Effects of the Roasting-Assisted Aqueous Ethanol Extraction of Peanut Oil on the Structure and Functional Properties of Dreg Proteins
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Reagents
2.2. Peanut Roasting and Grinding
2.3. Oil Extraction and Dreg Preparation
2.4. Protein Functional Properties
2.5. X-ray Diffraction (XRD)
2.6. Differential Scanning Calorimeter (DSC)
2.7. Ultraviolet (UV) and Intrinsic Fluorescence Spectra
2.8. Fourier-Transform Infrared Spectroscopy (FTIR)
2.9. Raman Spectroscopy
2.10. Microscopy Observation
2.11. Statistical Analysis
3. Results and Discussion
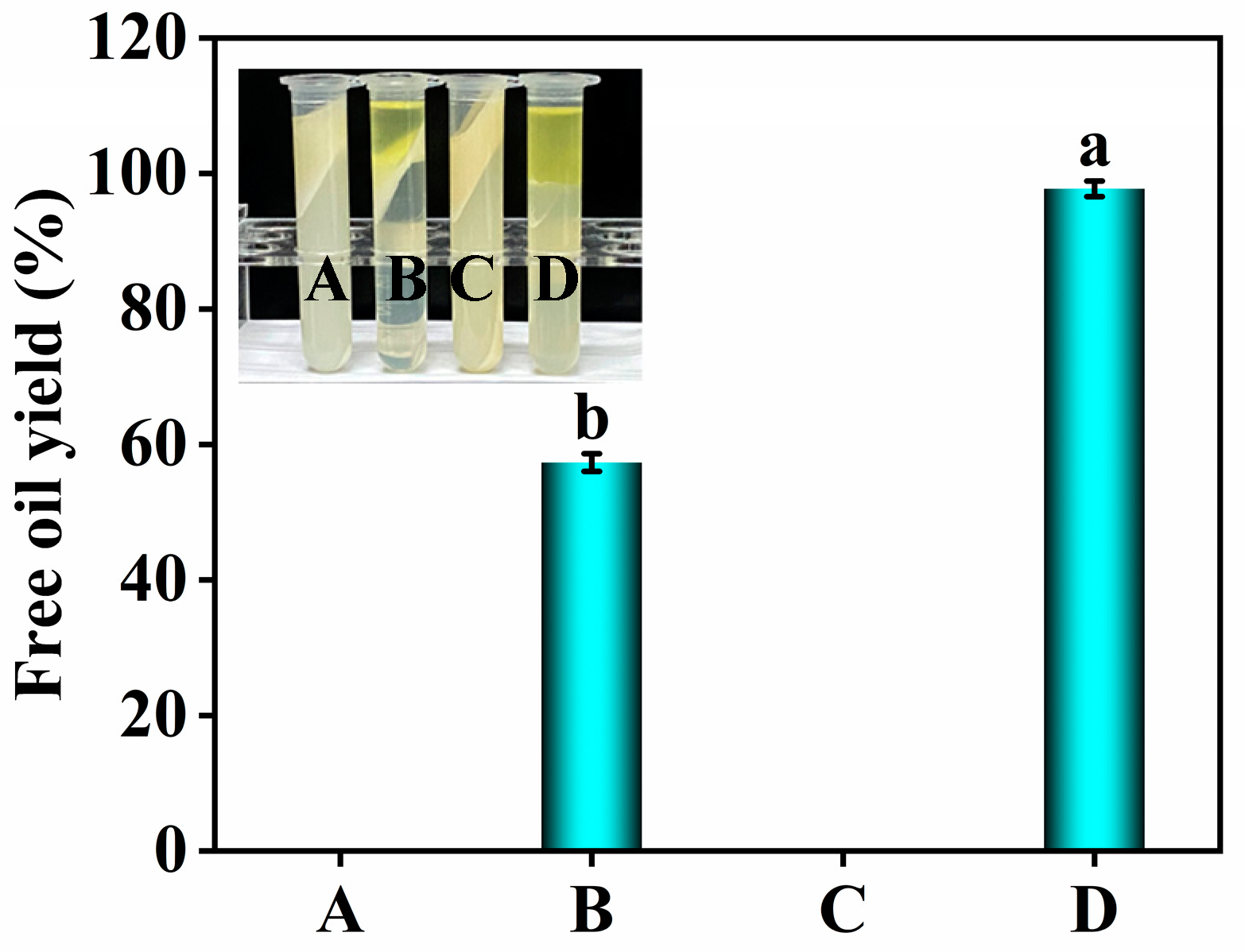
3.1. Effects of Roasting and Ethanol on the Free Oil Yield
3.2. Effect of Roasting on the Material Distribution during Oil Extraction
3.3. Effect of Roasting on the Functional Properties of Dreg Proteins
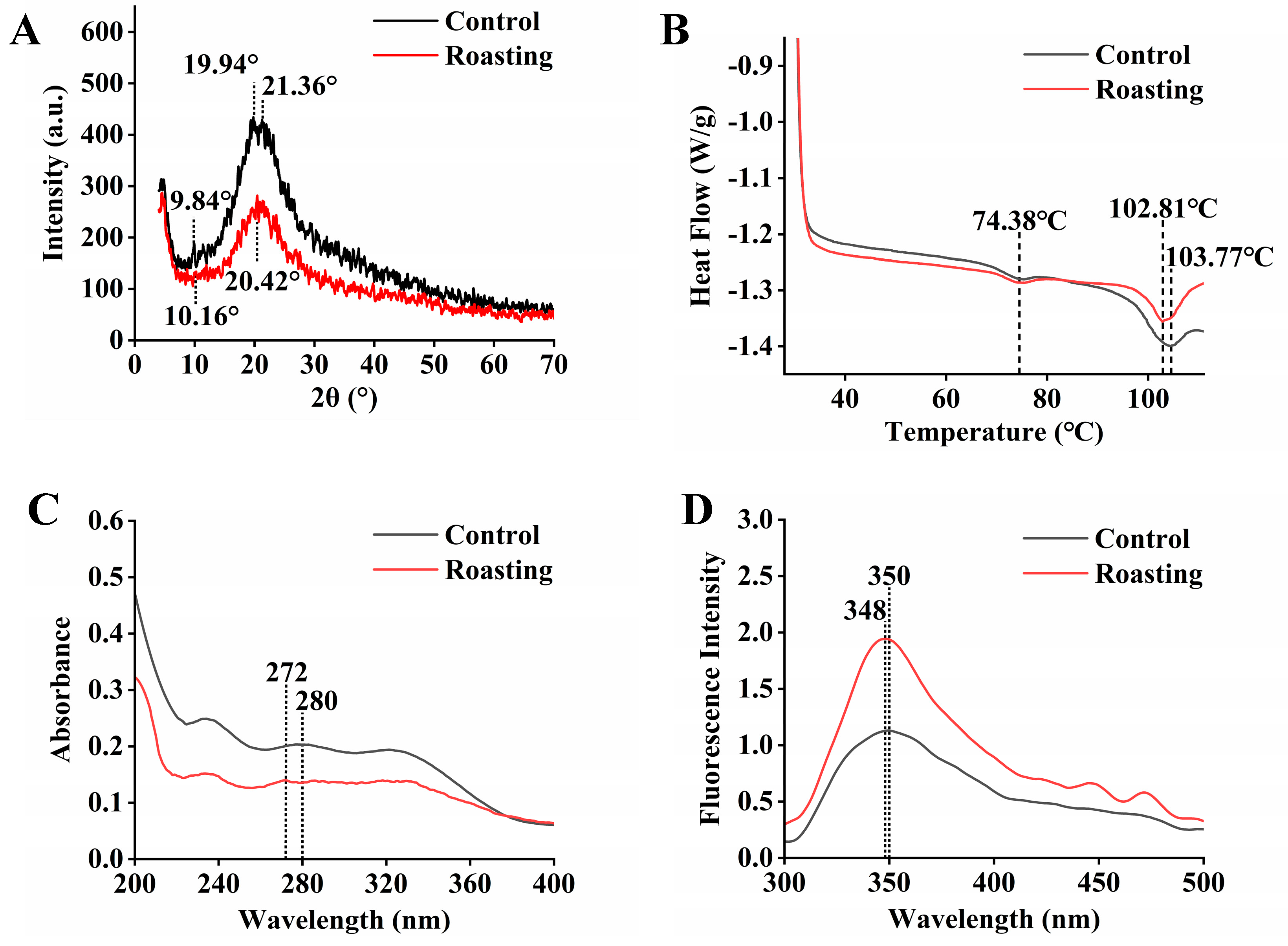
3.4. Effect of Roasting on the XRD Pattern
3.5. Effect of Roasting on the Thermal Properties
