Exploring Nutrient Profiles, Phytochemical Composition, and the Antiproliferative Activity of Ganoderma lucidum and Ganoderma leucocontextum: A Comprehensive Comparative Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Mushroom Species
2.2. Cell Lines and Chemicals
2.3. Proximate and Mineral Analysis
2.4. Analysis of Amino Acids
2.5. Fatty Acid Analysis
2.6. Determination of Total Phenol
2.7. Determination of Total Flavonoids
2.8. Determination of Triterpenes
2.9. Analysis of Polysaccharide
2.10. Antioxidant Activity Assay
2.10.1. DPPH Radical Scavenging Activity
2.10.2. ABTS Radical Scavenging Activity
2.10.3. Reducing Power (Fe3+) Assay
2.11. Determination of Cell Viability
2.12. TUNEL Assay
2.13. Flow Cytometric Assay
2.14. Statistical Analysis
3. Results and Discussion
3.1. Proximate Analysis and Mineral Composition of GLU and GLE
3.2. Fatty Acid Composition of GLU and GLE
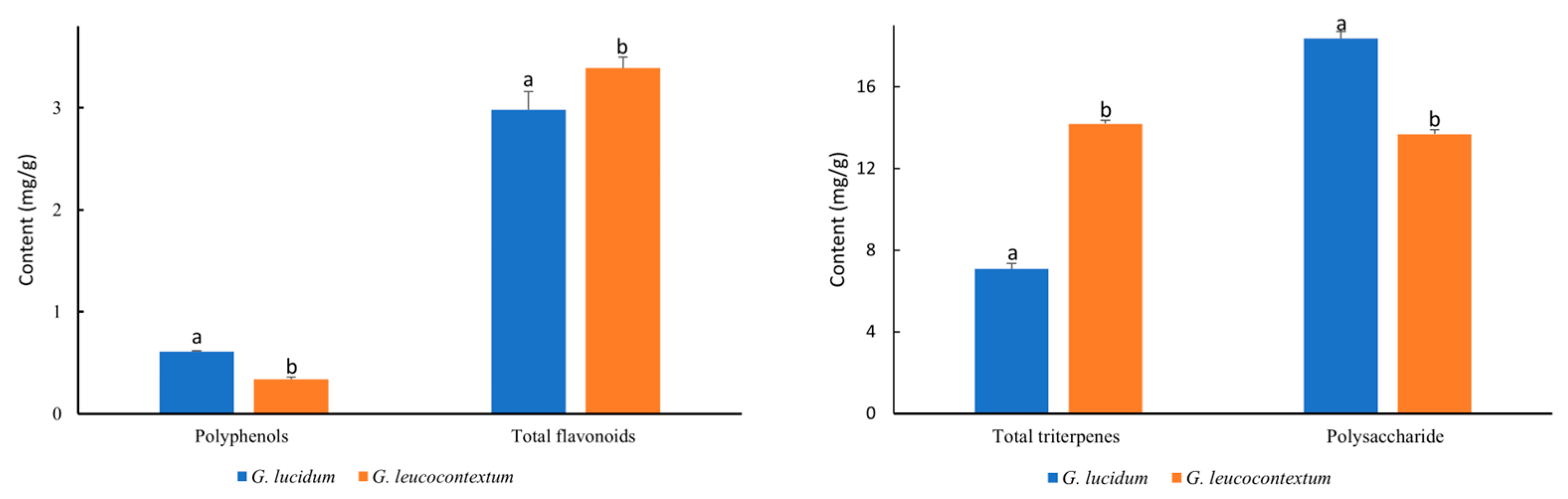
3.3. Bioactive Compounds of GLU and GLE
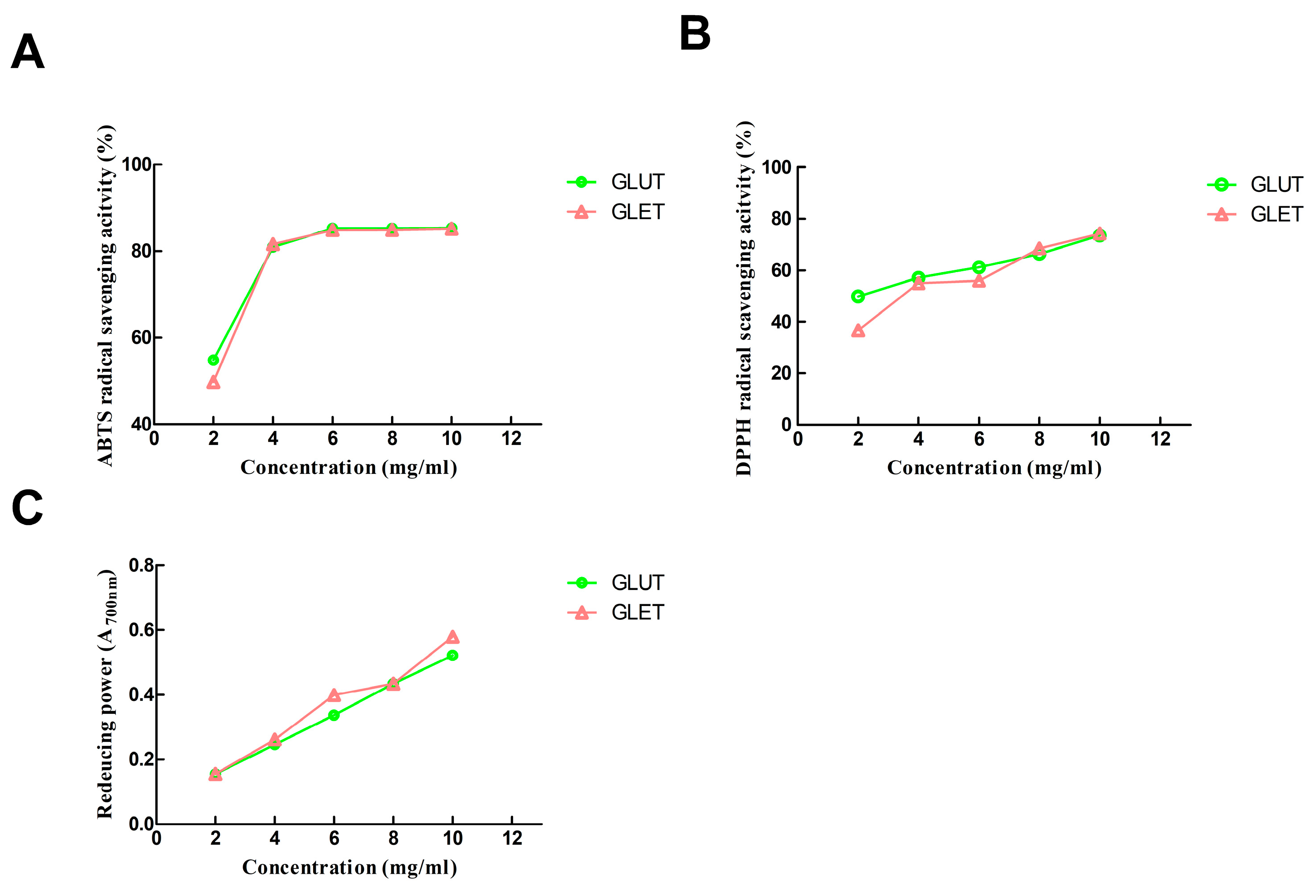
3.4. Antioxidant Activity of the Ethanol Extract of G. lucidum and G. leucocontextum
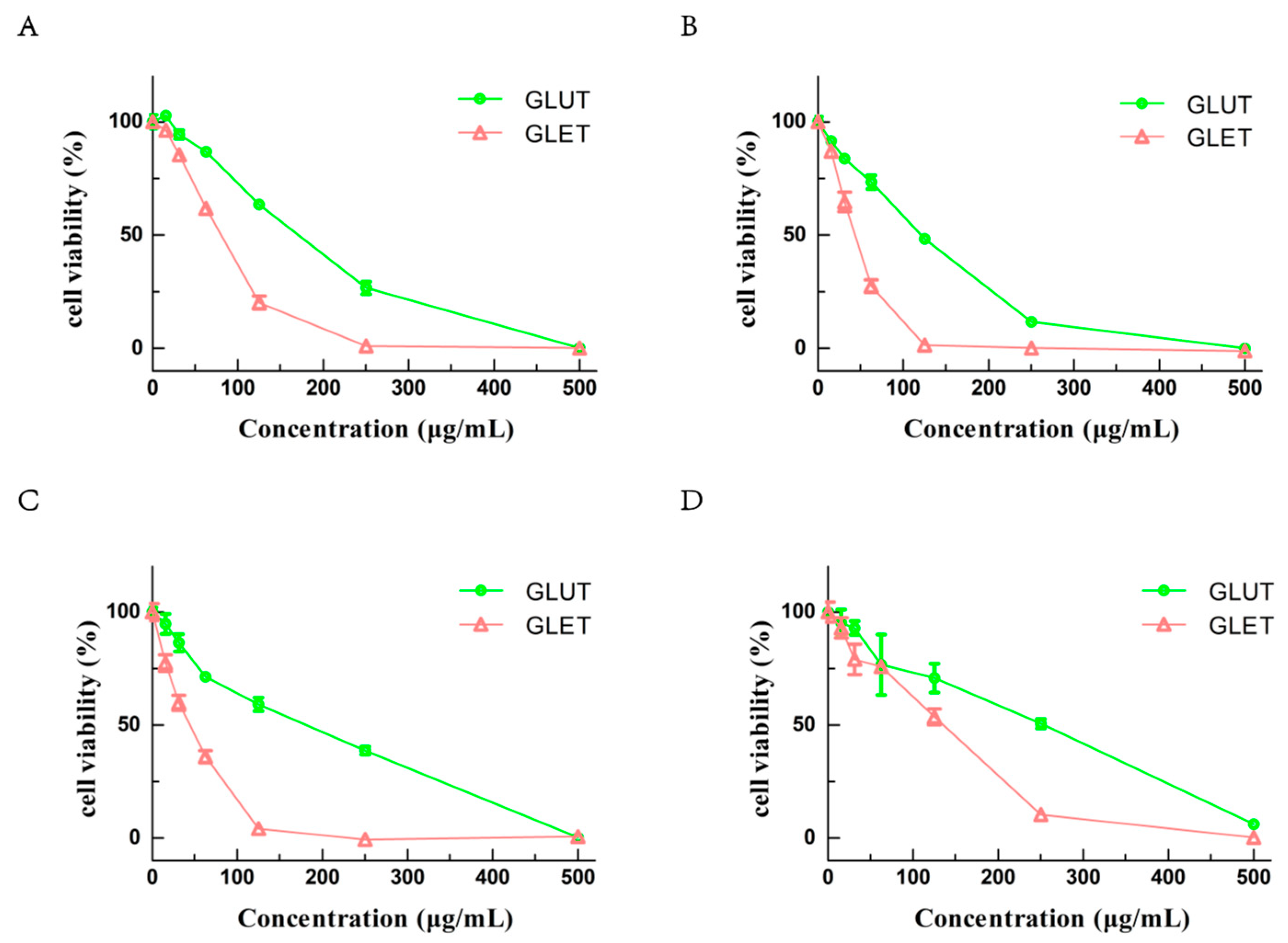
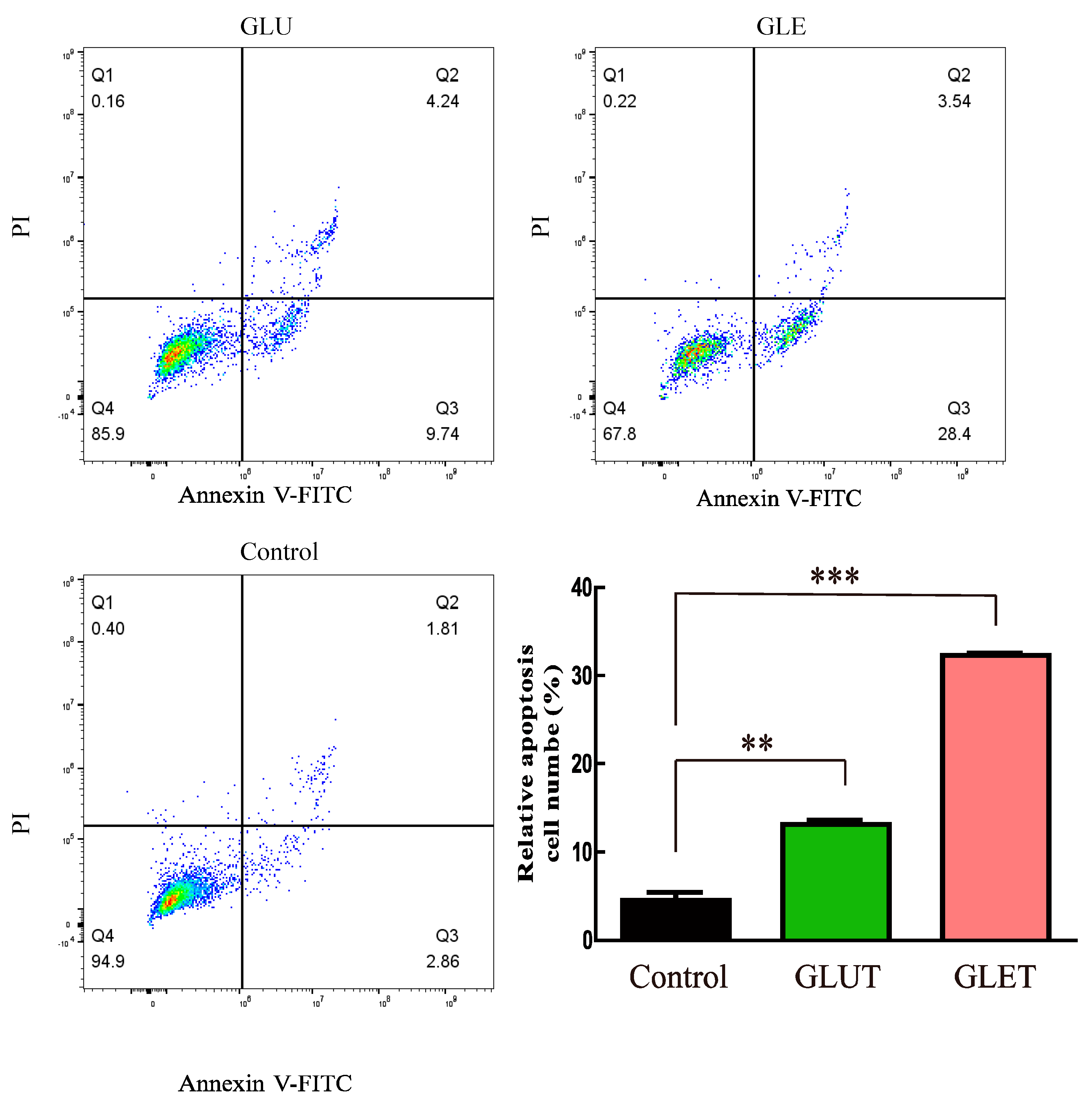
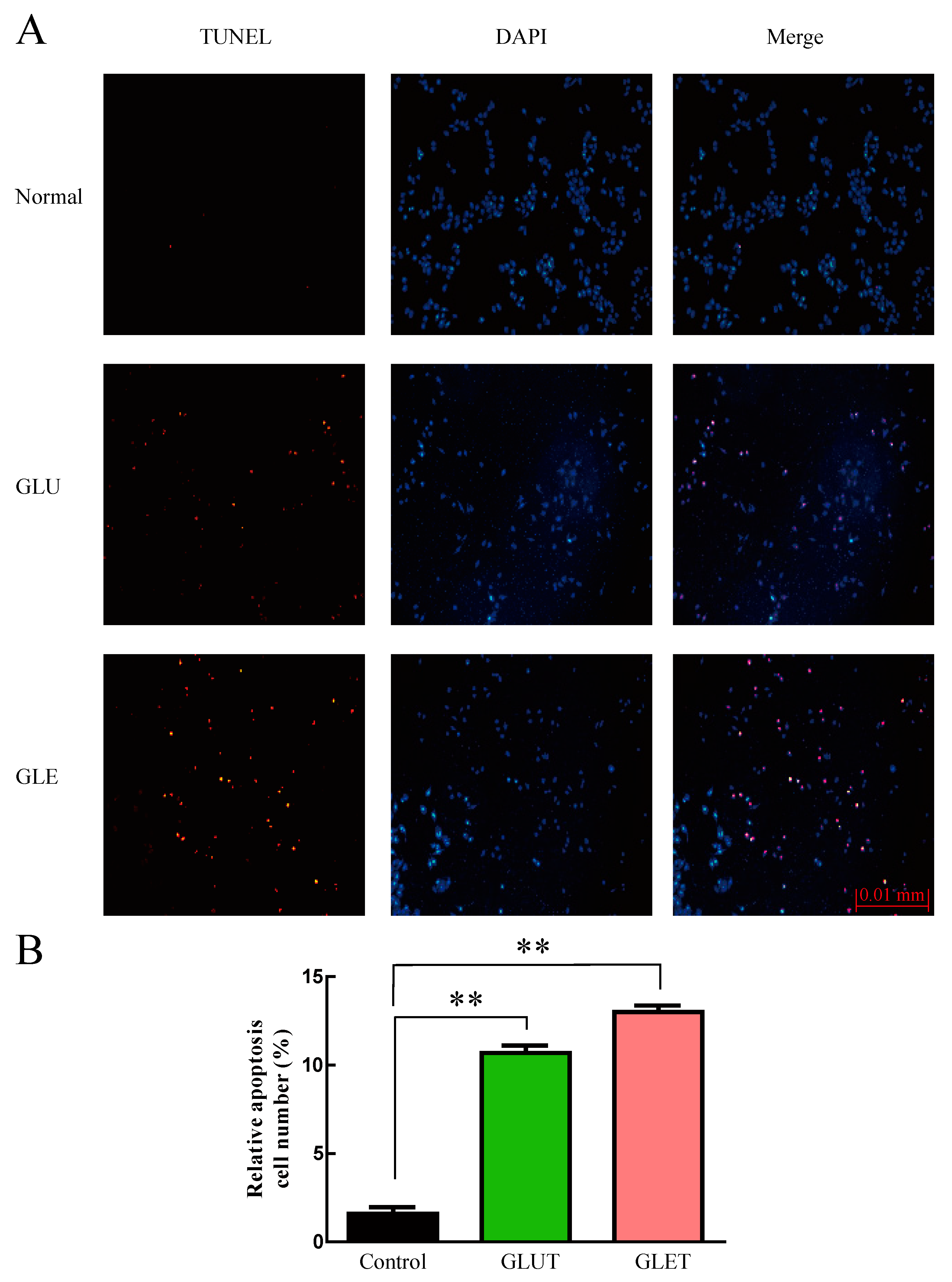
3.5. The Ethanol Extracts of Ganoderma sp. Suppress the Growth of Tumor Cells via Apoptosis
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Blundell, R.; Camilleri, E.; Baral, B.; Karpinski, T.M.; Neza, E.; Atrooz, O.M. The Phytochemistry of Ganoderma Species and their Medicinal Potentials. Am. J. Chin. Med. 2023, 51, 859–882. [Google Scholar] [CrossRef] [PubMed]
- Cor Andrejc, D.; Knez, Z.; Knez Marevci, M. Antioxidant, antibacterial, antitumor, antifungal, antiviral, anti-inflammatory, and nevro-protective activity of Ganoderma lucidum: An overview. Front. Pharmacol. 2022, 13, 934982. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, M.F. Ganoderma lucidum: Persuasive biologically active constituents and their health endorsement. Biomed. Pharmacother. Biomed. Pharmacother. 2018, 107, 507–519. [Google Scholar] [CrossRef] [PubMed]
