The Bioaccessibility and Bioavailability of Pentachlorophenol in Five Animal-Derived Foods Measured by Simulated Gastrointestinal Digestion
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sample Collection
2.2. Preparation of Spiked Samples and Culinary Treatments
2.3. In Vitro Digestion Procedure
2.4. In Vitro Intestinal Model
2.4.1. Model Building
2.4.2. Model Verification
Transmembrane Resistance
Alkaline Phosphatase
Lucifer Yellow Permeability Assay
2.4.3. Bioavailability Measurement
CCK-8 Experiment
Bioavailability Assay
2.5. Sample Pretreatment and Instrumental Analysis
2.5.1. Pretreatment
2.5.2. Instrumentation
2.5.3. Quality Control and Assurance
2.6. Bioaccessibility and Bioavailability Calculation
2.7. Exposure Assessment
2.8. Statistical Analysis
3. Results and Discussion
3.1. Quality Evaluation of Sample-Spiking Procedures
3.2. Effects of Culinary Treatments on PCP Bioaccessibility in the Five Foods
3.3. Influence of Food Matrices on the Bioaccessibility of PCP
3.4. In Vitro Bioavailability Modeling and Validation
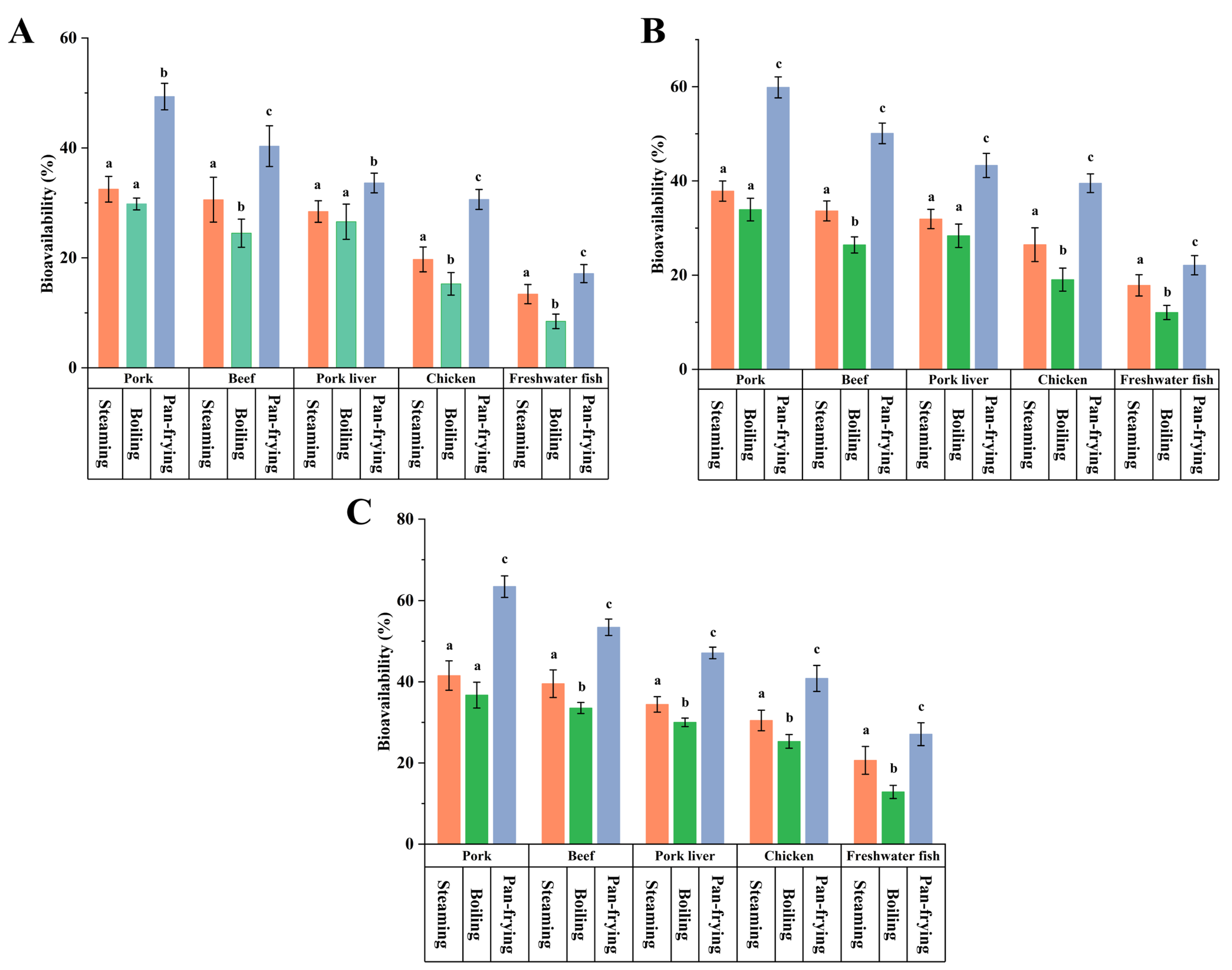
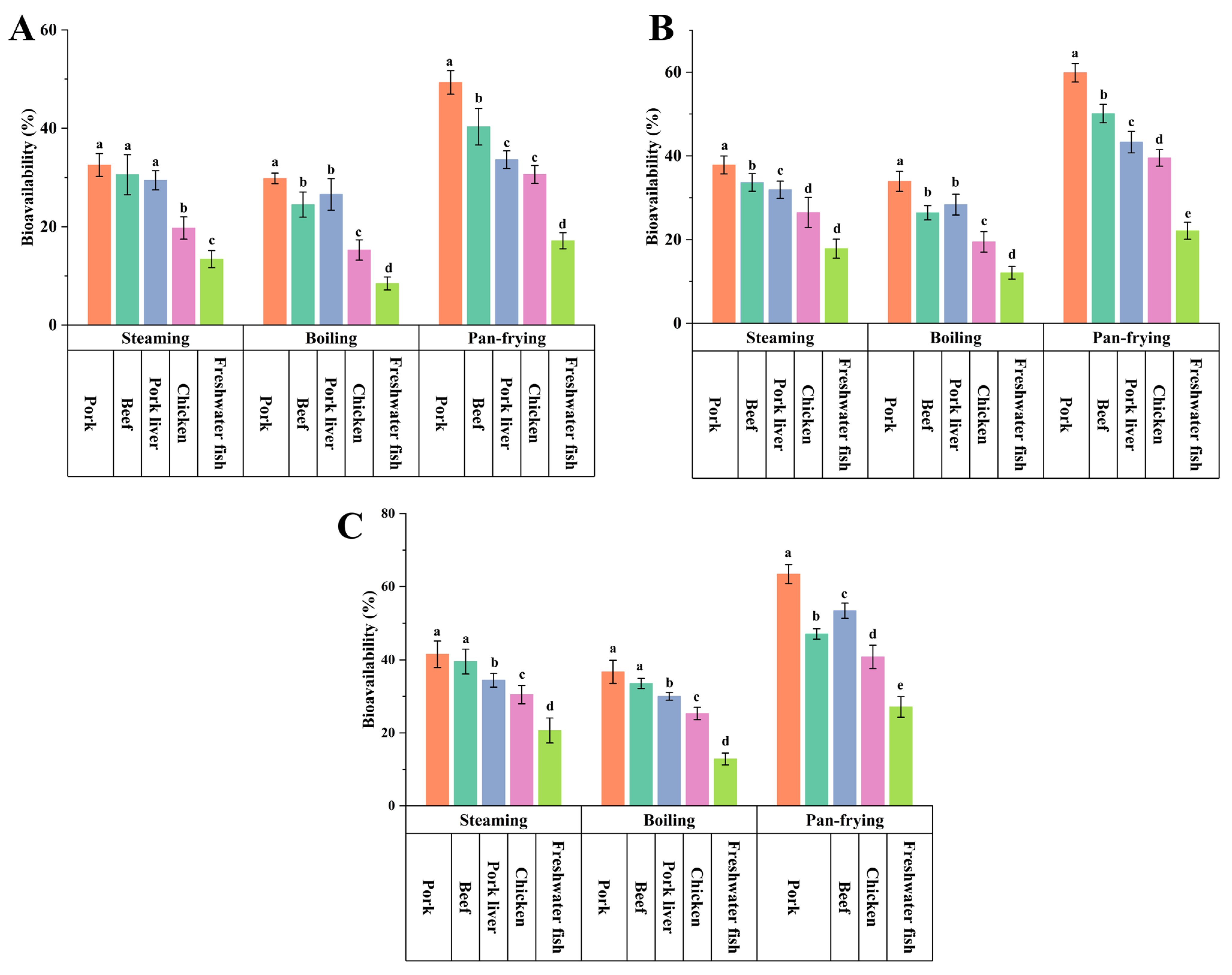
3.5. Effects of Cooking Methods on the Bioavailability of PCP in the Five Foods
3.6. Effects of Food Matrices on the Bioavailability of PCP
3.7. Dietary Exposure Assessment Based on Bioaccessibility and Bioavailability
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhou, Q.; Wu, W.L.; Lin, C.Q.; Liang, H.; Long, C.Y.; Lv, F.; Pan, J.L.; Liu, Z.T.; Wang, B.Y.; Yang, X.F.; et al. Occurrence and dietary exposure assessment of pentachlorophenol in livestock, poultry, and aquatic foods marketed in Guangdong Province, China: Based on food monitoring data from 2015 to 2018. J. Food Sci. 2021, 86, 1132–1143. [Google Scholar] [CrossRef]
- Piskorska-Pliszczynska, J.; Strucinski, P.; Mikolajczyk, S.; Maszewski, S.; Rachubik, J.; Pajurek, M. 3 Pentachlorophenol from an old henhouse as a dioxin source in eggs and related human exposure. Environ. Pollut. 2016, 208 Pt B, 404–412. [Google Scholar] [CrossRef]
