Comprehensive Elemental Profiling of Romanian Honey: Exploring Regional Variance, Honey Types, and Analyzed Metals for Sustainable Apicultural and Environmental Practices
Abstract
:1. Introduction
2. Materials and Methods
2.1. Research Location
2.2. Collection of Honey Samples
Honey Sampling
2.3. Sample Preparation and Microwave Digestion Procedure
2.3.1. Analytical Approaches for Heavy Metal Analysis
2.3.2. The Microwave-Assisted Digestion Methodology
2.4. Basic ICP-MS Analytical Instrumental Parameters
2.5. Chemical and Apparatus
2.6. Chemical Analysis Quality Control
2.7. Statistical Analysis
3. Results and Discussion
3.1. Analyzing Elemental Distribution in Various Honey Types—Mineral Profiles
3.2. Elemental Dispersion According to the Types of Honey
- i.
- Sunflower honey: Sunflower honey exhibited a relatively low concentration of potassium (K): 45.35 ± 30.40 mg/kg. This finding suggests that sunflower honey has a distinct mineral composition compared to other honey types. This lower concentration might be due to factors such as soil characteristics and agricultural practices in the sunflower production region.
- ii.
- Chestnut honey: Chestnut honey, on the other hand, displayed the highest concentration of potassium (K): 2116.41 ± 183.26 mg/kg. This high K content contributes significantly to the overall mineral composition of chestnut honey. The specific values indicate considerable variability in chestnut honey, potentially influenced by the chestnut trees’ unique soil and nutrient requirements.
- iii.
- Rape: The honey derived from rape in Tecuci showed noteworthy concentrations of potassium (K), with the highest values recorded in autumn rape (1012.05 ± 20.89 mg/kg) and spring rape (1284.66 ± 52.33 mg/kg). These results underline the impact of geographical origin on the mineral content of honey, with variations attributed to seasonal and agricultural factors.
- iv.
- Lavender honey: Lavender honey from the Vaslui region demonstrated elevated concentrations of magnesium (Mg), namely, 84.45 ± 7.75 mg/kg, and iron (Fe), namely, 75.91 ± 5.98 mg/kg. These high values suggest that lavender honey has distinct mineral characteristics, potentially influenced by the specific soil and environmental conditions in the Vaslui region.
- v.
- Honeydew: Honeydew honey displayed the highest sodium (Na) concentrations among the samples, amounting to 69.42 ± 6.32 mg/kg. The mineral composition of honey is significantly influenced by its floral source, as evidenced by the elevated sodium content in honeydew honey compared to other honey varieties.
- vi.
- Heather honey: Heather honey exhibited elevated potassium (K) concentrations, showcasing the impact of floral source on this honey’s mineral profile. The results suggest that heather honey has a distinctive composition with higher potassium levels.
- vii.
- Acacia Honey: Acacia honey was discussed in relation to variations in potassium (K) concentrations across different regions. This highlights the influence of geographical factors, including soil characteristics and agricultural practices, on the mineral content of acacia honey.
- viii.
- Linden Honey: Linden honey was mentioned in the context of potassium (K) concentrations, with values reported in previous studies. This suggests that the mineral composition of linden honey can vary across different regions, with the specific concentration reflecting the unique characteristics of linden nectar.
- ix.
- Multifloral honey: Multifloral honey exhibited fluctuations in calcium (Ca) concentrations, indicating that even within the category of multifloral honey, there can be variations in mineral content. This highlights the need for detailed analyses to understand the specific mineral profile of multifloral honey.
- x.
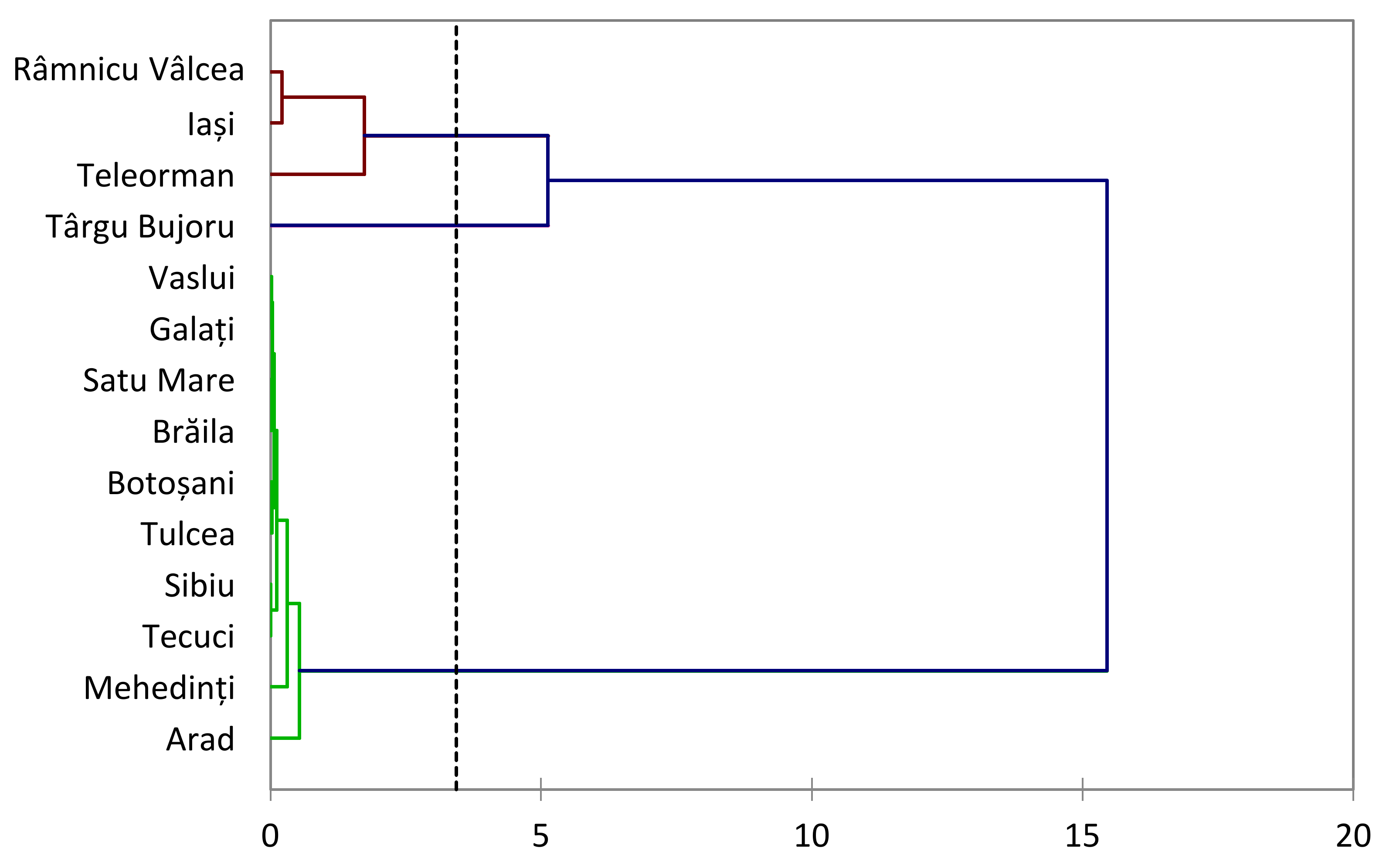
- Honey from various regions—geographical influence: This study analyzed honey samples from various regions, including Botoșani, Râmnicu Vâlcea, Târgu Bujoru, Vaslui, Brăila, Satu Mare, Iași, Tulcea, Mehedinți, Sibiu, and Tecuci. The geographical origins of honey significantly impacted the concentrations of various metals, underscoring the diverse mineral profiles of honey from different regions. This variability necessitates region-specific analyses to accurately assess honey quality and potential health implications.
3.3. Elemental Dispersion According to the Origin of Honey
- i.
- Potassium (K): The substantial variation in potassium (K) concentrations underscores the intricate relationship between honey composition and geographical factors. Honey from Tecuci, particularly that derived from rape, exhibited remarkably high potassium (K) values, emphasizing the regional influence on mineral content. The distribution of potassium (K) is intricately tied to honey’s geographical origin, involving soil characteristics and agricultural practices. This points to the significance of local environmental conditions in shaping the mineral profile of honey.
- ii.
- Calcium (Ca): The diverse concentrations of calcium (Ca) across different honey types and regions highlight the nuanced nature of mineral content in honey. Multifloral honey from Botoșani and Râmnicu Vâlcea displayed extreme values, showcasing variability influenced by regional factors. Târgu Bujoru emerged with the highest concentration of calcium (Ca), while Râmnicu Vâlcea followed closely. This regional disparity manifests the impact of local conditions on the mineral makeup of honey.
- iii.
- Magnesium (Mg), Sodium (Na), and Iron (Fe): The regional and varietal disparities in magnesium (Mg), sodium (Na), and iron (Fe) concentrations highlight the dynamic nature of honey composition. This variability necessitates a comprehensive understanding of the provenance and type of honey for conducting accurate quality assessments and determining potential health implications. Lavender honey samples from the Vaslui region exhibited the highest concentrations of magnesium (Mg) and iron (Fe), while honeydew stood out with elevated sodium (Na) values. Regional variations in mineral content, particularly the documented low values in Botoșani, Iași, and Râmnicu Vâlcea, underscore the regional specificity of honey composition. These variations are attributed to factors such as soil composition and floral sources, highlighting the importance of considering geographical provenance when evaluating honey quality and potential health implications.
