Qualitative and Quantitative Metabolite Comparison of Korean Traditional Alcoholic Beverages: Takju, Yakju, and Traditional-Soju
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
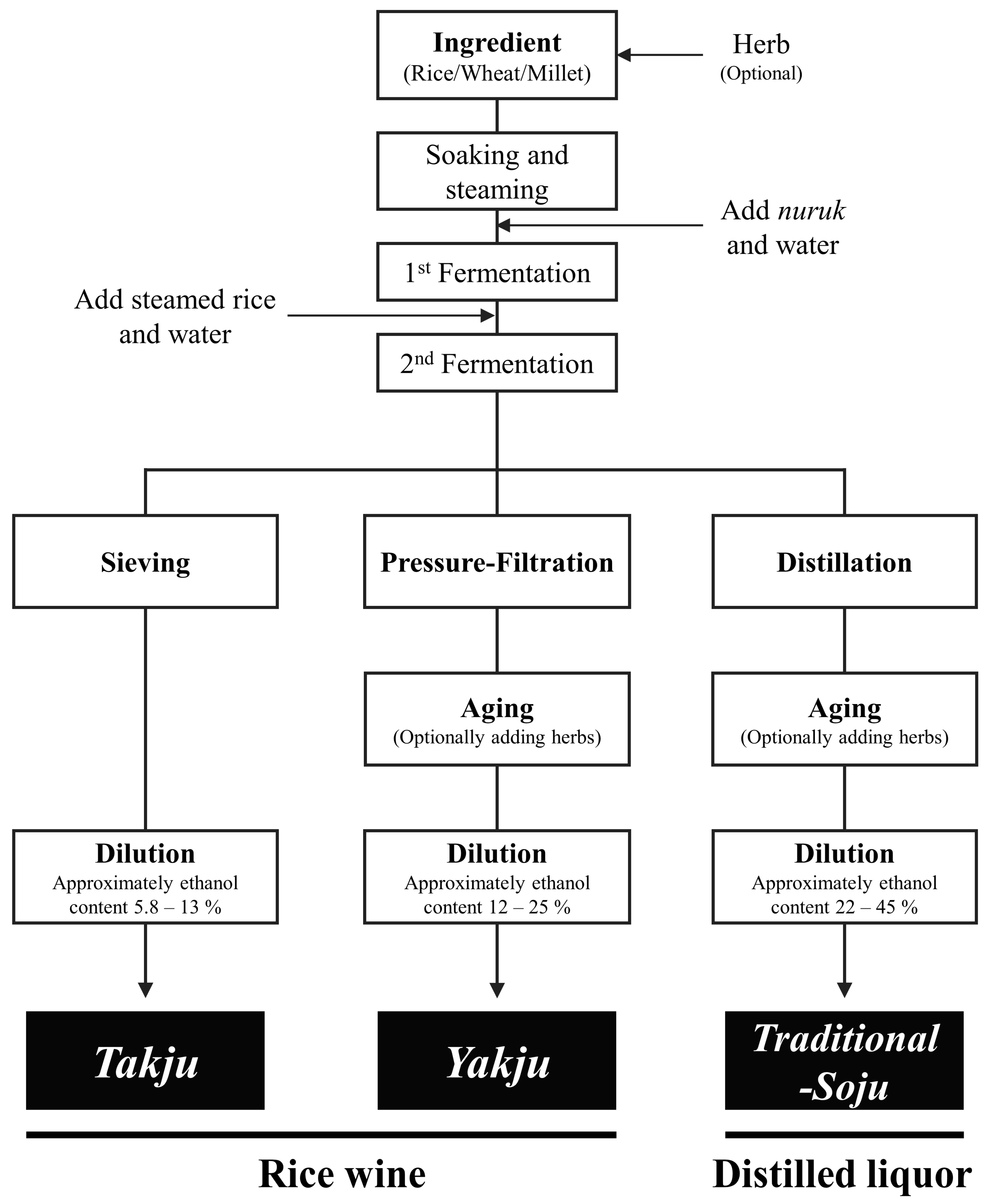
2.2. Alcoholic Beverage Samples
2.3. Sample Extraction for Non-Targeted Metabolomic Analysis
2.4. GC-TOF-MS Analysis for Non-Targeted Analysis
2.5. Quantification of Organic Acids
2.6. Quantification of Amino Acids
2.7. Quantification of Fatty Acids
2.8. Data Processing and Multivariate Statistical Analysis
3. Results
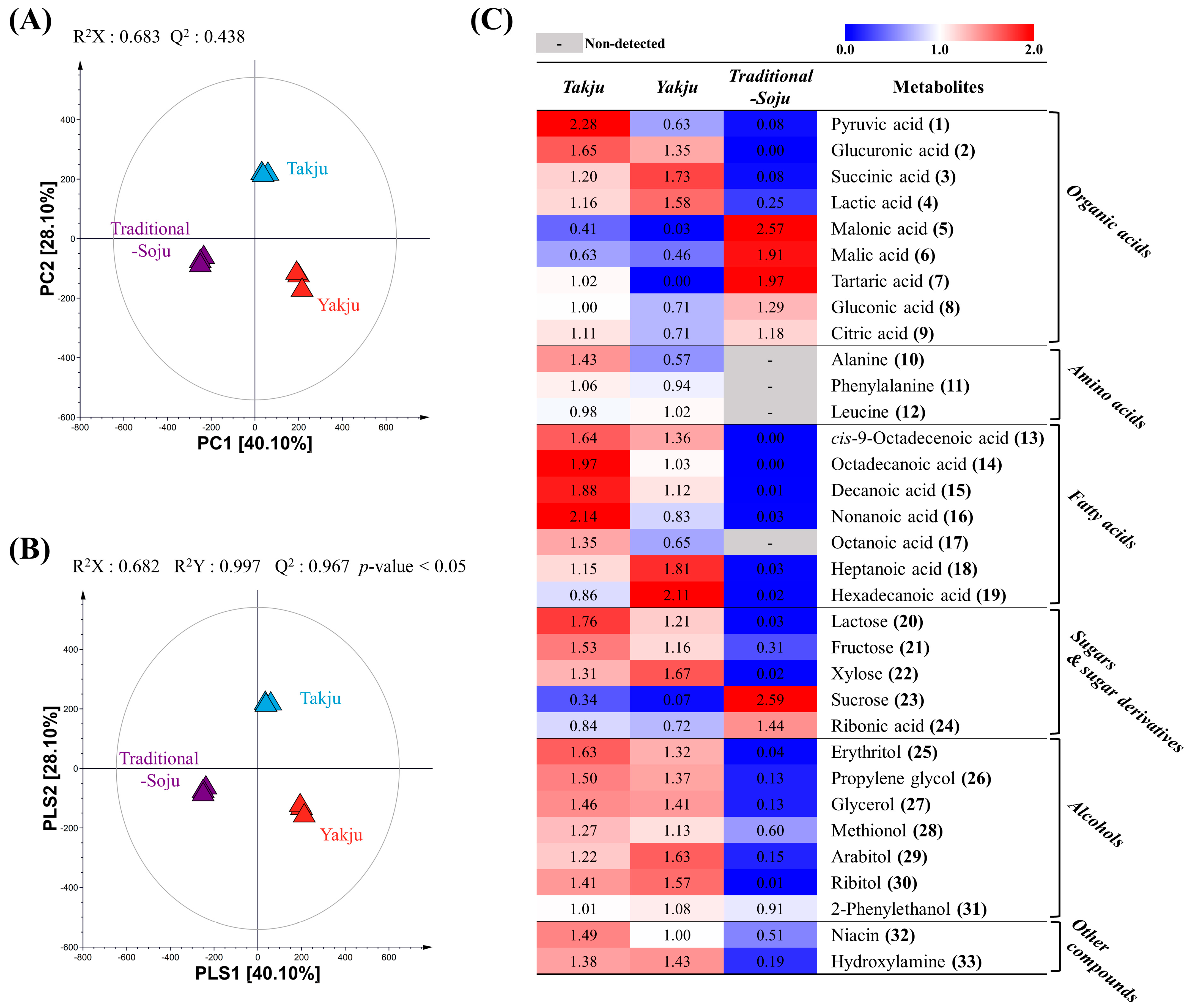
3.1. Non-Targeted Metabolite Profiling of Takju, Yakju, and Traditional-Soju
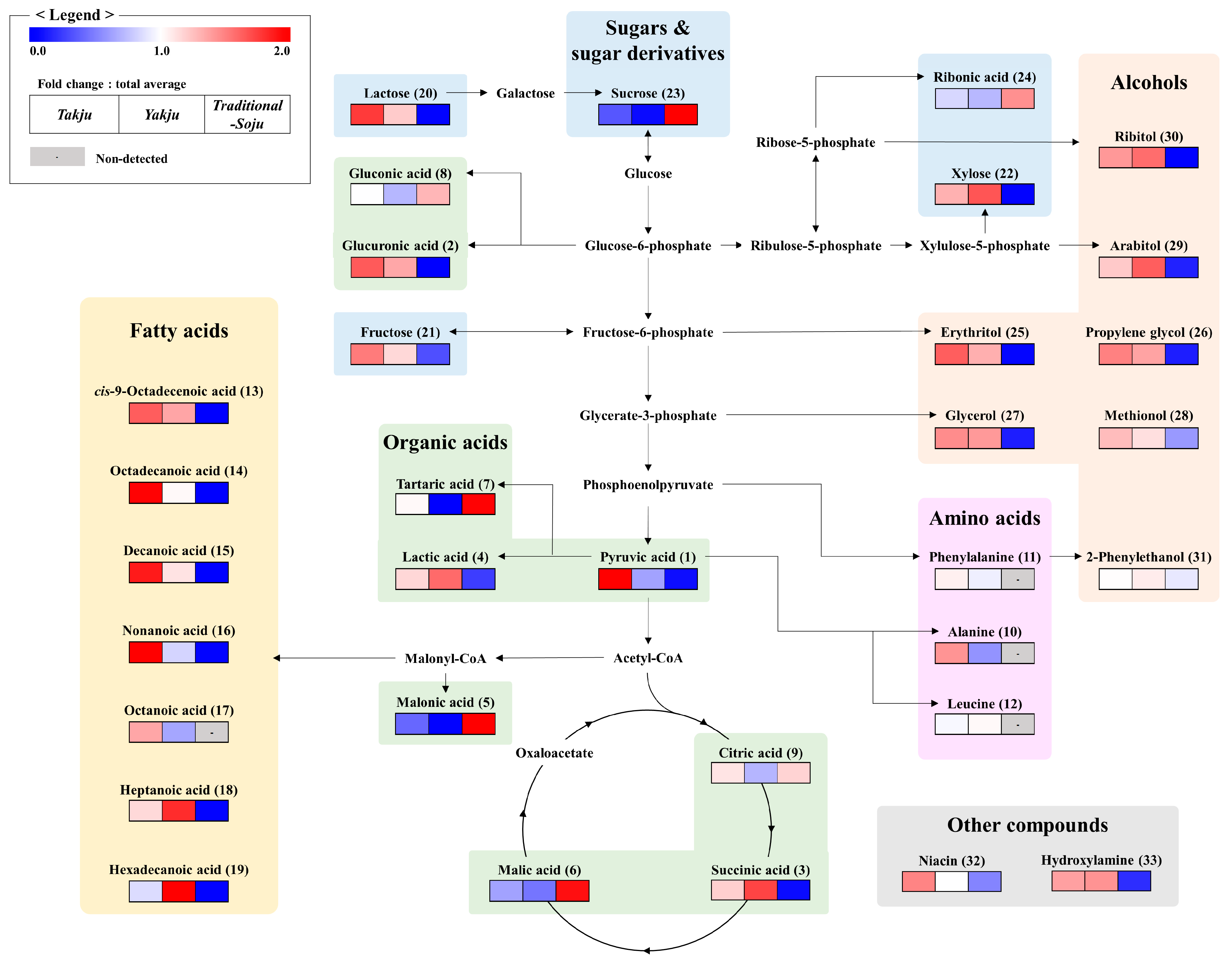
3.2. Metabolic Pathways of Three Korean Traditional Alcoholic Beverages
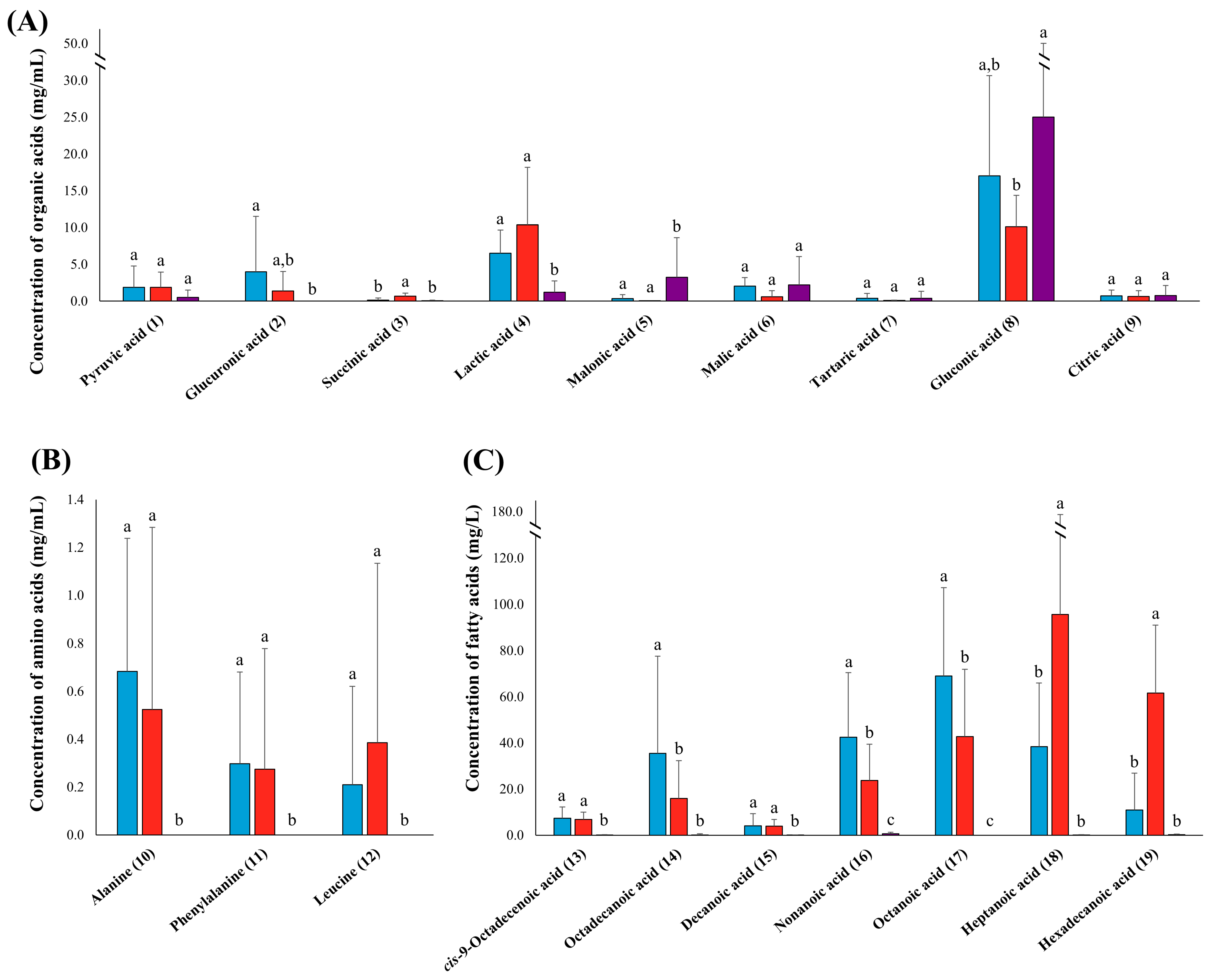
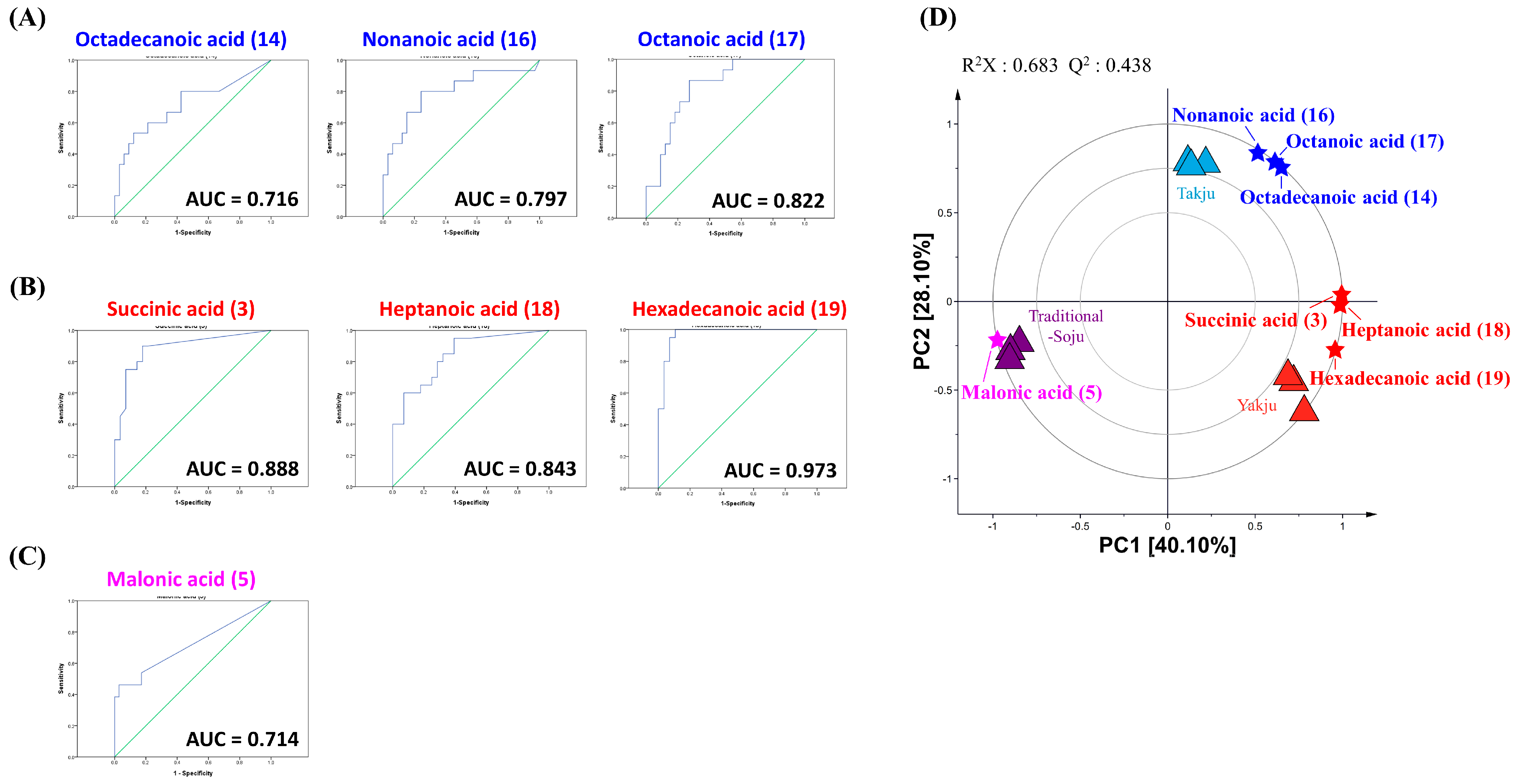
3.3. Quantitative Analysis of Organic Acids, Amino Acids, and Fatty Acids
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Manthey, J.; Shield, K.D.; Rylett, M.; Hasan, O.S.M.; Probst, C.; Rehm, J. Global Alcohol Exposure between 1990 and 2017 and Forecasts until 2030: A Modelling Study. Lancet 2019, 393, 2493–2502. [Google Scholar] [CrossRef]
- Jernigan, D.H. The Global Alcohol Industry: An Overview. Addiction 2009, 104, 6–12. [Google Scholar] [CrossRef] [PubMed]
- Shim, D.-H.; Han, K.-M. A Study on the Export of Korean Traditional Liquors in Respect of Institutionalism. JTLR 2020, 32, 253–265. [Google Scholar] [CrossRef]
