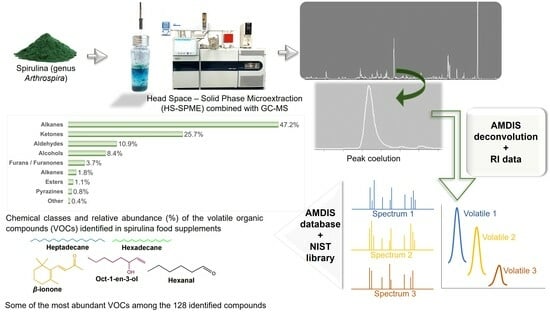
Volatile Profiling of Spirulina Food Supplements
Abstract
:1. Introduction
2. Materials and Methods
2.1. Spirulina Samples, Reference Substances, and Solvents
2.2. Sample Preparation
2.3. HS-SPME-GC/MS Analysis
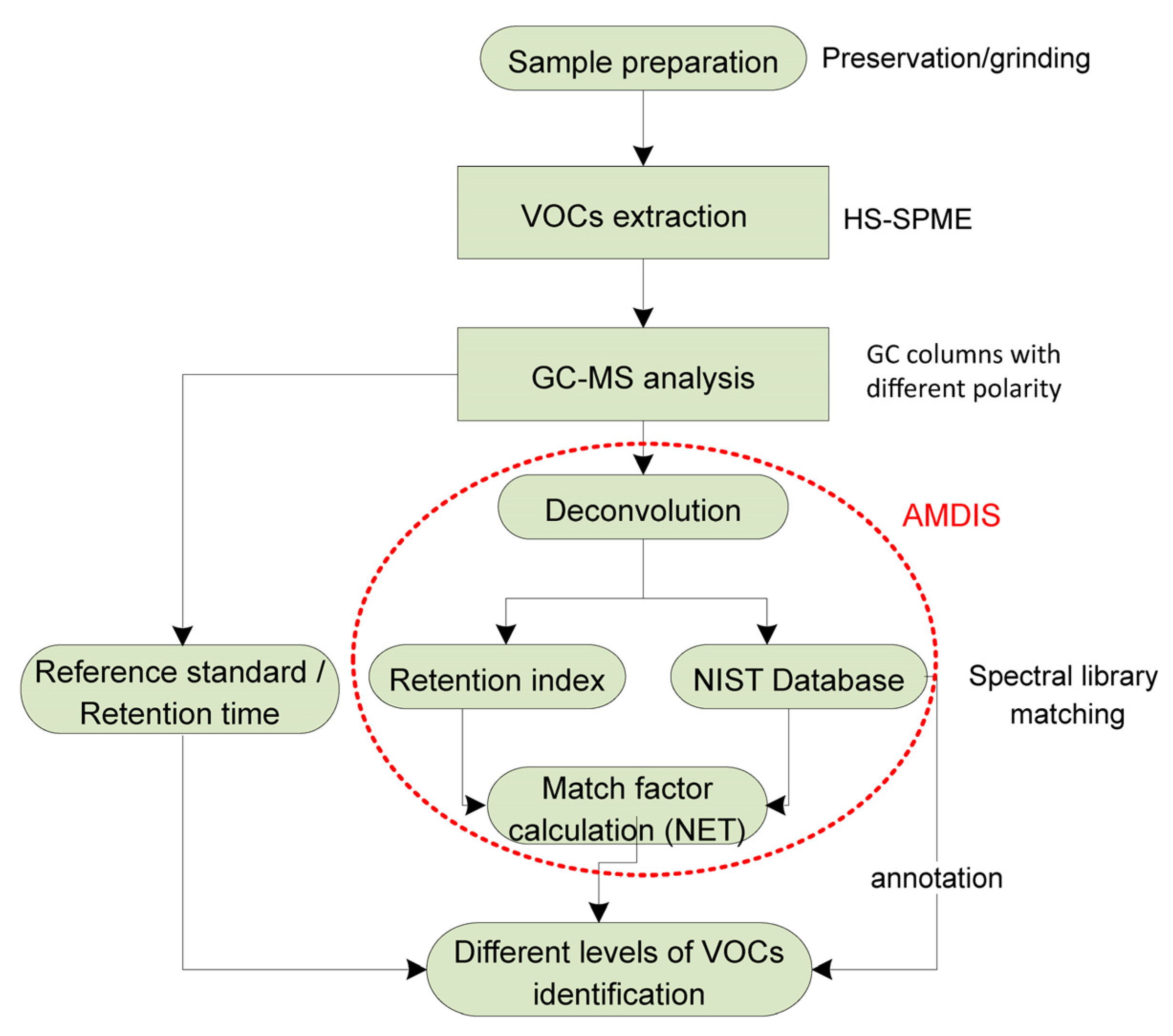
2.4. Data Analysis
3. Results and Discussion
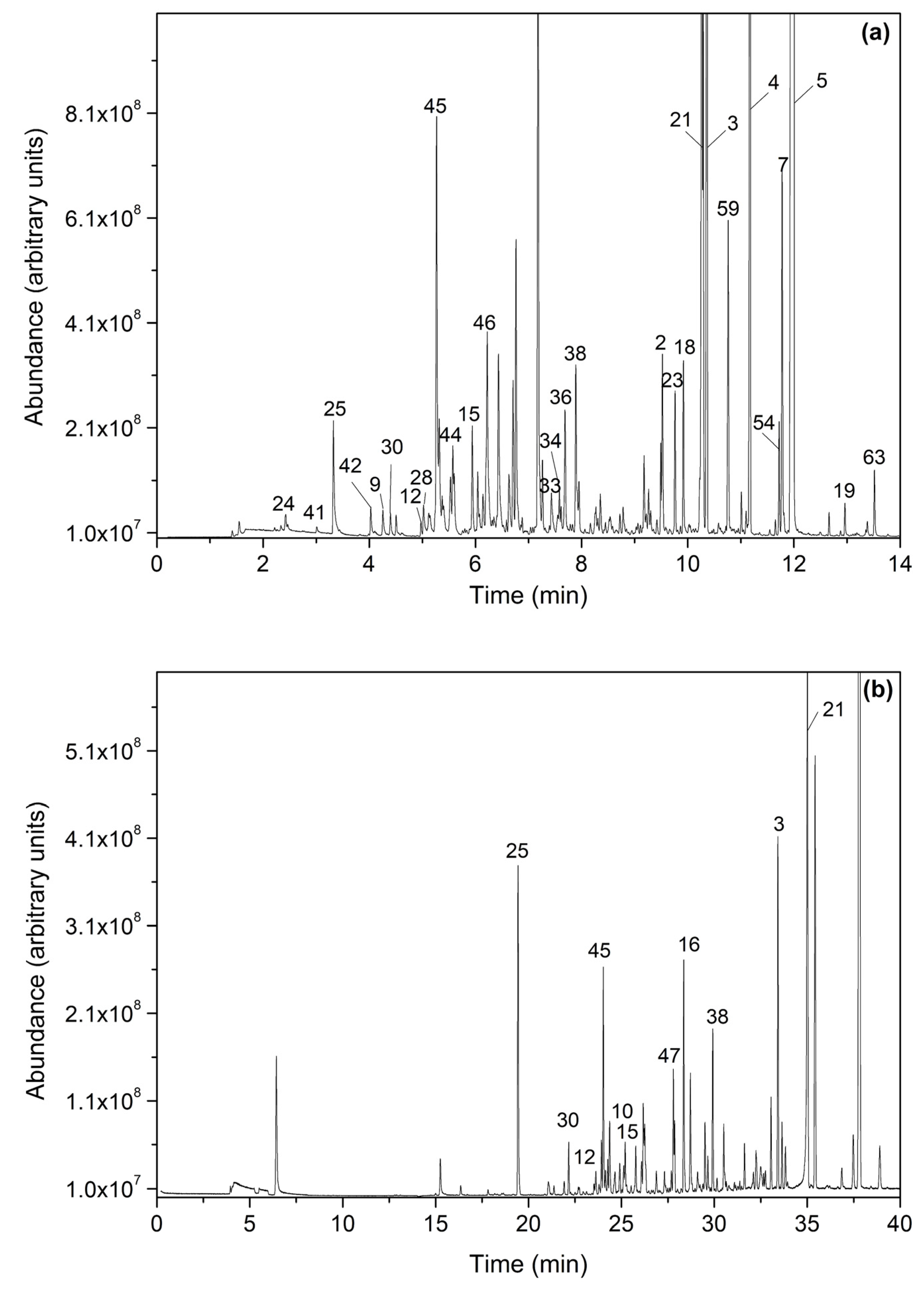
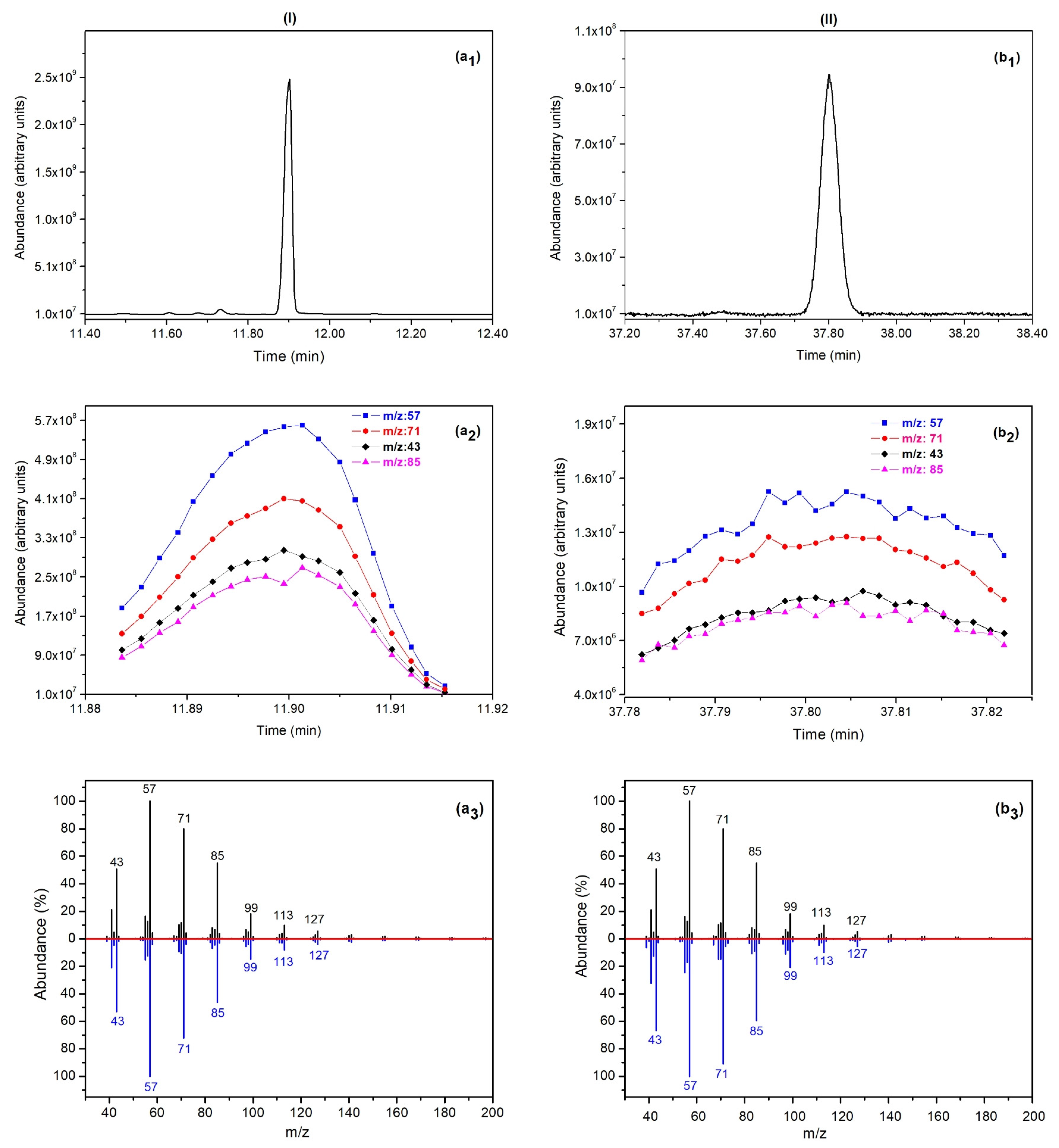
3.1. Identification of VOCs
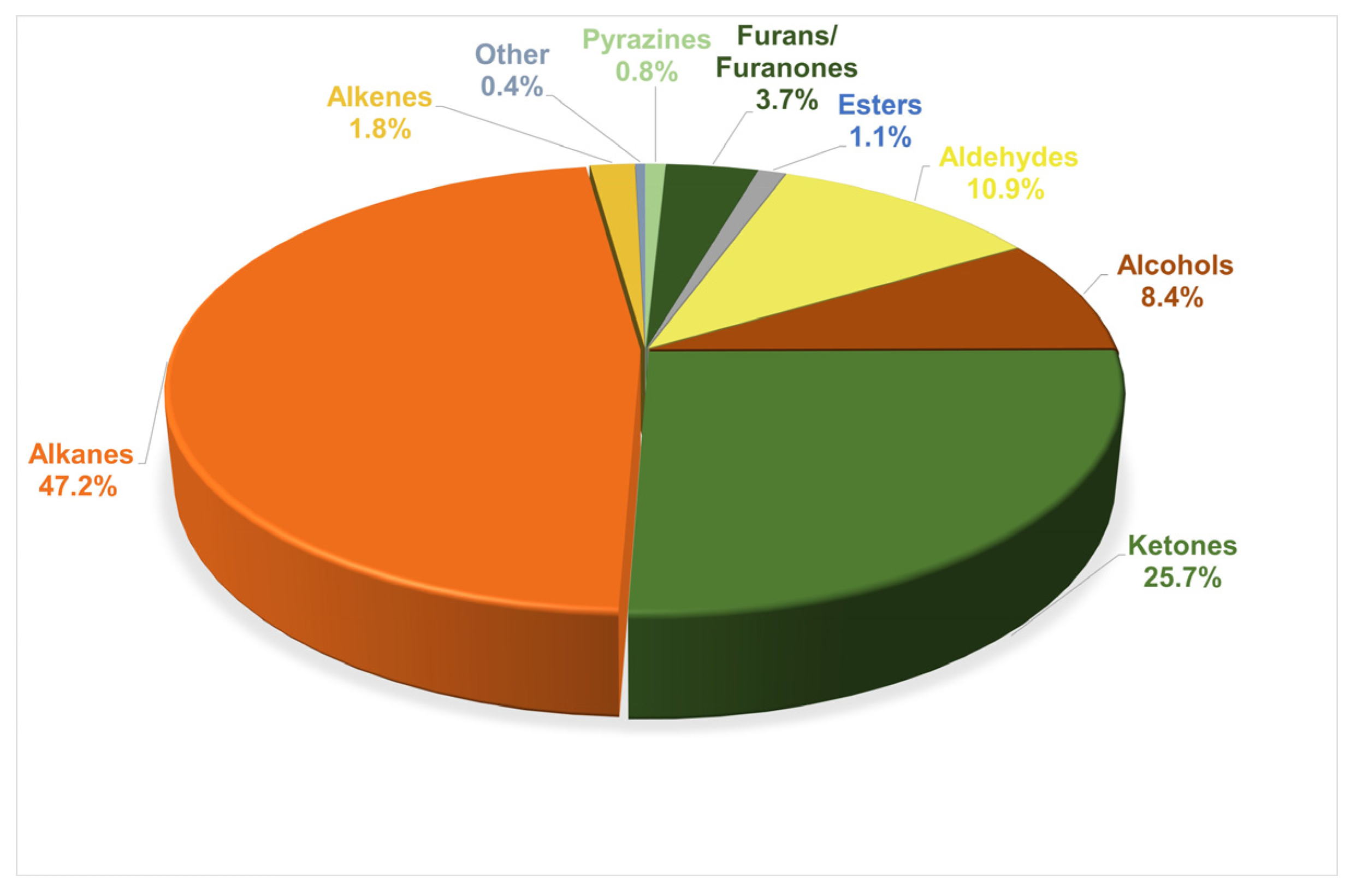
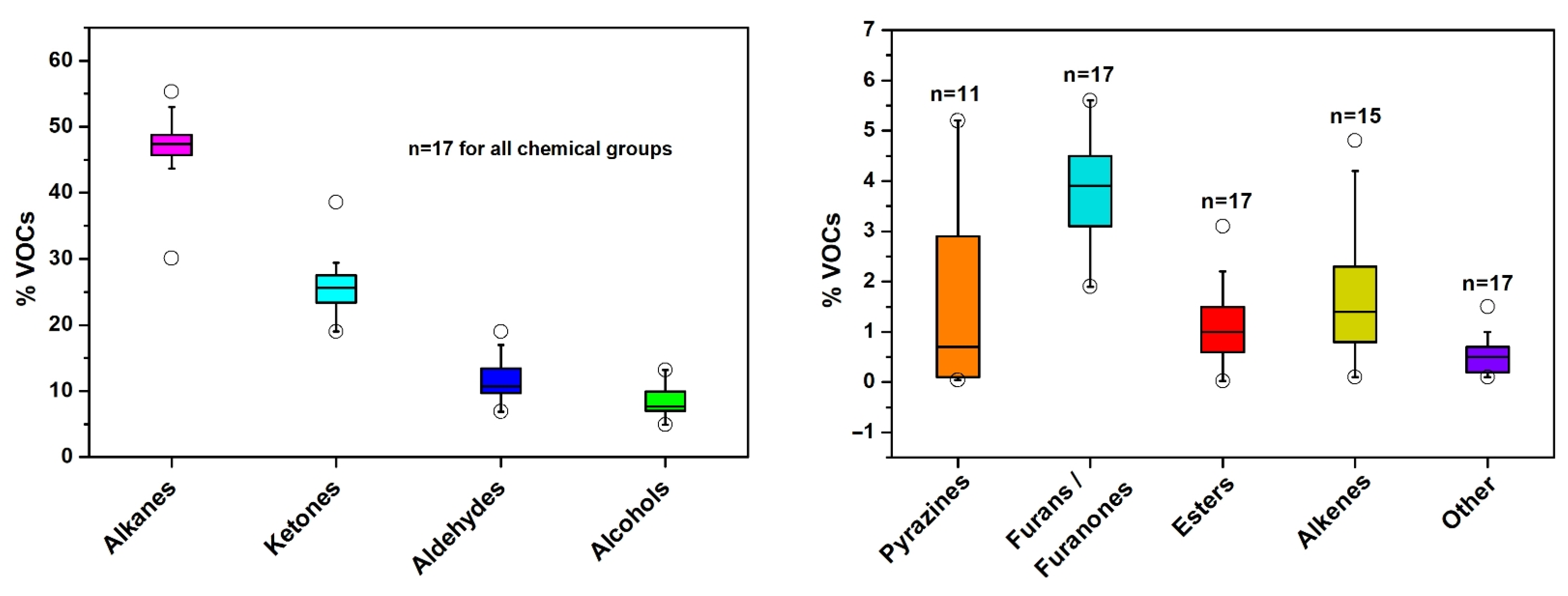
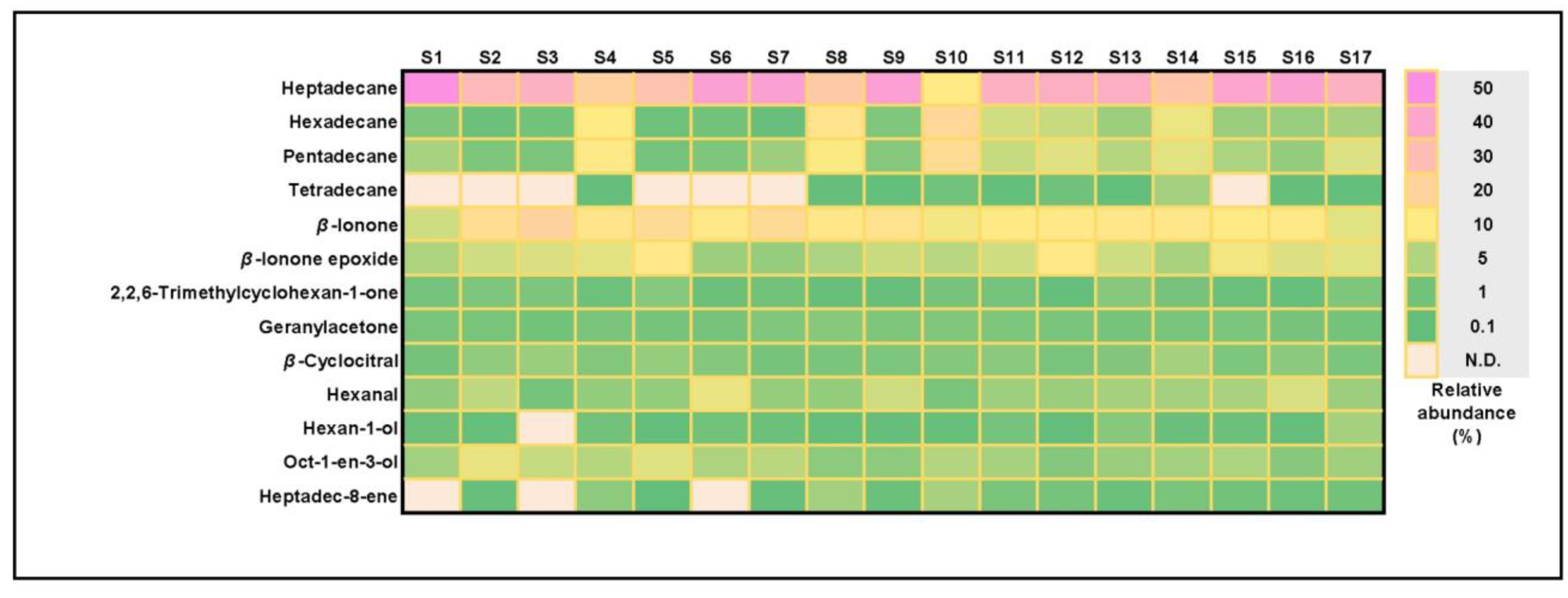
3.2. VOCs in Commercial Spirulina Samples
3.3. Variation in Major Volatiles among Spirulina Supplements
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Vardaka, E.; Kormas, K.A.; Katsiapi, M.; Genitsaris, S.; Moustaka-Gouni, M. Molecular diversity of bacteria in commercially available “Spirulina” food supplements. PeerJ 2016, 4, e1610. [Google Scholar] [CrossRef] [PubMed]
- Andrade, L.; Andrade, C.; Dias, M.; Nascimento, C.; Mendes, M. Chlorella and spirulina microalgae as sources of functional foods. MOJ Food Process. Technol. 2018, 6, 45–58. [Google Scholar] [CrossRef]
- de Jesus Raposo, M.F.; de Morais, R.M.S.C.; de Morais, A.M.M.B. Health applications of bioactive compounds from marine microalgae. Life Sci. 2013, 93, 479–486. [Google Scholar] [CrossRef] [PubMed]