3.6. Effect of Roasting on Ultraviolet and Intrinsic Fluorescence Spectra
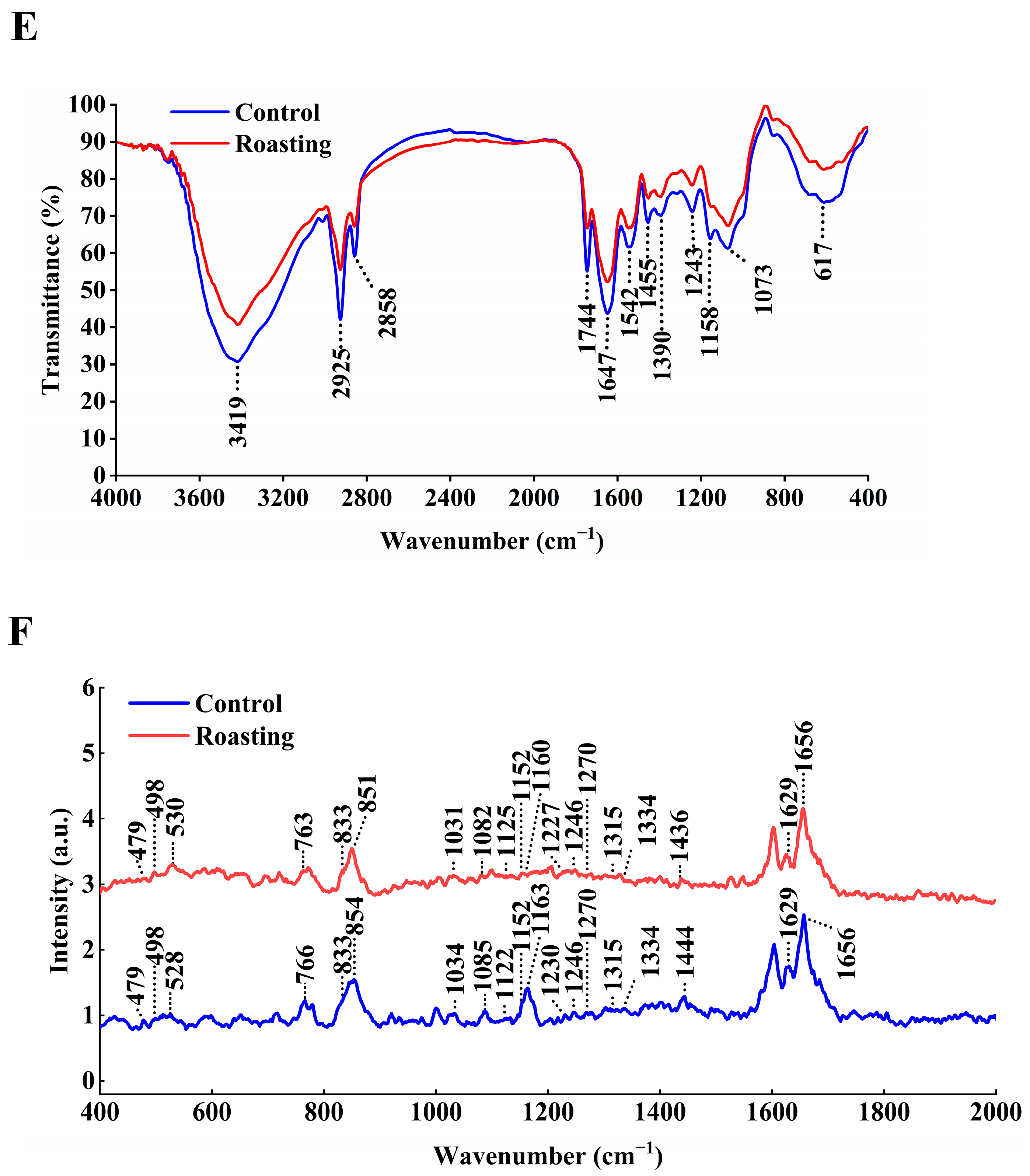
3.7. Effect of Roasting on FTIR Spectroscopy
3.8. Effect of Roasting on Raman Spectroscopy
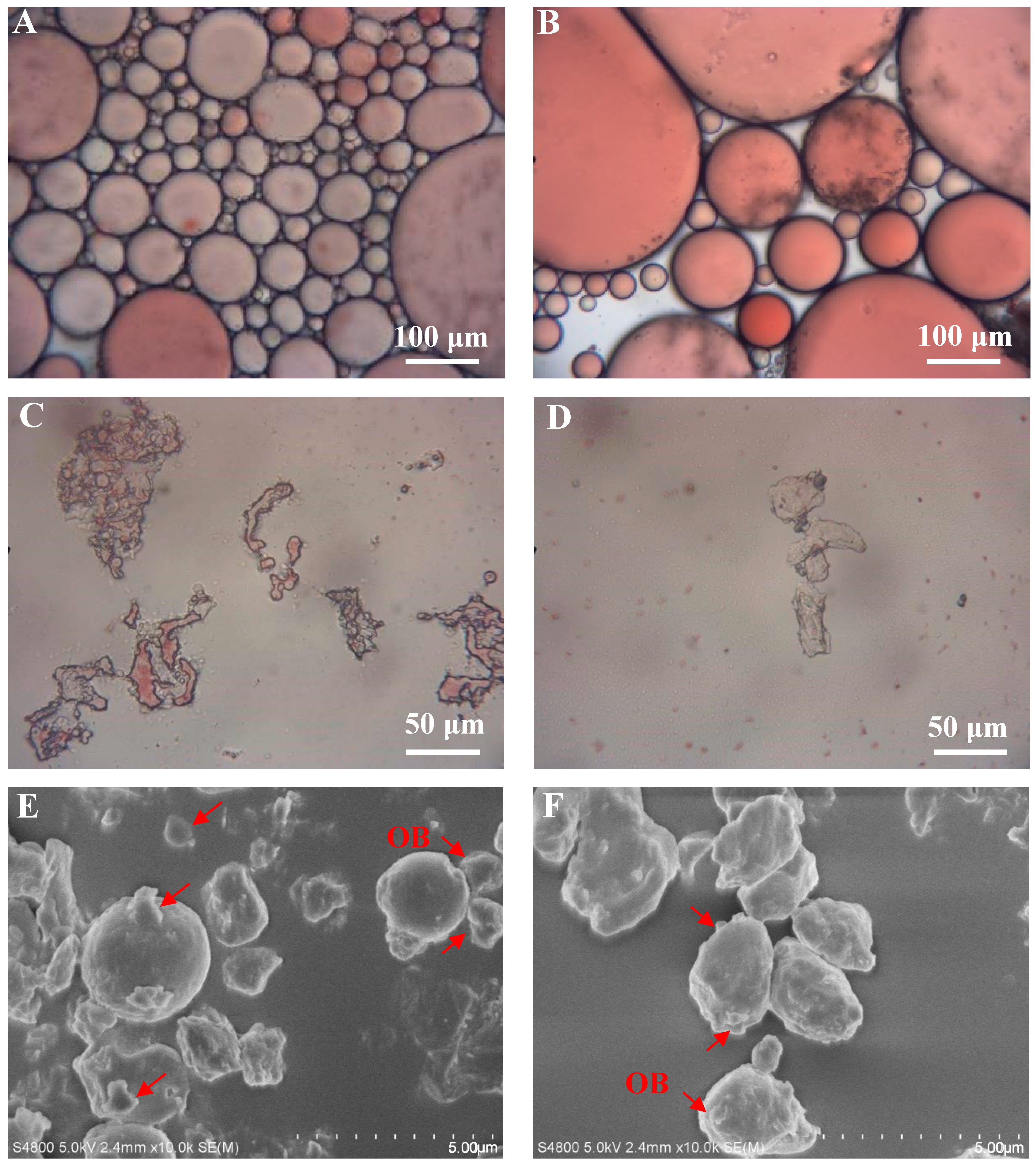
3.9. Effect of Roasting on the Microemulsion Morphology of Peanut Paste
3.10. Effect of Roasting on the Micromorphology of Peanut Dregs
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Adebiyi, A.P.; Adeyemi, I.A.; Olorunda, A.O. Effects of processing conditions and packaging material on the quality attributes of dry-roasted peanuts. J. Sci. Food Agric. 2002, 82, 1465–1471. [Google Scholar] [CrossRef]
- Lewis, S.; Touger-Decker, R.; Brody, R.; Parrott, J. Feeding tube use in adults with head and neck cancer for whom chemoradiation is the primary mode of treatment. J. Acad. Nutr. Diet. 2012, 112, A9. [Google Scholar] [CrossRef]
- Davis, J.P.; Dean, L.L. Peanuts: Genetics, Processing, and Utilization; Academic Press: Cambridge, MA, USA, 2016; pp. 289–345. [Google Scholar]