- iv.
- Manganese (Mn): The elevated manganese (Mn) concentrations in the honey samples from Botoșani, Brăila, and Arad underscore the regional variability in honey’s mineral composition. This regional specificity highlights the importance of considering geographical provenance when evaluating honey’s quality and potential health implications, as Mn content can influence honey’s sensory and nutritional properties. A notable proportion of samples exhibited manganese concentrations below the detection limit, indicating variations in honey composition even within the analyzed set.
- v.
- Zinc (Zn), Copper (Cu), and Chromium (Cr): The encroachment of the legally permitted maximum concentrations of zinc (Zn) and copper (Cu) in some samples raises concerns about the impact of environmental factors, agricultural practices, and regional differences on honey quality. Râmnicu Vâlcea stood out with the highest zinc concentrations, and several samples exhibited elevated copper values, emphasizing the need for detailed analyses at the regional level. Chromium (Cr) concentrations, though lower in magnitude, displayed regional disparities, with Sibiu, Iași, and Mehedinți showing higher values. This suggests localized influences on honey composition.
- vi.
- Lead (Pb) and Cadmium (Cd): The alarmingly high prevalence of lead (Pb) and cadmium (Cd) that exceeded the legal limits in honey samples from Brăila, Tulcea, Botoșani, Iași, and Mehedinți regions raises significant food safety concerns. These findings warrant immediate attention from regulatory bodies and apicultural stakeholders to implement effective mitigation strategies and safeguard public health. Regions with concentrations below the detection limits, such as Tecuci, Braila, Satu Mare, Sibiu, and Râmnicu Vâlcea, exhibit a more favorable profile, emphasizing the need for stringent monitoring and regulation.
- vii.
- Lithium (Li), Strontium (Sr), Nichel (Ni), and Aluminum (Al): The highest concentrations of lithium (Li), strontium (Sr), nickel (Ni), and aluminum (Al) in the honey samples from Tulcea, Botoșani, Iași, and Sibiu regions suggest potential atmospheric pollution in these areas. Further investigation into pollution sources in these specific regions is warranted to understand and address the factors contributing to elevated levels of these elements in honey.
3.4. Elemental Dispersion According to the Origins of Honey
- i.
- Na/K (R2 = 0.444 **): Explanation: The moderate positive correlation between sodium (Na) and potassium (K) may be attributed to commonalities in environmental factors or plant sources, as both elements are influenced by soil composition.
- ii.
- Mg/K (R2 = 0.309 **): Explanation: The moderate positive correlation between magnesium (Mg) and potassium (K) suggests a shared influence from factors like soil composition and agricultural practices, impacting the concentrations of both minerals.
- iii.
- Mg/Na (R2 = 0.289 **): Explanation: The moderate positive correlation between magnesium (Mg) and sodium (Na) indicates a potential linkage in their uptake from the environment, possibly through similar floral or soil sources.
- iv.
- Al/Na (R2 = 0.288 **): Explanation: The moderate positive correlation between aluminum (Al) and sodium (Na) could be influenced by environmental factors, with both elements showing parallel variations in concentration.
- v.
- Cd/Na (R2 = 0.377 **): Explanation: The moderate positive correlation between cadmium (Cd) and sodium (Na) might be due to commonalities in the sources of contamination or shared pathways of uptake from the environment.
- vi.
- Zn/Ca (R2 = 0.498 **): Explanation: The strong positive correlation between zinc (Zn) and calcium (Ca) suggests a potential relationship influenced by soil characteristics or specific floral sources common to the regions.
- vii.
- Cd/Li (R2 = 0.252 **): Explanation: The weak positive correlation between cadmium (Cd) and lithium (Li) could be indicative of shared geological or anthropogenic sources, influencing their presence in honey.
- viii.
- Cd/Cu (R2 = 0.282 **): Explanation: The weak positive correlation between cadmium (Cd) and copper (Cu) may reflect similar environmental pathways or contamination sources affecting both elements.
- ix.
- Ni/Cr (R2 = 0.61 **): Explanation: The strong positive correlation between nickel (Ni) and chromium (Cr) suggests a substantial commonality in their environmental sources or geochemical influences on honey composition in the respective regions.
- x.
- Al/K (R2 = 0.232 *): Explanation: The positive correlation between Aluminum (Al) and Potassium (K) suggests a shared influence or common environmental factors affecting their presence in honey. This may be linked to soil composition or agricultural practices.
- xi.
- Li/Na (R2 = 0.248 *): Explanation: The positive correlation between Lithium (Li) and Sodium (Na) indicates a potential common source or similar uptake mechanisms. Environmental factors influencing Na concentrations may also impact Li levels in honey.
- xii.
- Cu/Al (R2 = 0.231 *): Explanation: The positive correlation between Copper (Cu) and Aluminum (Al) suggests a potential shared environmental influence or similar pathways of uptake. This could be related to soil composition or other regional factors.
- i.
- Pb/K (R2 = −0.273 **): Explanation: A negative correlation between lead (Pb) and potassium (K) may indicate that regions with higher potassium levels tend to have lower lead concentrations, possibly influenced by soil characteristics or agricultural practices.
- ii.
- Zn/Na (R2 = −0.311 **): Explanation: The negative correlation between zinc (Zn) and sodium (Na) suggests an inverse relationship, implying that areas with higher zinc concentrations may exhibit lower sodium levels, possibly reflecting variations in floral sources or environmental conditions.
- iii.
- Sr/Mg (R2 = −0.213 **): Explanation: The negative correlation between strontium (Sr) and magnesium (Mg) implies an opposing trend. Regions with elevated magnesium content may show reduced strontium levels, highlighting potential geological or environmental influences.
- iv.
- Cr/Mg (R2 = −0.203 **) and Cr/Zn (R2 = −0.273 **): Explanation: Negative correlations between chromium (Cr) and magnesium (Mg), as well as chromium (Cr) and zinc (Zn), suggest that higher magnesium or zinc levels may be associated with lower chromium concentrations, indicating potential interactions influenced by regional factors.
- v.
- Mn/Ca (R2 = −0.206 *) and Cd/Zn (R2 = −0.251 *): Explanation: Negative correlations between manganese (Mn) and calcium (Ca), as well as cadmium (Cd) and zinc (Zn), hint at potential antagonistic relationships. Regions with higher manganese or zinc levels might exhibit lower calcium or cadmium concentrations, respectively, emphasizing the complexity of mineral interactions in honey.
