Investigation of Structural Characteristics and Solubility Mechanism of Edible Bird Nest: A Mucin Glycoprotein
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Chemicals
2.2. Preparation of Extracts of EBN Samples
2.2.1. EBN Extraction
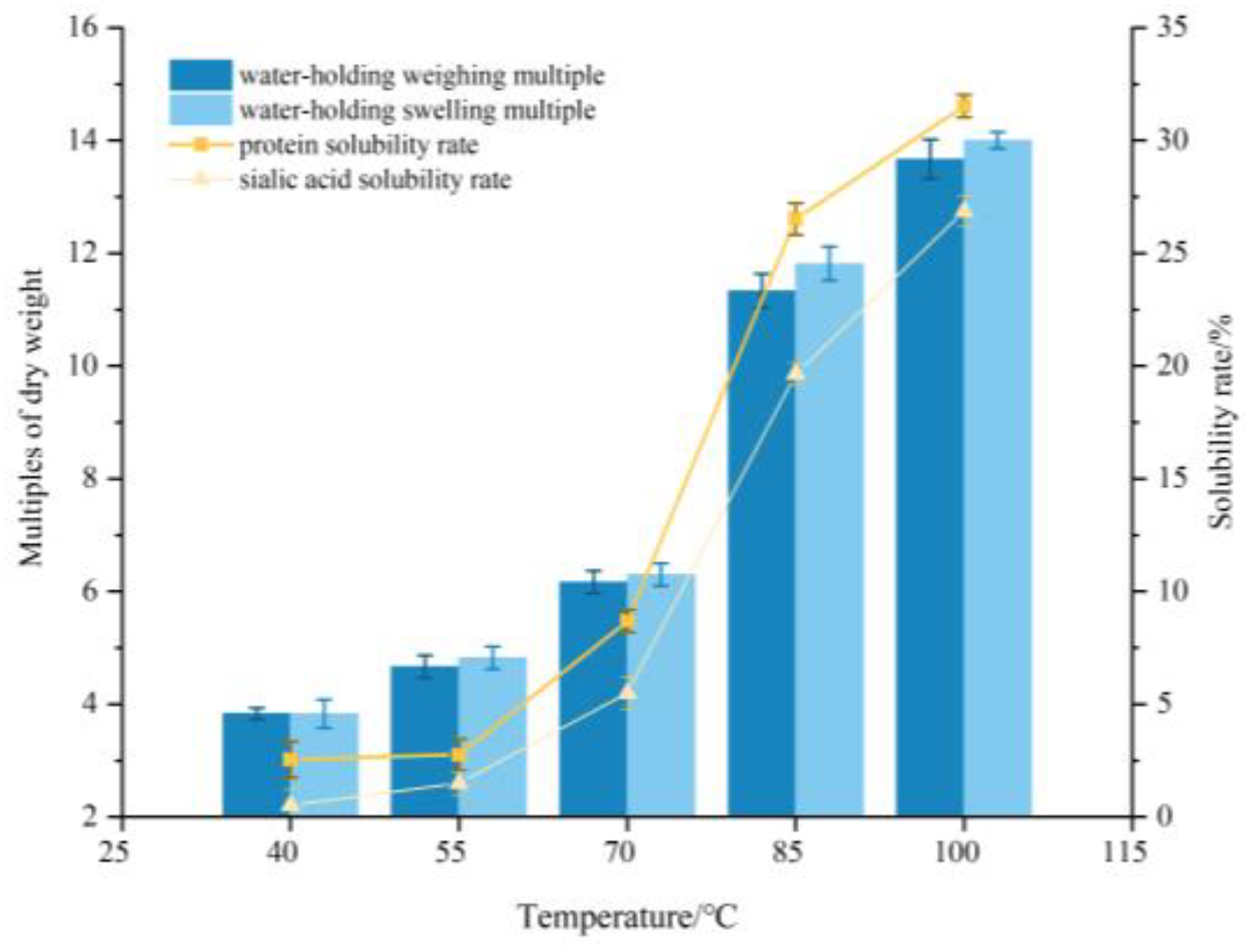
2.2.2. Protein and Sialic Acid Extraction Rate
2.2.3. Water-Holding Capacity
2.3. Soluble Compositions Analysis
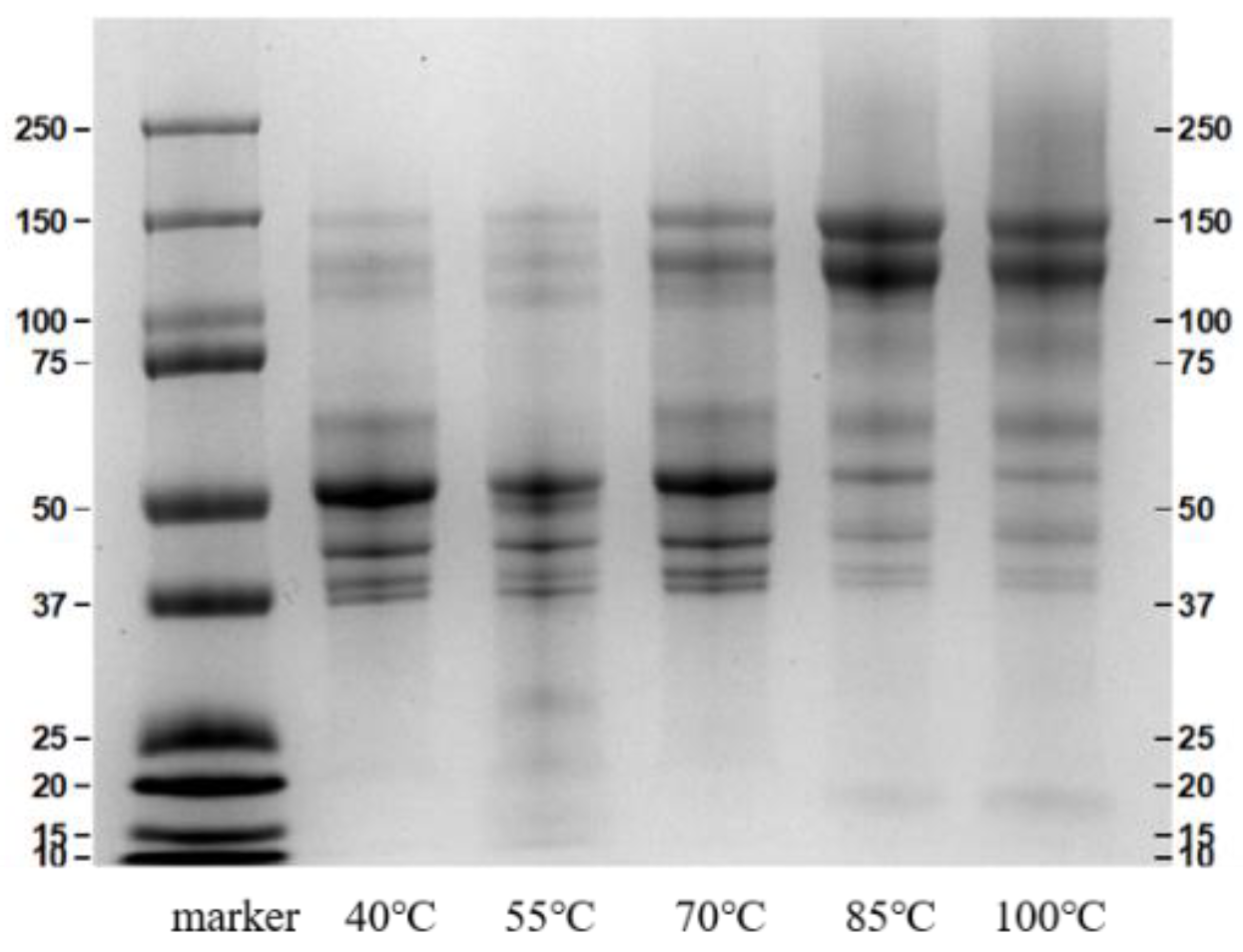
2.3.1. SDS-PAGE Analysis
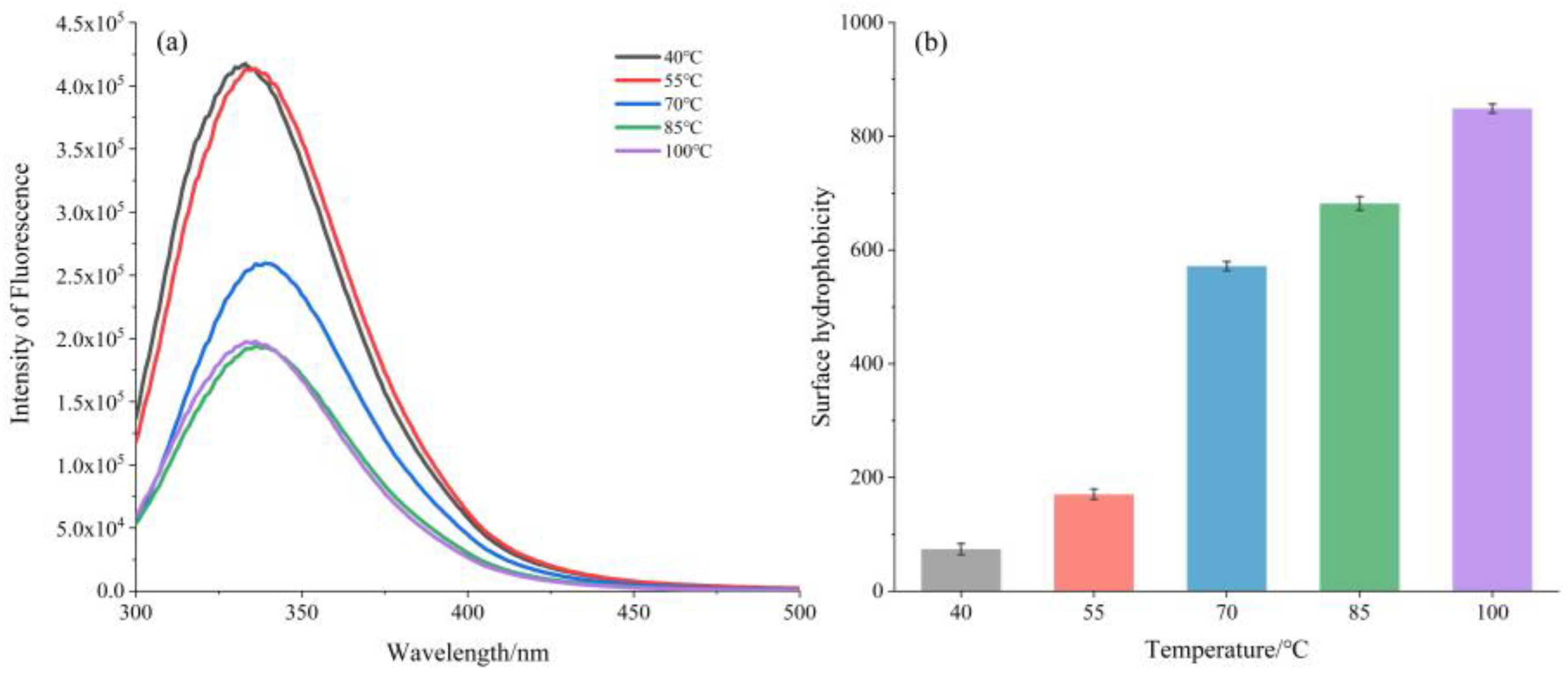
2.3.2. Intrinsic Fluorescence Spectroscopy Analysis
2.3.3. Surface Hydrophobicity Analysis
2.3.4. Secondary Structure Analysis
2.4. Insoluble Compositions Analysis
2.4.1. Amino Acid Composition Analysis
2.4.2. Secondary Structure Analysis
2.4.3. Relative Crystallinity Analysis
2.5. Different Combination of Cross-Linking Agents
2.6. Statistical Analysis
3. Results
3.1. Solubility and Water-Holding Capacity of EBN
3.2. Structure Analysis of EBN Soluble Fraction
3.2.1. SDS-PAGE Analysis
3.2.2. Intrinsic Fluorescence and Surface Hydrophobicity (S0)
3.2.3. CD Spectroscopy Analysis
3.3. Structure and Crystallinity Analysis of EBN Insoluble Fraction
3.3.1. Amino Acids Analysis
3.3.2. FTIR Analysis
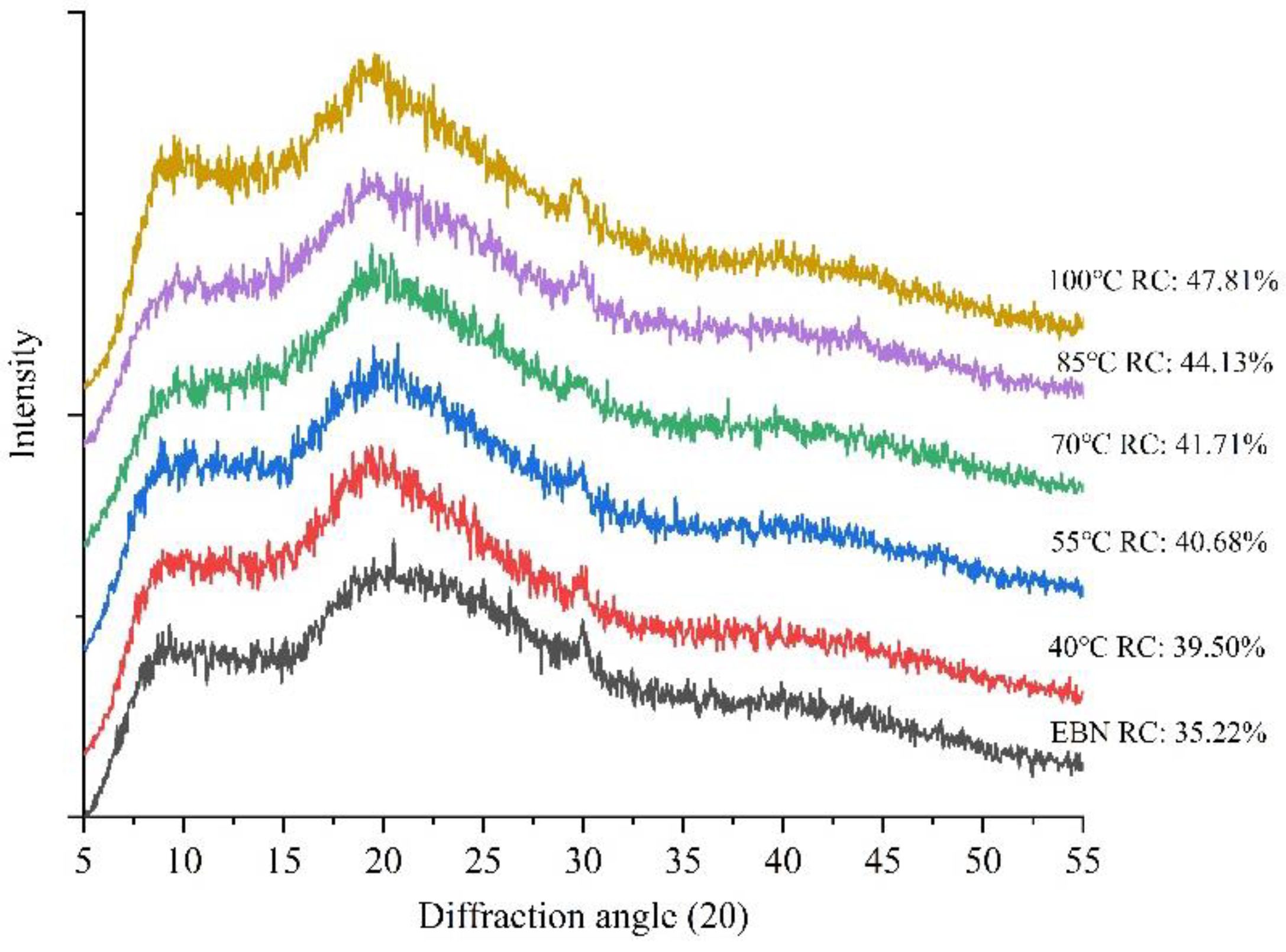
3.3.3. Relative Crystallinity Analysis
3.4. Chemical Bond Analysis
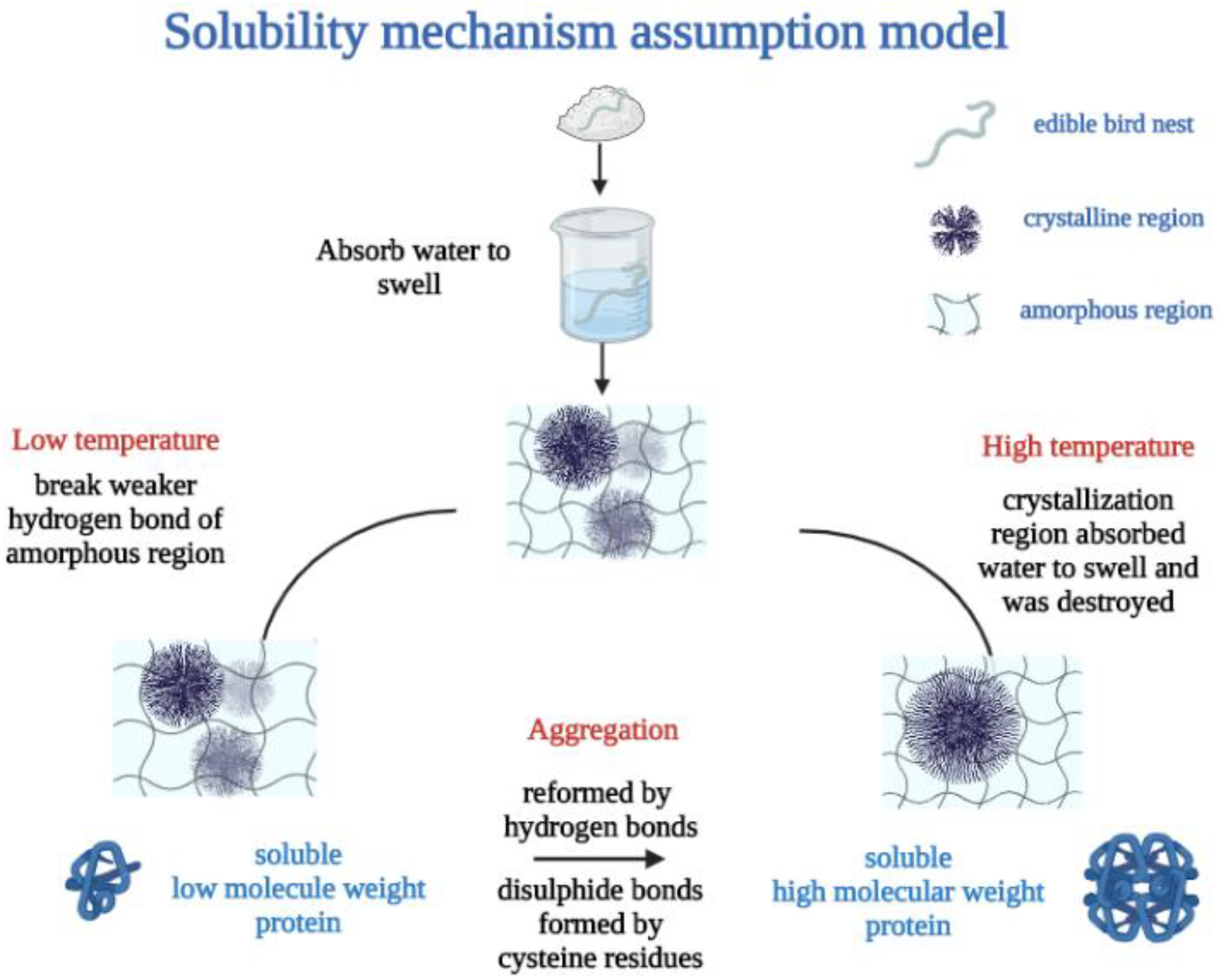
3.5. Solubility and Water-Holding Capacity Mechanism Assumption
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Dai, Y.; Cao, J.; Wang, Y.; Chen, Y.; Jiang, L. A comprehensive review of edible bird’s nest. Food Res. Int. 2021, 140, 109875. [Google Scholar] [CrossRef]
- Tai, S.K.; Hamzah, Z.; Ng, Q.H.; Tan, C.S. Surface Morphology Study on Unclean, Commercial and Bromelain Treated Edible Bird Nest (EBN) Using Scanning Electron Microscope. In IOP Conference Series: Materials Science and Engineering; IOP Publishing: Bristol, UK, 2020; Volume 932. [Google Scholar]
