Study on the Effect of Different Concentrations of SO2 on the Volatile Aroma Components of ‘Beibinghong’ Ice Wine
Abstract
:1. Introduction
1.1. Materials and Reagents
1.1.1. Experimental Materials
1.1.2. Reagents
1.2. Instruments and Equipment
1.3. Methods
1.3.1. Process Flow of Brewing ‘Beibinghong’ Ice Wine
1.3.2. Sample Labeling
1.3.3. Detection Methods of Basic Physical and Chemical Indexes
1.3.4. Quantification of Volatile Compounds in ‘Beibinghong’ Ice Wine by HS-GC-IMS
1.3.5. Odor Activity Value (OAV) Calculation
1.4. Sensory Evaluation
1.5. Statistical Analysis of Data
2. Results and Analysis
2.1. Fermentation Process and Basic Indexes of ‘Beibinghong’ Ice Wine with Different SO2 Additions
2.2. Changes in Anthocyanin Content of ‘Beibinghong’ Ice Wine Brewed with Different SO2 Additions
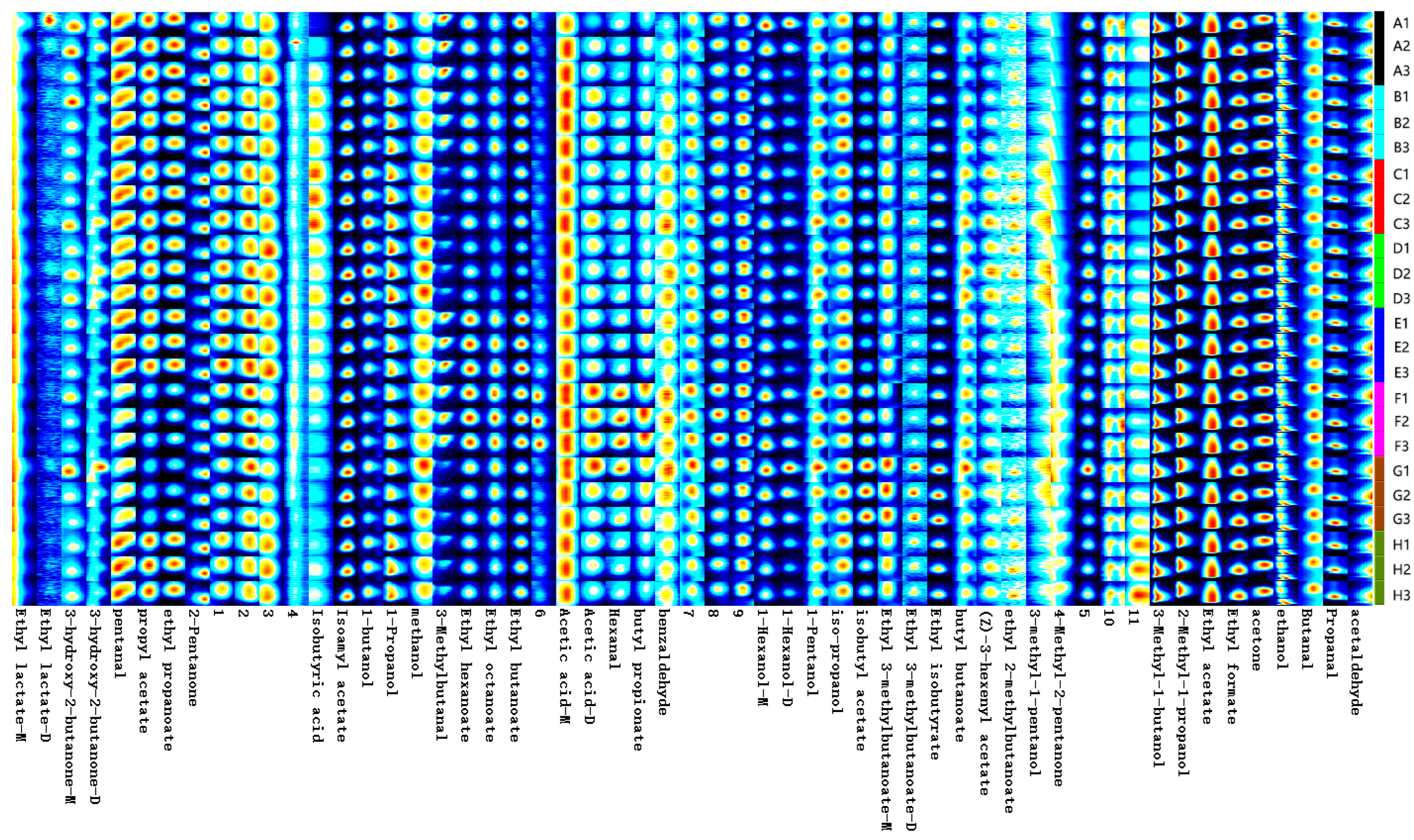
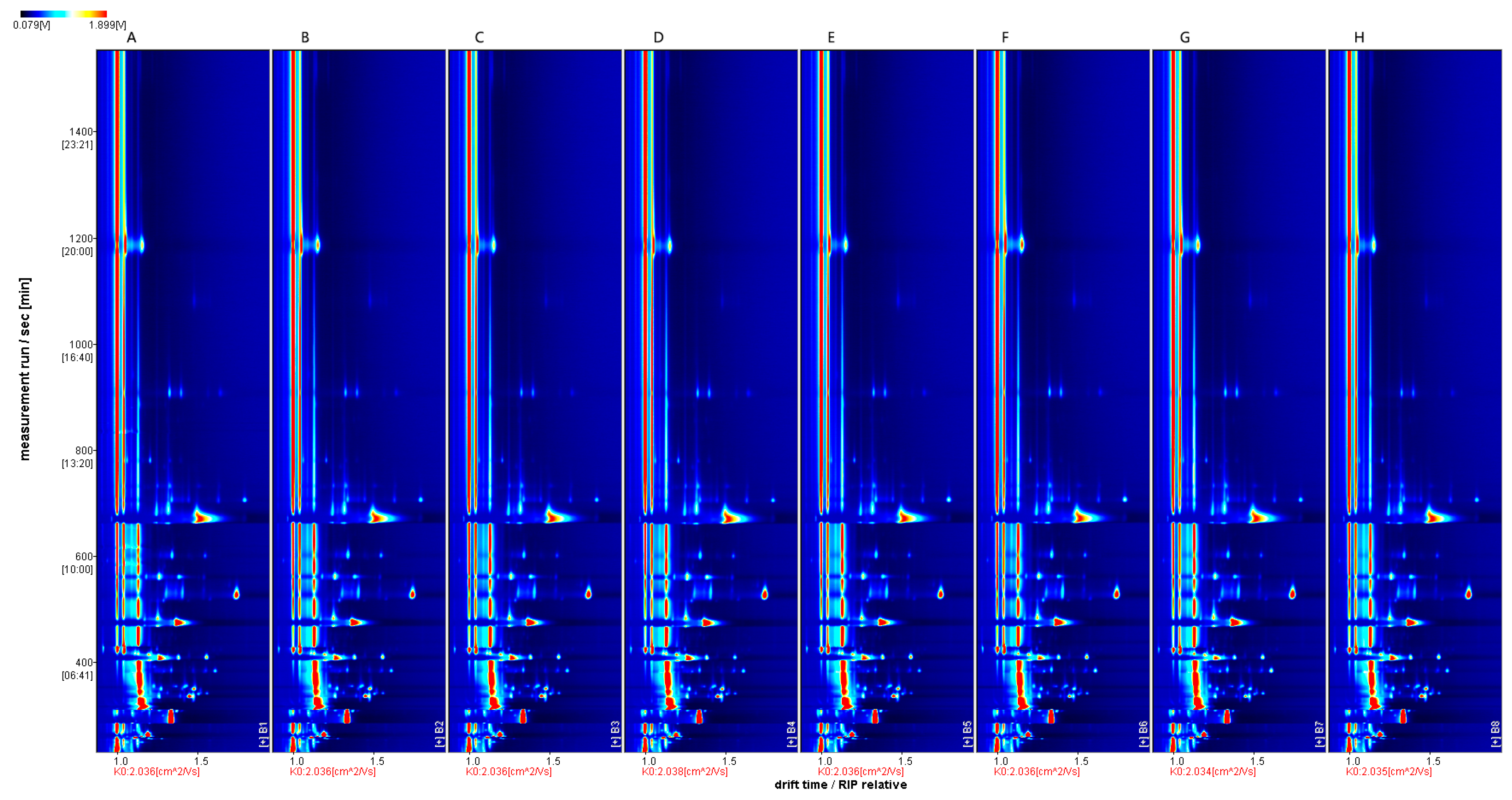
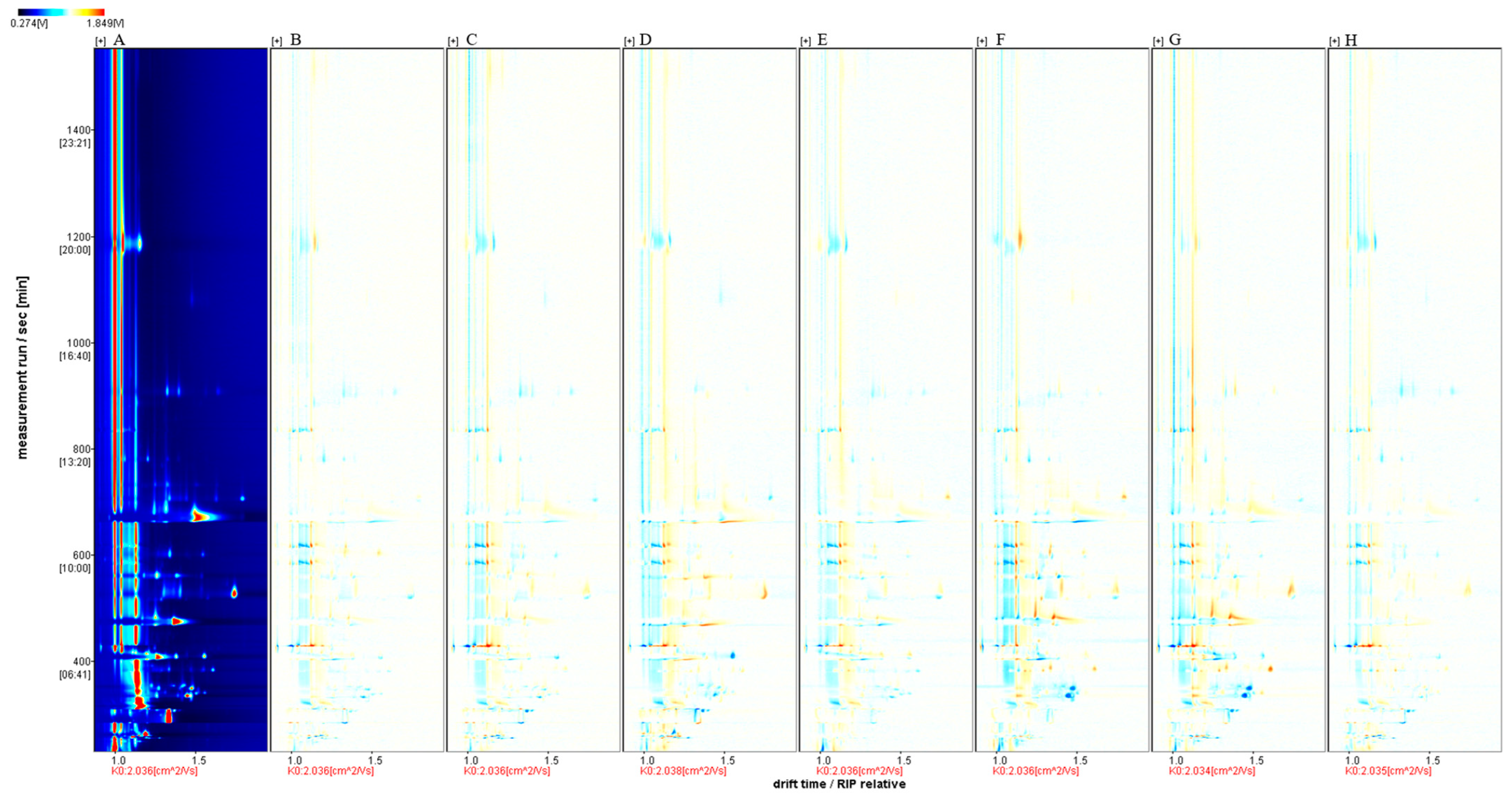
2.3. HS-GC-IMS Analysis of ‘Beibinghong’ Ice Wine Brewed with Different SO2 Additions
Fingerprints of Volatile Components of ‘Beibinghong’ Ice Wine Brewed with Different SO2 Additives
2.4. Analysis of Volatile Components
2.4.1. Esters
2.4.2. Alcohols
2.4.3. Others
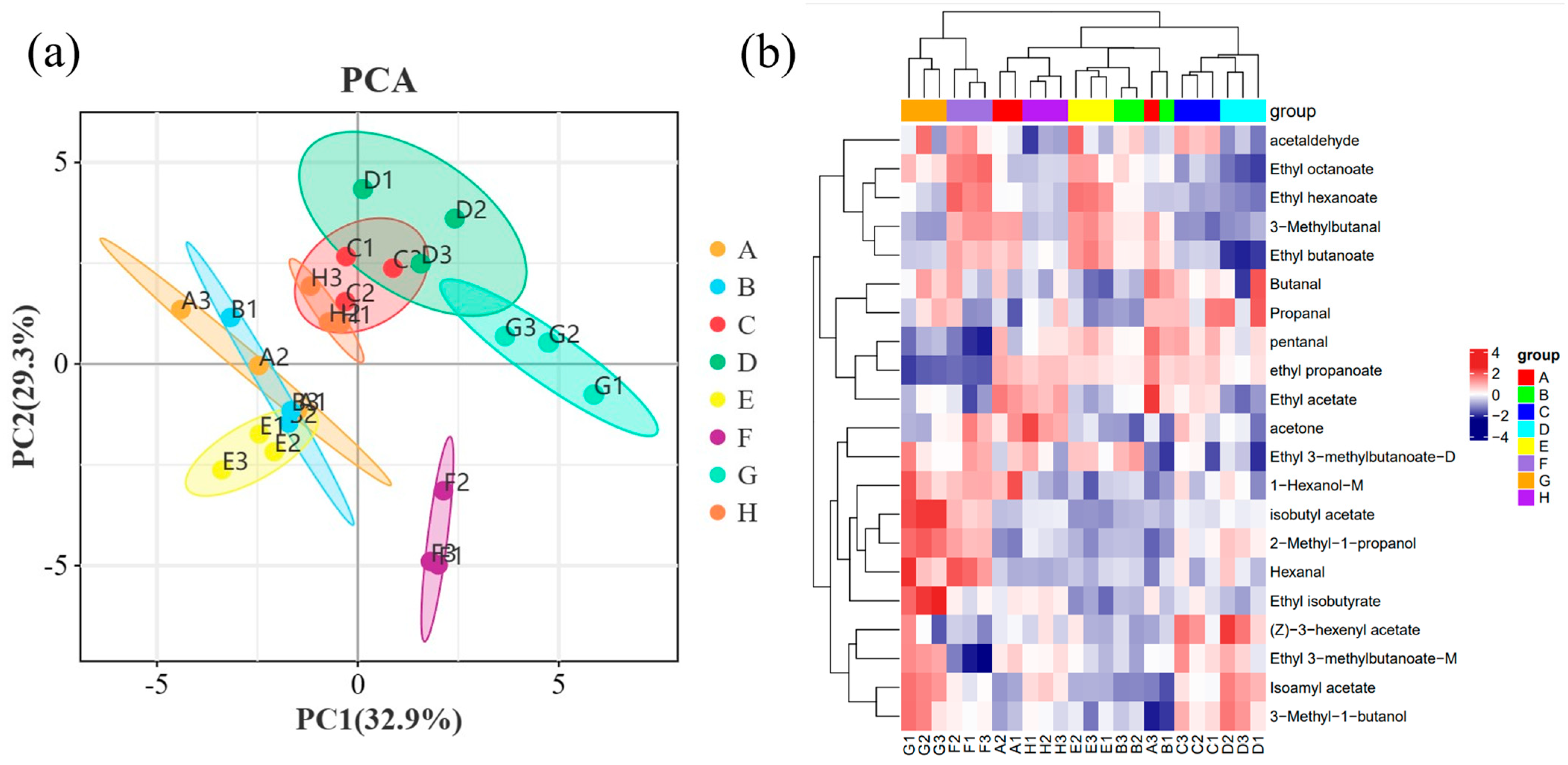
2.5. Principal Component Analysis (PCA) of Wine Samples
2.5.1. OAV Analysis of Major Aroma Compounds of Different Wine Samples
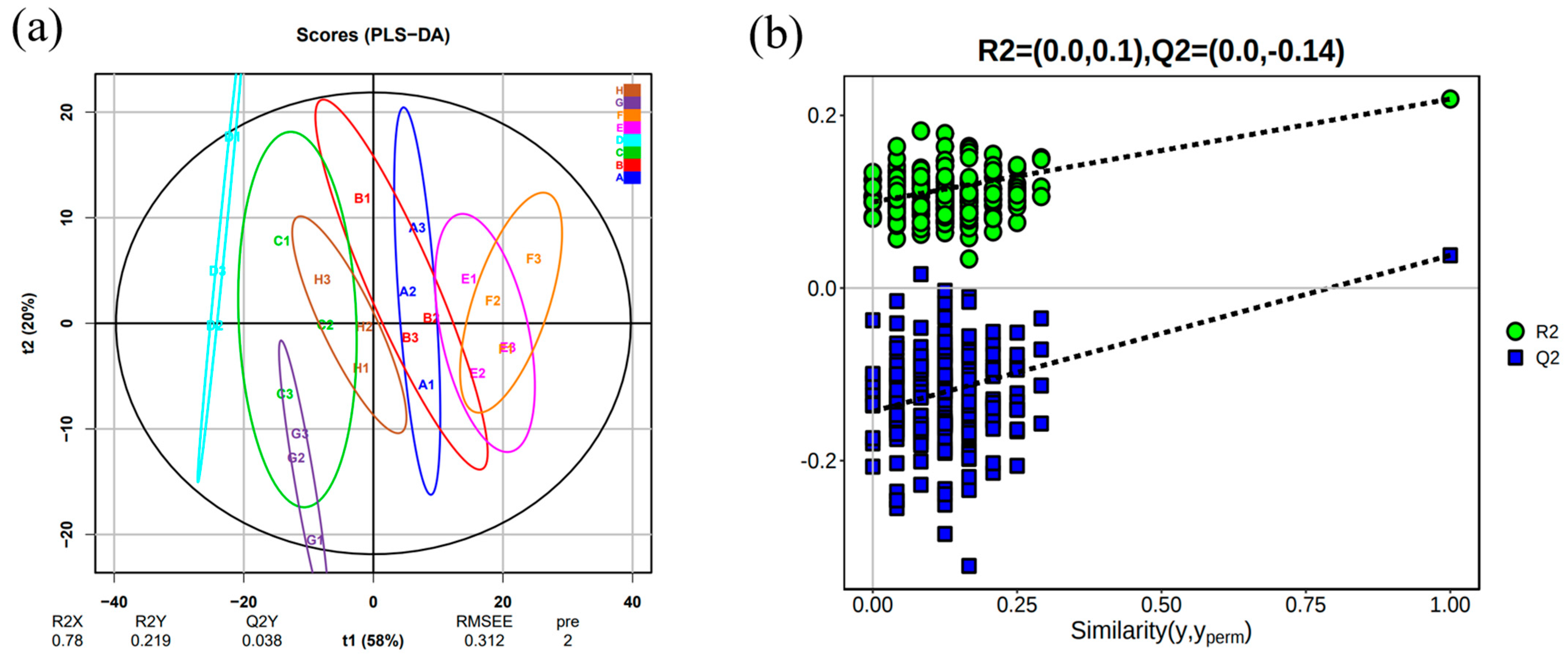
2.5.2. Analysis of Volatile Compounds 0PLS-DA in Wine
2.6. Sensory Evaluation of ‘Beibinghong’ Ice Wine Brewed with Different Concentrations of SO2
3. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Penghui, L.; Yuanlong, J.; Donglin, C.; Xinyuan, W.; Jiahua, L.; Rongchen, Z.; Zhitong, W.; Yang, H.; Liankui, W. Study on the relationship between flavor components and quality of ice wine during freezing and brewing of ‘beibinghong’ grapes. Food Chem. X 2023, 20, 101016. [Google Scholar]
- Lan, Y.-B.; Qian, X.; Yang, Z.-J.; Xiang, X.-F.; Yang, W.-X.; Liu, T.; Zhu, B.-Q.; Pan, Q.-H.; Duan, C.-Q. Striking changes in volatile profiles at sub-zero temperatures during over-ripening of ‘Beibinghong’ grapes in Northeastern China. Food Chem. 2016, 212, 172–182. [Google Scholar] [CrossRef] [PubMed]
- Song, R.; Lu, W.; Shen, Y. New Grape Winemaking Variety-Beibinghong. J. Acta Hortic. Sin. 2008, 35, 1085. [Google Scholar]