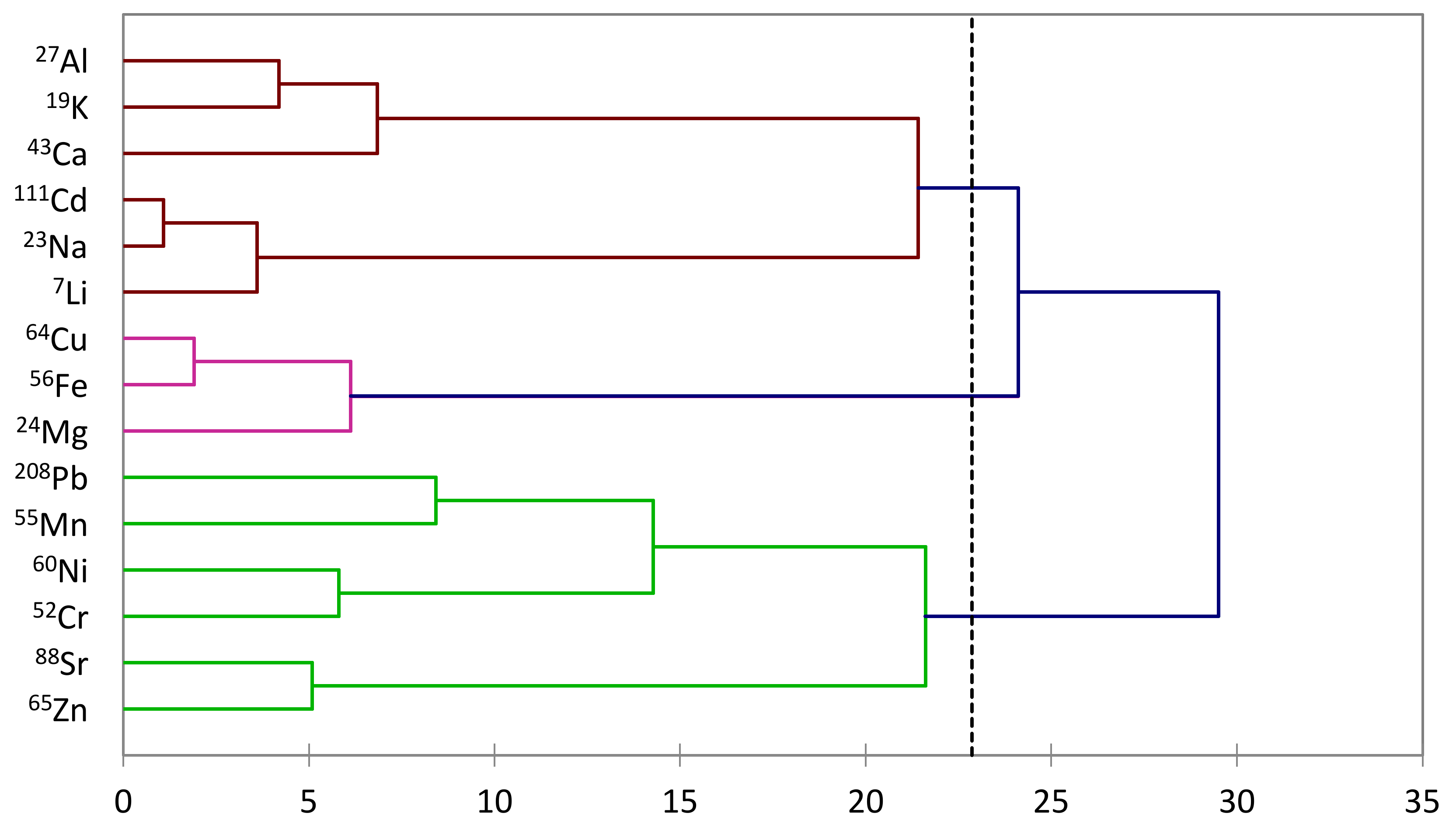
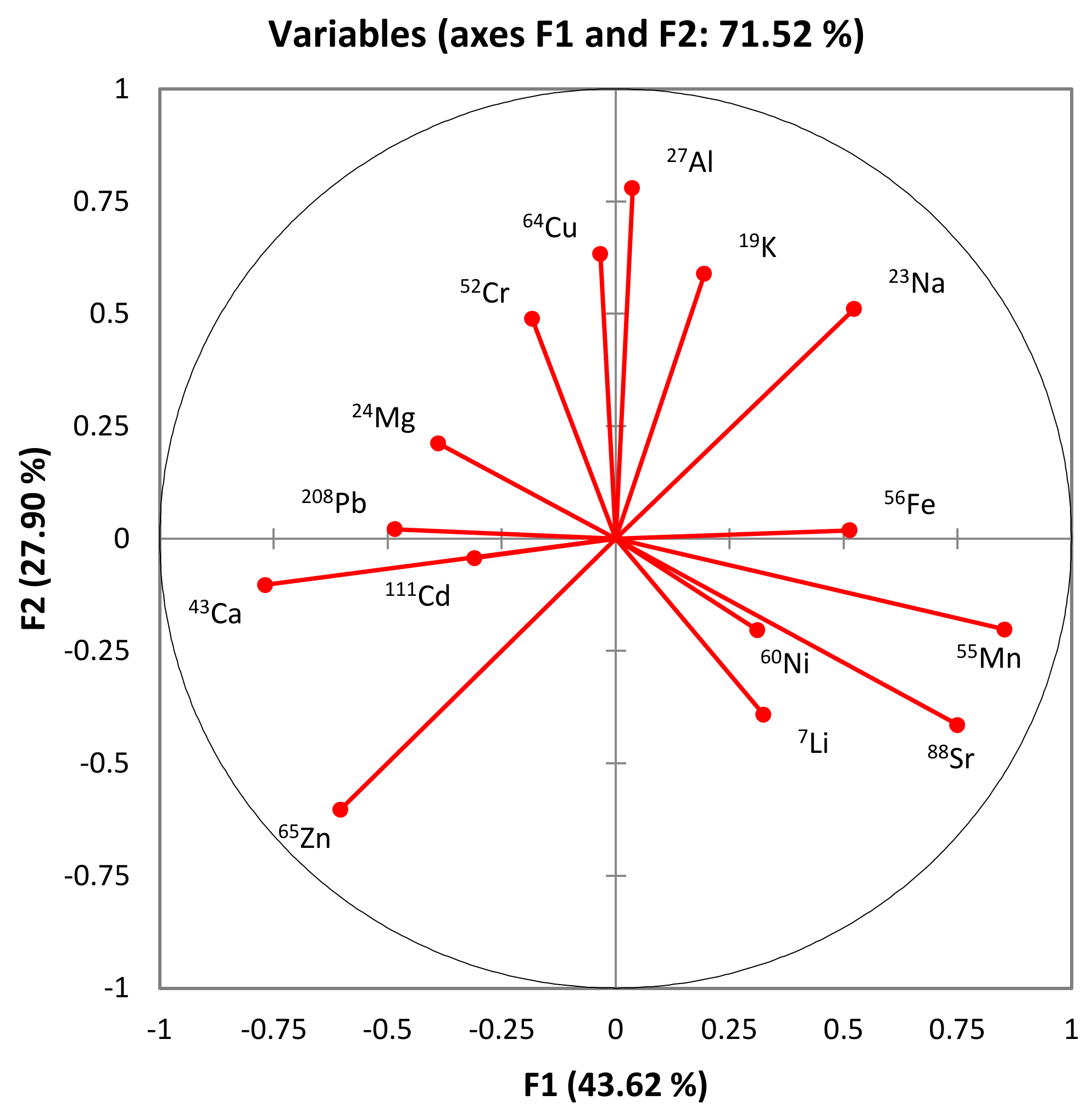
3.5. Hierarchical Clustering and Principal Component Analysis Unveiling Elemental Profiles in Honey
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kastrati, G.; Sopaj, F.; Tašev, K.; Stafilov, T.; Šajn, R.; Paçarizi, M. Analysis of Chemical Elements in Honey Samples in the Territory of Kosovo. J. Food Compos. Anal. 2023, 124, 105505. [Google Scholar] [CrossRef]
- El Sohaimy, S.A.; Masry, S.H.D.; Shehata, M.G. Physicochemical Characteristics of Honey from Different Origins. Ann. Agric. Sci. 2015, 60, 279–287. [Google Scholar] [CrossRef]
- Bogdanov, S.; Ruoff, K.; Persano Oddo, L. Physico-Chemical Methods for the Characterisation of Unifloral Honeys: A Review. Apidologie 2004, 35, S4–S17. [Google Scholar] [CrossRef]
- Wang, J.; Li, Q.X. Chemical Composition, Characterization, and Differentiation of Honey Botanical and Geographical Origins. Adv. Food Nutr. Res. 2011, 62, 89–137. [Google Scholar] [CrossRef] [PubMed]
- Bogdanov, S.; Charrière, J.-D.; Imdorf, A.; Kilchenmann, V.; Fluri, P. Determination of Residues in Honey after Treatments with Formic and Oxalic Acid under Field Conditions. Apidologie 2022, 33, 399–409. [Google Scholar] [CrossRef]
- Ouchemoukh, S.; Schweitzer, P.; Bachir Bey, M.; Djoudad-Kadji, H.; Louaileche, H. HPLC Sugar Profiles of Algerian Honeys. Food Chem. 2010, 121, 561–568. [Google Scholar] [CrossRef]
- Mejías, E.; Garrido, T.; Mejías, E.; Garrido, T. Analytical Procedures for Determining Heavy Metal Contents in Honey: A Bioindicator of Environmental Pollution. In Honey Analysis; IntechOpen Limited: London, UK, 2017. [Google Scholar] [CrossRef]
- Finola, M.S.; Lasagno, M.C.; Marioli, J.M. Microbiological and Chemical Characterization of Honeys from Central Argentina. Food Chem. 2007, 100, 1649–1653. [Google Scholar] [CrossRef]
- Jandrić, Z.; Haughey, S.A.; Frew, R.D.; McComb, K.; Galvin-King, P.; Elliott, C.T.; Cannavan, A. Discrimination of Honey of Different Floral Origins by a Combination of Various Chemical Parameters. Food Chem. 2015, 189, 52–59. [Google Scholar] [CrossRef] [PubMed]
- Donarski, J.A.; Jones, S.A.; Harrison, M.; Driffield, M.; Charlton, A.J. Identification of Botanical Biomarkers Found in Corsican Honey. Food Chem. 2010, 118, 987–994. [Google Scholar] [CrossRef]
- Mureșan, C.I.; Cornea-Cipcigan, M.; Suharoschi, R.; Erler, S.; Mărgăoan, R. Honey Botanical Origin and Honey-Specific Protein Pattern: Characterization of Some European Honeys. LWT 2022, 154, 112883. [Google Scholar] [CrossRef]
- Solayman, M.; Islam, M.A.; Paul, S.; Ali, Y.; Khalil, M.I.; Alam, N.; Gan, S.H. Physicochemical Properties, Minerals, Trace Elements, and Heavy Metals in Honey of Different Origins: A Comprehensive Review. Compr. Rev. Food Sci. Food Saf. 2016, 15, 219–233. [Google Scholar] [CrossRef] [PubMed]
- Mckee, B. Prevention of Residues in Honey: A Future Perspective. 2003; Volume 2. Available online: https://www.fiitea.org/foundation/files/2003/MCKEE.pdf (accessed on 10 January 2024).