- Boh, B.; Berovic, M.; Zhang, J.; Lin, Z.-B. Ganoderma lucidum and its pharmaceutically active compounds. Biotechnol. Annu. Rev. 2007, 13, 265–301. [Google Scholar] [CrossRef] [PubMed]
- Obodai, M.; Mensah, D.L.; Fernandes, A.; Kortei, N.K.; Dzomeku, M.; Teegarden, M.; Schwartz, S.J.; Barros, L.; Prempeh, J.; Takli, R.K.; et al. Chemical Characterization and Antioxidant Potential of Wild Ganoderma Species from Ghana. Molecules 2017, 22, 196. [Google Scholar] [CrossRef] [PubMed]
- Saltarelli, R.; Ceccaroli, P.; Buffalini, M.; Vallorani, L.; Casadei, L.; Zambonelli, A.; Iotti, M.; Badalyan, S.; Stocchi, V. Biochemical characterization and antioxidant and antiproliferative activities of different Ganoderma collections. J. Mol. Microbiol. Biotechnol. 2015, 25, 16–25. [Google Scholar] [CrossRef]
- Mohammadifar, S.; Fallahi Gharaghoz, S.; Asef Shayan, M.R.; Vaziri, A. Comparison between antioxidant activity and bioactive compounds of Ganoderma applanatum (Pers.) Pat. and Ganoderma lucidum (Curt.) P. Karst from Iran. Iran. J. Plant Physiol. 2020, 11, 3417–3424. [Google Scholar]
- Lv, G.P.; Zhao, J.; Duan, J.A.; Tang, Y.P.; Li, S.P. Comparison of sterols and fatty acids in two species of Ganoderma. Chem. Cent. J. 2012, 6, 10. [Google Scholar] [CrossRef]
- Gill, B.S.; Navgeet; Mehra, R.; Kumar, V.; Kumar, S. Ganoderic acid, lanostanoid triterpene: A key player in apoptosis. Investig. New Drugs 2018, 36, 136–143. [Google Scholar] [CrossRef]
- Xu, J.; Li, P. Researches and Application of Ganoderma Spores Powder. Adv. Exp. Med. Biol. 2019, 1181, 157–186. [Google Scholar] [CrossRef]
- Li, L.D.; Mao, P.W.; Shao, K.D.; Bai, X.H.; Zhou, X.W. Ganoderma proteins and their potential applications in cosmetics. Appl. Microbiol. Biotechnol. 2019, 103, 9239–9250. [Google Scholar] [CrossRef]