- Moon, J.-S.; Kong, T.-I.; Cheong, C. Characteristics of Fermentation and Aging by Different Adding of Brewing Water in Korean Traditional Cheongju. J. Korea Acad. Ind. Coop. Soc. 2015, 16, 5468–5475. [Google Scholar] [CrossRef]
- Yang, S.; Lee, J.; Kwak, J.; Kim, K.; Seo, M.; Lee, Y.-W. Fungi Associated with the Traditional Starter Cultures Used for Rice Wine in Korea. J. Korean Soc. Appl. Biol. Chem. 2011, 54, 933–943. [Google Scholar] [CrossRef]
- Lee, J.-H.; Yu, T.-S. Identification and Characteristics of Lactic Acid Bacteria Isolated from Nuruk. Korean J. Biotechnol. Bioeng. 2000, 15, 359–365. [Google Scholar]
- Kim, J.-W.; Kang, J.-E.; Choi, H.-S.; Kim, C.-W.; Jeong, S.-T. Analysis of the Physicochemical Characteristics and Sensory Properties in Makgeolli. J. East Asian Soc. Diet Life 2017, 27, 491–499. [Google Scholar] [CrossRef]
- Kim, H.-E.; Kim, Y.-S.; Kim, Y.-S. Comparison of Quality Characteristics and Instrumental Analysis on Korean Makgeolli. Int. J. Bio-Sci. Bio-Technol. 2014, 6, 123–128. [Google Scholar] [CrossRef]
- Lee, J.-E.; Kim, J.H. Chemistry of the Korean Traditional Alcoholic Beverage Makgeolli. Chem. Korean Foods Beverages 2019, 1303, 97–103. [Google Scholar] [CrossRef]
- Lee, S.; Kwak, H.S.; Jung, J.; Kim, S.S.; Lee, Y. Identifying Drivers of Liking for Korean Traditional Rice Wine (Yakju) across Different Age Groups by Penalty Analysis Based on the CATA Method. J. Inst. Brew. 2021, 127, 286–295. [Google Scholar] [CrossRef]
- Kim, T.W. Korean Soju. In Whisky and Other Spirits, 3rd ed.; Academic Press: Cambridge, MA, USA, 2022; pp. 63–74. [Google Scholar] [CrossRef]