- Grosshagauer, S.; Kraemer, K.; Somoza, V. The True Value of Spirulina. J. Agric. Food Chem. 2020, 68, 4109–4115. [Google Scholar] [CrossRef]
- Hosseini, S.M.; Shahbazizadeh, S.; Khosravi-Darani, K.; Mozafari, M.R. Spirulina paltensis: Food and function. Curr. Nutr. Food Sci. 2013, 9, 189–193. [Google Scholar] [CrossRef]
- Hussein, A.; Ibrahim, G.; Kamil, M.; El-Shamarka, M.; Mostafa, S.; Mohamed, D. Spirulina-enriched pasta as functional food rich in protein and antioxidant. Biointerface Res. Appl. Chem. 2021, 11, 14736–14750. [Google Scholar] [CrossRef]
- Winarni Agustini, T.; Farid Ma’ruf, W.; Widayat, W.; Suzery, M.; Hadiyanto, H.; Benjakul, S. Application of Spirulina platensis on ice cream and soft cheese with respect to their nutritional and sensory perspectives. J. Teknol. 2016, 78, 245–251. [Google Scholar] [CrossRef]
- Byeon, S.Y.; Cho, M.K.; Shim, K.H.; Kim, H.J.; Song, H.G.; Shin, H.S. Development of a Spirulina Extract/Alginate-Imbedded PCL Nanofibrous Cosmetic Patch. J. Microbiol. Biotechnol. 2017, 27, 1657–1663. [Google Scholar] [CrossRef] [PubMed]
- Dianursanti; Sari, P.R.; Alifia, K.C.H. Utilization of microalgae Spirulina platensis as a raw material for making capsule shell. In Proceedings of the The 4th International Tropical Renewable Energy Conference (i-TREC 2019), Bali, Indonesia, 14–16 August 2019. [Google Scholar]