- Liu, Y.; Fan, S.; Yang, Y.; Zhang, B.; Qin, H.; Lu, W. Effects of Different Fertilization Amounts on Nutrient Elements Accumulation, Yield and Quality of ‘Beibinghong’ Grape. North. Hortic. 2023, 7, 46–51. [Google Scholar]
- Li, C.; Liu, Y.; Fan, S.; Yang, Y.; Lu, W. Current Situation and Preview of ‘Beibinghongs’ Cultivation. Spec. Wild Econ. Anim. Plant 2019, 41, 125–128. [Google Scholar] [CrossRef]
- Na, L.; Guanyu, L.; Aihua, L.; Yongsheng, T. Synergy Effect between Fruity Esters and Potential Odorants on the Aroma of Hutai-8 Rose Wine Revealed by Threshold, S-Curve, and σ-τ Plot Methods. J. Agric. Food Chem. 2023, 71, 13869–13879. [Google Scholar]
- Peng, X.; Yang, X.; Aisaiti, A.; Yang, F.; Li, Z.; Li, H. Effects of different concentrations of free SO2 and dissolved oxygen on wine color and anthocyanins content. Food Mach. 2022, 38, 11–16+23. [Google Scholar] [CrossRef]
- Li, Q.; Zhang, X.; Niu, G.; Dang, Y.; Sun, S. Determination of Wine by Inductively Coupled Plasma Emission Spectrometry (ICP-OES) Study on Sulfur Dioxide Method. Food Nutr. China 2023, 29, 17–20. [Google Scholar] [CrossRef]
- Sara, W.; Pascaline, R.; Soizic, L.; Laura, F.; Georgia, L.; Margaux, C.; Jean-Christophe, B.; Joana, C.; Joana, T.; Isabelle, M.-P. Non-Saccharomyces yeasts as bioprotection in the composition of red wine and in the reduction of sulfur dioxide. LWT 2021, 149, 111781. [Google Scholar]
- Mu, J.; Wang, Y.; Liu, J.; Zhao, X. The Changes of the SO2 during the Alcohol Fermentation Process in Wine. Food Res. Dev. 2011, 32, 53–55. [Google Scholar]
- Yildirim, H.K. Alternative methods of sulfur dioxide used in wine production. J. Microbiol. Biotechnol. Food Sci. 2020, 9, 675–687. [Google Scholar] [CrossRef]
- The National Standard for Food Safety Packaged Drinking Water (GB19298-2014), the National Standard for Food Safety Use of Food Additives (GB2760-2014) and 37 other national food safety standards were issued. Beverage Ind. 2014, 17, 46–47.
- GB 7718-2011; General Principles for the Labelling of Prepackaged Foods Promulgated and Implemented. Ministry of Health: Wellington, New Zealand, 2011.
- Lin, Z. Analysis and Prospects of Sulfur Dioxide Residues in Wine. Food Ind. 2023, 12, 104–106. [Google Scholar]
- Wang, H.; Tian, X.; Yang, C.; Han, Y.; Shi, X.; Li, H. Wine and health. China Brew. 2022, 41, 1–5. [Google Scholar]
- Du, F.; Huang, Y.; Liu, Z.; Miao, J.; Lai, K. Effects of pH, linoleic acid, and reheating on volatile compounds in glucose-lysine model system. Food Biosci. 2024, 58, 103631. [Google Scholar] [CrossRef]