- Oliveira, S.S.; Alves, C.N.; Boa Morte, E.S.; de Freitas Santos Júnior, A.; Araujo, R.G.O.; Santos, D.C.M.B. Determination of Essential and Potentially Toxic Elements and Their Estimation of Bioaccessibility in Honeys. Microchem. J. 2019, 151, 104221. [Google Scholar] [CrossRef]
- González-Miret, M.L.; Terrab, A.; Hernanz, D.; Fernández-Recamales, M.Á.; Heredia, F.J. Multivariate Correlation between Color and Mineral Composition of Honeys and by Their Botanical Origin. J. Agric. Food Chem. 2005, 53, 2574–2580. [Google Scholar] [CrossRef] [PubMed]
- Pohl, P. Determination of Metal Content in Honey by Atomic Absorption and Emission Spectrometries. TrAC Trends Anal. Chem. 2009, 28, 117–128. [Google Scholar] [CrossRef]
- Paul, S.; Hossen, M.S.; Tanvir, E.M.; Afroz, R.; Hossen, D.; Das, S.; Bhoumik, N.C.; Karim, N.; Juliana, F.M.; Gan, S.H.; et al. Minerals, Toxic Heavy Metals, and Antioxidant Properties of Honeys from Bangladesh. J. Chem. 2017, 2017, 6101793. [Google Scholar] [CrossRef]
- Bilandžić, N.; Sedak, M.; Đokić, M.; Bošković, A.G.; Florijančić, T.; Bošković, I.; Kovačić, M.; Puškadija, Z.; Hruškar, M. Element Content in Ten Croatian Honey Types from Different Geographical Regions during Three Seasons. J. Food Compos. Anal. 2019, 84, 103305. [Google Scholar] [CrossRef]
- Stankovska, E.; Stafilov, T.; Šajn, R. Monitoring of Trace Elements in Honey from the Republic of Macedonia by Atomic Absorption Spectrometry. Environ. Monit. Assess. 2008, 142, 117–126. [Google Scholar] [CrossRef] [PubMed]
- Cordella, C.B.Y.; Militão, J.S.L.T.; Clément, M.C.; Cabrol-Bass, D. Honey Characterization and Adulteration Detection by Pattern Recognition Applied on HPAEC-PAD Profiles. 1. Honey Floral Species Characterization. J. Agric. Food Chem. 2003, 51, 3234–3242. [Google Scholar] [CrossRef]
- Lees, M.; Humber Institute of Food and Fisheries. Food Authenticity and Traceability; Woodhead Publishing: Sawston, UK, 2003; Volume 612. [Google Scholar]
- Sivakesave, S.; Irudayaraj, J. Prediction of Inverted Cane Sugar Adulteration of Honey by Fourier Transform Infrared Spectroscopy. J. Food Sci. 2001, 66, 972–978. [Google Scholar] [CrossRef]
- Tewari, J.; Irudayaraj, J. Quantification of Saccharides in Multiple Floral Honeys Using Fourier Transform Infrared Microattenuated Total Reflectance Spectroscopy. J. Agric. Food Chem. 2004, 52, 3237–3243. [Google Scholar] [CrossRef]
- Odeh, I.; Abu-Lafi, S.; Dewik, H.; Al-Najjar, I.; Imam, A.; Dembitsky, V.M.; Hanuš, L.O. A Variety of Volatile Compounds as Markers in Palestinian Honey from Thymus Capitatus, Thymelaea Hirsuta, and Tolpis Virgata. Food Chem. 2007, 101, 1393–1397. [Google Scholar] [CrossRef]
- Kelly, J.F.D.; Downey, G.; Fouratier, V. Initial Study of Honey Adulteration by Sugar Solutions Using Midinfrared (MIR) Spectroscopy and Chemometrics. J. Agric. Food Chem. 2003, 52, 33–39. [Google Scholar] [CrossRef] [PubMed]
- Won, S.R.; Lee, D.C.; Ko, S.H.; Kim, J.W.; Rhee, H.I. Honey Major Protein Characterization and Its Application to Adulteration Detection. Food Res. Int. 2008, 41, 952–956. [Google Scholar] [CrossRef]
- Aghamirlou, H.M.; Khadem, M.; Rahmani, A.; Sadeghian, M.; Mahvi, A.H.; Akbarzadeh, A.; Nazmara, S. Heavy Metals Determination in Honey Samples Using Inductively Coupled Plasma-Optical Emission Spectrometry. J. Environ. Health Sci. Eng. 2015, 13, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Rashed, M.N.; El-Haty, M.T.A.; Mohamed, S.M. Bee Honey as Environmental Indicator for Pollution with Heavy Metals. Toxicol. Environ. Chem. 2009, 91, 389–403. [Google Scholar] [CrossRef]
- Da Silveira, T.A.; Araújo, D.F.D.; Marchini, L.C.; Moreti, A.C.C.C.; Olinda, R.A. Detection of Metals by Differential Pulse Anodic Stripping Voltammetry (DPASV) in Pollen Collected from a Fragment of the Atlantic Forest in Piracicaba/SP. Ecotoxicol. Environ. Contam. 2013, 8, 31–36. [Google Scholar] [CrossRef]
- Geana, E.I.; Ciucure, C.T. Establishing Authenticity of Honey via Comprehensive Romanian Honey Analysis. Food Chem. 2020, 306, 125595. [Google Scholar] [CrossRef] [PubMed]
- Dobre, I.; Escuredo, O.; Rodriguez-Flores, S.; Seijo, M.C. Evaluation of Several Romanian Honeys Based on Their Palynological and Biochemical Profiles. Int. J. Food Prop. 2014, 17, 1850–1860. [Google Scholar] [CrossRef]
- Pătruică, S.; Hărmănescu, M.; Bura, M.; Jivan, A.; Ciobănaş, C. Researches Concerning of the Mineral Content of Acacia Honey Derived on Different Country. Sci. Pap. Anim. Sci. Biotechnol. 2008, 41, 325–327. [Google Scholar]
- Pătruică, S.; Hărmănescu, M.; Jivan, A.; Cioabă, C.; Simiz, E.; Călamar, C.D. Research Regarding the Mineral Trace Content of Some Honey Types Harvested from Southern Romania during 2007 and 2008. Sci. Pap. Anim. Sci. Biotechnol. 2009, 42, 178–181. [Google Scholar]
- Bartha, S.; Taut, I.; Goji, G.; Andravlad, I.; Dinulică, F. Heavy Metal Content in PolyfloralHoney and Potential Health Risk. A Case Study of Copșa Mică, Romania. Int. J. Environ. Res. Public Health 2020, 17, 1507. [Google Scholar] [CrossRef]
- Ciobanu, O.; Rădulescu, H. Monitoring of heavy metals residues in honey. Res. J. Agric. Sci. 2016, 48, 9–13. [Google Scholar]
- Pătruică, S.; Alexa, E.; Obiștioiu, D.; Cocan, I.; Radulov, I.; Berbecea, A.; Lazăr, R.N.; Simiz, E.; Vicar, N.M.; Hulea, A.; et al. Chemical Composition, Antioxidant and Antimicrobial Activity of Some Types of Honey from Banat Region, Romania. Molecules 2022, 27, 4179. [Google Scholar] [CrossRef] [PubMed]