- Tolpeznikaite, E.; Bartkevics, V.; Ruzauskas, M.; Pilkaityte, R.; Viskelis, P.; Urbonaviciene, D.; Zavistanaviciute, P.; Zokaityte, E.; Ruibys, R.; Bartkiene, E. Characterization of macro-and microalgae extracts bioactive compounds and micro-and macroelements transition from algae to extract. Foods 2021, 10, 2226. [Google Scholar] [CrossRef]
- Mahmoud, Y.I.; Shehata, A.M.M.; Fares, N.H.; Mahmoud, A.A. Spirulina inhibits hepatocellular carcinoma through activating p53 and apoptosis and suppressing oxidative stress and angiogenesis. Life Sci. 2021, 265, 118827. [Google Scholar] [CrossRef]
- Agustina, S.; Aidha, N.N.; Oktarina, E.; Kurniati, N.F. Evaluation of Antioxidant and Wound Healing Activities of Spirulina sp. Extract. Egypt. J. Chem. 2021, 64, 4601–4610. [Google Scholar] [CrossRef]
- Cuellar-Bermúdez, S.P.; Barba-Davila, B.; Serna-Saldivar, S.O.; Parra-Saldivar, R.; Rodriguez-Rodriguez, J.; Morales-Davila, S.; Goiris, K.; Muylaert, K.; Chuck-Hernández, C. Deodorization of Arthrospira platensis biomass for further scale-up food applications. J. Sci. Food Agric. 2017, 97, 5123–5130. [Google Scholar] [CrossRef] [PubMed]
- Nunes, M.C.; Ferreira, J.; Raymundo, A. Volatile fingerprint impact on the sensory properties of microalgae and development of mitigation strategies. Curr. Opin. Food Sci. 2023, 51, 101040. [Google Scholar] [CrossRef]