- Ogbe, A.O.; Obeka, A.D. Proximate, Mineral and Anti-Nutrient Composition of Wild Ganoderma lucidum: Implication on Its Utilization in Poultry Production. Iran. J. Appl. Anim. Sci. 2013, 3, 161–166. [Google Scholar]
- Fraile-Fabero, R.; Ozcariz-Fermoselle, M.V.; Oria-de-Rueda-Salgueiro, J.A.; Garcia-Recio, V.; Cordoba-Diaz, D.; Del, P.J.-L.M.; Girbes-Juan, T. Differences in Antioxidants, Polyphenols, Protein Digestibility and Nutritional Profile between Ganoderma lingzhi from Industrial Crops in Asia and Ganoderma lucidum from Cultivation and Iberian Origin. Foods 2021, 10, 1750. [Google Scholar] [CrossRef]
- Li, T.-H.; Hu, H.-P.; Deng, W.-Q.; Wu, S.-H.; Wang, D.-M.; Tsering, T. Ganoderma leucocontextum, a new member of the G. lucidum complex from southwestern China. Mycoscience 2015, 56, 81–85. [Google Scholar] [CrossRef]
- Chen, H.; Zhang, J.; Ren, J.; Wang, W.; Xiong, W.; Zhang, Y.; Bao, L.; Liu, H. Triterpenes and Meroterpenes with Neuroprotective Effects from Ganoderma leucocontextum. Chem. Biodivers. 2018, 15, e1700567. [Google Scholar] [CrossRef]
- Zhang, J.; Ma, K.; Han, J.; Wang, K.; Chen, H.; Bao, L.; Liu, L.; Xiong, W.; Zhang, Y.; Huang, Y.; et al. Eight new triterpenoids with inhibitory activity against HMG-CoA reductase from the medical mushroom Ganoderma leucocontextum collected in Tibetan plateau. Fitoterapia 2018, 130, 79–88. [Google Scholar] [CrossRef] [PubMed]
- Gao, X.; Qi, J.; Ho, C.T.; Li, B.; Xie, Y.; Chen, S.; Hu, H.; Chen, Z.; Wu, Q. Purification, Physicochemical Properties, and Antioxidant Activities of Two Low-Molecular-Weight Polysaccharides from Ganoderma leucocontextum Fruiting Bodies. Antioxidants 2021, 10, 1145. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.; Wang, K.; Kuang, S.; Cao, R.; Bao, L.; Liu, R.; Liu, H.; Sun, C. The natural compound GL22, isolated from Ganoderma mushrooms, suppresses tumor growth by altering lipid metabolism and triggering cell death. Cell Death Dis. 2018, 9, 689. [Google Scholar] [CrossRef] [PubMed]