- Zhao, G.; Liu, Y.; Ren, J.; Zhao, M.; Yang, B. Effect of protease pretreatment on the functional properties of protein concentrate from defatted peanut flour. J. Food Process Eng. 2013, 36, 9–17. [Google Scholar] [CrossRef]
- Moure, A.; Sineiro, J.; Domínguez, H.; Parajó, J.C. Functionality of oilseed protein products: A review. Food Res. Int. 2006, 39, 945–963. [Google Scholar] [CrossRef]
- Zhao, Y.; Chen, F.; Liu, C. Peanut oil and protein extraction using an aqueous enzymatic method and analysis of the characteristics of the emulsions produced. Cereal Chem. 2023, 100, 762–774. [Google Scholar] [CrossRef]
- Jimsheena, V.K.; Gowda, L.R. Arachin derived peptides as selective angiotensin I-converting enzyme (ACE) inhibitors: Structure-activity relationship. Peptides 2010, 31, 1165–1176. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, Z.; Cheng, S.; Hai, F. Aqueous enzymatic extraction of peanut oil and protein hydrolysates. Food Sci. Technol Res. 2008, 14, 533–540. [Google Scholar] [CrossRef]
- Zhang, W.; Li, P.; Yang, R. Enzymes in Food and Beverage Processing; CRC Press: Boca Raton, FL, USA, 2015; pp. 133–154. [Google Scholar]
- Li, P.; Gasmalla, M.A.A.; Liu, J.; Zhang, W.; Yang, R.; Aboagarib, E.A.A. Characterization and demusification of cream emulsion from aqueous extraction of peanut. J. Food Eng. 2016, 185, 62–71. [Google Scholar] [CrossRef]
- Li, P.; Zhang, W.; Han, X.; Liu, J.; Liu, Y.; Gasmalla, M.A.A.; Yang, R. Demulsification of oil-rich emulsion and characterization of protein hydrolysates from peanut cream emulsion of aqueous extraction processing. J. Food Eng. 2017, 204, 64–72. [Google Scholar] [CrossRef]
- Jing, B.; Guo, R.; Wang, M.; Zhang, L.; Yu, X. Influence of seed roasting on the quality of glucosinolate content and flavor in virgin rapeseed oil. LWT-Food Sci. Technol. 2020, 126, 109301. [Google Scholar] [CrossRef]