- Nader, C.; Cella, H.; Lopes, R.G.; Oliveira, C.Y.B.; D’Alessandro, E.B.; Filho, N.R.A.; Derner, R.B. Effect of different cultivation conditions on the production of volatile organic compounds by the microalgae Arthrospira platensis and Chlorella sp. J. Appl. Phycol. 2022, 34, 203–217. [Google Scholar] [CrossRef]
- Lee, J.; Rai, P.K.; Jeon, Y.J.; Kim, K.-H.; Kwon, E.E. The role of algae and cyanobacteria in the production and release of odorants in water. Environ. Pollut. 2017, 227, 252–262. [Google Scholar] [CrossRef] [PubMed]
- Martelli, F.; Cirlini, M.; Lazzi, C.; Neviani, E.; Bernini, V. Solid-state fermentation of Arthrospira platensis to implement new food products: Evaluation of stabilization treatments and bacterial growth on the volatile fraction. Foods 2021, 10, 67. [Google Scholar] [CrossRef] [PubMed]
- Yuliani, A.T.W.; Dewi, E.N. Evaluation of the volatile compound basil leaf (Ocimum basilicum) intervention on Spirulina platensis. Food Res. 2021, 5, 48–53. [Google Scholar] [CrossRef]
- Pinheiro, P.N.; Vieira, K.R.; Santos, A.B.; Jacob-Lopes, E.; Zepka, L.Q. Biogeneration of volatile organic compounds in microalgae-based systems. In Handbook of Algal Technologies and Phytochemicals; Ravishankar, G.A., Ambati, R.R., Eds.; CRC Press: Boca Raton, FL, USA, 2019; pp. 89–96. [Google Scholar]