- Purcarea, C.; Dzugan, M.; Wesolowska, M.; Chis, A.M.; Zagula, G.; Teusdea, A.C.; Puchalski, C. A Comparative Study of Metal Content in Selected Polish and Roamnia Honey Samples. Rev. Chim. 2017, 68, 1163–1169. [Google Scholar] [CrossRef]
- Berinde, Z.M.; Michnea, A.M. Comparative Study of the Evolution of Environmental and Honey Pollution with Heavy Metals. J. Sci. Art. 2013, 2, 173–180. [Google Scholar]
- Conti, M.E.; Canepari, S.; Finoia, M.G.; Mele, G.; Astolfi, M.L. Characterization of Italian Multifloral Honeys on the Basis of Their Mineral Content and Some Typical Quality Parameters. J. Food Compos. Anal. 2018, 74, 102–113. [Google Scholar] [CrossRef]
- Atanassova, J.; Yurukova, L.; Lazarova, M. Pollen and Inorganic Characteristics of Bulgarian Unifloral Honeys. Czech J. Food Sci. 2012, 30, 520–526. [Google Scholar] [CrossRef]
- Devillers, J.; Doré, J.C.; Marenco, M.; Poirier-Duchêne, F.; Galand, N.; Viel, C. Chemometrical Analysis of 18 Metallic and Nonmetallic Elements Found in Honeys Sold in France. J. Agric. Food Chem. 2002, 50, 5998–6007. [Google Scholar] [CrossRef]
- Louppis, A.P.; Karabagias, I.K.; Kontakos, S.; Kontominas, M.G.; Papastephanou, C. Botanical Discrimination of Greek Unifloral Honeys Based on Mineral Content in Combination with Physicochemical Parameter Analysis, Using a Validated Chemometric Approach. Microchem. J. 2017, 135, 180–189. [Google Scholar] [CrossRef]
- Bereksi-Reguig, D.; Bouchentouf, S.; Allali, H.; Adamczuk, A.; Kowalska, G.; Kowalski, R. Trace Elements and Heavy Metal Contents in West Algerian Natural Honey. J. Anal. Methods Chem. 2022, 2022, 7890856. [Google Scholar] [CrossRef] [PubMed]
- Sajtos, Z.; Herman, P.; Harangi, S.; Baranyai, E. Elemental Analysis of Hungarian Honey Samples and Bee Products by MP-AES Method. Microchem. J. 2019, 149, 103968. [Google Scholar] [CrossRef]
- Bogdanov, S. Harmonised Methods of the International IHC. Bee Prod. Sci. 2009, 5, 1–62. [Google Scholar]
- Tibebe, D.; Hussen, M.; Mulugeta, M.; Yenealem, D.; Moges, Z.; Gedefaw, M.; Kassa, Y. Assessment of Selected Heavy Metals in Honey Samples Using Flame Atomic Absorption Spectroscopy (FAAS), Ethiopia. BMC Chem. 2022, 16, 87. [Google Scholar] [CrossRef] [PubMed]
- Tesfaye, B. Evaluation of Physico-Chemical Properties of Honey Produced in Bale Natural Forest, Southeastern Ethiopia. Int. J. Agric. Sci. Food Technol. 2016, 2, 021–027. [Google Scholar] [CrossRef]
- Bora, F.D.; Babeș, A.C.; Călugăr, A.; Jitea, M.I.; Hoble, A.; Filimon, R.V.; Bunea, A.; Nicolescu, A.; Bunea, C.I. Unravelling Heavy Metal Dynamics in Soil and Honey: A Case Study from Maramureș Region, Romania. Foods 2023, 12, 3577. [Google Scholar] [CrossRef] [PubMed]
- Chudzinska, M.; Baralkiewicz, D. Application of ICP-MS Method of Determination of 15 Elements in Honey with Chemometric Approach for the Verification of Their Authenticity. Food Chem. Toxicol. 2011, 49, 2741–2749. [Google Scholar] [CrossRef] [PubMed]
| Seasons | Region (No Samples) | Honey Details | Area | 19K (mg/kg) M.P.L. | 23Na (mg/kg) M.P.L. | 24Mg (mg/kg) M.P.L. | 43Ca (mg/kg) M.P.L. | 7Li (mg/kg) M.P.L. | 27Al (mg/kg) M.P.L. | 56Fe (mg/kg) M.P.L. | 64Cu (mg/kg) M.P.L. | 65Zn (mg/kg) M.P.L. | 88Sr (µg/kg) M.P.L. |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Maximum Permissible Levels (M.P.Ls) | – | – | – | – | – | – | – | 0.50 mg/kg | 1.00 mg/kg | – | |||
| 2020 | Southeast (2) | Multifloral | Galați | 895.16 ± 43.74 ef | 19.12 ± 1.90 efghi | 32.37 ± 12.42 defg | 101.89 ± 9.29 f | 0.12 ± 0.03 fgh | <LoQ e | 2.19 ± 0.34 hij | 0.64 ± 0.44 ef | 0.29 ± 0.17 hi | <LoQ d |
| 2021 | Southeast (3) | Linde | 697.64 ± 51.41 ghi | 23.41 ± 41 ef | 27.13 ± 13.04 efghi | 80.95 ± 9.51 fgh | 0.16 ± 0.05 efgh | 0.01 ± 0.01 e | 61.65 ± 11.72 b | 0.17 ± 0.06 f | 0.16 ± 0.15 h | <LoQ d | |
| 2021 | Southeast (1) | Acacia | 275.34 ± 18.07 jk | 6.89 ± 2.06 î | 30.95 ± 2.90 efg | 26.77 ± 7.35 lmn | 0.06 ± 0.01 gh | 0.02 ± 0.03 e | 15.98 ± 6.57 efg | 0.17 ± 0.02 f | <LoQ h | <LoQ d | |
| 2022 | Southeast (2) | Sunflower | Târgu Bujoru | 75.09 ± 6.08 l | 3.02 ± 0.71 î | 12.47 ± 0.72 îjk | 195.53 ± 16.45 cd | 0.33 ± 0.08 cdef | <LoQ e | 6.21 ± 3.50 hij | 0.43 ± 0.23 f | 2.63 ± 0.49 dc | <LoQ d |
| 2020 | Southeast (2) | Spring rape | Tecuci | 1284.66 ± 52.33 cd | 14.11 ± 5.69 fghiî | 13.69 ± 1.62 iîjk | 42.87 ± 6.48 jklm | <LoQ h | <LoQ e | 1.64 ± 0.58 ij | <LoQ f | 0.63 ± 0.13 ghi | 0.10 ± 0.04 cd |
| 2020 | Southeast (1) | Autumn rape | 1012.05 ± 20.89 e | 3.11 ± 1.71 î | 20.83 ± 12.47 ghiîj | 31.37 ± 2.20 klmn | <LoQ h | <LoQ e | 3.55 ± 0.52 hij | <LoQ f | 1.29 ± 0.25 fg | 0.17 ± 0.05 c | |
| 2019 | East (2) | Sunflower | Vaslui | 630.96 ± 115.09 hiî | 9.85 ± 1.48 hiî | 44.88 ± 7.34 bcd | 78.67 ± 13.00 ghi | 0.28 ± 0.21 cdefg | 0.01 ± 0.01 e | 5.71 ± 1.70 hij | 1.83 ± 0.55 bcd | 3.23 ± 0.33 bc | <LoQ d |
| 2018 | East (2) | Linden | 196.64 ± 8.42 kl | 11.86 ± 0.46 ghiî | 27.23 ± 9.52 efghi | 58.92 ± 14.98 iîj | 0.13 ± 0.02 fgh | 0.10 ± 0.07 cd | 11.50 ± 1.95 fgh | 0.86 ± 0.41 def | <LoQ h | <LoQ d | |
| 2019 | East (3) | Lavender | 1004.16 ± 101.91 e | 25.66 ± 3.44 de | 84.45 ± 7.75 a | 27.81 ± 7.26 lmn | 0.06 ± 0.01 gh | 0.11 ± 0.04 c | 75.91 ± 5.98 a | 2.40 ± 1.25 b | <LoQ h | <LoQ d | |
| 2021 | Southeast (3) | Multifloral | Brăila | 1842.76 ± 92.93 b | 35.46 ± 8.56 cd | 24.87 ± 5.54 fghiî | 151.44 ± 25.41 e | 0.62 ± 0.20 ab | <LoQ e | 15.83 ± 2.88 efg | 0.79 ± 0.38 def | 2.18 ± 1.36 de | <LoQ d |
| 2022 | Southeast (1) | Linden | 124.57 ± 5.95 l | 9.49 ± 2.60 iî | 24.87 ± 10.05 fghiî | 81.42 ± 6.65 fgh | 0.21 ± 0.15 defgh | <LoQ e | 33.23 ± 9.52 c | <LoQ f | 0.91 ± 0.69 ghi | <LoQ d | |