- Makeri, M.U.; Bala, S.M.; Kassum, A.S. The effects of roasting temperatures on the rate of extraction and quality of locally-processed oil from two Nigerian peanut (Arachis hypogea L.) cultivars. Afric. J. Food Sci. 2011, 5, 194–199. [Google Scholar] [CrossRef]
- Zhang, S.; Lu, Q.; Yang, H.; Li, Y.; Wang, S. Aqueous enzymatic extraction of oil and protein hydrolysates from roasted peanut seeds. J. Am. Oil Chem. Soc. 2010, 88, 727–732. [Google Scholar] [CrossRef]
- Hua, Y.; Huang, Y.; Qiu, A.; Liu, X. Properties of soy protein isolate prepared from aqueous alcohol washed soy flakes. Food Res. Int. 2005, 38, 273–279. [Google Scholar] [CrossRef]
- Du, Y.; Zhang, W.; Yang, R.; Hua, X.; Zhao, W. Extraction of grape seed oil by aqueous ethanol method and its quality. Chin. Oils Fats. 2019, 44, 8–42. [Google Scholar] [CrossRef]
- AOAC Offcial Method 955.19; Fat in Cream Mojonnier ether Extraction Method. Association of Official Agricultural Chemists: Arlington, VA, USA, 1998.