- Perez-Garcia, O.; Bashan, Y. Microalgal Heterotrophic and Mixotrophic Culturing for Bio-refining: From Metabolic Routes to Techno-economics. In Algal Biorefineries: Volume 2: Products and Refinery Design; Prokop, A., Bajpai, R.K., Zappi, M.E., Eds.; Springer International Publishing: Cham, Switzerland, 2015; Volume 2, pp. 61–131. [Google Scholar]
- Chojnacka, K.; Noworyta, A. Evaluation of Spirulina sp. growth in photoautotrophic, heterotrophic and mixotrophic cultures. Enzym. Microb. Technol. 2004, 34, 461–465. [Google Scholar] [CrossRef]
- Bao, J.; Zhang, X.; Zheng, J.-H.; Ren, D.-F.; Lu, J. Mixed fermentation of Spirulina platensis with Lactobacillus plantarum and Bacillus subtilis by random-centroid optimization. Food Chem. 2018, 264, 64–72. [Google Scholar] [CrossRef]
- Milovanović, I.; Mišan, A.; Simeunović, J.; Kovač, D.; Jambrec, D.; Mandić, A. Determination of Volatile Organic Compounds in Selected Strains of Cyanobacteria. J. Chem. 2015, 2015, 969542. [Google Scholar] [CrossRef]
- Ozdemir, G.; Ulku Karabay, N.; Dalay, M.C.; Pazarbasi, B. Antibacterial activity of volatile component and various extracts of Spirulina platensis. Phytother. Res. 2004, 18, 754–757. [Google Scholar] [CrossRef] [PubMed]
- Ould Bellahcen, T.; Cherki, M.; Sánchez, J.A.C.; Cherif, A.; El Amrani, A. Chemical Composition and Antibacterial Activity of the Essential Oil of Spirulina platensis from Morocco. J. Essent. Oil Bear. Plants 2019, 22, 1265–1276. [Google Scholar] [CrossRef]