- Khushairay, E.S.I.; Ayub, M.K.; Babji, A.S. Effect of Enzymatic Hydrolysis of Pancreatin and Alcalase Enzyme on Some Properties of Edible Bird’s Nest Hydrolysate. In AIP Conference Proceedings; American Institute of Physics: College Park, MD, USA, 2014; pp. 427–432. [Google Scholar]
- Yida, Z.; Imam, M.U.; Ismail, M. In vitro bioaccessibility and antioxidant properties of edible bird’s nest following simulated human gastro-intestinal digestion. BMC Complement. Altern. Med. 2014, 14, 468. [Google Scholar] [CrossRef]
- Nasir, N.N.M.; Ibrahim, R.M.; Bakar, M.Z.A.; Mahmud, R.; Razak, N.A.A. Characterization and Extraction Influence Protein Profiling of Edible Bird’s Nest. Foods 2021, 10, 2248. [Google Scholar] [CrossRef] [PubMed]
- Wong, Z.C.F.; Chan, G.K.; Wu, L.; Lam, H.H.; Yao, P.; Dong, T.T.; Tsim, K.W. A comprehensive proteomics study on edible bird’s nest using new monoclonal antibody approach and application in quality control. J. Food Compos. Anal. 2018, 66, 145–151. [Google Scholar] [CrossRef]
- Ali, M.J.; Venugopal, A.; Ranganath, K.S.; Jagannadham, M.V.; Nadimpalli, S.K. Soluble glycoproteins of the lacrimal sac: Role in defense with special reference to prolactin-inducible protein (PIP). Orbit 2019, 38, 279–284. [Google Scholar] [CrossRef] [PubMed]
- Tong, S.R.; Lee, T.H.; Cheong, S.K.; Lim, Y.M. Untargeted metabolite profiling on the water-soluble metabolites of edible bird’s nest through liquid chromatography-mass spectrometry. Vet. World 2020, 13, 304–316. [Google Scholar] [CrossRef] [PubMed]
- Fan, Q.; Wang, P.; Zheng, X.; Hamzah, S.S.; Zeng, H.; Zhang, Y.; Hu, J. Effect of dynamic high pressure microfluidization on the solubility properties and structure profiles of proteins in water-insoluble fraction of edible bird’s nests. LWT 2020, 132, 109923. [Google Scholar] [CrossRef]