- AOAC Offcial Method Ca 2c-25; Moisture and Volatile Matter Air Oven Method. Association of Official Agricultural Chemists: Arlington, VA, USA, 2009.
- AOAC Offcial Method Ac 4-91; Nitrogen-Ammonia-Protein Modified Kjeldahl Method Titanium Dioxide + Copper Sulfate Catalyst. Association of Official Agricultural Chemists: Arlington, VA, USA, 2009.
- Liu, J.; Zhang, W.; Li, P.; Jiang, Z.; Yang, R. Isolation of peanut protein aggregates using aqueous extraction processing combined with membrane separation. Int. J. Food Sci. Technol. 2020, 55, 3203–3214. [Google Scholar] [CrossRef]
- Xu, Y.; Fan, H.; Zhao, D.; Su, W.; Feng, Y.; Xiao, F.; Zheng, J.; Wang, D. Optimization of extraction method for water-soluble protein determination by coomassie bright blue method. Soybean Sci. 2022, 41, 196–202. [Google Scholar] [CrossRef]
- Bencini, M.C. Functional properties of drum-dried chickpea (Cicer arietinum L.) flours. J. Food Sci. 1986, 51, 1518–1521. [Google Scholar] [CrossRef]
- Lassissi, T.A.; Hettiarachchy, N.S.; Rayaprolu, S.J.; Kannan, A.; Davis, M. Functional properties and Angiotensin-I converting enzyme inhibitory activity of soy-whey proteins and fractions. Food Res. Int. 2014, 64, 598–602. [Google Scholar] [CrossRef]
- Kato, A.; Takahashi, A.; Matsudomi, N.; Kobayashi, K. Determination of foaming properties of proteins by conductivity measurements. J. Food Sci. 1983, 48, 62–65. [Google Scholar] [CrossRef]
- Bertsch, M.; Mayburd, A.L.; Kassner, R.J. The identification of hydrophobic sites on the surface of proteins using absorption difference spectroscopy of bromophenol blue. Anal. Biochem. 2003, 313, 187–195. [Google Scholar] [CrossRef]
- Zhao, X.; Liu, H.; Zhang, X.; Zhu, H.; Ao, Q. Surface structure and volatile characteristic of peanut proteins obtained through AOT reverse micelles. Colloids Surf. B Biointerfaces 2019, 173, 860–868. [Google Scholar] [CrossRef]
- Tang, Y.; Yang, Y.; Wang, Q.; Tang, Y.; Ming, J. Combined effect of carboxymethylcellulose and salt on structural properties of wheat gluten proteins. Food Hydrocolloid. 2019, 97, 105189. [Google Scholar] [CrossRef]
- Zhang, W.; Boateng, I.D.; Zhang, W.; Jia, S.; Wang, T.; Huang, L. Effect of ultrasound-assisted ionic liquid pretreatment on the structure and interfacial properties of soy protein isolate. Process Biochem. 2022, 115, 160–168. [Google Scholar] [CrossRef]
- Liu, J.; Li, P.; Jiang, Z.; Yang, R.; Zhang, W. Characterisation of peanut protein concentrates from industrial aqueous extraction processing prepared by spray and freeze drying methods. Int. J. Food Sci. Technol. 2018, 54, 1597–1608. [Google Scholar] [CrossRef]
- Liu, R.; Shi, C.; Song, Y.; Wu, T.; Zhang, M. Impact of oligomeric procyanidins on wheat gluten microstructure and physicochemical properties. Food Chem. 2018, 260, 37–43. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Zhu, L.; Chen, Y.; Wang, B.; Liu, H.; He, Y.; Ma, T. The effect on secondary structure and emulsibility of peanut protein under different dry heat treatment. Food Ferment. Ind. 2016, 42, 86–90. [Google Scholar] [CrossRef]