- AOAC International. Official Methods of Analysis of AOAC International; AOAC International: Arlington, VA, USA, 1995; volume (loose-leaf). [Google Scholar]
- Lynch, J.M.; Barbano, D.M. Kjeldahl nitrogen analysis as a reference method for protein determination in dairy products. J. AOAC Int. 1999, 82, 1389–1398. [Google Scholar] [CrossRef]
- Tokul-Olmez, O.; Kaplaner, E.; Ozturk, M.; Ullah, Z.; Duru, M.E. Fatty acid profile of four Ganoderma species collected from various host trees with chemometric approach. Biochem. Syst. Ecol. 2018, 78, 91–97. [Google Scholar] [CrossRef]
- Sidhu, J.S.; Ali, M.; Al-Rashdan, A.; Ahmed, N. Onion (Allium cepa L.) is potentially a good source of important antioxidants. J. Food Sci. Technol. 2019, 56, 1811–1819. [Google Scholar] [CrossRef] [PubMed]
- Dat, T.D.; Viet, N.D.; Thanh, V.H.; Nhi, H.N.D.; Linh, N.T.T.; Ngan, N.T.K.; Nam, H.M.; Thanh Phong, M.; Hieu, N.H. Optimization of triterpenoid extracted from Vietnamese ganoderma lucidum via supercritical extraction method and biological tests. Sep. Sci. Technol. 2022, 57, 2211–2226. [Google Scholar] [CrossRef]
- Liu, S.-R.; Ke, B.-R.; Zhang, W.-R.; Liu, X.-R.; Wu, X.-P. Breeding of new Ganoderma lucidum strains simultaneously rich in polysaccharides and triterpenes by mating basidiospore-derived monokaryons of two commercial cultivars. Sci. Hortic. 2017, 216, 58–65. [Google Scholar] [CrossRef]
- Kebaili, F.F.; Tahar, N.; Esseddik, T.M.; Redouane, R.; Chawki, B.; Pablo, A.; Massimiliano, P. Antioxidant Activity and Phenolic Content of Extracts of Wild Algerian Lingzhi or Reishi Medicinal Mushroom, Ganoderma lucidum (Agaricomycetes). Int. J. Med. Mushrooms 2021, 23, 79–88. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.-F.; Guo, Y.-X.; Shangguan, X.; Zheng, G.; Wang, W.-J. Antioxidant and anti-proliferative activity of Rhizoma Smilacis Chinae extracts and main constituents. Food Chem. 2012, 133, 140–145. [Google Scholar] [CrossRef]
