijima, Y.; Nakamura, Y.; Ogata, Y.; Tanaka, K.; Sakurai, N.; Suda, K.; Suzuki, T.; Suzuki, H.; Okazaki, K.; Kitayama, M.; et al. Metabolite annotations based on the integration of mass spectral information. Plant J. 2008, 54, 949–962. [Google Scholar] [CrossRef] [PubMed]
- Krishnan, M.; Babu, S.; Thomas, S.A.; Surulivel, J.S.; Ayyanar, K. Molecular docking analysis of VEGF with compounds from tomato. Bioinformation 2022, 18, 478–481. [Google Scholar] [CrossRef] [PubMed]
- Spencer, J.P.; Kuhnle, G.G.; Hajirezaei, M.; Mock, H.P.; Sonnewald, U.; Rice-Evans, C. The genotypic variation of the antioxidant potential of different tomato varieties. Free Radic. Res. 2005, 39, 1005–1016. [Google Scholar] [CrossRef] [PubMed]
- Slimestad, R.; Fossen, T.; Verheul, M.J. The flavonoids of tomatoes. J. Agric. Food Chem. 2008, 56, 2436–2441. [Google Scholar] [CrossRef] [PubMed]
- Vallverdú-Queralt, A.; Jáuregui, O.; Di Lecce, G.; Andrés-Lacueva, C.; Lamuela-Raventós, R.M. Screening of the polyphenol content of tomato-based products through accurate-mass spectrometry (HPLC-ESI-QTOF). Food Chem. 2011, 129, 877–883. [Google Scholar] [CrossRef] [PubMed]
- Le Gall, G.; DuPont, M.S.; Mellon, F.A.; Davis, A.L.; Collins, G.J.; Verhoeyen, M.E.; Colquhoun, I.J. Characterization and content of flavonoid glycosides in genetically modified tomato (Lycopersicon esculentum) fruits. J. Agric. Food Chem. 2003, 51, 2438–2446. [Google Scholar] [CrossRef]
- Larbat, R.; Le Bot, J.; Bourgaud, F.; Robin, C.; Adamowicz, S. Organ-specific responses of tomato growth and phenolic metabolism to nitrate limitation. Plant Biol. 2012, 14, 760–769. [Google Scholar] [CrossRef]
- Morelli, C.F.; Cutignano, A.; Speranza, G.; Abbamondi, G.R.; Rabuffetti, M.; Iodice, C.; De Prisco, R.; Tommonaro, G. Taste Compounds and Polyphenolic Profile of Tomato Varieties Cultivated with Beneficial Microorganisms: A Chemical Investigation on Nutritional Properties and Sensory Qualities. Biomolecules 2023, 13, 117. [Google Scholar] [CrossRef]
- Wang, H.; Zhang, Z.; Song, J.; Tian, M.; Li, R.; Cui, X. Phenolic compound identification in tomato fruit by UPLC-QTOF-MS. LWT 2023, 182, 114791. [Google Scholar] [CrossRef]
- Iijima, Y.; Suda, K.; Suzuki, T.; Aoki, K.; Shibata, D. Metabolite Profiling of Chalcones and Flavanones in Tomato Fruit. J. Jpn. Soc. Hortic. Sci. 2008, 77, 94–102. [Google Scholar] [CrossRef]