- Wang, X.; Dang, C.; Liu, Y.; Ge, X.; Suo, R.; Ma, Q.; Wang, J. Effect of indigenous Saccharomyces cerevisiae strains on microbial community successions and volatile compounds changes during Longyan wine fermentation. Food Biosci. 2024, 57, 103595. [Google Scholar] [CrossRef]
- Prezioso, I.; Fioschi, G.; Rustioni, L.; Mascellani, M.; Natrella, G.; Venerito, P.; Gambacorta, G.; Paradiso, V.M. Influence of prolonged maceration on phenolic compounds, volatile profile and sensory properties of wines from Minutolo and Verdeca, two Apulian white grape varieties. Lebensm.-Wiss. Technol. 2024, 192, 115698. [Google Scholar] [CrossRef]
- Ju, Y.L.; Xu, X.L.; Yu, Y.K.; Liu, M.; Wang, W.N.; Wu, J.R.; Liu, B.C.; Zhang, Y.; Fang, Y.L. Effects of winemaking techniques on the phenolics, organic acids, and volatile compounds of Muscat wines. Food Biosci. 2023, 54, 102937. [Google Scholar] [CrossRef]
- Weiyu, C.; Nan, S.; Jinli, W.; Yiming, Y.; Yanli, W.; Wenpeng, L. Widely Targeted Metabolomics Was Used to Reveal the Differences between Non-Volatile Compounds in Different Wines and Their Associations with Sensory Properties. Foods 2023, 12, 290. [Google Scholar] [CrossRef]
- Shu, N. Study on Fermentation Characteristics and Dry Red WineBrewing Technology of New Vitis amurensis Cultivar ‘Beiguohong’. Master’s Thesis, Chinese Academy of Agricultural Sciences, Beijing, China, 2019. [Google Scholar]
- Yonghong, Y.; Songyan, Z.; Yuanxing, W. Analysis of aroma components changes in Gannan navel orange at different growth stages by HS-SPME-GC–MS, OAV, and multivariate analysis. Food Res. Int. 2024, 175, 113622. [Google Scholar]
- Qianqian, L.; Bei, L.; Rong, Z.; Shuyan, L.; Shupeng, Y.; Yi, L.; Jianxun, L. Flavoromics Approach in Critical Aroma Compounds Exploration of Peach: Correlation to Origin Based on OAV Combined with Chemometrics. Foods 2023, 12, 837. [Google Scholar] [CrossRef] [PubMed]
- Yijin, Y.; Lianzhong, A.; Zhiyong, M.; Haodong, L.; Xin, Y.; Li, N.; Hui, Z.; Yongjun, X. Flavor compounds with high odor activity values (OAV > 1) dominate the aroma of aged Chinese rice wine (Huangjiu) by molecular association. Food Chem. 2022, 383, 132370. [Google Scholar]
- ISO 8586-2:2008; Sensory analysis. General Guidelines for the Selection, Training and Monitoring of Assessors. Part 2: Professional Sensory Assessors. ISO: Geneva, Switzerland, 2008.