- Liu, T.; Li, Y.; Yang, Y.; Yi, H.; Zhang, L.; He, G. The Influence of Different Lactic Acid Bacteria on Sourdough Flavor and a Deep Insight into Sourdough Fermentation through RNA Sequencing. Food Chem. 2020, 307, 125529. [Google Scholar] [CrossRef] [PubMed]
- Kim, B.K.; Kang, M.S.; Jeon, M.-J.; Lee, S.-H.; Kim, M. Effects of Makgeolli and Makgeolli Precipitate on Hepatotoxicity and Serum Lipid Content in Rats. J. Life Sci. 2013, 23, 282–289. [Google Scholar] [CrossRef]
- Namgung, H.J.; Park, H.J.; Cho, I.H.; Choi, H.K.; Kwon, D.Y.; Shim, S.M.; Kim, Y.S. Metabolite Profiling of Doenjang, Fermented Soybean Paste, during Fermentation. J. Sci. Food Agric. 2010, 90, 1926–1935. [Google Scholar] [CrossRef]
- Ye, M.; Yue, T.; Yuan, Y. Evolution of Polyphenols and Organic Acids during the Fermentation of Apple Cider. J. Sci. Food Agric. 2014, 94, 2951–2957. [Google Scholar] [CrossRef]
- Alañón, M.E.; Pérez-Coello, M.S.; Marina, M.L. Wine Science in the Metabolomics Era. TrAC Trends Anal. Chem. 2015, 74, 1–20. [Google Scholar] [CrossRef]
- Tomita, S.; Saito, K.; Nakamura, T.; Sekiyama, Y.; Kikuchi, J. Rapid Discrimination of Strain-Dependent Fermentation Characteristics among Lactobacillus Strains by NMR-Based Metabolomics of Fermented Vegetable Juice. PLoS ONE 2017, 12, e0182229. [Google Scholar] [CrossRef]
- Soung, S.H.; Lee, S.; Lee, S.H.; Kim, H.-J.; Lee, N.-R.; Lee, C.-H. Metabolomic-Based Comparison of Traditional and Industrial Doenjang Samples with Antioxidative Activities. Foods 2021, 10, 1377. [Google Scholar] [CrossRef]
- Gao, Y.; Hou, L.; Gao, J.; Li, D.; Tian, Z.; Fan, B.; Wang, F.; Li, S. Metabolomics Approaches for the Comprehensive Evaluation of Fermented Foods: A Review. Foods 2021, 10, 2294. [Google Scholar] [CrossRef]