- Guo, Y.; Cai, W.; Tu, K.; Tu, S.; Wang, S.; Zhu, X.; Zhang, W. Infrared and Raman spectroscopic characterization of structural changes in albumin, globulin, glutelin, and prolamin during rice aging. J. Agric. Food Chem. 2013, 61, 185–192. [Google Scholar] [CrossRef]
- Ni, S.; Yang, R.; Zhang, W.; Zhao, W.; Hua, X. Process optimization for extraction of corn germ oil by aqueous ethanol. Trans. Chin. Soc. Agric. Eng. 2016, 32, 283–289. [Google Scholar] [CrossRef]
- Xiong, Y.L.; Guo, A. Animal and plant protein oxidation: Chemical and functional property significance. Foods 2020, 10, 40. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Ahmedna, M.; Goktepe, I. Peanut protein concentrate: Production and functional properties as affected by processing. Food Chem. 2007, 103, 121–129. [Google Scholar] [CrossRef]
- Martelli-Tosi, M.; Masson, M.M.; Silva, N.C.; Esposto, B.S.; Barros, T.T.; Assis, O.B.G.; Tapia-Blácido, D.R. Soybean straw nanocellulose produced by enzymatic or acid treatment as a reinforcing filler in soy protein isolate films. Carbohyd. Polym. 2018, 198, 61–68. [Google Scholar] [CrossRef]
- Li, J.; Luo, J.; Li, X.; Yi, Z.; Gao, Q.; Li, J. Soybean meal-based wood adhesive enhanced by ethylene glycol diglycidyl ether and diethylenetriamine. Ind. Crop. Prod. 2015, 74, 613–618. [Google Scholar] [CrossRef]
- Sonda, T.S.; Kallon, S. Analysis of functional properties of peanut protein isolates as affected by oil extraction methods. Food Sci. Res. J. 2016, 7, 148–155. [Google Scholar] [CrossRef]
- Trnkova, L.; Bousova, I.; Kubicek, V.; Drsata, J. Binding of naturally occurring hydroxycinnamic acids to bovine serum albumin. Nat. Sci. 2010, 2, 563–570. [Google Scholar] [CrossRef]
- Cao, X.; He, Y.; Kong, Y.; Mei, X.; Huo, Y.; He, Y.; Liu, J. Elucidating the interaction mechanism of eriocitrin with β-casein by multi-spectroscopic and molecular simulation methods. Food Hydrocolloid. 2019, 94, 63–70. [Google Scholar] [CrossRef]
- Joye, I.J.; Davidov-Pardo, G.; Ludescher, R.D.; Mcclements, D.J. Fluorescence quenching study of resveratrol binding to zein and gliadin: Towards a more rational approach to resveratrol encapsulation using water-insoluble proteins. Food Chem. 2015, 185, 261–267. [Google Scholar] [CrossRef] [PubMed]
- Jiang, S.; Zhang, J.; Li, S.; Zhang, C. Effect of enzymatic hydrolysis on the formation and structural properties of peanut protein gels. Int. J. Food Eng. 2021, 17, 167–176. [Google Scholar] [CrossRef]
- Wang, C.; Ma, Y.; Zhang, Z.; Zhang, L.; Chi, Y.; Chi, Y. Effects of different processing methods on the immunogenicity and structure of egg white protein. Food Sci. 2022, 43, 93–100. [Google Scholar] [CrossRef]
- Vanga, S.K.; Singh, A.; Kalkan, F.; Gariepy, Y.; Orsat, V.; Raghavan, V. Effect of thermal and high electric fields on secondary structure of peanut protein. Int. J. Food Prop. 2015, 19, 1259–1271. [Google Scholar] [CrossRef]
- Eissa, A.S.; Puhl, C.; Kadla, J.F.; Khan, S.A. Enzymatic cross-linking of beta-lactoglobulin: Conformational properties using FTIR spectroscopy. Biomacromolecules 2006, 7, 1707–1713. [Google Scholar] [CrossRef]