- ISO 8586:2012; Sensory Analysis. General Guidelines for the Selection, Training and Monitoring of Selected Assessors and Specialised Sensory Assessors. ISO: Geneva, Switzerland, 2012.
- Jin, Y.; Shu, N.; Xie, S.; Cao, W.; Xiao, J.; Zhang, B.; Lu, W. Comparison of ‘Beibinghong’ dry red wines from six producing areas based on volatile compounds analysis, mineral content analysis, and sensory evaluation analysis. Eur. Food Res. Technol. 2021, 247, 1461–1475. [Google Scholar] [CrossRef]
- Kim, B.H.; Park, S.K. Volatile aroma and sensory analysis of black raspberry wines fermented by different yeast strains. J. Inst. Brew. 2015, 121, 87–94. [Google Scholar] [CrossRef]
- Sun, H.; Wu, Y. Sulphur dioxide in the wine industry. Deciduous Fruits 1996, S1, 45–46. [Google Scholar] [CrossRef]
- Pelonnier-Magimel, E.; Mangiorou, P.; Philippe, D.; De Revel, G.; Jourdes, M.; Marchal, A.; Marchand, S.; Pons, A.; Riquier, L.; Tesseidre, P.L.; et al. Sensory characterisation of Bordeaux red wines produced without added sulfites. Oeno One 2020, 54, 687–697. [Google Scholar] [CrossRef]
- Tian, B.; Yuan, M.; Yuan, X. Determination of Dry Leachate in Wine by FOSS Instrument—Specific Gravity Bottle Method. Sino-Overseas Grapevine Wine 2016, 4, 39–41. [Google Scholar] [CrossRef]
- Liu, X.; Xing, J.; Feng, J.; Chen, J.; Jiao, Y.; Yang, B. Research progress on copigmentation of red wine. China Brew. 2023, 42, 9–14. [Google Scholar]
- Margherita, M.; Gianmarco, A.; Anna, M.; Roberto, F.; Serena, F.; Milena, P.; Isabella, T.; Fabio, M.; Andrea, B. Using ethanol as postharvest treatment to increase polyphenols and anthocyanins in wine grape. Heliyon 2024, 10, e26067. [Google Scholar] [CrossRef]
- Ferreira, V.; Carrascon, V.; Bueno, M.; Ugliano, M.; Fernandez-Zurbano, P. Oxygen Consumption by Red Wines. Part I: Consumption Rates, Relationship with Chemical Composition, and Role of SO2. J. Agric. Food Chem. 2015, 63, 10928–10937. [Google Scholar] [CrossRef]
- Christofi, S.; Malliaris, D.; Katsaros, G.; Panagou, E.; Kallithraka, S. Limit SO2 content of wines by applying High Hydrostatic Pressure. Innov. Food Sci. Emerg. Technol. 2020, 62, 102342. [Google Scholar] [CrossRef]
- Oliveira, C.M.; Ferreira, A.C.S.; Freitas, V.D.; Silva, A.M.S. Oxidation mechanisms occurring in wines. Food Res. Int. 2011, 44, 1115–1126. [Google Scholar] [CrossRef]
- Ribéreau-Gayon, P.; Dubourdieu, D.; Donèche, B.; Lonvaud, A. Handbook of Enology, Volume 1: The Microbiology of Wine and Vinifications; John Wiley & Sons: Hoboken, NJ, USA, 2006; Volume 1. [Google Scholar]
- Dallas, C.; Laureano, O. Effects of pH, sulphur dioxide, alcohol content, temperature and storage time on colour composition of a young Portuguese red table wine. J. Sci. Food Agric. 1994, 65, 477–485. [Google Scholar] [CrossRef]
- Sáenz-Navajas, M.-P.; Henschen, C.; Cantu, A.; Watrelot, A.A.; Waterhouse, A.L. Understanding microoxygenation: Effect of viable yeasts and sulfur dioxide levels on the sensory properties of a Merlot red wine. Food Res. Int. 2018, 108, 505–515. [Google Scholar] [CrossRef] [PubMed]
- Bakker, J.; Bridle, P.; Bellworthy, S.; Garcia-Viguera, C.; Reader, H.; Watkins, S. Effect of sulphur dioxide and must extraction on colour, phenolic composition and sensory quality of red table wine. J. Sci. Food Agric. 1998, 78, 297–307. [Google Scholar] [CrossRef]
- Miao, L.; Zhao, X.; Han, A.; Jiang, K. The Main Influencing Factors of Anthocyanins in Red Wine. Liquor-Mak. Sci. Technol. 2016, 2, 40–46. [Google Scholar] [CrossRef]
- Coetzee, C.; Lisjak, K.; Nicolau, L.; Kilmartin, P.; du Toit, W.J. Oxygen and sulfur dioxide additions to Sauvignon blanc must: Effect on must and wine composition. Flavour Fragr. J. 2013, 28, 155–167. [Google Scholar] [CrossRef]
- Liu, P.-T.; Zhang, B.-Q.; Duan, C.-Q.; Yan, G.-L. Pre-fermentative supplementation of unsaturated fatty acids alters the effect of overexpressing ATF1 and EEB1 on esters biosynthesis in red wine. Lebensm.-Wiss. Technol. 2020, 120, 108925. [Google Scholar] [CrossRef]
- Yang, Z.; Zhang, Z.; He, Y.; Zhu, C.; Hu, B. Effect of fermentation with peel on volatile compounds in Kiwi wines based on Headspace Solid Phase Microextraction-Gas Chromatography-Mass Spectrometry and Gas Chromatography-Ion Mobility Spectrometry. Food Sci. 2024, 1–14. [Google Scholar]
- Wei, G.; Yang, X.; Zhou, Y.; Zeng, F.; Zhang, H. Review on research progress of esters in wine. Sci. Technol. Food Ind. 2015, 36, 394–399. [Google Scholar] [CrossRef]
- Garde-Cerdán, T.; Ancín-Azpilicueta, C. Effect of SO2 on the formation and evolution of volatile compounds in wines. Food Control 2007, 18, 1501–1506. [Google Scholar] [CrossRef]
- Liu, J.; Zhang, B.; Zhu, B.; Duan, C.; Yan, G. Effect of pretreatment of sulfur dioxide preservative on aroma and biogenic amines contents in wines. China Brew. 2020, 39, 32–39. [Google Scholar]
- Santos, T.P.M.; Alberti, A.; Judacewski, P.; Zielinski, A.A.F.; Nogueira, A. Effect of sulphur dioxide concentration added at different processing stages on volatile composition of ciders. J. Inst. Brew. 2018, 124, 261–268. [Google Scholar] [CrossRef]
- Daniel, M.A.; Elsey, G.M.; Capone, D.L.; Perkins, M.V.; Sefton, M.A. Fate of damascenone in wine: The role of SO2. J. Agric. Food Chem. 2004, 52, 8127–8131. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Li, Y.; Pan, L.; Guan, M.; Yuan, X.; Li, S.; Ren, D.; Gu, Y.; Liang, M.; Yi, L. Aroma and taste analysis of pickled tea from spontaneous and yeast-enhanced fermentation by mass spectrometry and sensory evaluation. Food Chem. 2024, 442, 138472. [Google Scholar] [CrossRef] [PubMed]
- Weiyu, C.; Nan, S.; Jinli, W.; Yiming, Y.; Yuning, J.; Wenpeng, L. Characterization of the Key Aroma Volatile Compounds in Nine Different Grape Varieties Wine by Headspace Gas Chromatography–Ion Mobility Spectrometry (HS-GC-IMS), Odor Activity Values (OAV) and Sensory Analysis. Foods 2022, 11, 2767. [Google Scholar] [CrossRef] [PubMed]