- Cavallini, N.; Savorani, F.; Bro, R.; Cocchi, M. A Metabolomic Approach to Beer Characterization. Molecules 2021, 26, 1472. [Google Scholar] [CrossRef]
- Seo, S.-H.; Park, S.-E.; Yoo, S.-A.; Lee, K.I.; Na, C.-S.; Son, H.-S. Metabolite Profiling of Makgeolli for the Understanding of Yeast Fermentation Characteristics during Fermentation and Aging. Process Biochem. 2016, 51, 1363–1373. [Google Scholar] [CrossRef]
- He, F.; Duan, J.; Zhao, J.; Li, H.; Sun, J.; Huang, M.; Sun, B. Different Distillation Stages Baijiu Classification by Temperature-Programmed Headspace-Gas Chromatography-Ion Mobility Spectrometry and Gas Chromatography-Olfactometry-Mass Spectrometry Combined with Chemometric Strategies. Food Chem. 2021, 365, 130430. [Google Scholar] [CrossRef]
- Rhee, S.Y.; Jung, E.S.; Suh, D.H.; Jeong, S.J.; Kim, K.; Chon, S.; Yu, S.-Y.; Woo, J.-T.; Lee, C.H. Plasma amino acids and oxylipins as potential multi-biomarkers for predicting diabetic macular edema. Sci. Rep. 2021, 11, 9727. [Google Scholar] [CrossRef]
- Hijazi, A.; Pisano, I.; Illek, P.; Leahy, J.J. A Rapid HPLC Method for the Simultaneous Determination of Organic Acids and Furans: Food Applications. Beverages 2022, 8, 6. [Google Scholar] [CrossRef]
- Jin, Z.; Cai, G.; Wu, C.; Hu, Z.; Xu, X.; Xie, G.; Wu, D.; Lu, J. Profiling the Key Metabolites Produced during the Modern Brewing Process of Chinese Rice Wine. Food Res. Int. 2021, 139, 109955. [Google Scholar] [CrossRef]