- Li, X.; Lin, J.; Gao, Y.; Han, W.; Chen, D. Antioxidant activity and mechanism of Rhizoma Cimicifugae. Chem. Cent. J. 2012, 6, 140. [Google Scholar] [CrossRef] [PubMed]
- Stojković, D.S.; Barros, L.; Calhelha, R.C.; Glamočlija, J.; Ćirić, A.; van Griensven, L.J.; Soković, M.; Ferreira, I.C. A detailed comparative study between chemical and bioactive properties of Ganoderma lucidum from different origins. Int. J. Food Sci. Nutr. 2014, 65, 42–47. [Google Scholar] [CrossRef] [PubMed]
- Soliman, G.A. Dietary Fiber, Atherosclerosis, and Cardiovascular Disease. Nutrients 2019, 11, 1155. [Google Scholar] [CrossRef]
- Sharif, S.; Rashid, S.; Atta, A.; Irshad, A.; Riaz, M.; Shahid, M.; Mustafa, G. Phenolics, tocopherols and fatty acid profiling of wild and commercial mushrooms from Pakistan. J. Biol. Regul. Homeost. Agents 2018, 32, 863–867. [Google Scholar]
- Froyen, E.; Burns-Whitmore, B. The Effects of Linoleic Acid Consumption on Lipid Risk Markers for Cardiovascular Disease in Healthy Individuals: A Review of Human Intervention Trials. Nutrients 2020, 12, 2329. [Google Scholar] [CrossRef]
- Kavishree, S.; Hemavathy, J.; Lokesh, B.R.; Shashirekha, M.N.; Rajarathnam, S. Fat and fatty acids of Indian edible mushrooms. Food Chem. 2008, 106, 597–602. [Google Scholar] [CrossRef]
- Barros, L.; Baptista, P.; Correia, D.M.; Casal, S.; Oliveira, B.; Ferreira, I.C.F.R. Fatty acid and sugar compositions, and nutritional value of five wild edible mushrooms from Northeast Portugal. Food Chem. 2007, 105, 140–145. [Google Scholar] [CrossRef]
- Taofiq, O.; Heleno, S.A.; Calhelha, R.C.; Alves, M.J.; Barros, L.; González-Paramás, A.M.; Barreiro, M.F.; Ferreira, I.C.F.R. The potential of Ganoderma lucidum extracts as bioactive ingredients in topical formulations, beyond its nutritional benefits. Food Chem. Toxicol. 2017, 108, 139–147. [Google Scholar] [CrossRef]
- Nakagawa, T.; Zhu, Q.; Tamrakar, S.; Amen, Y.; Mori, Y.; Suhara, H.; Kaneko, S.; Kawashima, H.; Okuzono, K.; Inoue, Y.; et al. Changes in content of triterpenoids and polysaccharides in Ganoderma lingzhi at different growth stages. J. Nat. Med. 2018, 72, 734–744. [Google Scholar] [CrossRef] [PubMed]