| 2021 | Southeast (2) | Acacia + Linden | 819.14 ± 210.14 fg | 11.09 ± 1.31 ghiî | 15.19 ± 5.74 hiîjk | 61.66 ± 14.63 hiîj | <LoQ h | <LoQ e | 29.02 ± 18.24 cd | <LoQ f | 0.86 ± 0.63 ghi | <LoQ d | |
| 2020 | Southeast (1) | Multifloral | 1310.57 ± 115.01 cd | 40.07 ± 7.50 bc | 74.43 ± 14.85 a | 22.59 ± 3.80 mn | <LoQ h | <LoQ e | 20.19 ± 3.61 ef | 1.72 ± 1.84 bcde | 0.90 ± 0.06 ghi | <LoQ d | |
| 2021 | Northwest (2) | Chestnut | Satu Mare | 2116.41 ± 183.26 a | 33.96 ± 8.90 cd | 47.64 ± 6.16 bc | 185.25 ± 15.64 d | <LoQ h | 0.21 ± 0.02 a | <LoQ j | <LoQ f | <LoQ h | 0.02 ± 0.01 d |
| 2020 | Northwest (1) | Acacia | 83.40 ± 7.34 l | 20.09 ± 1.82 efghi | 11.62 ± 0.71 îjk | 18.50 ± 3.16 n | <LoQ h | 0.07 ± 0.04 d | <LoQ j | 2.01 ± 1.71 bc | <LoQ h | <LoQ d | |
| 2020 | Southeast (1) | Multifloral | Tulcea | 318.77 ± 41.84 jk | 11.05 ± 1.97 ghiî | 18.52 ± 5.27 ghiîj | 48.66 ± 9.52 îjk | 0.47 ± 0.17 bc | <LoQ e | 15.02 ± 5.17 fg | 0.97 ± 0.39 cdef | <LoQ h | <LoQ d |
| 2020 | Southeast (2) | Sunflower | 585.91 ± 33.69 iî | 42.53 ± 30.72 bc | 40.38 ± 0.42 bcde | 32.66 ± 5.98 klmn | 0.23 ± 0.15 defgh | <LoQ e | 3.14 ± 2.31 hij | 4.31 ± 0.39 a | <LoQ h | <LoQ d | |
| 2019 | Southeast (1) | Linden | 85.09 ± 15.20 l | 8.79 ± 4.73 iî | 35.78 ± 1.13 bcde | 51.22 ± 3.88 îjk | 0.75 ± 0.21 a | <LoQ e | 2.47 ± 0.74 hij | 0.60 ± 0.10 f | 0.22 ± 0.25 h | <LoQ d | |
| 2021 | Northeast (2) | Honeydew | Botoșani | 1395.08 ± 77.99 c | 69.42 ± 6.34 a | 71.09 ± 8.22 a | 84.83 ± 13.41 fg | 0.69 ± 0.03 a | <LoQ e | 3.09 ± 2.50 hij | <LoQ f | 0.37 ± 0.18 hi | <LoQ d |
| 2019 | Northeast (3) | Multifloral | 405.59 ± 16.04 j | 20.51 ± 3.10 efgh | 78.62 ± 6.09 a | 14.87 ± 3.45 n | 0.36 ± 0.17 cde | <LoQ e | 16.23 ± 3.11 efg | <LoQ f | <LoQ h | <LoQ d | |
| 2019 | Northeast (2) | Linden | 76.17 ± 12.08 l | 8.61 ± 1.76 iî | 13.52 ± 1.03 iîjk | 17.69 ± 9.48 n | 0.11 ± 0.02 fgh | <LoQ e | <LoQ j | <LoQ f | 0.18 ± 0.04 h | <LoQ d | |
| 2019 | Northeast (1) | Sunflower | 45.35 ± 30.40 l | 4.15 ± 0.64 î | 18.14 ± 4.48 ghiîj | 27.86 ± 10.71 lmn | 0.40 ± 0.23 cd | <LoQ e | 3.61 ± 1.90 hij | <LoQ f | 3.43 ± 0.27 b | 0.87 ± 0.27 a | |
| 2021 | Northeast (2) | Acacia + Linden | Iași | 62.73 ± 9.26 l | 10.38 ± 1.13 ghiî | 28.38 ± 5.03 efgh | 222.31 ± 4.39 b | <LoQ h | <LoQ e | <LoQ j | 0.69 ± 0.12 ef | <LoQ h | 0.04 ± 0.01 d |
| 2020 | Northeast (2) | Acacia | 354.07 ± 59.45 j | 21.42 ± 2.83 efg | 2.17 ± 1.48 k | 33.52 ± 3.76 klmn | 0.81 ± 0.32 a | 0.02 ± 0.03 e | <LoQ j | 1.00 ± 0.14 cdef | <LoQ h | 0.03 ± 0.01 d | |
| 2020 | Northeast (2) | Sunflower | 133.41 ± 70.41 l | 47.70 ± 13.21 b | 18.73 ± 4.10 ghiîj | 70.39 ± 3.36 ghiî | 0.39 ± 0.39 cd | 0.14 ± 0.02 b | 1.59 ± 0.40 ij | 2.03 ± 1.18 bc | <LoQ h | <LoQ d | |
| 2021 | Center (3) | Acacia | Sibiu | 544.79 ± 24.04 î | 5.30 ± 2.98 î | 3.73 ± 0.19 k | 33.38 ± 5.03 klmn | 0.37 ± 0.15 cde | <LoQ e | 32.05 ± 5.51 cd | 0.69 ± 0.36 ef | 0.36 ± 0.13 hi | <LoQ d |
| 2020 | Center (2) | Sunflower | 1216.83 ± 103.83 d | 5.91 ± 2.55 î | 52.49 ± 15.17 b | 36.41 ± 11.80 klmn | 0.13 ± 0.03 fgh | <LoQ e | 10.52 ± 2.47 ghi | <LoQ f | 0.31 ± 0.23 hi | <LoQ d | |
| 2019 | Southwest (1) | Sunflower | Râmnicu Vâlcea | 623.97 ± 133.70 hiî | 3.68 ± 1.22 î | 48.50 ± 13.56 bc | 22.28 ± 6.08 mn | <LoQ h | <LoQ e | 5.46 ± 3.48 hij | <LoQ f | 1.76 ± 1.27 ef | <LoQ d |
| 2018 | Southwest (1) | Multifloral | 95.64 ± 23.64 l | 2.48 ± 1.48 î | 35.87 ± 5.59 cdef | 303.13 ± 19.57 a | <LoQ h | <LoQ e | 4.42 ± 4.86 hij | <LoQ f | 6.46 ± 1.41 a | <LoQ d | |
| 2018 | West (2) | Acacia | Arad | 139.16 ± 44.70 l | 23.35 ± 3.32 ef | 7.92 ± 2.17 jk | 24.36 ± 7.72 mn | 0.47 ± 0.03 bc | <LoQ e | 24.30 ± 11.87 ed | 0.10 ± 0.01 f | 0.34 ± 0.16 hi | 0.35 ± 0.13 b |
| 2020 | South (3) | Sunflower | Teleorman | 75.41 ± 9.19 l | 8.49 ± 5.52 iî | 45.51 ± 5.21 bcd | 64.03 ± 15.39 ghiîj | 0.46 ± 0.16 bc | <LoQ e | 2.13 ± 0.37 hij | <LoQ f | 2.45 ± 1.22 de | <LoQ d |
| 2020 | Southwest (2) | Acacia | Mehedinți | 736.61 ±147.58 gh | 19.09 ± 0.78 efghi | 14.71 ± 3.78 hiîjk | 31.15 ± 3.03 klmn | <LoQ h | <LoQ e | <LoQ j | <LoQ f | <LoQ h | <LoQ d |
| 2022 | Southwest (1) | Sunflower | 305.38 ± 199.82 jk | 8.79 ± 0.69 iî | 73.42 ± 3.02 a | 213.28 ± 19.01 bc | <LoQ h | <LoQ e | <LoQ j | <LoQ f | 1.54 ± 1.30 fgh | <LoQ d | |
| 1 F | 146.015 | 19.782 | 28.339 | 109.750 | 12.675 | 22.107 | 39.021 | 8.600 | 29.028 | 20.438 | |||
| 2 Sig. | *** | *** | *** | *** | *** | *** | *** | *** | *** | *** | |||
| Minimum–Maximum Values | 45.35 - | 2.48 - | 2.17 - | 14.87 - | <LoQ - | <LoQ - | <LoQ - | <LoQ - | <LoQ - | <LoQ - | |||
| 2116.41 | 69.42 | 84.45 | 303.13 | 0.81 | 0.21 | 75.91 | 4.31 | 6.46 | 0.87 | ||||
| AVERAGE | 809.41 ± 577.92 | 20.76 ± 17.20 | 37.14 ± 22.39 | 77.49 ± 48.39 | 0.19 ± 0.20 | 0.03 ± 0.06 | 14.60 ± 20.72 | 0.55 ± 0.68 | 0.71 ± 0.68 | 0.05 ± 0.06 | |||
| Seasons | Region (No Samples) | Honey Details | Area | 9Be (µg/kg) M.P.L. | 51V(µg/kg) M.P.L. | 52Cr (mg/kg) M.P.L. | 55Mn (mg/kg) M.P.L. | 59Co (mg/kg) M.P.L. | 60Ni (mg/kg) M.P.L. | 70Ga (µg/kg) M.P.L. | 79Se (µg/kg) M.P.L. | 85Rb (µg/kg) M.P.L. | 204Tl (µg/kg) M.P.L. |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Maximum Permissible Levels (M.P.Ls) | – | – | – | – | – | – | – | ||||||
| 2020 | Southeast (2) | Multifloral | Galați | <LoQ | <LoQ | 0.13 ± 0.03 ef | 1.12 ± 0.20 fgh | <LoQ | 0.13 ± 0.02 cde | <LoQ | <LoQ | <LoQ | <LoQ |
| 2021 | Southeast (3) | Linde | <LoQ | <LoQ | 0.13 ± 0.03 ef | 3.53 ± 1.06 cd | <LoQ | 0.07 ± 0.05 de | <LoQ | <LoQ | <LoQ | <LoQ | |