- Raffo, A.; Leonardi, C.; Fogliano, V.; Ambrosino, P.; Salucci, M.; Gennaro, L.; Bugianesi, R.; Giuffrida, F.; Quaglia, G. Nutritional value of cherry tomatoes (Lycopersicon esculentum Cv. Naomi F1) harvested at different ripening stages. J. Agric. Food Chem. 2002, 50, 6550–6556. [Google Scholar] [CrossRef] [PubMed]
- Muir, S.R.; Collins, G.J.; Robinson, S.; Hughes, S.; Bovy, A.; Ric De Vos, C.H.; van Tunen, A.J.; Verhoeyen, M.E. Overexpression of petunia chalcone isomerase in tomato results in fruit containing increased levels of flavonols. Nat. Biotechnol. 2001, 19, 470–474. [Google Scholar] [CrossRef] [PubMed]
- Le Gall, G.; Colquhoun, I.J.; Davis, A.L.; Collins, G.J.; Verhoeyen, M.E. Metabolite profiling of tomato (Lycopersicon esculentum) using 1H NMR spectroscopy as a tool to detect potential unintended effects following a genetic modification. J. Agric. Food Chem. 2003, 51, 2447–2456. [Google Scholar] [CrossRef]
- Mintz-Oron, S.; Mandel, T.; Rogachev, I.; Feldberg, L.; Lotan, O.; Yativ, M.; Wang, Z.; Jetter, R.; Venger, I.; Adato, A.; et al. Gene expression and metabolism in tomato fruit surface tissues. Plant Physiol. 2008, 147, 823–851. [Google Scholar] [CrossRef]



| Index | Compounds | WT-GR vs. NOR-like1-GR | WT-BR3 vs. NOR-like1-BR3 | WT-BR9 vs. NOR-like1-BR9 | NOR-like1-GR vs. NOR-like1-BR3 | NOR-like1-BR3 vs. NOR-like1-BR9 | WT-GR vs. WT-BR3 | WT-BR3 vs. WT-BR9 | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| VIP | Log2FC | VIP | Log2FC | VIP | Log2FC | VIP | Log2FC | VIP | Log2FC | VIP | Log2FC | VIP | Log2FC | ||
| 1 | Astilbin | - | - | - | - | - | - | - | - | - | - | - | - | - | - |
| 2 | Miquelianin | - | - | - | - | - | - | - | - | - | - | - | - | - | - |
| 9 | Genistein | - | - | - | - | - | - | 1.48 | inf | 1.41 | 1.38 | 1.38 | inf | - | - |
| 106 | Typhaneoside | - | - | 1.38 | −1.27 | - | - | - | - | - | - | - | - | - | - |
| 11 | Hydroxysafflor yellow A | - | - | - | - | 1.24 | 1.11 | 1.19 | 1.35 | - | - | - | - | - | - |
| 115 | Quercitrin | 1.08 | 2.77 | - | - | - | - | 1.24 | −4.87 | - | - | - | - | - | - |
| 117 | Engeletin | - | - | 1.35 | −1.18 | - | - | - | - | - | - | 1.39 | inf | - | - |
| 118 | Narcissin | - | - | - | - | - | - | - | - | - | - | - | - | 1.04 | −1.71 |
| 119 | Astragalin | 1.25 | −1.22 | - | - | - | - | - | - | - | - | 1.19 | −1.19 | - | - |
| 124 | Silychristin | - | - | - | - | - | - | - | - | - | - | - | - | - | - |