- Yue, M.; Noëlle, B.; Ke, T.; Yuanyi, L.; Marie, S.; Yan, X.; Thierry, T.-D. Assessing the contribution of odor-active compounds in icewine considering odor mixture-induced interactions through gas chromatography–olfactometry and Olfactoscan. Food Chem. 2022, 388, 132991. [Google Scholar]
- Lan, Y.-B.; Xiang, X.-F.; Qian, X.; Wang, J.-M.; Ling, M.-Q.; Zhu, B.-Q.; Liu, T.; Sun, L.-B.; Shi, Y.; Reynolds, A.G.; et al. Characterization and differentiation of key odor-active compounds of ‘Beibinghong’ icewine and dry wine by gas chromatography-olfactometry and aroma reconstitution. Food Chem. 2019, 287, 186–196. [Google Scholar] [CrossRef] [PubMed]
- Xu, Q.; Mengqi, L.; Yanfeng, S.; Fuliang, H.; Ying, S.; Changqing, D.; Yibin, L. Decoding the aroma characteristics of icewine by partial least-squares regression, aroma reconstitution, and omission studies. Food Chem. 2024, 440, 138226. [Google Scholar]
- Marine, T.; Marina, B.; Warren, A.; Isabelle, M.; Benoit, C.; Philippe, M.; JeanChristophe, B. Impact of Grape Maturity on Ester Composition and Sensory Properties of Merlot and Tempranillo Wines. J. Agric. Food Chem. 2022, 70, 11520–11530. [Google Scholar]
- Sebzalli, Y.M.; Wang, X.Z. Knowledge discovery from process operational data using PCA and fuzzy clustering. Eng. Appl. Artif. Intell. 2001, 14, 607–616. [Google Scholar] [CrossRef]
- Yi, F.; Ma, N.; Zhu, J. Identification of Characteristic Aroma Compounds in Soy Sauce Aroma Type Xi Baijiu Using Gas Chromatography-Olfactometry, Odor Activity Value and Feller’s Additive Model. Food Sci. 2022, 43, 242–256. [Google Scholar]
- Xiangwu, H.; Lihong, Z.; Sheng, P.; Yijun, L.; Jianrong, L.; Meiqian, Z. Effects of Varieties, Cultivation Methods, and Origins of Citrus sinensis ‘hongjiang’ on Volatile Organic Compounds: HS-SPME-GC/MS Analysis Coupled with OPLS-DA. Agriculture 2022, 12, 1725. [Google Scholar] [CrossRef]
- Li, C.; Wang, Y.; Lv, Y.; Qiu, X.; Wu, B.; Ma, T.; Fang, Y.; Sun, X. Physicochemical characterization and antioxidant capacity analysis of commercial Marselan wines from Ningxia and Hebei regions based on OPLS-DA. Food Ferment. Ind. 2023, 49, 283–292. [Google Scholar] [CrossRef]
- Qi, Y.; Wang, M.; Wan, N.; Yin, D.; Wei, M.; Sun, X.; Fang, Y.; Ma, T. Sensory characteristics of “Shine Muscat” grapes based on consumer reviews and human and intelligent sensory evaluation. Lebensm.-Wiss. Technol. 2024, 195, 115810. [Google Scholar] [CrossRef]
- Valásek, P.; Mlcek, J.; Fisera, M.; Fiserová, L.; Sochor, J.; Baron, M.; Juríková, T. Effect of various sulphur dioxide additions on amount of dissolved oxygen, total antioxidant capacity and sensory properties of white wines. Mitteilungen Klosterneubg. 2014, 64, 193–200. [Google Scholar]

| Time (Min:Sec) | E1 | E2 | Recording |
|---|---|---|---|
| 00:00,000 | 150 mL/min | 2 mL/min | rec |
| 02:00,000 | 150 mL/min | 2 mL/min | - |
| 10:00,000 | 150 mL/min | 10 mL/min | - |
| 20:00,000 | 150 mL/min | 100 mL/min | - |
| 30:00,000 | 150 mL/min | 100 mL/min | stop |
| Item | Percentage | Features | Full Marks |
|---|---|---|---|
| Color | 10% | Chroma and hue | 10 |
| Clarification | 10% | Degree of clarification | 10 |
| Aroma | 30% | Finesse | 5 |
| Richness | 5 | ||
| Coherence | 5 | ||
| Variety characteristics | 5 | ||
| Duration | 5 | ||
| Variation and complexity (multiple levels of aroma) | 5 | ||
| Taste | 40% | Balance and coordination | 10 |
| Body and fullness (weightiness in the mouth) | 10 | ||
| Texture and structure | 5 | ||
| Continuity and layers | 5 | ||
| Flavor quality and persistence | 5 | ||
| Lingering flavor | 5 | ||
| Typicality | 10% | Synthesize and evaluate | 10 |
| Totals | 100 | ||
| SO2 Concentration (mg/L) | Start of Fermentation (h) | End of Fermentation (days) | Soluble Solids (%) | Total Sugar (g/L) | Total Acid (g/L) | Dry Extract (g/L) | Alcohol Content (v/v) |
|---|---|---|---|---|---|---|---|
| 10 | 24 ± 0 f | 23 ± 1 e | 26.3 ± 0.5 b | 162.66 ± 13.2 d | 11.36 ± 0.41 bc | 151.24 ± 15.61 d | 11.5 ± 0.50 bc |
| 20 | 72 ± 0 d | 34 ± 3 d | 26.5 ± 2.0 b | 163.57 ± 8.06 c | 11.28 ± 1.10 c | 150.33 ± 7.58 e | 11.5 ± 0.36 bc |
| 30 | 48 ± 0 e | 34 ± 3 d | 25.4 ± 1.7 c | 162.19 ± 5.13 d | 11.26 ± 1.65 c | 153.71 ± 6.42 b | 12 ± 1.0 a |
| 40 | 48 ± 0 e | 23 ± 1 e | 26.5 ± 1.0 b | 160.96 ± 10.7 e | 11.32 ± 0.26 bc | 156.94 ± 13.33 a | 11.5 ± 0.72 bc |
| 50 | 48 ± 0 e | 21 ± 2 f | 26.7 ± 2.0 b | 161.08 ± 4.5 e | 11.48 ± 0.15 abc | 152.82 ± 17.21 c | 11.5 ± 0.21 bc |
| 60 | 96 ± 0 c | 35 ± 4 c | 27.8 ± 1.0 a | 164.69 ± 5.57 b | 11.65 ± 1.03 abc | 149.21 ± 4.31 f | 11 ± 0.06 cd |
| 80 | 168 ± 0 b | 64 ± 9 a | 28.3 ± 0.8 a | 168.73 ± 11.02 a | 12.06 ± 1.02 ab | 145.17 ± 15.30 g | 10.5 ± 0.42 d |
| 100 | 192 ± 0 a | 38 ± 5 b | 28.5 ± 1.7 a | 168.92 ± 19.20 a | 12.15 ± 0.78 a | 144.98 ± 7.06 g | 10.5 ± 0.11 d |
| SO2 (mg/L) | 10 | 20 | 30 | 40 | 50 | 60 | 80 | 100 |
|---|---|---|---|---|---|---|---|---|
| Total anthocyanin (µg/L) | 126.63 ± 11.26 h | 133.87 ± 7.48 f | 155.86 ± 2.51 c | 135.26 ± 9.06 e | 140.27 ± 3.29 d | 172.56 ± 11.38 b | 194.26 ± 26.93 a | 132.76 ± 18.71 g |
| Serial No. | Retention Time (sec) | Substances | Aroma Description | Substance Content (µg/L) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Sample No. 1 | Sample No. 2 | Sample No. 3 | Sample No. 4 | Sample No. 5 | Sample No. 6 | Sample No. 7 | Sample No. 8 | |||||
| 1 | 1080.89 | Ethyl octanoate | Apricot, Brandy, Fat, Floral, Pineapple | 322.722 ± 19.384 | 347.309 ± 13.027 | 296.373 ± 17.554 | 248.987 ± 13.003 | 376.587 ± 32.87 | 419.786 ± 8.103 | 360.532 ± 17.447 | 310.012 ± 6.096 | |