- Skalicka-Woźniak, K.S.J.Ł.R.S.M.S.K.G.K.M.A. Evaluation of polysaccharides content in fruit bodies and their antimicrobial activity of four Ganoderma lucidum (W Curt.: Fr.) P. Karst. strains cultivated on different wood type substrates. Acta Soc. Bot. Pol. 2012, 81, 17–21. [Google Scholar] [CrossRef]
- Xia, Q.; Zhang, H.; Sun, X.; Zhao, H.; Wu, L.; Zhu, D.; Yang, G.; Shao, Y.; Zhang, X.; Mao, X.; et al. A comprehensive review of the structure elucidation and biological activity of triterpenoids from Ganoderma spp. Molecules 2014, 19, 17478–17535. [Google Scholar] [CrossRef] [PubMed]
- Cadar, E.; Negreanu-Pirjol, T.; Pascale, C.; Sirbu, R.; Prasacu, I.; Negreanu-Pirjol, B.S.; Tomescu, C.L.; Ionescu, A.M. Natural Bio-Compounds from Ganoderma lucidum and Their Beneficial Biological Actions for Anticancer Application: A Review. Antioxidants 2023, 12, 1907. [Google Scholar] [CrossRef]
- Bacallao-Escudero, A.; Guerrero-German, P.; Torres-Moreno, H.; Vidal-Gutierrez, M.; Lopez-Romero, J.C.; Tejeda-Mansir, A.; Esqueda, M.; Robles-Zepeda, R.E. Biological Activity of Ganoderma Species (Agaricomycetes) from Sonoran Desert, Mexico. Int. J. Med. Mushrooms 2023, 25, 65–76. [Google Scholar] [CrossRef]
- Cör, D.; Botić, T.; Gregori, A.; Pohleven, F.; Knez, Ž. The effects of different solvents on bioactive metabolites and “in vitro” antioxidant and anti-acetylcholinesterase activity of Ganoderma lucidum fruiting body and primordia extracts. Maced. J. Chem. Chem. Eng. 2017, 36, 129–141. [Google Scholar] [CrossRef]
- Cilerdzic, J.; Vukojevic, J.; Stajic, M.; Stanojkovic, T.; Glamoclija, J. Biological activity of Ganoderma lucidum basidiocarps cultivated on alternative and commercial substrate. J. Ethnopharmacol. 2014, 155, 312–319. [Google Scholar] [CrossRef]