| 126 | Eriodictyol | - | - | - | - | 1.37 | 1.34 | 1.35 | 6.46 | - | - | 1.38 | 8.24 | - | - |
| 13 | Isoorientin | - | - | - | - | 1.32 | 2.84 | - | - | 1.83 | inf | - | - | - | - |
| 135 | Theaflavin 3,3′-digallate | - | - | - | - | - | - | - | - | - | - | - | - | - | - |
| 136 | 6,2′-Dihydroxyflavone | - | - | - | - | 1.22 | 1.05 | 1.05 | inf | - | - | 1.39 | inf | 1.27 | −1.38 |
| 138 | Baimaside | - | - | - | - | - | - | - | - | - | - | - | - | 1.15 | −6.44 |
| 139 | Dihydrokaempferol | - | - | - | - | - | - | 1.47 | 6.66 | - | - | 1.38 | 7.25 | 1.57 | −1.78 |
| 145 | Procyanidin B2 | 1.1 | 4.5 | - | - | - | - | 1.02 | −3.51 | - | - | - | - | - | - |
| 147 | (-)-Epicatechin | 1.29 | 3.11 | - | - | - | - | 1.32 | −3.74 | - | - | - | - | - | - |
| 150 | (-)-Catechin gallate | 1.35 | 6.76 | - | - | - | - | 1.2 | −6.36 | - | - | - | - | - | - |
| 152 | Ononin | 1.13 | −inf | - | - | - | - | - | - | - | - | - | - | - | - |
| 153 | Glycitin | 1.63 | inf | 1.77 | 1.94 | 1.72 | 1.78 | 1.12 | 1.62 | - | - | 1.39 | inf | - | - |
| 157 | Isomangiferin | - | - | 1.63 | 1.49 | - | - | - | - | - | - | - | - | - | - |
| 160 | Apigenin 7-glucoside | - | - | - | - | - | - | - | - | 1.83 | inf | - | - | 1.2 | inf |
| 169 | Quercetin 3-O-(6″-galloyl)-β-D-galactopyranoside | - | - | - | - | - | - | - | - | - | - | - | - | - | - |
| 176 | Naringenin−7-glucoside | - | - | - | - | - | - | 1.39 | 4.9 | 1.46 | 1.39 | 1.32 | 5.07 | - | - |
| 177 | Phloretin | 1.08 | inf | - | - | - | - | 1.34 | 7.64 | 1.07 | −1.11 | 1.39 | inf | 1.48 | −2.26 |
| 183 | 2′-Hydroxygenistein | - | - | - | - | - | - | 1.05 | inf | - | - | 1.38 | inf | - | - |
| 184 | Afzelechin | - | - | - | - | - | - | - | - | - | - | - | - | - | - |
| 191 | Naringenin chalcone | 1.15 | 4.01 | - | - | 1.31 | 1.02 | 1.38 | 7.85 | - | - | 1.38 | 11.8 | 1.41 | −1.42 |
| 195 | (-)-Gallocatechin | 1.15 | 2.18 | - | - | - | - | 1.13 | −2.11 | - | - | - | - | - | - |
| 197 | Isorhamnetin 3-O-glucoside | - | - | - | - | - | - | - | - | - | - | - | - | - | - |
| 201 | Trilobatin | - | - | - | - | - | - | 1.48 | inf | 1.26 | 1.4 | 1.39 | inf | 1.67 | 1.68 |
| 202 | Sieboldin | - | - | 1.36 | 1.04 | - | - | - | - | 1.82 | 3.19 | - | - | 1.49 | 4.91 |
| 23 | Quercetin | - | - | - | - | - | - | 1.05 | inf | - | - | 1.39 | inf | - | - |
| 25 | Apigenin-7-glucuronide | 1.15 | inf | 1.98 | inf | 1.24 | inf | - | - | 1.27 | −1.34 | - | - | - | - |