| 2021 | Southeast (1) | Acacia | <LoQ | <LoQ | 0.14 ± 0.06 ef | 0.57 ± 0.25 h | <LoQ | <LoQ e | <LoQ | <LoQ | <LoQ | <LoQ | |
| 2022 | Southeast (2) | Sunflower | Târgu Bujoru | <LoQ | <LoQ | 0.45 ± 0.10 defg | 0.85 ± 0.09 gh | <LoQ | 0.08 ± 0.07 de | <LoQ | <LoQ | <LoQ | <LoQ |
| 2020 | Southeast (2) | Spring rape | Tecuci | <LoQ | <LoQ | <LoQ g | 2.12 ± 0.41 ef | <LoQ | <LoQ e | <LoQ | <LoQ | <LoQ | <LoQ |
| 2020 | Southeast (1) | Autumn rape | <LoQ | <LoQ | <LoQ g | 0.89 ± 0.33 gh | <LoQ | <LoQ e | <LoQ | <LoQ | <LoQ | <LoQ | |
| 2019 | East (2) | Sunflower | Vaslui | <LoQ | <LoQ | 0.20 ± 0.04 efg | <LoQ h | <LoQ | 0.18 ± 0.06 abcd | <LoQ | <LoQ | <LoQ | <LoQ |
| 2018 | East (2) | Linden | <LoQ | <LoQ | 1.74 ± 0.73 bc | 2.65 ± 1.01 de | <LoQ | 0.24 ± 0.09 abcd | <LoQ | <LoQ | <LoQ | <LoQ | |
| 2019 | East (3) | Lavender | <LoQ | <LoQ | 0.67 ± 0.48 defg | <LoQ h | <LoQ | <LoQ e | <LoQ | <LoQ | <LoQ | <LoQ | |
| 2021 | Southeast (3) | Multifloral | Brăila | <LoQ | <LoQ | 0.18 ± 0.04 efg | 2.33 ± 1.01 e | <LoQ | <LoQ e | <LoQ | <LoQ | <LoQ | <LoQ |
| 2022 | Southeast (1) | Linden | <LoQ | <LoQ | 0.34 ± 0.18 defg | 5.42 ± 0.42 b | <LoQ | <LoQ e | <LoQ | <LoQ | <LoQ | <LoQ | |
| 2021 | Southeast (2) | Acacia + Linden | <LoQ | <LoQ | 0.13 ± 0.02 ef | 3.97 ± 1.24 c | <LoQ | <LoQ e | <LoQ | <LoQ | <LoQ | <LoQ | |
| 2020 | Southeast (1) | Multifloral | <LoQ | <LoQ | 0.78 ± 0.01 defg | <LoQ h | <LoQ | <LoQ e | <LoQ | <LoQ | <LoQ | <LoQ | |
| 2021 | Northwest (2) | Chestnut | Satu Mare | <LoQ | <LoQ | 1.04 ± 0.42 cde | <LoQ h | <LoQ | 0.07 ± 0.01 de | <LoQ | <LoQ | <LoQ | <LoQ |
| 2020 | Northwest (1) | Acacia | <LoQ | <LoQ | 0.89 ± 0.23 cdef | 2.54 ± 0.90 de | <LoQ | <LoQ e | <LoQ | <LoQ | <LoQ | <LoQ | |
| 2020 | Southeast (1) | Multifloral | Tulcea | <LoQ | <LoQ | <LoQ g | <LoQ h | <LoQ | <LoQ e | <LoQ | <LoQ | <LoQ | <LoQ |
| 2020 | Southeast (2) | Sunflower | <LoQ | <LoQ | <LoQ g | <LoQ h | <LoQ | <LoQ e | <LoQ | <LoQ | <LoQ | <LoQ | |
| 2019 | Southeast (1) | Linden | <LoQ | <LoQ | <LoQ g | <LoQ h | <LoQ | <LoQ e | <LoQ | <LoQ | <LoQ | <LoQ | |
| 2021 | Northeast (2) | Honeydew | Botoșani | <LoQ | <LoQ | 0.14 ± 0.04 ef | 2.00 ± 0.47 efg | <LoQ | <LoQ e | <LoQ | <LoQ | <LoQ | <LoQ |
| 2019 | Northeast (3) | Multifloral | <LoQ | <LoQ | 0.37 ± 0.15 defg | 0.89 ± 0.29 gh | <LoQ | <LoQ e | <LoQ | <LoQ | <LoQ | <LoQ | |
| 2019 | Northeast (2) | Linden | <LoQ | <LoQ | <LoQ g | 7.17 ± 2.81 a | <LoQ | <LoQ e | <LoQ | <LoQ | <LoQ | <LoQ | |
| 2019 | Northeast (1) | Sunflower | <LoQ | <LoQ | <LoQ g | 0.12 ± 0.11 h | <LoQ | <LoQ e | <LoQ | <LoQ | <LoQ | <LoQ | |
| 2021 | Northeast (2) | Acacia + Linden | Iași | <LoQ | <LoQ | 2.34 ± 0.71 b | <LoQ h | <LoQ | <LoQ e | <LoQ | <LoQ | <LoQ | <LoQ |
| 2020 | Northeast (2) | Acacia | <LoQ | <LoQ | 2.17 ± 0.17 b | 1.99 ± 0.20 efg | <LoQ | 0.29 ± 0.06 a | <LoQ | <LoQ | <LoQ | <LoQ | |
| 2020 | Northeast (2) | Sunflower | <LoQ | <LoQ | 0.89 ± 0.23 defg | <LoQ h | <LoQ | 0.09 ± 0.03 de | <LoQ | <LoQ | <LoQ | <LoQ | |
| 2021 | Center (3) | Acacia | Sibiu | <LoQ | <LoQ | 3.29 ± 0.57 a | 0.63 ± 0.16 h | <LoQ | 0.30 ± 0.09 a | <LoQ | <LoQ | <LoQ | <LoQ |
| 2020 | Center (2) | Sunflower | <LoQ | <LoQ | <LoQ g | 1.92 ± 0.50 efg | <LoQ | 0.22 ± 0.09 abc | <LoQ | <LoQ | <LoQ | <LoQ | |
| 2019 | Southwest (1) | Sunflower | Râmnicu Vâlcea | <LoQ | <LoQ | <LoQ g | 0.52 ± 0.08 h | <LoQ | 0.09 ± 0.04 de | <LoQ | <LoQ | <LoQ | <LoQ |
| 2018 | Southwest (1) | Multifloral | <LoQ | <LoQ | <LoQ g | <LoQ h | <LoQ | <LoQ e | <LoQ | <LoQ | <LoQ | <LoQ | |
| 2018 | West (2) | Acacia | Arad | <LoQ | <LoQ | <LoQ g | 4.39 ± 1.34 bc | <LoQ | 0.26 ± 0.24 ab | <LoQ | <LoQ | <LoQ | <LoQ |
| 2020 | South (3) | Sunflower | Teleorman | <LoQ | <LoQ | <LoQ g | 0.09 ± 0.04 h | <LoQ | 0.15 ± 0.03 bcd | <LoQ | <LoQ | <LoQ | <LoQ |
| 2020 | Southwest (2) | Acacia | Mehedinți | <LoQ | <LoQ | 3.66 ± 1.63 a | <LoQ h | <LoQ | 0.15 ± 0.09 bcd | <LoQ | <LoQ | <LoQ | <LoQ |
| 2022 | Southwest (1) | Sunflower | <LoQ | <LoQ | 1.15 ± 0.18 cd | <LoQ h | <LoQ | 0.18 ± 0.08 bcd | <LoQ | <LoQ | <LoQ | <LoQ | |
| F | - | - | 13.810 | 23.345 | - | 6.183 | - | - | - | - | |||
| Sig. | - | - | *** | *** | - | *** | - | - | - | - | |||
| Minimum–Maximum Values | - | - | <LoQ | <LoQ | - | <LoQ | - | - | - | - | |||
| 3.66 | 7.17 | 0.30 | |||||||||||
| AVERAGE | - | - | 0.54 ± 0.53 | 1.48 ± 1.31 | - | 0.05 ± 0.06 | - | - | - | - | |||
| Seasons | Region (No Samples) | Honey Details | Area | 208Ag (µg/kg) M.P.L. | 209Bi(µg/kg) M.P.L. | 115In (µg/kg) M.P.L. | 133Cs (µg/kg) M.P.L. | 137Ba (µg/kg) M.P.L. | 75As (µg/kg) M.P.L. | 111Cd (mg/kg) M.P.L. | 201Hg (µg/kg) M.P.L. | 208Pb (mg/kg) M.P.L. | 238U (µg/kg) M.P.L. |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Maximum Permissible Levels (M.P.Ls) | – | – | – | – | – | – | 0.02 mg/kg | – | 0.20 mg/kg | – | |||
| 2020 | Southeast (2) | Multifloral | Galați | <LoQ | <LoQ | <LoQ | <LoQ | <LoQ | <LoQ | 0.02 ± 0.01 b | <LoQ | 0.19 ± 0.04 bcde | <LoQ |
| 2021 | Southeast (3) | Linde | <LoQ | <LoQ | <LoQ | <LoQ | <LoQ | <LoQ | 0.01 ± 0.01 b | <LoQ | 0.21 ± 0.04 bcd | <LoQ | |
| 2021 | Southeast (1) | Acacia | <LoQ | <LoQ | <LoQ | <LoQ | <LoQ | <LoQ | <LoQ | <LoQ | 0.10 ± 0.05 efg | <LoQ | |
| 2022 | Southeast (2) | Sunflower | Târgu Bujoru | <LoQ | <LoQ | <LoQ | <LoQ | <LoQ | <LoQ | 0.08 ± 0.06 b | <LoQ | 0.12 ± 0.03 def | <LoQ |
| 2020 | Southeast (2) | Spring rape | Tecuci | <LoQ | <LoQ | <LoQ | <LoQ | <LoQ | <LoQ | <LoQ | <LoQ | <LoQ | <LoQ |
| 2020 | Southeast (1) | Autumn rape | <LoQ | <LoQ | <LoQ | <LoQ | <LoQ | <LoQ | <LoQ | <LoQ | <LoQ | <LoQ | |