- Gan, S.H.; Ong, S.P.; Chin, N.L.; Law, C.L. A comparative quality study and energy saving on intermittent heat pump drying of Malaysian edible bird’s nest. Dry. Technol. 2016, 35, 4–14. [Google Scholar] [CrossRef]
- Sissolak, B.; Zabik, C.; Saric, N.; Sommeregger, W.; Vorauer-Uhl, K.; Striedner, G. Application of the Bradford Assay for Cell Lysis Quantification: Residual Protein Content in Cell Culture Supernatants. Biotechnol. J. 2019, 14, 1800714. [Google Scholar] [CrossRef]
- Zukefli, S.N.; Chua, L.S.; Rahmat, Z. Protein Extraction and Identification by Gel Electrophoresis and Mass Spectrometry from Edible bird’s Nest Samples. Food Anal. Methods 2016, 10, 387–398. [Google Scholar] [CrossRef]
- Liu, L.; Li, X.; Du, L.; Zhang, X.; Yang, W.; Zhang, H. Effect of ultrasound assisted heating on structure and antioxidant activity of whey protein peptide grafted with galactose. LWT 2019, 109, 130–136. [Google Scholar] [CrossRef]
- Huang, S.; Zhao, L.; Chen, Q.; Qin, Z.; Zhou, J.; Qiu, Y.; Zhang, Y.; Ma, M. Physicochemical Characteristics of Edible Bird’s Nest Proteins and Their Cooking Processing Properties. Int. J. Food Eng. 2018, 14, 3. [Google Scholar] [CrossRef]
- Li, X.-J.; Dong, G.P.; Fang, J.M.; Liu, H.J.; Guo, W.L. Parasitic behaviour of Dastarcus helophoroides (Fairmaire) (Coleoptera: Bothrideridae) induced changes in free animo acid pools in hemolymph of host Monochamus alternatus Hope (Coleoptera: Cerambycidae). Orient. Insects 2017, 52, 329–341. [Google Scholar] [CrossRef]
- Guo, L.; Wu, Y.; Liu, M.; Ge, Y.; Chen, Y. Rapid authentication of edible bird’s nest by FTIR spectroscopy combined with chemometrics. J. Sci. Food Agric. 2018, 98, 3057–3065. [Google Scholar] [CrossRef]