| 28 | Scutellarin | - | - | - | - | - | - | - | - | - | - | - | - | - | - |
| 30 | Isoliquiritigenin | - | - | - | - | 1.65 | 2.2 | 1.05 | inf | - | - | 1.38 | inf | 1.61 | _2.77 |
| 42 | Nicotiflorin | - | - | - | - | - | - | - | - | - | - | - | - | - | - |
| 48 | Narirutin | - | - | 1.89 | −5.14 | 1.27 | −1.89 | - | - | 1.72 | 4.39 | 1.39 | 17.52 | - | - |
| 50 | Luteolin | - | - | - | - | 1.02 | 1.36 | 1.05 | inf | - | - | 1.39 | inf | - | - |
| 55 | (-)-Catechin | - | - | 1.81 | 1.43 | 1.57 | 1.33 | - | - | - | - | - | - | - | - |
| 56 | Taxifolin | - | - | - | - | - | - | 1.48 | inf | - | - | 1.38 | inf | - | - |
| 57 | Rutin | - | - | - | - | - | - | - | - | - | - | - | - | - | - |
| 58 | Isorhamnetin | - | - | - | − | - | - | - | - | - | - | - | - | - | - |
| 65 | Phlorizin | 1.35 | −1.60 | - | - | - | - | 1.35 | 3.18 | 1.61 | 1.85 | 1.28 | 2.05 | 1.6 | 1.05 |
| 69 | Liquiritin | - | - | - | - | - | - | 1.36 | 1.37 | - | - | 1.21 | 1.5 | - | - |
| 80 | (-)-Gallocatechin gallate | 1.4 | 4.27 | - | - | - | - | 1.13 | −3.33 | - | - | - | - | - | - |
| 84 | (-)-Epigallocatechin | 1.42 | 3.19 | - | - | - | - | 1.29 | −3.36 | - | - | - | - | - | - |
| 90 | Cynaroside | 1.16 | 1.73 | 1.38 | inf | - | - | - | - | 1.27 | −inf | - | - | - | - |
| 96 | Calycosin-7-O-β-D-glucoside | 1.64 | inf | - | - | 1.74 | inf | - | - | - | - | - | - | 1.23 | −inf |
| Index | Compounds | WT-(mg/kg) | WT-BR3 (mg/kg) | WT-BR9 (mg/kg) | NOR-like1-GR (mg/kg) | NOR-like1-BR3 (mg/kg) | NOR-like1-BR9 (mg/kg) |
|---|---|---|---|---|---|---|---|
| 1 | Astilbin | 0.021 ± 0.01 | 0.018 ± 0 | 0.013 ± 0 | 0.03 ± 0.01 | 0.024 ± 0 | 0.022 ± 0 |
| 2 | Miquelianin | 0.011 ± 0 | 0.018 ± 0 | 0.021 ± 0.01 | 0.013 ± 0 | 0.014 ± 0 | 0.06 ± 0.09 |
| 9 | Genistein | - | 0.013 ± 0 | 0.018 ± 0 | - | 0.011 ± 0.01 | 0.03 ± 0.01 |
| 106 | Typhaneoside | 0.012 ± 0.01 | 0.09 ± 0.04 | 0.03 ± 0.03 | 0.018 ± 0.03 | 0.04 ± 0.07 | 0.04 ± 0.04 |
| 11 | Hydroxysafflor yellow A | 0.08 ± 0.06 | 0.17 ± 0.09 | 0.10 ± 0.03 | 0.08 ± 0.04 | 0.21 ± 0.02 | 0.22 ± 0.11 |
| 115 | Quercitrin | 0.015 ± 0.02 | 0.004 ± 0 | 0.002 ± 0 | 0.1 ± 0.09 | 0.004 ± 0.01 | 0.006 ± 0.01 |
| 117 | Engeletin | - | 0.016 ± 0.01 | 0.008 ± 0.01 | - | 0.007 ± 0.01 | 0.008 ± 0.01 |
| 118 | Narcissin | 1.36 ± 0.31 | 1.76 ± 1.87 | 0.54 ± 0.12 | 0.8 ± 0.16 | 1.14 ± 0.28 | 0.77 ± 0.08 |
| 119 | Astragalin | 0.27 ± 0.06 | 0.12 ± 0.04 | 0.09 ± 0.07 | 0.12 ± 0.06 | 0.1 ± 0.02 | 0.23 ± 0.24 |