- Qu, W.; Zhang, X.; Chen, W.; Wang, Z.; He, R.; Ma, H. Effects of ultrasonic and graft treatments on grafting degree, structure, functionality, and digestibility of rapeseed protein isolate-dextran conjugates. Ultrason. Sonochem. 2018, 42, 250–259. [Google Scholar] [CrossRef] [PubMed]
- Ning, J.; Guo, Y.; Song, R.; Zhu, S.; Dong, P. Spectral analysis of glutelin changes during rice aging and its effects on glutelin functional properties. Spectrosc. Spect. Anal. 2021, 41, 3431–3437. [Google Scholar] [CrossRef]
- Liu, F.; Ma, C.; Mcclements, D.J.; Gao, Y. A comparative study of covalent and non-covalent interactions between zein and polyphenols in ethanol-water solution. Food Hydrocoll. 2017, 63, 625–634. [Google Scholar] [CrossRef]
- Stathopulos, P.B.; Scholz, G.A.; Hwang, Y.-M.; Rumfeldt, J.A.O.; Lepock, J.R.; Meiering, E.M. Sonication of proteins causes formation of aggregates that resemble amyloid. Protein Sci. 2004, 13, 3017–3027. [Google Scholar] [CrossRef]
- Ellepola, S.W.; Choi, S.-M.; Phillips, D.L.; Ma, C.-Y. Raman spectroscopic study of rice globulin. J. Cereal Sci. 2006, 43, 85–93. [Google Scholar] [CrossRef]
- Shao, J.; Zou, Y.; Xu, X.; Wu, J.; Zhou, G. Evaluation of structural changes in raw and heated meat batters prepared with different lipids using Raman spectroscopy. Food Res. Int. 2011, 44, 2955–2961. [Google Scholar] [CrossRef]
| Treatment | FO (g) | EO (g) | APO (g) | APS (g) | DRO (g) | DP (g) | DC (g) | DM (g) |
|---|---|---|---|---|---|---|---|---|
| Control | 27.99 ± 0.07 b | 16.32 ± 0.11 a | 1.36 ± 0.17 a | 9.40 ± 0.71 a | 3.74 ± 0.21 a | 19.37 ± 0.22 a | 20.19 ± 0.47 b | 1.64 ± 0.02 a |
| Roasting | 47.34 ± 0.16 a | 0.33 ± 0.19 b | 0.63 ± 0.11 b | 8.27 ± 0.12 b | 1.10 ± 0.11 b | 17.78 ± 0.05 b | 22.87 ± 0.10 a | 1.68 ± 0.01 a |
| Treatment | SP (%) | WHC (g/g) | OHC (g/g) | EA (m2/g) | ES (m2/g) | FA (%) | FS (%) | S0 (μg) |
|---|---|---|---|---|---|---|---|---|
| Control | 19.28 ± 0.02 a | 5.69 ± 0.12 b | 5.06 ± 0.03 a | 8.98 ± 0.32 a | 68.34 ± 0.15 a | 16.25 ± 0.42 a | 55.18 ± 0.13 a | 72.15 ± 4.94 a |
| Roasting | 19.18 ± 0.15 a | 7.70 ± 0.03 a | 4.82 ± 0.10 b | 7.16 ± 0.09 b | 42.76 ± 0.52 b | 14.92 ± 0.63 b | 42.20 ± 0.37 b | 38.57 ± 1.46 b |
| Treatment | α-Helix (%) | β-Sheet (%) | β-Turn (%) | Random Coil (%) |
|---|---|---|---|---|
| Control | 17.52 ± 3.52 a | 30.12 ± 2.09 b | 28.24 ± 4.08 b | 24.12 ± 1.99 b |
| Roasting | 1.29 ± 0.25 b | 34.79 ± 3.12 a | 31.19 ± 0.85 a | 32.73 ± 2.31 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, S.; Guo, Y.; Zhu, X.; Xie, D.; Wang, Z. Effects of the Roasting-Assisted Aqueous Ethanol Extraction of Peanut Oil on the Structure and Functional Properties of Dreg Proteins. Foods 2024, 13, 758. https://doi.org/10.3390/foods13050758
Wang S, Guo Y, Zhu X, Xie D, Wang Z. Effects of the Roasting-Assisted Aqueous Ethanol Extraction of Peanut Oil on the Structure and Functional Properties of Dreg Proteins. Foods. 2024; 13(5):758. https://doi.org/10.3390/foods13050758
Chicago/Turabian StyleWang, Sicheng, Yubao Guo, Xiuling Zhu, Dan Xie, and Zhenzhen Wang. 2024. "Effects of the Roasting-Assisted Aqueous Ethanol Extraction of Peanut Oil on the Structure and Functional Properties of Dreg Proteins" Foods 13, no. 5: 758. https://doi.org/10.3390/foods13050758