- Moran, L.; Bou, G.; Aldai, N.; Ciardi, M.; Morillas-España, A.; Sánchez-Zurano, A.; Barron, L.J.R.; Lafarga, T. Characterisation of the volatile profile of microalgae and cyanobacteria using solid-phase microextraction followed by gas chromatography coupled to mass spectrometry. Sci. Rep. 2022, 12, 3661. [Google Scholar] [CrossRef]
- Aguero, J.; Lora, J.; Estrada, K.; Concepcion, F.; Nunez, A.; Rodriguez, A.; Pino, J.A. Volatile Components of a Commercial Sample of the Blue-Green Algae Spirulina platensis. J. Essent. Oil Res. 2003, 15, 114–117. [Google Scholar] [CrossRef]
- Ramasamy, V.; Gopalakrishnan, V.K. Chemical Composition of Spirulina by Gas Chromatography Coupled with Mass Spectrophotometer (GC-MS). Int. J. Pharm. Phytopharm. Res. 2013, 3, 239–244. Available online: https://eijppr.com/s3gVvln (accessed on 9 March 2024).
- Van Durme, J.; Goiris, K.; De Winne, A.; De Cooman, L.; Muylaert, K. Evaluation of the volatile composition and sensory properties of five species of microalgae. J. Agric. Food Chem. 2013, 61, 10881–10890. [Google Scholar] [CrossRef] [PubMed]
- Steinhaus, M. Gas Chromatography–Olfactometry: Principles, Practical Aspects and Applications in Food Analysis. In Advanced Gas Chromatography in Food Analysis; Tranchida, P.Q., Ed.; Food Chemistry, Function and Analysis; The Royal Society of Chemistry: London, UK, 2019; pp. 337–399. [Google Scholar]