| 2019 | East (2) | Sunflower | Vaslui | <LoQ | <LoQ | <LoQ | <LoQ | <LoQ | <LoQ | 0.03 ± 0.02 b | <LoQ | 0.08 ± 0.02 fg | <LoQ |
| 2018 | East (2) | Linden | <LoQ | <LoQ | <LoQ | <LoQ | <LoQ | <LoQ | 0.01 ± 0.01 b | <LoQ | <LoQ | <LoQ | |
| 2019 | East (3) | Lavender | <LoQ | <LoQ | <LoQ | <LoQ | <LoQ | <LoQ | <LoQ | <LoQ | 0.12 ± 0.03 def | <LoQ | |
| 2021 | Southeast (3) | Multifloral | Brăila | <LoQ | <LoQ | <LoQ | <LoQ | <LoQ | <LoQ | <LoQ | <LoQ | 0.06 ± 0.05 fg | <LoQ |
| 2022 | Southeast (1) | Linden | <LoQ | <LoQ | <LoQ | <LoQ | <LoQ | <LoQ | <LoQ | <LoQ | 0.20 ± 0.17 bcde | <LoQ | |
| 2021 | Southeast (2) | Acacia + Linden | <LoQ | <LoQ | <LoQ | <LoQ | <LoQ | <LoQ | <LoQ | <LoQ | 0.12 ± 0.04 def | <LoQ | |
| 2020 | Southeast (1) | Multifloral | <LoQ | <LoQ | <LoQ | <LoQ | <LoQ | <LoQ | <LoQ | <LoQ | 0.20 ± 0.06 bcde | <LoQ | |
| 2021 | Northwest (2) | Chestnut | Satu Mare | <LoQ | <LoQ | <LoQ | <LoQ | <LoQ | <LoQ | 0.04 ± 0.03 ab | <LoQ | <LoQ | <LoQ |
| 2020 | Northwest (1) | Acacia | <LoQ | <LoQ | <LoQ | <LoQ | <LoQ | <LoQ | 0.02 ± 0.01 ab | <LoQ | <LoQ | <LoQ | |
| 2020 | Southeast (1) | Multifloral | Tulcea | <LoQ | <LoQ | <LoQ | <LoQ | <LoQ | <LoQ | 0.17 ± 0.04 a | <LoQ | 0.26 ± 0.13 abc | <LoQ |
| 2020 | Southeast (2) | Sunflower | <LoQ | <LoQ | <LoQ | <LoQ | <LoQ | <LoQ | 0.12 ± 0.01 a | <LoQ | 0.17 ± 0.02 bcdef | <LoQ | |
| 2019 | Southeast (1) | Linden | <LoQ | <LoQ | <LoQ | <LoQ | <LoQ | <LoQ | <LoQ | <LoQ | 0.11 ± 0.01 defg | <LoQ | |
| 2021 | Northeast (2) | Honeydew | Botoșani | <LoQ | <LoQ | <LoQ | <LoQ | <LoQ | <LoQ | 0.13 ± 0.14 a | <LoQ | <LoQ | <LoQ |
| 2019 | Northeast (3) | Multifloral | <LoQ | <LoQ | <LoQ | <LoQ | <LoQ | <LoQ | 0.05 ± 0.01 ab | <LoQ | 0.19 ± 0.02 bcde | <LoQ | |
| 2019 | Northeast (2) | Linden | <LoQ | <LoQ | <LoQ | <LoQ | <LoQ | <LoQ | 0.01 ± 0.01 b | <LoQ | 0.31 ± 0.29 a | <LoQ | |
| 2019 | Northeast (1) | Sunflower | <LoQ | <LoQ | <LoQ | <LoQ | <LoQ | <LoQ | 0.01 ± 0.01 b | <LoQ | 0.16 ± 0.03 cdef | <LoQ | |
| 2021 | Northeast (2) | Acacia + Linden | Iași | <LoQ | <LoQ | <LoQ | <LoQ | <LoQ | <LoQ | 0.01 ± 0.01 b | <LoQ | 0.14 ± 0.03 def | <LoQ |
| 2020 | Northeast (2) | Acacia | <LoQ | <LoQ | <LoQ | <LoQ | <LoQ | <LoQ | 0.02 ± 0.01 b | <LoQ | <LoQ | <LoQ | |
| 2020 | Northeast (2) | Sunflower | <LoQ | <LoQ | <LoQ | <LoQ | <LoQ | <LoQ | 0.04 ± 0.03 ab | <LoQ | 0.27 ± 0.05 ab | <LoQ | |
| 2021 | Center (3) | Acacia | Sibiu | <LoQ | <LoQ | <LoQ | <LoQ | <LoQ | <LoQ | <LoQ | <LoQ | <LoQ | <LoQ |
| 2020 | Center (2) | Sunflower | <LoQ | <LoQ | <LoQ | <LoQ | <LoQ | <LoQ | <LoQ | <LoQ | <LoQ | <LoQ | |
| 2019 | Southwest (1) | Sunflower | Râmnicu Vâlcea | <LoQ | <LoQ | <LoQ | <LoQ | <LoQ | <LoQ | <LoQ | <LoQ | 0.19 ± 0.04 bcde | <LoQ |
| 2018 | Southwest (1) | Multifloral | <LoQ | <LoQ | <LoQ | <LoQ | <LoQ | <LoQ | <LoQ | <LoQ | 0.14 ± 0.04 def | <LoQ | |
| 2018 | West (2) | Acacia | Arad | <LoQ | <LoQ | <LoQ | <LoQ | <LoQ | <LoQ | <LoQ | <LoQ | <LoQ | <LoQ |
| 2020 | South (3) | Sunflower | Teleorman | <LoQ | <LoQ | <LoQ | <LoQ | <LoQ | <LoQ | 0.03 ± 0.01 ab | <LoQ | <LoQ | <LoQ |
| 2020 | Southwest (2) | Acacia | Mehedinți | <LoQ | <LoQ | <LoQ | <LoQ | <LoQ | <LoQ | 0.02 ± 0.01 b | <LoQ | 0.20 ± 0.05 bcde | <LoQ |
| 2022 | Southwest (1) | Sunflower | <LoQ | <LoQ | <LoQ | <LoQ | <LoQ | <LoQ | <LoQ | <LoQ | 0.21 ± 0.05 bcd | <LoQ | |
| F | - | - | - | - | - | - | 7.380 | - | 9.202 | - | |||
| Sig. | - | - | - | - | - | - | *** | - | *** | - | |||
| Minimum–Maximum Values | - | - | - | - | - | - | <LoQ - | - | <LoQ - | - | |||
| 0.17 | 0.31 | ||||||||||||
| AVERAGE | - | - | - | - | - | - | 0.03 ± 0.04 | - | 0.09 ± 0.07 | - | |||
| Elements | K | Na | Mg | Ca | Li | Al | Fe | Cu | Zn | Sr | Cr | Mn | Ni | Cd | Pb |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| K | 1.000 | ||||||||||||||
| Na | 0.444 ** | 1.000 | |||||||||||||
| Mg | 0.309 ** | 0.289 ** | 1.000 | ||||||||||||
| Ca | 1.000 | ||||||||||||||
| Li | 0.248 * | 1.000 | |||||||||||||
| Al | 0.232 * | 0.288 ** | 1.000 | ||||||||||||
| Fe | 1.000 | ||||||||||||||
| Cu | 0.231 * | 1.000 | |||||||||||||
| Zn | −0.311 ** | 0.498 ** | −0.251 * | 1.000 | |||||||||||
| Sr | −0.213 ** | 1.000 | |||||||||||||
| Cr | −0.203 ** | −0.273 ** | 1.000 | ||||||||||||
| Mn | −0.345 ** | −0.206 * | −0.230 * | 1.000 | |||||||||||
| Ni | 0.361 ** | 1.000 | |||||||||||||
| Cd | 0.377 ** | 0.252 ** | 0.282 ** | 1.000 | |||||||||||
| Pb | −0.273 ** | −0.292 ** | 1.000 | ||||||||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bora, F.D.; Andrecan, A.F.; Călugăr, A.; Bunea, C.I.; Popescu, M.; Petrescu-Mag, I.V.; Bunea, A. Comprehensive Elemental Profiling of Romanian Honey: Exploring Regional Variance, Honey Types, and Analyzed Metals for Sustainable Apicultural and Environmental Practices. Foods 2024, 13, 1253. https://doi.org/10.3390/foods13081253
Bora FD, Andrecan AF, Călugăr A, Bunea CI, Popescu M, Petrescu-Mag IV, Bunea A. Comprehensive Elemental Profiling of Romanian Honey: Exploring Regional Variance, Honey Types, and Analyzed Metals for Sustainable Apicultural and Environmental Practices. Foods. 2024; 13(8):1253. https://doi.org/10.3390/foods13081253
Chicago/Turabian StyleBora, Florin Dumitru, Andreea Flavia Andrecan, Anamaria Călugăr, Claudiu Ioan Bunea, Maria Popescu, Ioan Valentin Petrescu-Mag, and Andrea Bunea. 2024. "Comprehensive Elemental Profiling of Romanian Honey: Exploring Regional Variance, Honey Types, and Analyzed Metals for Sustainable Apicultural and Environmental Practices" Foods 13, no. 8: 1253. https://doi.org/10.3390/foods13081253