- Adenan, M.N.H.; Moosa, S.; Muhammad, S.A.; Abrahim, A.; Jandric, Z.; Islam, M.; Rodionova, O.; Pomerantsev, A.; Perston, B.; Cannavan, A.; et al. Screening Malaysian edible bird’s nests for structural adulterants and geographical origin using Mid-Infrared—Attenuated Total Reflectance (MIR-ATR) spectroscopy combined with chemometric analysis by Data-Driven—Soft Independent Modelling of Class Analogy (DD-SIMCA). Forensic Chem. 2020, 17, 100197. [Google Scholar]
- Chantakun, K.; Benjakul, S. Qualities of dried edible bird’s nest flakes from different drying methods and properties of their beverage. Dry. Technol. 2020, 40, 243–253. [Google Scholar] [CrossRef]
- Fang, S.; Chen, C.; Yao, Y.; Nsor-Atindana, J.; Liu, F.; Chen, M.; Zhong, F. Study on the Pasting Properties of Indica and Japonica Waxy Rice. Foods 2022, 11, 1132. [Google Scholar] [CrossRef]
- Meenmanee, S.; Rattananukrom, A.; Thaiphanit, S.; Suppavorasatit, I. Improvement of solubility, foaming, and emulsification properties of coconut (Cocos nucifera L.) protein by non-enzymatic deamidation. LWT 2022, 153, 112493. [Google Scholar] [CrossRef]
- Tang, S.Q.; Du, Q.H.; Fu, Z. Ultrasonic treatment on physicochemical properties of water-soluble protein from Moringa oleifera seed. Ultrason. Sonochem. 2021, 71, 105357. [Google Scholar] [CrossRef]
- Wagner, J.R.; AÑON, M.C. Influence of denaturation, hydrophobicity and sulfhydryl content on solubility and water absorbing capacity of soy protein isolates. J. Food Sci. 1990, 55, 765–770. [Google Scholar] [CrossRef]
- Tang, M.Q.; Gao, Q.; Xu, Y.; Zhong, L.; Wang, X.W.; Zhang, J.W.; Peng, X.; Tanokura, M.; Xue, Y.L. Solubility and emulsifying activity of yam soluble protein. J. Food Sci. Technol. 2020, 57, 1619–1627. [Google Scholar] [CrossRef] [PubMed]
- Luo, L.; Wang, Z.; Deng, Y.; Wei, Z.; Zhang, Y.; Tang, X.; Liu, G.; Zhou, P.; Zhao, Z.; Zhang, M.; et al. High-pressure homogenization: A potential technique for transforming insoluble pea protein isolates into soluble aggregates. Food Chem. 2022, 397, 133684. [Google Scholar] [CrossRef]