- Zhou, Y.; Yin, G.; Asplund, L.; Stewart, K.; Rantakokko, P.; Bignert, A.; Ruokojärvi, P.; Kiviranta, H.; Qiu, Y.; Ma, Z.; et al. Human exposure to PCDDs and their precursors from heron and tern eggs in the Yangtze River Delta indicate PCP origin. Environ. Pollut. 2017, 225, 184–192. [Google Scholar] [CrossRef] [PubMed]
- Zheng, W.; Yu, H.; Wang, X.; Qu, W. Systematic review of pentachlorophenol occurrence in the environment and in humans in China: Not a negligible health risk due to the re-emergence of schistosomiasis. Environ. Int. 2012, 42, 105–116. [Google Scholar] [CrossRef] [PubMed]
- Jiang, P.; Wang, J.; Zhang, J.; Dai, J. Effect of pentachlorophenol on the detoxification system in white-rumped munia (Lonchura striata). J. Environ. Sci. 2016, 44, 224–234. (In Chinese) [Google Scholar] [CrossRef] [PubMed]
- Campbell, L.; Muir, D.; Whittle, D.; Whittle, D.M.; Backus, S.; Norstrom, R.J.; Fisk, A.T. Hydroxylated PCBs and other chlorinated phenolic compounds in lake trout (Salvelinus namaycush) blood plasma from the Great Lakes region. Environ. Sci. Technol. 2003, 37, 1720–1725. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, A.R.; Mortimer, D.; Rose, M.; Smith, F.; Steel, Z.; Panton, S. Recently listed Stockholm convention POPs: Analytical methodology, occurrence in food and dietary exposure. Sci. Total Environ. 2019, 678, 793–800. [Google Scholar] [CrossRef] [PubMed]
- Feng, P.; Ye, Z.; Kakade, A.; Virk, A.K.; Li, X.; Liu, P. A review on gut remediation of selected environmental contaminants: Possible roles of probiotics and gut microbiota. Nutrients 2019, 11, 22. [Google Scholar] [CrossRef] [PubMed]
- Man, Y.B.; Lopez, B.N.; Wang, H.S.; Leung, A.Q.W.; Chow, K.L.; Wang, M.H. Cancer risk assessment of polybrominated diphenyl ethers (PBDEs) and polychlorinated biphenyls (PCBs) in former agricultural soils of Hong Kong. J. Hazard. Mater. 2011, 195, 92–99. [Google Scholar] [CrossRef]
- Dougherty, C.P.; Holtz, S.H.; Reinert, J.C.; Panyacosit, L.; Axelrad, D.A.; Woodruff, T.J. Dietary exposures to food contaminants across the United States. Environ. Res. 2000, 84, 170–185. [Google Scholar] [CrossRef]