| 2 | 886.48 | Ethyl lactate-M | Cheese, Floral, Fruit, Pungent, Rubber | 357.994 ± 50.581 | 330.748 ± 9.635 | 345.173 ± 6.24 | 393.777 ± 9.815 | 378.219 ± 11.958 | 302.744 ± 2.979 | 333.986 ± 37.971 | 318.373 ± 5.942 | |
| 3 | 885.16 | Ethyl lactate-D | Cheese, Floral, Fruit, Pungent, Rubber | 41.248 ± 10.967 | 27.997 ± 2.465 | 30.767 ± 4.564 | 33.067 ± 6.445 | 28.271 ± 3.16 | 29.047 ± 5.952 | 31.753 ± 3.379 | 29.832 ± 3.881 | |
| 4 | 707.83 | Ethyl hexanoate | Cheese, Floral, Fruit, Pungent, Rubber | 408.356 ± 25.405 | 414.364 ± 31.845 | 360.863 ± 18.269 | 322.325 ± 11.466 | 548.61 ± 17.016 | 555.558 ± 18.26 | 394.243 ± 29.17 | 382.963 ± 9.191 | |
| 5 | 526.01 | Isoamyl acetate | Apple, Banana, Pear | 2867.294 ± 58.401 | 2775.802 ± 94.577 | 3217.441 ± 64.118 | 3539.672 ± 96.581 | 2949.724 ± 2.712 | 3181.046 ± 71.388 | 3621.83 ± 67.244 | 3319.403 ± 54.505 | |
| 6 | 409.12 | Ethyl butanoate | pineapple flavor | 712.02 ± 17.695 | 653.213 ± 6.981 | 577.538 ± 7.574 | 417.204 ± 11.829 | 765.006 ± 29.341 | 725.577 ± 20.563 | 580.78 ± 6.748 | 617.956 ± 16.818 | |
| 7 | 383.33 | isobutyl acetate | Apple, Banana, Floral, Herb | 266.451 ± 3.555 | 255.106 ± 3.543 | 297.941 ± 9.633 | 308.365 ± 5.877 | 224.468 ± 3.165 | 377.387 ± 14.584 | 512.87 ± 10.248 | 302.304 ± 2.072 | |
| 8 | 350.14 | propyl acetate | Celery, Floral, Pear, Red Fruit | 589.059 ± 20.017 | 535.422 ± 7.295 | 571.309 ± 0.436 | 561.966 ± 4.026 | 554.909 ± 11.265 | 327.439 ± 5.638 | 295.958 ± 12.592 | 600.24 ± 9.152 | |
| 9 | 336.31 | ethyl propanoate | Apple, Pineapple, Rum, Strawberry | 904.927 ± 50.425 | 783.426 ± 25.267 | 827.193 ± 14.304 | 760.554 ± 30.164 | 802.542 ± 14.539 | 479.4 ± 17.647 | 460.67 ± 32.085 | 845.108 ± 17.746 | |
| 10 | 340.92 | Ethyl isobutyrate | fruit | 189.551 ± 5.722 | 173.682 ± 1.248 | 184.696 ± 6.095 | 174.112 ± 6.533 | 163.053 ± 6.197 | 187.054 ± 6.442 | 233.276 ± 8.061 | 192.519 ± 1.879 | |
| 11 | 292.51 | Ethyl acetate | Aromatic, Brandy, Grape | 10,270.327 ± 79.702 | 10,043.96 ± 35.944 | 10,070.65 ± 23.923 | 9856.912 ± 39.493 | 9926.147 ± 32.15 | 9879.314 ± 112.062 | 9994.921 ± 63.219 | 10,158.871 ± 27.223 | |
| 12 | 263.7 | Ethyl formate | Pungent | 2485.568 ± 36.304 | 2385.056 ± 11.981 | 2380.013 ± 13.785 | 2164.664 ± 5.431 | 2485.498 ± 36.882 | 2487.519 ± 7.555 | 2639.144 ± 16.111 | 2418.238 ± 6.044 | |
| 13 | 732.02 | Butyl butanoate | Apple, pineapple flavor | 314.625 ± 20.591 | 339.229 ± 24.721 | 346.936 ± 5.068 | 377.007 ± 20.819 | 372.044 ± 14.43 | 362.305 ± 5.044 | 343.359 ± 12.26 | 331.742 ± 9.67 | |
| 14 | 767.45 | (Z)-3-Hexenyl acetate | Banana, floral | 51.247 ± 2.123 | 50.296 ± 1.656 | 57.74 ± 4.47 | 60.325 ± 4.389 | 50.99 ± 3.059 | 49.451 ± 1.981 | 52.637 ± 7.373 | 52.258 ± 2.257 | |
| 15 | 516.1 | Butyl propionate | Fruit | 88.274 ± 3.05 | 99.196 ± 7.891 | 98.357 ± 5.902 | 88.606 ± 7.85 | 100.681 ± 5.435 | 140.951 ± 4.581 | 114.616 ± 7.301 | 90.438 ± 1.154 | |
| 16 | 424.71 | Ethyl3-methylbutanoate-M | Apple, Mulberry Aroma | 119.992 ± 2.944 | 113.215 ± 4.203 | 123.41 ± 5.895 | 121.37 ± 5.868 | 114.138 ± 3.706 | 98.191 ± 6.187 | 128.856 ± 1.569 | 119.214 ± 1.785 | |
| 17 | 424.09 | Ethyl3-methylbutanoate-D | Apple, Mulberry Aroma | 63.356 ± 8.178 | 66.816 ± 14.996 | 61.928 ± 9.264 | 58.284 ± 8.347 | 70.701 ± 3.646 | 73.035 ± 5.266 | 71.903 ± 6.921 | 67.135 ± 8.382 | |
| 18 | 444.3 | Ethyl 2-methylbutanoate | Apple, Ester, Green Apple, Kiwi, Strawberry | 36.115 ± 0.756 | 43.242 ± 5.611 | 49.203 ± 7.374 | 45.902 ± 3.926 | 54.997 ± 4.229 | 58.865 ± 4.477 | 46.289 ± 4.716 | 44.875 ± 2.32 | |
| No. of ester species | 18 | Total | 20,089.12584 | 19,438.08048 | 19,897.53192 | 19,533.09736 | 19,966.58664 | 19,734.66768 | 20,217.62344 | 20,201.48088 | ||
| % | 25.82 | 24.63 | 24.51 | 24 | 25.09 | 24.27 | 24.68 | 25.5 | ||||
| 1 | 908.81 | 1-Hexanol-M | Banana, Flower, Grass, Herb | 761.642 ± 160.852 | 664.524 ± 32.62 | 709.027 ± 50.629 | 695.845 ± 76.075 | 637.454 ± 59.986 | 802.564 ± 14.503 | 823.121 ± 75.245 | 650.641 ± 30.475 | |
| 2 | 907.5 | 1-Hexanol-D | Banana, Flower, Grass, Herb | 230.981 ± 77.158 | 172.311 ± 15.172 | 194.661 ± 16.176 | 197.904 ± 33.232 | 181.147 ± 34.356 | 246.892 ± 10.754 | 286.767 ± 50.847 | 173.555 ± 14.727 | |
| 3 | 672.36 | 3-Methyl-1-butanol | brandy | 11,466.999 ± 322.872 | 11,589.634 ± 344.147 | 12,161.029 ± 151.633 | 12,360.758 ± 264.311 | 11,737.75 ± 270.254 | 11,990.457 ± 38.299 | 12,405.587 ± 280.962 | 11,910.102 ± 94.116 | |
| 4 | 560.94 | 1-Butanol | Fruit | 566.218 ± 48.597 | 603.987 ± 42.987 | 747.541 ± 51.003 | 813.903 ± 68.365 | 606.207 ± 54.248 | 552.356 ± 4.052 | 549.573 ± 59.427 | 656.573 ± 8.621 | |
| 5 | 472.65 | 2-Methyl-1-propanol | pungent odor | 5164.863 ± 71.084 | 5345.103 ± 89.543 | 5632.972 ± 81.471 | 5768.918 ± 91.136 | 5318.293 ± 89.947 | 5916.619 ± 59.966 | 6204.353 ± 51.918 | 5508.228 ± 54.195 | |
| 6 | 408.34 | 1-Propanol | Alcohol, Candy, Pungent | 2975.777 ± 47.018 | 3039.996 ± 39.433 | 3196.466 ± 54.537 | 3380.095 ± 61.027 | 3033.742 ± 44.128 | 2616.758 ± 31.35 | 2550.967 ± 71.881 | 3153.482 ± 10.22 | |
| 7 | 355.13 | Iso-propanol | pungent odor | 205.871 ± 14.473 | 209.079 ± 14.817 | 222.704 ± 19.452 | 202.013 ± 14.932 | 183.215 ± 10.323 | 187.335 ± 19.493 | 207.814 ± 30.254 | 204.116 ± 8.551 | |
| 8 | 316.72 | Ethanol | alcoholic flavor | 27,126.098 ± 103.789 | 27,986.877 ± 144.839 | 28,515.642 ± 295.583 | 28,715.711 ± 181.66 | 28,281.016 ± 139.473 | 29,096.813 ± 127.829 | 28,853.397 ± 710.693 | 27,325.985 ± 86.132 | |
| 9 | 304.42 | Methanol | alcoholic flavor | 226.817 ± 10.88 | 260.779 ± 9.167 | 270.235 ± 1.287 | 295.837 ± 5.807 | 277.732 ± 4.16 | 285.157 ± 7.154 | 288.702 ± 19.969 | 255.843 ± 5.281 | |
| 10 | 856.39 | 3-methyl-1-pentanol | Fruit | 19.148 ± 1.42 | 20.134 ± 0.737 | 24.437 ± 1.327 | 26.431 ± 2.355 | 20.43 ± 1.558 | 20.347 ± 1.472 | 22.287 ± 1.954 | 21.038 ± 0.308 | |