- Han, G.; Xu, J.; Chen, Q.; Xia, X.; Liu, H.; Kong, B. Improving the solubility of myofibrillar proteins in water by destroying and suppressing myosin molecular assembly via glycation. Food Chem. 2022, 395, 133590. [Google Scholar] [CrossRef] [PubMed]
- Chiang, J.H.; Loveday, S.M.; Hardacre, A.K.; Parker, M.E. Effects of soy protein to wheat gluten ratio on the physicochemical properties of extruded meat analogues. Food Struct. 2019, 19, 100102. [Google Scholar] [CrossRef]
- Li, S.; He, Z.; Qu, C.; Yu, S.; Li, M.; Li, H. Insights into the structural characteristic of rabbit glycated myofibrillar protein with high solubility in low ionic strength medium. LWT 2021, 137, 110387. [Google Scholar] [CrossRef]
- Zhao, M.; Xiong, W.; Chen, B.; Zhu, J.; Wang, L. Enhancing the solubility and foam ability of rice glutelin by heat treatment at pH12: Insight into protein structure. Food Hydrocoll. 2020, 103, 105626. [Google Scholar] [CrossRef]
- Yan, S.; Xu, J.; Zhang, S.; Li, Y. Effects of flexibility and surface hydrophobicity on emulsifying properties: Ultrasound-treated soybean protein isolate. LWT 2021, 142, 110881. [Google Scholar] [CrossRef]
- Yu, J.J.; Ji, H.; Chen, Y.; Zhang, Y.F.; Zheng, X.C.; Li, S.H.; Chen, Y. Analysis of the glycosylation products of peanut protein and lactose by cold plasma treatment: Solubility and structural characteristics. Int. J. Biol. Macromol. 2020, 158, 1194–1203. [Google Scholar] [CrossRef]
- Kumar, V.; Sharma, V.K.; Kalonia, D.S. Effect of polyols on polyethylene glycol (PEG)-induced precipitation of proteins: Impact on solubility, stability and conformation. Int. J. Pharm. 2009, 366, 38–43. [Google Scholar] [CrossRef]