| 124 | Silychristin | - | 0.013 ± 0.01 | 0.03 ± 0.01 | 0.003 ± 0.01 | 0.003 ± 0.01 | 0.017 ± 0.02 |
| 126 | Eriodictyol | 0.016 ± 0 | 4.89 ± 2.31 | 4.67 ± 2.54 | 0.08 ± 0.06 | 7.19 ± 5.16 | 11.84 ± 3.07 |
| 13 | Isoorientin | - | - | 0.005 ± 0.01 | 0.008 ± 0.01 | - | 0.04 ± 0.02 |
| 135 | Theaflavin 3,3′-digallate | - | 0.05 ± 0.09 | - | - | - | - |
| 136 | 6,2′-Dihydroxyflavone | - | 0.019 ± 0 | 0.007 ± 0 | - | 0.014 ± 0.01 | 0.015 ± 0 |
| 138 | Baimaside | 0.59 ± 1 | 0.5 ± 0.53 | 0.006 ± 0 | 0.12 ± 0.08 | 0.07 ± 0.1 | 0.023 ± 0.03 |
| 139 | Dihydrokaempferol | 0.004 ± 0 | 0.63 ± 0.1 | 0.19 ± 0.06 | 0.005 ± 0 | 0.51 ± 0.26 | 0.32 ± 0.15 |
| 145 | Procyanidin B2 | 0.016 ± 0.02 | 0.023 ± 0.02 | 0.04 ± 0.03 | 0.37 ± 0.17 | 0.03 ± 0.03 | 0.028 ± 0.05 |
| 147 | (-)-Epicatechin | 0.05 ± 0.03 | 0.04 ± 0.02 | 0.05 ± 0.02 | 0.45 ± 0.44 | 0.03 ± 0.01 | 0.04 ± 0.03 |
| 150 | (-)-Catechin gallate | 0.015 ± 0.01 | 0.01 ± 0.01 | 0.021 ± 0.03 | 1.57 ± 2.43 | 0.019 ± 0.02 | 0.017 ± 0.01 |
| 152 | Ononin | 0.04 ± 0.05 | - | - | - | - | - |
| 153 | Glycitin | - | 0.05 ± 0.01 | 0.04 ± 0 | 0.06 ± 0.05 | 0.19 ± 0.1 | 0.13 ± 0.03 |
| 157 | Isomangiferin | 0.005 ± 0 | 0.002 ± 0 | 0.003 ± 0 | 0.005 ± 0 | 0.005 ± 0 | 0.006 ± 0 |
| 160 | Apigenin 7-glucoside | - | - | 0.011 ± 0.01 | - | - | 0.013 ± 0.01 |
| 169 | Quercetin 3-O-(6″-galloyl)-β-D-galactopyranoside | 0.003 ± 0 | - | - | - | - | - |
| 176 | Naringenin-7-glucoside | 0.08 ± 0.08 | 2.58 ± 0.75 | 4.05 ± 1.64 | 0.05 ± 0.03 | 1.42 ± 0.91 | 3.72 ± 0.31 |
| 177 | Phloretin | - | 1.80 ± 0.44 | 0.37 ± 0.17 | 0.005 ± 0.01 | 1.07 ± 0.68 | 0.50 ± 0.27 |
| 183 | 2′-Hydroxygenistein | - | 0.006 ± 0 | 0.003 ± 0 | - | 0.005 ± 0 | 0.005 ± 0 |
| 184 | Afzelechin | 0.04 ± 0.04 | 0.013 ± 0.02 | 0.019 ± 0.02 | 0.02 ± 0.04 | - | 0.009 ± 0.02 |
| 191 | Naringenin chalcone | 0.008 ± 0 | 28.46 ± 5.57 | 10.67 ± 4.94 | 0.13 ± 0.15 | 29.60 ± 22.19 | 21.62 ± 5.88 |
| 195 | (-)-Gallocatechin | 0.04 ± 0.04 | 0.05 ± 0.01 | 0.08 ± 0.03 | 0.20 ± 0.19 | 0.05 ± 0.01 | 0.04 ± 0.01 |
| 197 | Isorhamnetin 3-O-glucoside | 0.004 ± 0 | 0.004 ± 0.01 | 0.002 ± 0 | 0.002 ± 0 | 0.001 ± 0 | - |
| 201 | Trilobatin | - | 0.008 ± 0 | 0.03 ± 0 | - | 0.007 ± 0 | 0.018 ± 0.01 |
| 202 | Sieboldin | 0.008 ± 0.01 | 0.007 ± 0.01 | 0.22 ± 0.05 | 0.017 ± 0 | 0.015 ± 0.01 | 0.14 ± 0.04 |
| 23 | Quercetin | - | 0.13 ± 0.04 | 0.09 ± 0.05 | - | 0.09 ± 0.08 | 0.09 ± 0.03 |