- Radwan, F.F.; Perez, J.M.; Haque, A. Apoptotic and Immune Restoration Effects of Ganoderic Acids Define a New Prospective for Complementary Treatment of Cancer. J. Clin. Cell. Immunol. 2011, S3, 4. [Google Scholar] [CrossRef]
- Kolniak-Ostek, J.; Oszmianski, J.; Szyjka, A.; Moreira, H.; Barg, E. Anticancer and Antioxidant Activities in Ganoderma lucidum Wild Mushrooms in Poland, as Well as Their Phenolic and Triterpenoid Compounds. Int. J. Mol. Sci. 2022, 23, 9359. [Google Scholar] [CrossRef]
- Veljovic, S.; Veljovic, M.; Nikicevic, N.; Despotovic, S.; Radulovic, S.; Niksic, M.; Filipovic, L. Chemical composition, antiproliferative and antioxidant activity of differently processed Ganoderma lucidum ethanol extracts. J. Food Sci. Technol. 2017, 54, 1312–1320. [Google Scholar] [CrossRef]
- Hu, H.; Ahn, N.S.; Yang, X.; Lee, Y.S.; Kang, K.S. Ganoderma lucidum extract induces cell cycle arrest and apoptosis in MCF-7 human breast cancer cell. Int. J. Cancer 2002, 102, 250–253. [Google Scholar] [CrossRef]
- Wu, G.; Qian, Z.; Guo, J.; Hu, D.; Bao, J.; Xie, J.; Xu, W.; Lu, J.; Chen, X.; Wang, Y. Ganoderma lucidum extract induces G1 cell cycle arrest, and apoptosis in human breast cancer cells. Am. J. Chin. Med. 2012, 40, 631–642. [Google Scholar] [CrossRef] [PubMed]
- Smina, T.P.; Nitha, B.; Devasagayam, T.P.; Janardhanan, K.K. Ganoderma lucidum total triterpenes induce apoptosis in MCF-7 cells and attenuate DMBA induced mammary and skin carcinomas in experimental animals. Mutat. Res. Genet. Toxicol. Environ. Mutagen. 2017, 813, 45–51. [Google Scholar] [CrossRef] [PubMed]
- Deng, S.; Aga, E.B.; Xie, H.; Xiong, H.; Ye, B. Evaluation of the acute toxicity and 28-days subacute toxicity of the alcoholic extract from Ganoderma leucocontextum. Food Sci. Nutr. 2023, 11, 434–442. [Google Scholar] [CrossRef] [PubMed]

| Chemical Composition | G. lucidum | G. leucocontextum |
|---|---|---|
| Total gross fat (g/100 g) | 2.02 ± 0.07 a | 3.54 ± 0.11 b |
| Crude protein (g/100 g) | 14.0 ± 0.36 a | 21.2 ± 0.31 b |
| Moisture (g/100 g) | 9.56 ± 0.43 a | 8.93 ± 0.28 a |