- Wang, W.Q.; Bao, Y.H.; Chen, Y. Characteristics and antioxidant activity of water-soluble Maillard reaction products from interactions in a whey protein isolate and sugars system. Food Chem. 2013, 139, 355–361. [Google Scholar] [CrossRef]
- Grossmann, L.; Hinrichs, J.; Weiss, J. Solubility of extracted proteins from Chlorella sorokiniana, Phaeodactylum tricornutum, and Nannochloropsis oceanica: Impact of pH-value. LWT 2019, 105, 408–416. [Google Scholar] [CrossRef]
- Ali, A.A.; Noor, H.S.; Chong, P.K.; Babji, A.S.; Lim, S.J. Comparison of amino acids profile and antioxidant activities between edible bird nest and chicken egg. Malays. Appl. Biol. 2019, 48, 63–69. [Google Scholar]
- Nomoto, A.; Nishinami, S.; Shiraki, K. Affinity of aromatic amino acid side chains in amino acid solvents. Biophys. Chem. 2022, 287, 106831. [Google Scholar] [CrossRef] [PubMed]
- Hirano, A.; Wada, M.; Sato, T.K.; Kameda, T. The solubility of N-acetyl amino acid amides in organic acid and alcohol solutions: Mechanistic insight into structural protein solubilization. Int. J. Biol. Macromol. 2021, 178, 607–615. [Google Scholar] [CrossRef]
- Yan, C.; Zhou, Z. Solubility and emulsifying properties of phosphorylated walnut protein isolate extracted by sodium trimetaphosphate. LWT 2021, 143. [Google Scholar] [CrossRef]
- Li, Y.; Cheng, Y.; Zhang, Z.; Wang, Y.; Mintah, B.K.; Dabbour, M.; Jiang, H.; He, R.; Ma, H. Modification of rapeseed protein by ultrasound-assisted pH shift treatment: Ultrasonic mode and frequency screening, changes in protein solubility and structural characteristics. Ultrason. Sonochem. 2020, 69, 105240. [Google Scholar] [CrossRef]
- Wang, N.; Zhou, X.; Wang, W.; Wang, L.; Jiang, L.; Liu, T.; Yu, D. Effect of high intensity ultrasound on the structure and solubility of soy protein isolate-pectin complex. Ultrason. Sonochem. 2021, 80, 105808. [Google Scholar] [CrossRef]