- Basheer, C.; Lee, H.K.; Tan, K.S. Endocrine disrupting alkylphenols and bisphenol-A in coastal waters and supermarket seafood from Singapore. Mar. Pollut. Bull. 2004, 48, 1161–1167. [Google Scholar] [CrossRef] [PubMed]
- Ge, J.; Pan, J.; Fei, Z.; Wu, G.; Giesy, J. Concentrations of pentachlorophenol (PCP) in fish and shrimp in Jiangsu Province, China. Chemosphere 2007, 69, 164–169. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Mhungu, F.; Zhang, W.; Wang, Y.; Li, H.; Liu, Y.; Li, Y.; Gan, P.; Pan, X.; Huang, J.; et al. Probabilistic risk assessment of dietary exposure to pentachlorophenol in Guangzhou, China. Food Addit. Contam. A 2023, 40, 262–270. [Google Scholar] [CrossRef] [PubMed]
- Ozaki, A.; Yamaguchi, Y.; Fujita, T.; Kuroda, K.; Endo, G. Chemical analysis and genotoxicological safety assessment of paper and paperboard used for food packaging. Food Chem. Toxicol. 2004, 42, 1323–1337. [Google Scholar] [CrossRef] [PubMed]
- European Commission. Corrigendum to Regulation (EC) No 1907/2006 of the European Parliament and of the Council of 18 December 2006 concerning the Registration, Evaluation, Authorisation and Restriction of Chemicals (REACH), establishing a European Chemicals Agency, amending Directive 1999/45/EC and repealing Council Regulation (EEC) No 793/93 and Commission Regulation (EC) No 1488/94 as well as Council Directive 76/769/EEC and Commission Directives 91/155/EEC, 93/67/EEC, 93/105/EC and 2000/21/EC. Off. J. Eur. Union L 2007, 136, 3. [Google Scholar]
- Marques, A.; Lourenço, H.M.; Nunes, M.L.; Roseiro, C.; Santos, C.; Barranco, A.; Rainieri, S.; Langerholc, T.; Cencic, A. New tools to assess toxicity, bioaccessibility and uptake of chemical contaminants in meat and seafood. Food Res. Int. 2011, 44, 510–522. [Google Scholar] [CrossRef]
- Shen, H.; Henkelmann, B.; Rambeck, W.A.; Mayer, R.; Wehr, U.; Schramm, K.W. Physiologically based persistent organic pollutant accumulation in pig tissues and their edible safety differences: An in vivo study. Food Chem. 2012, 132, 1830–1835. [Google Scholar] [CrossRef]
- U.S. Department of Health and Human Services. Toxicological Profile for Pentachlorophenol; U.S. Department of Health and Human Services: Atlanta, GA, USA, 2022.