| 11 | 734.1 | 1-Pentanol | alcoholic flavor | 207.371 ± 15.316 | 214.742 ± 8.932 | 226.28 ± 9.042 | 230.289 ± 15.029 | 209.406 ± 6.591 | 221.346 ± 10.837 | 226.752 ± 13.725 | 207.941 ± 5.393 | |
| No. of alcohol species | 11 | Total | 48,951.784 | 50,107.1648 | 51,900.99208 | 52,687.70472 | 50,486.39344 | 51,936.64448 | 52,419.32192 | 50,067.50616 | ||
| % | 62.9 | 63.49 | 63.93 | 64.73 | 63.45 | 63.86 | 63.98 | 63.2 | ||||
| 1 | 1184.66 | Acetic acid-M | Acid, Fruit, Pungent, Sour, Vinegar | 2054.497 ± 79.013 | 2128.91 ± 70.701 | 2107.461 ± 50.139 | 2023.938 ± 65.237 | 2100.214 ± 30.593 | 2214.206 ± 13.902 | 2077.372 ± 158.519 | 2037.589 ± 46.317 | |
| 2 | 1187.29 | Acetic acid-D | Acid, Fruit, Pungent, Sour, Vinegar | 1451.248 ± 398.813 | 1903.677 ± 96.528 | 1755.035 ± 222.202 | 1736.671 ± 70.548 | 1608.712 ± 60.058 | 2177.888 ± 122.534 | 1989.475 ± 388.328 | 1604.389 ± 116.831 | |
| 3 | 1528.26 | Isobutyric acid | Burnt, Butter, Cheese, Sweat | 175.997 ± 74.937 | 277.775 ± 5.854 | 311.54 ± 1.733 | 261.381 ± 20.018 | 246.378 ± 18.058 | 212.109 ± 35.442 | 179.723 ± 16.096 | 206.798 ± 15.601 | |
| No. of acid species | 3 | Total | 3681.7424 | 4310.36144 | 4174.0356 | 4021.99056 | 3955.30352 | 4604.20464 | 4246.57128 | 3848.77752 | ||
| % | 4.73 | 5.46 | 5.14 | 4.94 | 4.97 | 5.66 | 5.18 | 4.86 | ||||
| 1 | 518.25 | Hexanal | Apple, Fat, Fresh, Green, Oil | 81.55 ± 4.566 | 87.068 ± 0.793 | 87.762 ± 6.23 | 90.012 ± 7.245 | 84.554 ± 2.101 | 108.163 ± 4.545 | 103.644 ± 10.978 | 82.262 ± 0.496 | |
| 2 | 355.52 | Pentanal | pungent odor | 234.398 ± 19.219 | 230.222 ± 8.562 | 236.699 ± 6.672 | 225.716 ± 6.372 | 234.86 ± 3.745 | 193.785 ± 7.765 | 208.26 ± 7.021 | 227.682 ± 3.167 | |
| 3 | 230.52 | Acetaldehyde | Floral, Green Apple | 591.445 ± 10.713 | 610.715 ± 20.006 | 638.589 ± 7.464 | 562.302 ± 17.218 | 614.15 ± 53.505 | 638.151 ± 24.136 | 609.98 ± 55.699 | 552.894 ± 24.171 | |
| 4 | 265.32 | Propanal | pungent odor | 492 ± 27.458 | 491.237 ± 23.69 | 517.149 ± 20.744 | 526.488 ± 27.194 | 481.319 ± 11.761 | 489.426 ± 23.396 | 506.841 ± 16.626 | 504.963 ± 11.44 | |
| 5 | 299.69 | Butanal | lemon scent | 89.624 ± 4.037 | 87.713 ± 3.454 | 89.178 ± 1.06 | 88.058 ± 6.826 | 83.719 ± 1.95 | 87.988 ± 3.23 | 89.737 ± 2.049 | 86.741 ± 1.879 | |
| 6 | 1306.57 | Benzaldehyde | Bitter Almond, Burnt Sugar, Cherry, Malt, Roasted Pepper | 115.298 ± 12.179 | 136.866 ± 11.171 | 155.016 ± 16.2 | 136.091 ± 9.857 | 134.335 ± 5.562 | 139.781 ± 10.206 | 134.194 ± 7.058 | 129.152 ± 3.039 | |
| 7 | 309.35 | 3-Methylbutanal | apple flavor | 72.15 ± 0.211 | 57.976 ± 0.993 | 34.274 ± 3.734 | 38.078 ± 3.222 | 73.824 ± 4.135 | 73.536 ± 3.911 | 39.255 ± 3.173 | 44.275 ± 1.451 | |
| No. of aldehyde species | 7 | Total | 1676.46248 | 1701.79744 | 1758.6688 | 1666.7448 | 1706.76016 | 1730.82896 | 1691.9112 | 1627.96816 | ||
| % | 2.15 | 2.16 | 2.17 | 2.05 | 2.14 | 2.13 | 2.07 | 2.06 | ||||
| 1 | 782.71 | 3-Hydroxy-2-butanone-M | Buttery | 166.582 ± 8.812 | 151.271 ± 30.591 | 143.925 ± 20.161 | 144.25 ± 14.122 | 130.308 ± 3.805 | 128.757 ± 18.812 | 131.074 ± 31.528 | 125.517 ± 5.321 | |
| 2 | 781.39 | 3-Hydroxy-2-butanone-D | Buttery | 106.295 ± 15.869 | 97.755 ± 7.259 | 117.172 ± 15.478 | 133.046 ± 15.239 | 93.92 ± 9.742 | 93.471 ± 4.586 | 123.232 ± 21.554 | 102.916 ± 3.284 | |
| 3 | 258.7 | Acetone | Butter, Creamy, Green Pepper | 1019.321 ± 12.794 | 988.156 ± 5.67 | 1014.408 ± 10.468 | 1002.652 ± 12.949 | 1002.012 ± 6.228 | 1025.531 ± 11.472 | 1002.946 ± 8.749 | 1035.26 ± 10.713 | |
| 4 | 381.32 | 4-Methyl-2-pentanone | ketone odor | 73.862 ± 2.163 | 79.874 ± 1.468 | 80.804 ± 1.759 | 88.907 ± 4.275 | 101.28 ± 4.113 | 103.437 ± 1.217 | 102.252 ± 2.736 | 104.469 ± 1.763 | |
| 5 | 356.18 | 2-Pentanone | Fruit, Pungent | 209.642 ± 4.206 | 202.415 ± 1.836 | 193.008 ± 4.084 | 160.568 ± 5.345 | 184.14 ± 2.373 | 170.299 ± 4.524 | 190.478 ± 3.963 | 204.548 ± 3.183 | |
| No. of ketone species | 5 | Total | 1575.7028 | 1519.4704 | 1549.31728 | 1529.42328 | 1511.65784 | 1521.49704 | 1549.97976 | 1572.70792 | ||
| % | 2.02 | 1.93 | 1.91 | 1.88 | 1.9 | 1.87 | 1.89 | 1.99 | ||||
| 1 | 350.14 | 1 | 216.928 ± 6.115 | 233.045 ± 1.731 | 247.923 ± 7.816 | 247.363 ± 4.28 | 248.273 ± 5.335 | 202.989 ± 9.454 | 193.582 ± 8.193 | 233.392 ± 2.338 | ||
| 2 | 335.54 | 2 | 353.637 ± 9.007 | 379.626 ± 7.302 | 399.756 ± 16.515 | 395.541 ± 21.29 | 407.613 ± 1.878 | 375.575 ± 10.84 | 362.538 ± 14.597 | 379.164 ± 5.413 | ||
| 3 | 335.92 | 3 | 378.483 ± 11.402 | 377.544 ± 1.911 | 379.778 ± 14.451 | 386.267 ± 18.497 | 377.753 ± 17.064 | 302.47 ± 5.513 | 314.602 ± 16.398 | 385.479 ± 8.25 | ||
| 4 | 846.77 | 4 | 45.751 ± 10.74 | 39.73 ± 3.065 | 36.079 ± 1.483 | 42.046 ± 3.424 | 42.802 ± 1.533 | 41.685 ± 3.221 | 36.582 ± 8.861 | 42.381 ± 3.375 | ||
| 5 | 780.1 | 5 | 316.261 ± 31.987 | 277.719 ± 49.572 | 291.323 ± 53.825 | 315.245 ± 28.833 | 278.636 ± 8.194 | 252.474 ± 40.115 | 301.364 ± 77.458 | 253.721 ± 10.992 | ||
| 6 | 438.27 | 6 | 45.653 ± 2.394 | 46.319 ± 1.665 | 50.52 ± 3.754 | 49.329 ± 2.226 | 59.937 ± 0.194 | 78.5 ± 5.716 | 50.037 ± 0.974 | 50.825 ± 0.44 | ||
| 7 | 391.08 | 7 | 125.533 ± 8.327 | 133.177 ± 4.903 | 143.463 ± 11.165 | 141.022 ± 3.036 | 124.45 ± 2.218 | 133.933 ± 15.61 | 136.195 ± 8.149 | 130.383 ± 2.378 | ||
| 8 | 589.68 | 8 | 73.228 ± 2.132 | 77.55 ± 2.724 | 75.599 ± 3.334 | 74.943 ± 1.968 | 76.667 ± 0.702 | 84.907 ± 3.385 | 73.308 ± 3.223 | 72.36 ± 3.653 | ||
| 9 | 668.96 | 9 | 115.615 ± 1.826 | 120.402 ± 6.387 | 122.84 ± 4.661 | 123.177 ± 4.685 | 126.067 ± 4.301 | 128.344 ± 4.744 | 120.47 ± 5.171 | 119.037 ± 0.98 | ||
| 10 | 254.41 | 10 | 71.712 ± 6.866 | 66.46 ± 3.262 | 69.208 ± 3.714 | 67.92 ± 3.427 | 66.642 ± 1.678 | 69.151 ± 1.258 | 70.994 ± 2.404 | 71.019 ± 1.878 | ||