- Liu, M.; Zhou, S.; Li, Y.; Tian, J.; Zhang, C. Structure, physicochemical properties and effects on nutrients digestion of modified soluble dietary fiber extracted from sweet potato residue. Food Res. Int. 2021, 150 Pt A, 110761. [Google Scholar] [CrossRef]
- Kebede, T.G.; Dube, S.; Nindi, M.M. Characterisation of water-soluble protein powder and optimisation of process parameters for the removal of sulphonamides from wastewater. Environ. Sci. Pollut. Res. Int. 2019, 26, 21450–21462. [Google Scholar] [CrossRef]
- Su, Y.; Chen, Y.; Zhang, L.; Adhikari, B.; Xu, B.; Li, J.; Zheng, T. Synthesis and characterization of lotus seed protein-based curcumin microcapsules with enhanced solubility, stability, and sustained release. J. Sci. Food Agric. 2022, 102, 2220–2231. [Google Scholar] [CrossRef]
- Chen, F.L.; Wei, Y.M.; Zhang, B. Chemical cross-linking and molecular aggregation of soybean protein during extrusion cooking at low and high moisture content. LWT Food Sci. Technol. 2011, 44, 957–962. [Google Scholar] [CrossRef]
- Nick Pace, C.; Trevino, S.; Prabhakaran, E.; Martin Scholtz, J. Protein structure, stability and solubility in water and other solvents. Philos. Trans. R. Soc. Lond. Ser. B Biol. Sci. 2004, 359, 1225–1235. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, N.; Ma, Y.; Chen, N.; Tang, X.; Lv, S. Effect of Microwave Irradiation on Dipeptides and Proteins Derived from Silk during Solvation. Adv. Fiber Mater. 2022, 4, 448–456. [Google Scholar] [CrossRef]

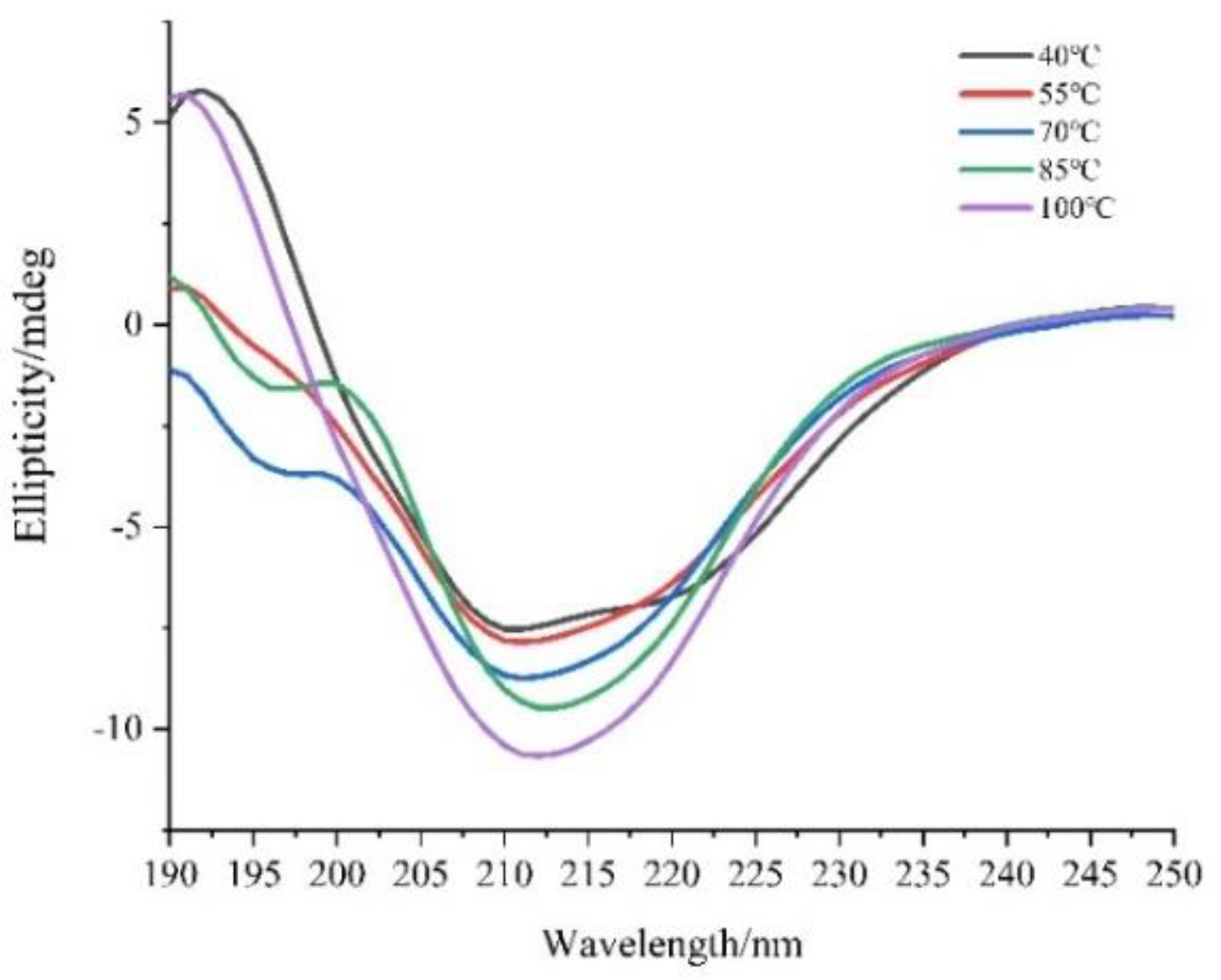
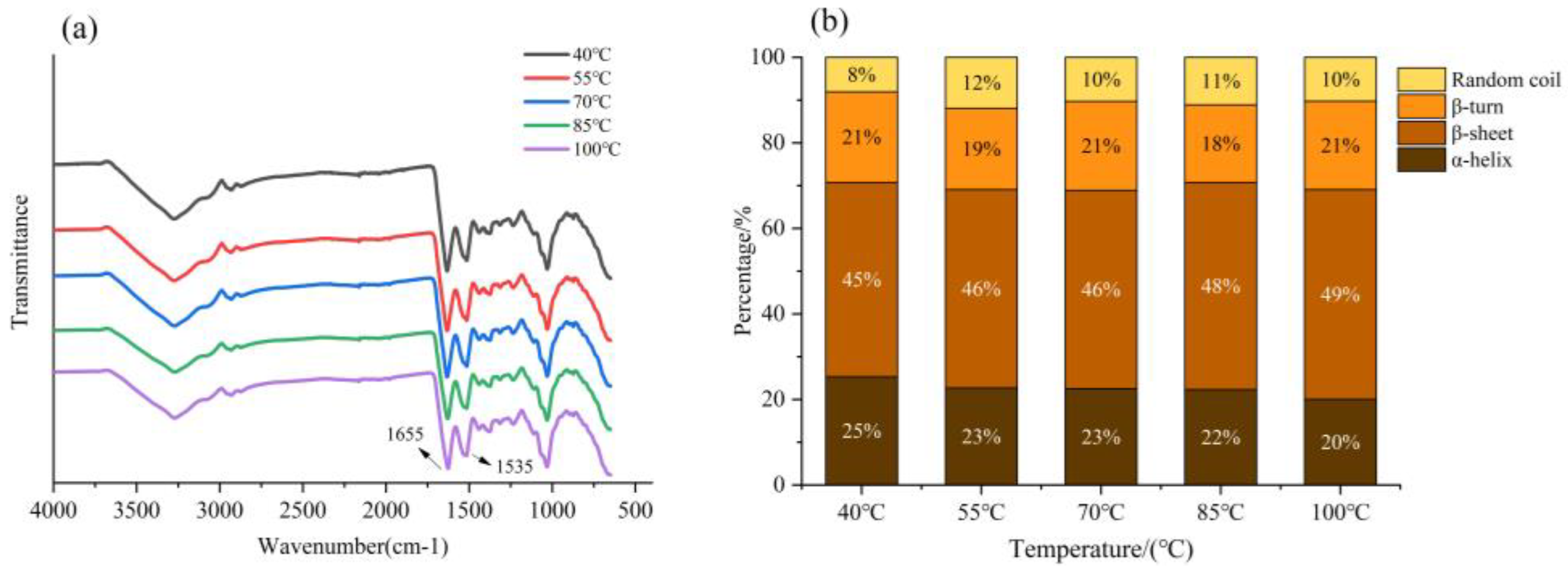

| Temperature(°C) | Protein Secondary Structure Content (%) | ||||
|---|---|---|---|---|---|
| β-sheet | Random Coil | α-Helix | β-Turn | NRMSD 1 | |
| 40 | 31.90 | 36.30 | 8.50 | 23.30 | 0.053 |
| 55 | 34.00 | 33.70 | 8.40 | 24.00 | 0.073 |