- Mallikarjun Gouda, K.G.; Udaya Sankar, K.; Sarada, R.; Ravishankar, G.A. Supercritical CO2 extraction of functional compounds from Spirulina and their biological activity. J. Food Sci. Technol. 2015, 52, 3627–3633. [Google Scholar] [CrossRef]
- Kaur, K.; Sharma, R.; Singh, S. Bioactive composition and promising health benefits of natural food flavors and colorants: Potential beyond their basic functions. Pigment. Resin Technol. 2020, 49, 110–118. [Google Scholar] [CrossRef]
- van Den Dool, H.; Kratz, P.D. A generalization of the retention index system including linear temperature programmed gas-liquid partition chromatography. J. Chromatogr. A 1963, 11, 463–471. [Google Scholar] [CrossRef]
- Mallard, W.G. AMDIS in the Chemical Weapons Convention. Anal. Bioanal. Chem. 2014, 406, 5075–5086. [Google Scholar] [CrossRef]
- Babushok, V.I.; Andriamaharavo, N.R. Use of Large Retention Index Database for Filtering of GC–MS False Positive Identifications of Compounds. Chromatographia 2012, 75, 685–692. [Google Scholar] [CrossRef]
- Popek, E.P. Sampling and Analysis of Environmental Chemical Pollutants: A Complete Guide; Academic Press: Cambridge, MA, USA, 2003. [Google Scholar]
- Acree, T.; Arn, H. Flavornet and Human Odor Space. Available online: http://www.flavornet.org (accessed on 9 March 2024).