| 25 | Apigenin-7-glucuronide | - | - | - | 0.002 ± 0 | 0.007 ± 0 | 0.003 ± 0 |
| 28 | Scutellarin | - | - | - | 0.019 ± 0.03 | - | 0.005 ± 0.01 |
| 30 | Isoliquiritigenin | - | 0.005 ± 0 | 0.001 ± 0 | - | 0.006 ± 0.01 | 0.003 ± 0 |
| 42 | Nicotiflorin | 102.77 ± 14.56 | 65.96 ± 25.76 | 53.63 ± 13.28 | 68.37 ± 45.17 | 46.47 ± 13.75 | 60.35 ± 23.33 |
| 48 | Narirutin | - | 0.04 ± 0.01 | 0.09 ± 0.05 | - | 0.001 ± 0 | 0.025 ± 0.02 |
| 50 | Luteolin | - | 0.009 ± 0 | 0.013 ± 0.01 | - | 0.015 ± 0.01 | 0.03 ± 0.01 |
| 55 | (-)-Catechin | 0.07 ± 0.03 | 0.06 ± 0.01 | 0.05 ± 0 | 0.34 ± 0.43 | 0.16 ± 0.06 | 0.12 ± 0.04 |
| 56 | Taxifolin | - | 0.04 ± 0.01 | 0.06 ± 0.02 | - | 0.05 ± 0.04 | 0.09 ± 0.01 |
| 57 | Rutin | 175.26 ± 44.54 | 168.46 ± 14.85 | 147.79 ± 18.69 | 101.18 ± 47.22 | 125.43 ± 29.2 | 145.01 ± 15.91 |
| 58 | Isorhamnetin | - | 0.004 ± 0.01 | - | - | - | - |
| 65 | Phlorizin | 0.012 ± 0.01 | 0.05 ± 0.01 | 0.11 ± 0.01 | 0.004 ± 0 | 0.04 ± 0.02 | 0.13 ± 0.02 |
| 69 | Liquiritin | 0.004 ± 0 | 0.011 ± 0 | 0.013 ± 0.01 | 0.004 ± 0 | 0.01 ± 0 | 0.013 ± 0 |
| 80 | (-)-Gallocatechin gallate | 0.09 ± 0.07 | 0.06 ± 0.04 | 0.11 ± 0.12 | 1.66 ± 1.57 | 0.17 ± 0.19 | 0.06 ± 0.02 |
| 84 | (-)-Epigallocatechin | 0.09 ± 0.06 | 0.06 ± 0.03 | 0.10 ± 0.11 | 0.85 ± 0.52 | 0.08 ± 0.06 | 0.04 ± 0.02 |
| 90 | Cynaroside | 0.05 ± 0.09 | - | - | 0.17 ± 0.03 | 0.06 ± 0.05 | - |
| 96 | Calycosin-7-O-β-D-glucoside | - | 0.009 ± 0.01 | - | 0.025 ± 0.01 | 0.025 ± 0.02 | 0.03 ± 0 |
| total amount | 281.02 ± 28.02 | 276.25 ± 22.95 | 223.37 ± 33.78 | 176.87 ± 66.48 | 214.39 ± 39.51 | 245.93 ± 35.24 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guan, D.; Zhao, Y.; Zhao, X.; Fu, D. Metabolomics Study of the Effect of Transcription Factor NOR-like1 on Flavonoids in Tomato at Different Stages of Maturity Using UPLC-MS/MS. Foods 2023, 12, 4445. https://doi.org/10.3390/foods12244445
Guan D, Zhao Y, Zhao X, Fu D. Metabolomics Study of the Effect of Transcription Factor NOR-like1 on Flavonoids in Tomato at Different Stages of Maturity Using UPLC-MS/MS. Foods. 2023; 12(24):4445. https://doi.org/10.3390/foods12244445
Chicago/Turabian StyleGuan, Di, Ying Zhao, Xiaodan Zhao, and Daqi Fu. 2023. "Metabolomics Study of the Effect of Transcription Factor NOR-like1 on Flavonoids in Tomato at Different Stages of Maturity Using UPLC-MS/MS" Foods 12, no. 24: 4445. https://doi.org/10.3390/foods12244445