| 70 | 35.80 | 31.30 | 8.80 | 24.00 | 0.049 |
| 85 | 27.30 | 34.10 | 15.60 | 23.00 | 0.094 |
| 100 | 15.90 | 26.20 | 33.80 | 24.20 | 0.056 |
| AA g/100 g | Raw Material | 40 °C | 55 °C | 70 °C | 85 °C | 100 °C |
|---|---|---|---|---|---|---|
| Asp | 5.31 | 5.72 | 5.76 | 5.70 | 5.60 | 5.49 |
| Glu | 4.26 | 4.57 | 4.59 | 4.56 | 4.58 | 4.51 |
| Ser | 3.49 | 4.03 | 3.78 | 3.94 | 3.68 | 3.75 |
| His | 1.98 | 2.17 | 2.28 | 2.16 | 2.09 | 2.05 |
| Gly | 2.07 | 2.22 | 2.24 | 2.20 | 2.21 | 2.16 |
| Thr | 3.44 | 3.82 | 3.73 | 3.76 | 3.67 | 3.66 |
| Arg | 3.60 | 3.90 | 3.96 | 3.89 | 3.87 | 3.75 |
| Ala | 1.69 | 1.94 | 1.78 | 1.83 | 1.76 | 1.78 |
| Tyr | 3.04 | 3.44 | 3.52 | 3.43 | 3.36 | 3.23 |
| Cys-s | 0.67 | 0.77 | 0.69 | 0.76 | 0.86 | 0.61 |
| Val | 4.21 | 4.47 | 4.53 | 4.47 | 4.77 | 4.40 |
| Met | 0.55 | 0.55 | 0.55 | 0.03 | 0.82 | 0.56 |
| Phe | 3.46 | 3.75 | 3.79 | 3.73 | 3.86 | 3.60 |
| Ile | 1.85 | 1.97 | 2.00 | 1.96 | 2.07 | 1.93 |
| Leu | 3.84 | 4.15 | 4.16 | 4.13 | 4.06 | 4.01 |
| Lys | 2.09 | 2.30 | 2.29 | 2.29 | 2.72 | 2.23 |
| Pro | 3.97 | 4.28 | 3.23 | 4.22 | 4.03 | 4.13 |
| Total | 56.02 | 61.23 | 60.18 | 60.23 | 61.23 | 60.68 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lv, Y.; Xu, F.; Liu, F.; Chen, M. Investigation of Structural Characteristics and Solubility Mechanism of Edible Bird Nest: A Mucin Glycoprotein. Foods 2023, 12, 688. https://doi.org/10.3390/foods12040688
Lv Y, Xu F, Liu F, Chen M. Investigation of Structural Characteristics and Solubility Mechanism of Edible Bird Nest: A Mucin Glycoprotein. Foods. 2023; 12(4):688. https://doi.org/10.3390/foods12040688
Chicago/Turabian StyleLv, Yating, Feifei Xu, Fei Liu, and Maoshen Chen. 2023. "Investigation of Structural Characteristics and Solubility Mechanism of Edible Bird Nest: A Mucin Glycoprotein" Foods 12, no. 4: 688. https://doi.org/10.3390/foods12040688