- Herbert, P.; Santos, L.; Alves, A. Simultaneous Quantification of Primary, Secondary Amino Acids, and Biogenic Amines in Musts and Wines Using OPA/3-MPA/FMOC-CI Fluorescent Derivatives. J. Food. Sci. 2001, 66, 1319–1325. [Google Scholar] [CrossRef]
- Jajic, I.; Krstovic, S.; Glamocic, D.; Jakšic, S.; Abramovic, B. Validation of an HPLC Method for the Determination of Amino Acids in Feed. J. Serbian Chem. Soc. 2013, 78, 839–850. [Google Scholar] [CrossRef]
- Sedgwick, G.W.; Fenton, T.W.; Thompson, J.R. Effect of Protein Precipitating Agents on the Recovery of Plasma Free Amino Acids. Can. J. Anim. Sci. 1991, 71, 953–957. [Google Scholar] [CrossRef]
- Rocha, S.; Araújo, A.M.; Almeida, A.; de Pinho, P.G.; Fernandes, E. Development and Validation of a GC-MS/MS Method for Cis-and Trans-Resveratrol Determination: Application to Portuguese Wines. Food Anal. Methods 2019, 12, 1536–1544. [Google Scholar] [CrossRef]
- Song, D.H.; Chun, B.H.; Lee, S.; Son, S.Y.; Reddy, C.K.; Mun, H.I.; Jeon, C.O.; Lee, C.H. Comprehensive Metabolite Profiling and Microbial Communities of Doenjang (Fermented Soy Paste) and Ganjang (Fermented Soy Sauce): A Comparative Study. Foods 2021, 10, 641. [Google Scholar] [CrossRef] [PubMed]
- Jeong, J.-H.; Chai, H.-S.; Lee, Y.-H.; Lee, K.-S.; Kim, J.-M.; Lee, J.-H. Quality Characteristics of Takju, Yakju, Spirit Made by Pulse Crop Nuruks. Culin. Sci. Hosp. Res. 2015, 21, 232–247. [Google Scholar] [CrossRef]
- Bujake, J.E. Beverage Spirits, Distilled. In Kirk-Othmer Encyclopedia of Chemical Technology; Wiley: New York, NY, USA, 2000. [Google Scholar] [CrossRef]