| Ash (g/100 g) | 1.20 ± 0.11 a | 2.20 ± 0.17 b |
| Dietary fiber (%) | 64.2 ± 2.30 a | 58.9 ± 1.30 a |
| Mineral (mg/kg) | ||
| Calcium | 1670 ± 0.01 a | 683 ± 21.3 b |
| Magnesium | 486 ± 23.0 a | 750 ± 28.0 b |
| Iron | 38.5 ± 1.30 a | 69.9 ± 3.5 b |
| Zinc | 18.3 ± 1.60 a | 42.9 ± 3.9 b |
| Amino acid (mg/g) | ||
| Aspartic acid | 8.90 ± 0.35 a | 12.6 ± 0.52 b |
| Threonine | 5.00 ± 0.12 a | 6.20 ± 0.35 b |
| Serine | 4.80 ± 0.06 a | 5.90 ± 0.36 b |
| Glutamic acid | 9.40 ± 0.29 a | 17.6 ± 0.43 b |
| Glycine | 4.60 ± 0.40 a | 6.30 ± 0.42 b |
| Alanine | 5.30 ± 0.46 a | 7.40 ± 0.41 b |
| Valine | 5.20 ± 0.40 a | 6.60 ± 0.29 b |
| Methionine | 0.81 ± 0.28 a | 1.50 ± 0.23 a |
| Isoleucine | 4.00 ± 0.52 a | 4.80 ± 0.35 a |
| Leucine | 6.10 ± 0.33 a | 8.40 ± 0.29 b |
| Tyrosine | 1.70 ± 0.44 a | 3.10 ± 0.23 b |
| Phenylalanine | 4.00 ± 0.46 a | 5.30 ± 0.23 a |
| Histidine | 9.10 ± 0.12 a | 12.6 ± 0.46 b |
| Lysine | 4.00 ± 0.11 a | 6.60 ± 0.24 b |
| Argnine | 3.10 ± 0.33 a | 8.00 ± 0.40 b |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Peng, G.; Xiong, C.; Zeng, X.; Jin, Y.; Huang, W. Exploring Nutrient Profiles, Phytochemical Composition, and the Antiproliferative Activity of Ganoderma lucidum and Ganoderma leucocontextum: A Comprehensive Comparative Study. Foods 2024, 13, 614. https://doi.org/10.3390/foods13040614
Peng G, Xiong C, Zeng X, Jin Y, Huang W. Exploring Nutrient Profiles, Phytochemical Composition, and the Antiproliferative Activity of Ganoderma lucidum and Ganoderma leucocontextum: A Comprehensive Comparative Study. Foods. 2024; 13(4):614. https://doi.org/10.3390/foods13040614
Chicago/Turabian StylePeng, Guoqin, Chuan Xiong, Xianfu Zeng, Ya Jin, and Wenli Huang. 2024. "Exploring Nutrient Profiles, Phytochemical Composition, and the Antiproliferative Activity of Ganoderma lucidum and Ganoderma leucocontextum: A Comprehensive Comparative Study" Foods 13, no. 4: 614. https://doi.org/10.3390/foods13040614