- IARC. Pentachlorophenol and some related compounds. In Monographs on the Evaluation of Carcinogenic Risks to Humans; International Agency for Research on Cancer: Lyon, France, 2019; Volume 117. [Google Scholar]
- Yang, W.J.; Wu, H.B.; Zhang, C.; Zhong, Q.; Hu, M.J.; He, J.L.; Li, G.A.; Zhu, Z.Y.; Zhu, J.L.; Zhao, H.H.; et al. Exposure to 2,4-dichlorophenol, 2,4,6-trichlorophenol, pentachlorophenol and risk of thyroid cancer: A case-control study in China. Environ. Sci. Pollut. Res. Int. 2021, 28, 61329–61343. [Google Scholar] [CrossRef] [PubMed]
- WHO. Pentachlorophenol in Drinking-Water: Background Document for Development of WHO Guidelines for Drinking-Water Quality; WHO/SDE/WSH/03.04/62; World Health Organization: Geneva, Switzerland, 2003.
- U.S. EPA. Table of Regulated Drinking Water Contaminants. National Primary Drinking Water Regulations. Ground Water and Drinking Water; United States Environmental PrEuropean Commission: Washington, DC, USA, 2005.
- Liao, W.; Wang, G.; Zhao, W.; Zhang, M.; Wu, Y.; Liu, X.; Li, K. Change in mercury speciation in seafood after cooking and gastrointestinal digestion. J. Hazard. Mater. 2019, 375, 130–137. [Google Scholar] [CrossRef]
- Sobral, M.M.C.; Romero-Gonzalez, R.; Faria, M.A.; Cunha, S.C.; Ferreira, I.M.P.L.V.O.; Garrido-Frenich, A. Stability of antibacterial and coccidiostat drugs on chicken meat burgers upon cooking and in vitro digestion. Food Chem. 2020, 316, 126–367. [Google Scholar] [CrossRef]
- Wang, P.; Yin, N.; Cai, X.; Du, H.; Li, Y.; Sun, G.; Cui, Y. Comparison of bioaccessibility and relative bioavailability of arsenic in rice bran: The in vitro with PBET/SHIME and in vivo with mice model. Chemosphere 2020, 259, 127443. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.N.; Wang, Z.H.; Jordan, R.W.; Jiang, S.J.; Gu, Y.G. Bioavailability and health risk assessment of Zn and Cd in edible tissues of two typical shellfish species using in vitro gastrointestinal digestion. Food Res. Int. 2023, 173 Pt 2, 113474. [Google Scholar] [CrossRef] [PubMed]
- Xu, F.F.; Song, J.; Li, Y.Q.; Lai, Y.F.; Lin, J.; Pan, J.L.; Chi, H.Q.; Wang, Y.; Li, Z.Y.; Zhang, G.Q.; et al. Bioaccessibility and bioavailability adjusted dietary exposure of cadmium for local residents from a high-level environmental cadmium region. J. Hazard. Mater. 2021, 420, 126550. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, P.; Sun, S.; Zhou, X.; Mao, P.; McBride, M.B.; Zhang, C.; Li, Y.; Xia, H.; Li, Z. Bioavailability and bioaccessibility of cadmium in contaminated rice by in vivo and in vitro bioassays. Sci. Total Environ. 2020, 719, 137453. [Google Scholar] [CrossRef] [PubMed]
- Ma, F.; Luo, Y.; Liu, Y.; Zhang, M.; Wu, J.; Chen, L.; Zhang, G. The disruption on gut microbiome of Decabromodiphenyl ethane exposure in the simulator of the human intestinal microbial ecosystem (SHIME). Toxicol. Appl. Pharmacol. 2022, 452, 116194. [Google Scholar] [CrossRef] [PubMed]
- Shen, H.; Starr, J.; Han, J.; Zhang, L.; Lu, D.; Guang, R.; Xu, X.; Wang, X.; Li, J.; Li, W.; et al. The bioaccessibility of polychlorinated biphenyls (PCBs) and polychlorinated dibenzo-p-dioxins/furans (PCDD/Fs) in cooked plant and animal origin foods. Environ. Int. 2016, 94, 33–42. [Google Scholar] [CrossRef] [PubMed]