| 11 | 383.56 | 11 | 101.099 ± 13.283 | 93.438 ± 2.733 | 85.338 ± 5.32 | 112.917 ± 11.088 | 133.728 ± 7.189 | 130.483 ± 9.075 | 145.343 ± 8.451 | 159.559 ± 1.114 | ||
| No. of other categories | 11 | Total | 1843.89744 | 1845.01072 | 1901.8272 | 1955.77032 | 1942.56776 | 1800.51088 | 1805.01496 | 1897.3192 | ||
| % | 2.37 | 2.34 | 2.34 | 2.4 | 2.44 | 2.26 | 2.2 | 2.4 | ||||
| Total | 77,818.71496 | 78,921.88528 | 81,182.37288 | 81,394.73104 | 79,569.26936 | 81,328.35368 | 81,930.42256 | 79,215.75984 | ||||
| Serial No. | Substance | A | B | C | D | E | F | G | H |
|---|---|---|---|---|---|---|---|---|---|
| 1 | Ethyl octanoate | 3.508 ± 0.211 | 3.775 ± 0.142 | 3.221 ± 0.191 | 2.706 ± 0.141 | 4.093 ± 0.357 | 4.563 ± 0.088 | 3.919 ± 0.19 | 3.37 ± 0.066 |
| 2 | 1-Hexanol-M | 1.523 ± 0.322 | 1.329 ± 0.065 | 1.418 ± 0.101 | 1.392 ± 0.152 | 1.275 ± 0.12 | 1.605 ± 0.029 | 1.646 ± 0.15 | 1.301 ± 0.061 |
| 3 | Ethyl hexanoate | 81.671 ± 5.081 | 82.873 ± 6.369 | 72.173 ± 3.654 | 64.465 ± 2.293 | 109.722 ± 3.403 | 111.112 ± 3.652 | 78.849 ± 5.834 | 76.593 ± 1.838 |
| 4 | 3-Methyl-1-butanol | 52.123 ± 1.468 | 52.68 ± 1.564 | 55.277 ± 0.689 | 56.185 ± 1.201 | 53.353 ± 1.228 | 54.502 ± 0.174 | 56.389 ± 1.277 | 54.137 ± 0.428 |
| 5 | Isoamyl acetate | 7.168 ± 0.146 | 6.94 ± 0.236 | 8.044 ± 0.16 | 8.849 ± 0.241 | 7.374 ± 0.007 | 7.953 ± 0.178 | 9.055 ± 0.168 | 8.299 ± 0.136 |
| 6 | Hexanal | 16.31 ± 0.913 | 17.414 ± 0.159 | 17.552 ± 1.246 | 18.002 ± 1.449 | 16.911 ± 0.42 | 19.817 ± 3.039 | 20.729 ± 2.196 | 16.452 ± 0.099 |
| 7 | 2-Methyl-1-propanol | 5.165 ± 0.071 | 5.345 ± 0.09 | 5.633 ± 0.081 | 5.769 ± 0.091 | 5.318 ± 0.09 | 5.696 ± 0.419 | 6.204 ± 0.052 | 5.508 ± 0.054 |
| 8 | Ethyl butanoate | 791.133 ± 19.661 | 725.792 ± 7.757 | 641.709 ± 8.416 | 463.56 ± 13.144 | 850.006 ± 32.601 | 838.845 ± 41.953 | 645.312 ± 7.497 | 686.617 ± 18.687 |
| 9 | isobutyl acetate | 10.658 ± 0.142 | 10.204 ± 0.142 | 11.918 ± 0.385 | 12.335 ± 0.235 | 8.979 ± 0.127 | 13.266 ± 3.644 | 20.515 ± 0.41 | 12.092 ± 0.083 |
| 10 | Pentanal | 19.533 ± 1.602 | 19.185 ± 0.714 | 19.725 ± 0.556 | 18.81 ± 0.531 | 19.572 ± 0.312 | 17.49 ± 2.186 | 17.355 ± 0.585 | 18.974 ± 0.264 |
| 11 | Ethyl propanoate | 90.493 ± 5.042 | 78.343 ± 2.527 | 82.719 ± 1.43 | 76.055 ± 3.016 | 80.254 ± 1.454 | 59.23 ± 19.215 | 46.067 ± 3.208 | 84.511 ± 1.775 |
| 12 | Ethyl isobutyrate | 12.637 ± 0.381 | 11.579 ± 0.083 | 12.313 ± 0.406 | 11.607 ± 0.436 | 10.87 ± 0.413 | 12.232 ± 0.841 | 15.552 ± 0.537 | 12.835 ± 0.125 |
| 13 | Ethyl acetate | 2.054 ± 0.016 | 2.009 ± 0.007 | 2.014 ± 0.005 | 1.971 ± 0.008 | 1.985 ± 0.006 | 1.984 ± 0.012 | 1.999 ± 0.013 | 2.032 ± 0.005 |
| 14 | Acetone | 1.225 ± 0.015 | 1.188 ± 0.007 | 1.219 ± 0.013 | 1.205 ± 0.016 | 1.204 ± 0.007 | 1.221 ± 0.011 | 1.205 ± 0.011 | 1.244 ± 0.013 |
| 15 | Acetaldehyde | 23.658 ± 0.429 | 24.429 ± 0.8 | 25.544 ± 0.299 | 22.492 ± 0.689 | 24.566 ± 2.14 | 24.719 ± 1.073 | 24.399 ± 2.228 | 22.116 ± 0.967 |
| 16 | Propanal | 6.074 ± 0.339 | 6.065 ± 0.292 | 6.385 ± 0.256 | 6.5 ± 0.336 | 5.942 ± 0.145 | 6.029 ± 0.301 | 6.257 ± 0.205 | 6.234 ± 0.141 |
| 17 | (Z)-3-hexenyl acetate | 1.653 ± 0.068 | 1.622 ± 0.053 | 1.863 ± 0.144 | 1.946 ± 0.142 | 1.645 ± 0.099 | 1.584 ± 0.059 | 1.698 ± 0.238 | 1.686 ± 0.073 |
| 18 | Ethyl 3-methylbutanoate-M | 1199.918 ± 29.439 | 1132.15 ± 42.03 | 1234.101 ± 58.948 | 1213.696 ± 58.676 | 1141.382 ± 37.058 | 1041.044 ± 103.454 | 1288.565 ± 15.688 | 1192.138 ± 17.852 |
| 19 | Ethyl 3-methylbutanoate-D | 633.563 ± 81.778 | 668.157 ± 149.962 | 619.284 ± 92.636 | 582.841 ± 83.472 | 707.005 ± 36.459 | 716.13 ± 41.233 | 719.029 ± 69.215 | 671.354 ± 83.818 |
| 20 | Butanal | 5.637 ± 0.254 | 5.517 ± 0.217 | 5.609 ± 0.067 | 5.538 ± 0.429 | 5.265 ± 0.123 | 5.451 ± 0.282 | 5.644 ± 0.129 | 5.455 ± 0.118 |
| 21 | 3-Methylbutanal | 180.374 ± 0.528 | 144.94 ± 2.482 | 85.685 ± 9.335 | 95.195 ± 8.055 | 184.561 ± 10.337 | 185.264 ± 11.209 | 98.137 ± 7.933 | 110.689 ± 3.628 |
| Substances | VIP Value |
|---|---|
| Ethyl butanoate | 2.741464 |
| ethyl propanoate | 1.077886 |
| Ethyl 3-methylbutanoate-M | 2.23449 |
| Ethyl 3-methylbutanoate-D | 1.761919 |
| 3-Methylbutanal | 1.550059 |
| Item | Percentage | A | B | C | D | E | F | G | H |
|---|---|---|---|---|---|---|---|---|---|
| Color | 10% | 9.0 ± 0.06 | 9.1 ± 0.1 | 9.7 ± 0.15 | 9.2 ± 0.06 | 9.3 ± 0.21 | 9.9 ± 0.15 | 9.8 ± 0.10 | 9.7 ± 0.10 |
| Clarification | 10% | 9.9 ± 0.06 | 10 ± 0 | 10 ± 0 | 10 ± 0 | 10 ± 0 | 10 ± 0 | 10 ± 0 | 10 ± 0 |
| Aroma | 30% | 26.4 ± 1.02 | 27.2 ± 1.25 | 29.3 ± 0.67 | 28.4 ± 1.02 | 28 ± 0.78 | 27 ± 0.21 | 26.7 ± 0.53 | 26.1 ± 1.02 |
| Taste | 40% | 36.1 ± 0.17 | 36.4 ± 2.08 | 39 ± 0.15 | 37 ± 1.0 | 38.1 ± 0.06 | 37.4 ± 1.03 | 38 ± 0.57 | 35.9±0.26 |
| Typicality | 10% | 9.4 ± 0.15 | 9.5 ± 1.21 | 10 ± 0.06 | 9.8 ± 1.15 | 9.6 ± 0.27 | 10 ± 0 | 9.4 ± 0.21 | 9.7 ± 0.06 |
| Totals | 100% | 90.7 ± 1.20 | 92.2 ± 1.27 | 98 ± 1.0 | 94.4 ± 0.57 | 95 ± 1.0 | 94.3 ± 2.07 | 93.9 ± 1.52 | 91.4 ± 0.70 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, B.; Cao, W.; Li, C.; Liu, Y.; Zhao, Z.; Qin, H.; Fan, S.; Xu, P.; Yang, Y.; Lu, W. Study on the Effect of Different Concentrations of SO2 on the Volatile Aroma Components of ‘Beibinghong’ Ice Wine. Foods 2024, 13, 1247. https://doi.org/10.3390/foods13081247
Zhang B, Cao W, Li C, Liu Y, Zhao Z, Qin H, Fan S, Xu P, Yang Y, Lu W. Study on the Effect of Different Concentrations of SO2 on the Volatile Aroma Components of ‘Beibinghong’ Ice Wine. Foods. 2024; 13(8):1247. https://doi.org/10.3390/foods13081247
Chicago/Turabian StyleZhang, Baoxiang, Weiyu Cao, Changyu Li, Yingxue Liu, Zihao Zhao, Hongyan Qin, Shutian Fan, Peilei Xu, Yiming Yang, and Wenpeng Lu. 2024. "Study on the Effect of Different Concentrations of SO2 on the Volatile Aroma Components of ‘Beibinghong’ Ice Wine" Foods 13, no. 8: 1247. https://doi.org/10.3390/foods13081247