- Shin, K.-J.; Kim, T.-W.; Lee, S.-J. Changes of Volatile Compositions in Soju Wash from Fermentation to Distillation Using Different Kinds of Fermentation Starters. ACS Symp. Ser. 2019, 1303, 57–76. [Google Scholar] [CrossRef]
- Ryu, L.-H.; Kim, Y.-M. Esterification of Alcohols with Organic Acids during Distilled Spirit Distillation. JKSFSN 2002, 15, 295–299. [Google Scholar]
- Christoph, N.; Bauer-Christoph, C. Flavour of Spirit Drinks: Raw Materials, Fermentation, Distillation, and Ageing. In Flavours and Fragrances; Springer: Berlin/Heidelberg, Germany, 2007; pp. 219–239. [Google Scholar] [CrossRef]
- Lee, Y.; Eom, T.; Cheong, C.; Cho, H.; Kim, I.; Lee, Y.; Kim, M.; Yu, S.; Jeong, Y. Quality Characteristics of Spirits by Different Distillation and Filtrations. J. Korean Soc. Food Sci. Nutr. 2013, 42, 2012–2018. [Google Scholar] [CrossRef]
- Badr, S.; Sakr, D.M.; Mahfouz, S.; Abdelfattah, M. Licorice (Glycyrrhiza glabra L.): Chemical Composition and Biological Impacts. Res. J. Pharm. Biol. Chem. Sci. 2013, 4, 606–621. [Google Scholar]
- Liu, Q.-Q.; Li, Y.; Zhang, M.-X.; Yu, X.-Y.; Yang, C.-Y.; Wu, G.-H. Differences in Total Phenol Content, Antioxidant Activity and Metabolic Fingerprint between Purple Mulberry and White Mulberry. Jiangsu J. Agric. Sci. 2022, 38, 813–820. [Google Scholar] [CrossRef]
- Wills, C. Regulation of Sugar and Ethanol Metabolism in Saccharomyces cerevisiae. Crit. Rev. Biochem. Mol. Biol. 1990, 25, 245–280. [Google Scholar] [CrossRef]
- Ma, Y.; Li, B.; Zhang, X.; Wang, C.; Chen, W. Production of Gluconic Acid and Its Derivatives by Microbial Fermentation: Process Improvement Based on Integrated Routes. Front. Bioeng. Biotechnol. 2022, 10, 864787. [Google Scholar] [CrossRef]