- Oomen, A.G.; Rompelberg, C.J.M.; Bruil, M.A.; Dobbe, C.J.G.; Pereboom, D.P.K.H.; Sips, A.J.A.M. Development of an in vitro digestion model for estimating the bioaccessibility of soil contaminants. Arch. Environ. Contam. Toxicol. 2003, 44, 281–287. [Google Scholar] [CrossRef]
- Fu, J.; Cui, Y. In vitro digestion/caco-2 cell model to estimate cadmium and lead bioaccessibility/bioavailability in two vegetables: The influence of cooking and additives. Food Chem. Toxicol. 2013, 59, 215–221. [Google Scholar] [CrossRef]
- Fedi, A.; Vitable, C.; Ponschin, G.; Ayehunie, S.; Fato, M.; Scaglione, S. In vitro models replicating the human intestinal epithelium for absorption and metabolism studies: A systematic review. J. Control. Release 2021, 335, 247–268. [Google Scholar] [CrossRef]
- Hubatsch, I.; Ragnarsson, E.G.E.; Artursson, P. Determination of drug permeability and prediction of drug absorption in Caco-2 monolayers. Nat. Protoc. 2007, 2, 2111–2119. [Google Scholar] [CrossRef]
- Pick, D.; Degen, C.; Leiterer, M.; Jahreis, G.; Einax, J.W. Transport of selenium species in Caco-2 cells: Analytical approach employing the Ussing chamber technique and HPLC-ICP-MS. Microchem. J. 2013, 110, 8–14. [Google Scholar] [CrossRef]
- Chen, C.; Li, T.; Chen, Z.; Wang, L.; Luo, X. Absorption rates and mechanisms of avenanthramides in a Caco-2 cell model and their antioxidant activity during absorption. J. Agric. Food Chem. 2020, 68, 2347–2356. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.H.; Xiao, J.J.; Liu, Y.Y.; Deng, Y.J.; Fen, W.Z.; Wei, D.; Liao, M.; Cao, H.Q. Gut microbiota influence on oral bioaccessibility and intestinal transport of pesticides in Chaenomeles speciose. Food Chem. 2021, 339, 127985. [Google Scholar] [CrossRef] [PubMed]
- Kondrashina, A.; Arranz, E.; Cilla, A.; Faria, M.A.; Santos-Hernández, M.; Miralles, B.; Hashemi, N.; Rasmussen, M.K.; Young, J.F.; Barberá, R.; et al. Coupling in vitro food digestion with in vitro epithelial absorption; recommendations for biocompatibility. Crit. Rev. Food Sci. 2023, 26, 1–19. [Google Scholar] [CrossRef] [PubMed]
- National Food Safety Standard. Determination of Pentachlorophenol Residues in Animal Origin Foods— Liquid Chromatography-Mass Spectrometry; Ministry of Agriculture and National Health Commission: Beijing, China, 2016.
- Zhang, Y.H.; Ma, W.J. Analysis for Ten-Years Changes of Dietary Nutrition and Health Status of Guangdong Residents, 1st ed.; Zhijian Publishing House: Beijing, China, 2016; pp. 102–115. (In Chinese) [Google Scholar]
- Clegg, H.; Riding, M.J.; Oliver, R.; Jones, K.C.; Semple, K.T. The impact of soil organic matter and soil sterilisation on the bioaccessibility of 14C-azoxystrobin determined by desorption kinetics. J. Hazard. Mater. 2014, 278, 336–342. [Google Scholar] [CrossRef] [PubMed]
- Xing, G.H.; Yang, Y.; Yan Chan, J.K.Y.; Tao, S.; Wong, M.H. Bioaccessibility of polychlorinated biphenyls in different foods using an in vitro digestion method. Environ. Pollut. 2008, 156, 1218–1226. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.X.; Huang, N.B.; Zhang, X.Y.; Li, J.L.; Yu, Z.Q.; Han, S.Y.; Lu, M.; Van de Wiele, T.; Wu, M.H.; Shen, G.Y.; et al. Polybrominated diphenyl ethers in food and associated human daily intake assessment considering bioaccessibility measured by simulated gastrointestinal digestion. Chemosphere 2011, 83, 152–160. [Google Scholar] [CrossRef]