- Ramachandran, S.; Fontanille, P.; Pandey, A.; Larroche, C. Gluconic Acid: Properties, Applications and Microbial Production. Food Technol. Biotechnol. 2006, 44, 185–195. [Google Scholar]
- Liu, S.Q. Practical Implications of Lactate and Pyruvate Metabolism by Lactic Acid Bacteria in Food and Beverage Fermentations. Int. J. Food Microbiol. 2003, 83, 115–131. [Google Scholar] [CrossRef]
- Kučerová, J.; Široky, J. Study of Changes Organic Acids in Red Wines during Malolactic Fermentation. Acta Univ. Agric. Silvic. Mendel. Brun. 2011, 59, 145–150. [Google Scholar] [CrossRef]
- Madžgalj, V.; Petrović, A.; Čakar, U.; Maraš, V.; Sofrenić, I.; Tešević, V. The Influence of Different Enzymatic Preparations and Skin Contact Time on Aromatic Profile of Wines Produced from Autochthonous Grape Varieties Krstač and Žižak. J. Serbian Chem. Soc. 2022, 88, 11–23. [Google Scholar] [CrossRef]
- Li, K.; Tang, J.; Zhang, Z.; Wu, Z.; Zhong, A.; Li, Z.; Wang, Y. Correlation between Flavor Compounds and Microorganisms of Chaling Natural Fermented Red Sufu. LWT 2022, 154, 112873. [Google Scholar] [CrossRef]
- Mao, X.; Yue, S.J.; Xu, D.Q.; Fu, R.J.; Han, J.Z.; Zhou, H.M.; Tang, Y.P. Research Progress on Flavor and Quality of Chinese Rice Wine in the Brewing Process. ACS Omega 2023, 8, 32311–32330. [Google Scholar] [CrossRef] [PubMed]
- Kaminarides, S.; Stamou, P.; Massouras, T. Changes of Organic Acids, Volatile Aroma Compounds and Sensory Characteristics of Halloumi Cheese Kept in Brine. Food Chem. 2007, 100, 219–225. [Google Scholar] [CrossRef]
- Dogan, I.S.; Javidipour, I.; Akan, T. Effects of Interesterified Palm and Cottonseed Oil Blends on Cake Quality. Int. J. Food Sci. Technol. 2007, 42, 157–164. [Google Scholar] [CrossRef]
- Bayraktar, V.N. Organic Acids Concentration in Wine Stocks after Saccharomyces cerevisiae Fermentation. Biotechnol. Acta 2013, 6, 97–106. [Google Scholar] [CrossRef]
- Ginane, C.; Baumont, R.; Favreau-Peigné, A. Perception and Hedonic Value of Basic Tastes in Domestic Ruminants. Physiol. Behav. 2011, 104, 666–674. [Google Scholar] [CrossRef]
- Wang, Y.; Nie, S.; Li, C.; Xiang, H.; Zhao, Y.; Chen, S.; Li, L.; Wu, Y. Application of Untargeted Metabolomics to Reveal the Taste-Related Metabolite Profiles during Mandarin Fish (Siniperca chuatsi) Fermentation. Foods 2022, 11, 944. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tak, H.-J.; Lee, N.-R.; Yang, S.; Kim, S.-Y.; Lee, C.-H. Qualitative and Quantitative Metabolite Comparison of Korean Traditional Alcoholic Beverages: Takju, Yakju, and Traditional-Soju. Foods 2024, 13, 956. https://doi.org/10.3390/foods13060956
Tak H-J, Lee N-R, Yang S, Kim S-Y, Lee C-H. Qualitative and Quantitative Metabolite Comparison of Korean Traditional Alcoholic Beverages: Takju, Yakju, and Traditional-Soju. Foods. 2024; 13(6):956. https://doi.org/10.3390/foods13060956
Chicago/Turabian StyleTak, Hyun-Ji, Na-Rae Lee, Sowon Yang, So-Young Kim, and Choong-Hwan Lee. 2024. "Qualitative and Quantitative Metabolite Comparison of Korean Traditional Alcoholic Beverages: Takju, Yakju, and Traditional-Soju" Foods 13, no. 6: 956. https://doi.org/10.3390/foods13060956