- Wang, H.S.; Zhao, Y.G.; Man, Y.B.; Wong, C.K.C.; Wong, M.H. Oral bioaccessibility and human risk assessment of organochlorine pesticides (OCPs) via fish consumption, using an in vitro gastrointestinal model. Food Chem. 2011, 127, 1673–1679. [Google Scholar] [CrossRef]
- Verheyen, L.; Degryse, F.; Niewold, T.; Smolders, E. Labile complexes facilitate cadmium uptake by Caco-2 cells. Sci. Total Environ. 2012, 426, 90–99. [Google Scholar] [CrossRef]
- Tefera, W.; Tang, L.; Lu, L.; Xie, R.; Seifu, W.; Tian, S. Rice cultivars significantly mitigate cadmium accumulation in grains and its bioaccessibility and toxicity in human HL-7702 cells. Environ. Pollut. 2021, 272, 116020. [Google Scholar] [CrossRef]
- United States National Research Council. Drinking Water and Health; The National Academies Press: Washington, DC, USA, 1977; Volume 1, pp. 19–62. [Google Scholar]
- Zheng, X.; Zhang, Z.; Chen, J.; Liang, H.; Chen, X.; Qin, Y.; Shohag, M.J.I.; Wei, Y.; Gu, M. Comparative evaluation of in vivo relative bioavailability and in vitro bioaccessibility of arsenic in leafy vegetables and its implication in human exposure assessment. J. Hazard. Mater. 2022, 423 Pt A, 126909. [Google Scholar] [CrossRef]
- Lu, M.; Li, G.; Yang, Y.; Yu, Y. A review on in-vitro oral bioaccessibility of organic pollutants and its application in human exposure assessment. Sci. Total Environ. 2021, 752, 142001. [Google Scholar] [CrossRef] [PubMed]
| Culinary Treatment | Condition |
|---|---|
| Steaming | After the water boils, the sample is added and steamed for 5 min |
| Boiling | After 500 mL of distilled water boils, the sample is added and boiled for 5 min |
| Pan-frying | Sample is fried in 20 mL peanut oil at 180 °C for 3 min |
| Groups | Background Levels (μg/kg ww) |
|---|---|
| Pork | <LOQ |
| Beef | <LOQ |
| Pork liver | 1.23 ± 0.16 |
| Chicken | <LOQ |
| Freshwater fish | <LOQ |
| Group | Culinary Treatment | Fortification | Bioaccessibility (%) | Bioavailability (%) |
|---|---|---|---|---|
| Pork | Steaming | Fortified | 60.52 ± 4.43 * | 38.47 ± 2.41 |
| Naturally contaminated | 57.55 ± 3.64 | 39.55 ± 3.14 | ||
| Boiling | Fortified | 54.48 ± 2.47 | 32.69 ± 2.12 | |
| Naturally contaminated | 53.77 ± 4.46 | 33.77 ± 2.84 | ||
| Pan-frying | Fortified | 85.66 ± 2.37 | 58.33 ± 2.41 | |
| Naturally contaminated | 86.74 ± 1.53 | 57.84 ± 3.32 | ||
| Beef | Steaming | Fortified | 55.49 ± 2.43 | 34.37 ± 3.12 |
| Naturally contaminated | 56.77 ± 3.15 | 35.22 ± 2.64 | ||
| Boiling | Fortified | 50.66 ± 2.84 | 28.38 ± 3.71 | |
| Naturally contaminated | 50.17 ± 2.11 | 29.52 ± 3.23 | ||
| Pan-frying | Fortified | 80.36 ± 2.69 * | 48.92 ± 3.71 | |
| Naturally contaminated | 78.22 ± 3.49 | 50.37 ± 4.03 | ||
| Pork liver | Steaming | Fortified | 52.17 ± 4.01 | 30.74 ± 2.52 |
| Naturally contaminated | 49.37 ± 2.46 | 29.47 ± 3.10 | ||
| Boiling | Fortified | 49.83 ± 2.61 | 26.54 ± 3.47 | |
| Naturally contaminated | 47.63 ± 3.23 | 27.67 ± 2.34 | ||
| Pan-frying | Fortified | 73.46 ± 3.58 | 42.76 ± 2.97 | |
| Naturally contaminated | 74.84 ± 2.79 | 41.57 ± 2.46 | ||
| Chicken | Steaming | Fortified | 47.67 ± 3.18 | 24.32 ± 3.47 |
| Naturally contaminated | 48.33 ± 2.73 | 23.69 ± 2.86 | ||
| Boiling | Fortified | 41.51 ± 3.52 | 20.73 ± 2.11 | |
| Naturally contaminated | 39.41 ± 2.44 | 22.34 ± 1.96 | ||
| Pan-frying | Fortified | 72.10 ± 3.29 | 37.62 ± 4.31 | |
| Naturally contaminated | 71.04 ± 2.91 | 39.13 ± 2.56 | ||
| Freshwater fish | Steaming | Fortified | 29.93 ± 3.37 | 16.62 ± 2.62 |
| Naturally contaminated | 30.45 ± 1.74 | 17.97 ± 3.13 | ||
| Boiling | Fortified | 24.94 ± 3.37 | 10.84 ± 2.85 | |
| Naturally contaminated | 22.62 ± 3.83 | 11.56 ± 1.43 | ||
| Pan-frying | Fortified | 67.64 ± 2.28 | 20.74 ± 2.18 | |
| Naturally contaminated | 69.16 ± 1.57 | 22.04 ± 2.63 |
| Group | Culinary Treatment | Bioaccessibility (%) | ||
|---|---|---|---|---|
| Oral Cavity | Stomach | Small Intestine | ||
| Pork | Steaming | 12.51 ± 1.56 aA | 51.05 ± 2.88 aB | 58.86 ± 2.43 aC |
| Boiling | 11.63 ± 2.08 aA | 46.39 ± 2.24 bB | 50.61 ± 2.17 bB | |
| Pan-frying | 16.80 ± 1.17 bA | 70.64 ± 3.59 cB | 81.37 ± 2.16 cC | |
| Beef | Steaming | 10.35 ± 1.77 abA | 42.51 ± 2.29 aB | 50.39 ± 2.08 aC |
| Boiling | 8.90 ± 2.06 aA | 38.74 ± 3.06 aB | 47.92 ± 1.46 aC | |
| Pan-frying | 13.83 ± 1.44 bA | 64.50 ± 2.31 bB | 72.09 ± 3.54 bC | |
| Pork liver | Steaming | 8.52 ± 2.01 abA | 37.89 ± 3.02 aB | 48.76 ± 2.49 aC |
| Boiling | 6.41 ± 1.74 aA | 33.54 ± 2.62 bB | 41.54 ± 2.18 bC | |
| Pan-frying | 10.08 ± 2.52 bA | 56.43 ± 3.08 cB | 69.11 ± 3.02 cC | |
| Chicken | Steaming | 7.09 ± 2.18 abA | 30.48 ± 1.96 aB | 40.71 ± 3.92 aC |
| Boiling | 6.73 ± 1.50 aA | 24.53 ± 2.27 bB | 31.32 ± 2.48 bC | |
| Pan-frying | 9.89 ± 1.63 bA | 51.08 ± 1.62 cB | 63.43 ± 3.27 cC | |
| Freshwater fish | Steaming | 7.72 ± 1.46 abA | 18.02 ± 2.51 aB | 27.31 ± 3.62 aC |
| Boiling | 5.62 ± 1.84 aA | 14.14 ± 2.46 aB | 21.82 ± 2.62 bC | |
| Pan-frying | 8.86 ± 2.04 bA | 45.72 ±3.14 bB | 60.27 ± 2.25 cC | |
| Group | Culinary Treatment | Bioaccessibility (%) | ||
|---|---|---|---|---|
| Oral Cavity | Stomach | Small Intestine | ||
| Pork | Steaming | 20.32 ± 1.83 aA | 53.92 ± 1.96 aB | 62.83 ± 3.52 aC |
| Boiling | 13.83 ± 1.27 bA | 46.49 ± 1.53 bB | 52.91 ± 3.19 bB | |
| Pan-frying | 27.16 ± 3.19 cA | 78.17 ± 3.12 cB | 84.27 ± 4.14 cB | |
| Beef | Steaming | 14.34 ± 2.51 aA | 50.84 ± 2.09 aB | 58.33 ± 2.48 aC |
| Boiling | 10.42 ± 2.16 aA | 46.35 ± 1.55 bB | 53.73 ± 2.16 bC | |
| Pan-frying | 23.61 ± 3.52 bA | 68.03 ± 2.41 cB | 79.62 ± 1.74 cC | |
| Pork liver | Steaming | 11.63 ± 1.64 aA | 44.83 ± 3.14 aB | 54.41 ± 2.66 aC |
| Boiling | 10.11 ± 1.47 aA | 47.41 ± 2.61 aB | 52.18 ± 2.07 aB | |
| Pan-frying | 18.19 ± 2.67 bA | 61.53 ± 1.74 bB | 71.25 ± 3.12 bC | |
| Chicken | Steaming | 8.23 ± 0.96 aA | 36.56 ± 2.09 aB | 48.02 ± 2.33 aC |
| Boiling | 8.05 ± 1.23 aA | 30.74 ± 2.08 bB | 40.09 ± 2.62 bC | |
| Pan-frying | 14.63 ± 2.83 bA | 63.58 ± 2.14 cB | 71.73 ± 2.76 cC | |
| Freshwater fish | Steaming | 7.03 ± 1.50 abA | 22.61 ± 3.46 aB | 30.45 ± 1.86 aC |
| Boiling | 4.93 ± 0.86 aA | 19.05 ± 2.78 aB | 23.19 ± 2.54 bB | |
| Pan-frying | 9.19 ± 1.26 bA | 53.46 ± 2.13 bB | 69.13 ± 2.47 cC | |
| Group | Culinary Treatment | Bioaccessibility (%) | ||
|---|---|---|---|---|
| Oral Cavity | Stomach | Small Intestine | ||
| Pork | Steaming | 23.41 ± 1.86 aA | 61.95 ± 3.48 aB | 70.53 ± 4.34 aC |
| Boiling | 17.05 ± 0.96 bA | 53.46 ± 2.47 bB | 62.39 ± 4.62 bC | |
| Pan-frying | 35.03 ± 1.85 cA | 81.57 ± 1.62 cB | 90.36 ± 3.38 cC | |
| Beef | Steaming | 19.39 ± 2.21 aA | 58.91 ± 4.11 aB | 66.41 ± 2.34 aC |
| Boiling | 15.18 ± 1.53 bA | 50.63 ± 3.18 bB | 60.16 ± 3.21 bC | |
| Pan-frying | 28.86 ± 3.16 cA | 73.89 ± 3.24 cB | 83.63 ± 4.01 cC | |
| Pork liver | Steaming | 15.71 ± 2.63 aA | 48.32 ± 2.54 aB | 60.53 ± 4.62 aC |
| Boiling | 12.08 ± 1.74 aA | 44.69 ± 1.86 aB | 56.32 ± 1.35 bC | |
| Pan-frying | 22.12 ± 3.18 bA | 70.46 ± 2.06 bB | 78.07 ± 2.37 cC | |
| Chicken | Steaming | 11.80 ± 2.76 aA | 44.56 ± 3.36 aB | 57.06 ± 3.14 aC |
| Boiling | 10.03 ± 1.76 aA | 35.09 ± 4.02 bB | 49.24 ± 3.39 bC | |
| Pan-frying | 18.09 ± 1.54 bA | 68.42 ± 3.83 cB | 75.52 ±1.74 cC | |
| Freshwater fish | Steaming | 9.26 ± 1.86 aA | 29.84 ± 2.65 aB | 36.24 ± 2.05 aC |
| Boiling | 8.52 ± 0.69 bA | 24.26 ± 2.78 bB | 30.36 ± 3.47 bC | |
| Pan-frying | 12.26 ± 1.41 cA | 62.48 ± 1.71 cB | 72.14 ± 2.73 cC | |
| Group | Culinary Treatments | Bioaccessibility (%) | Bioavailability (%) | Estimated Daily Intakes (μg/kg bw) | ||||
|---|---|---|---|---|---|---|---|---|
| General Population a | Boys b | Girls c | Male Adults d | Female Adults e | ||||
| Pork | Conventional assumption | 100 | 100 | 0.171 | 0.214 | 0.215 | 0.164 | 0.129 |
| Steaming | 58.86 | 32.54 | 0.033 | 0.041 | 0.041 | 0.031 | 0.025 | |
| Boiling | 50.61 | 29.81 | 0.026 | 0.032 | 0.032 | 0.025 | 0.019 | |
| Pan-frying | 81.37 | 49.34 | 0.069 | 0.086 | 0.086 | 0.066 | 0.052 | |
| Beef | Conventional assumption | 100 | 100 | 0.017 | 0.016 | 0.012 | 0.013 | 0.012 |
| Steaming | 50.39 | 30.58 | 0.003 | 0.002 | 0.002 | 0.002 | 0.002 | |
| Boiling | 47.92 | 24.49 | 0.002 | 0.002 | 0.001 | 0.002 | 0.001 | |
| Pan-frying | 72.09 | 40.32 | 0.005 | 0.005 | 0.004 | 0.004 | 0.004 | |
| Pork liver | Conventional assumption | 100 | 100 | 0.012 | 0.010 | 0.011 | 0.012 | 0.011 |
| Steaming | 48.76 | 28.43 | 0.002 | 0.001 | 0.001 | 0.002 | 0.001 | |
| Boiling | 41.54 | 26.57 | 0.001 | 0.001 | 0.001 | 0.001 | 0.001 | |
| Pan-frying | 69.11 | 33.63 | 0.003 | 0.002 | 0.002 | 0.003 | 0.003 | |
| Chicken | Conventional assumption | 100 | 100 | 0.065 | 0.075 | 0.065 | 0.062 | 0.067 |
| Steaming | 40.71 | 19.72 | 0.005 | 0.006 | 0.005 | 0.005 | 0.005 | |
| Boiling | 31.32 | 15.27 | 0.003 | 0.004 | 0.003 | 0.003 | 0.003 | |
| Pan-frying | 63.43 | 30.63 | 0.013 | 0.014 | 0.013 | 0.012 | 0.013 | |
| Freshwater fish | Conventional assumption | 100 | 100 | 0.092 | 0.076 | 0.105 | 0.098 | 0.099 |
| Steaming | 27.31 | 13.40 | 0.003 | 0.003 | 0.004 | 0.004 | 0.004 | |
| Boiling | 21.82 | 8.45 | 0.002 | 0.001 | 0.002 | 0.002 | 0.002 | |
| Pan-frying | 60.27 | 17.14 | 0.010 | 0.008 | 0.011 | 0.010 | 0.010 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, Q.; Chen, H.; Li, L.; Wu, Y.; Yang, X.; Jiang, A.; Wu, W. The Bioaccessibility and Bioavailability of Pentachlorophenol in Five Animal-Derived Foods Measured by Simulated Gastrointestinal Digestion. Foods 2024, 13, 1254. https://doi.org/10.3390/foods13081254
Zhou Q, Chen H, Li L, Wu Y, Yang X, Jiang A, Wu W. The Bioaccessibility and Bioavailability of Pentachlorophenol in Five Animal-Derived Foods Measured by Simulated Gastrointestinal Digestion. Foods. 2024; 13(8):1254. https://doi.org/10.3390/foods13081254
Chicago/Turabian StyleZhou, Quan, Huiming Chen, Liangliang Li, Yongning Wu, Xingfen Yang, Aimin Jiang, and Weiliang Wu. 2024. "The Bioaccessibility and Bioavailability of Pentachlorophenol in Five Animal-Derived Foods Measured by Simulated Gastrointestinal Digestion" Foods 13, no. 8: 1254. https://doi.org/10.3390/foods13081254