- Kreissl, J.; Mall, V.; Steinhaus, P.; Steinhaus, M. Leibniz-LSB@TUM Odorant Database, Version 1.2. Available online: https://www.leibniz-lsb.de/en/databases/leibniz-lsbtum-odorant-database (accessed on 9 March 2024).
- Babushok, V.I. Chromatographic retention indices in identification of chemical compounds. TrAC Trends Anal. Chem. 2015, 69, 98–104. [Google Scholar] [CrossRef]
- Watson, S.B. Cyanobacterial and eukaryotic algal odour compounds: Signals or by-products? A review of their biological activity. Phycologia 2003, 42, 332–350. [Google Scholar] [CrossRef]
- Nouri, E.; Abbasi, H. Effects of Different Processing Methods on Phytochemical Compounds and Antioxidant Activity of Spirulina platensis. Appl. Food Biotechnol. 2018, 5, 221–232. [Google Scholar] [CrossRef]
- Schirmer, A.; Rude, M.A.; Li, X.; Popova, E.; Del Cardayre, S.B. Microbial biosynthesis of alkanes. Science 2010, 329, 559–562. [Google Scholar] [CrossRef]
- Mendez-Perez, D.; Begemann, M.B.; Pfleger, B.F. Modular synthase-encoding gene involved in α-olefin biosynthesis in Synechococcus sp. strain PCC 7002. Appl. Environ. Microbiol. 2011, 77, 4264–4267. [Google Scholar] [CrossRef]
- Coates, R.C.; Podell, S.; Korobeynikov, A.; Lapidus, A.; Pevzner, P.; Sherman, D.H.; Allen, E.E.; Gerwick, L.; Gerwick, W.H. Characterization of Cyanobacterial Hydrocarbon Composition and Distribution of Biosynthetic Pathways. PLoS ONE 2014, 9, e85140. [Google Scholar] [CrossRef]
- Schneider, H.; Gelpi, E.; Bennett, E.O.; Oró, J. Fatty acids of geochemical significance in microscopic algae. Phytochemistry 1970, 9, 613–617. [Google Scholar] [CrossRef]
- Aloum, L.; Alefishat, E.; Adem, A.; Petroianu, G. Ionone Is More than a Violet’s Fragrance: A Review. Molecules 2020, 25, 5822. [Google Scholar] [CrossRef]
- Nawade, B.; Shaltiel-Harpaz, L.; Yahyaa, M.; Bosamia, T.C.; Kabaha, A.; Kedoshim, R.; Zohar, M.; Isaacson, T.; Ibdah, M. Analysis of apocarotenoid volatiles during the development of Ficus carica fruits and characterization of carotenoid cleavage dioxygenase genes. Plant Sci. 2020, 290, 110292. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, N.; Zhao, M.; Jing, T.; Jin, J.; Wu, B.; Wan, X.; Schwab, W.; Song, C. Carotenoid Cleavage Dioxygenase 4 Catalyzes the Formation of Carotenoid-Derived Volatile β-Ionone during Tea (Camellia sinensis) Withering. J. Agric. Food Chem. 2020, 68, 1684–1690. [Google Scholar] [CrossRef]
- Schulz, S.; Dickschat, J.S. Bacterial volatiles: The smell of small organisms. Nat. Prod. Rep. 2007, 24, 814–842. [Google Scholar] [CrossRef]
- Paparella, A.; Shaltiel-Harpaza, L.; Ibdah, M. β-Ionone: Its Occurrence and Biological Function and Metabolic Engineering. Plants 2021, 10, 754. [Google Scholar] [CrossRef]
- Yamamoto, M.; Baldermann, S.; Yoshikawa, K.; Fujita, A.; Mase, N.; Watanabe, N. Determination of Volatile Compounds in Four Commercial Samples of Japanese Green Algae Using Solid Phase Microextraction Gas Chromatography Mass Spectrometry. Sci. World J. 2014, 2014, 289780. [Google Scholar] [CrossRef]
- Jüttner, F. Dynamics of the Volatile Organic Substances Associated with Cyanobacteria and Algae in a Eutrophic Shallow Lake. Appl. Environ. Microbiol. 1984, 47, 814–820. [Google Scholar] [CrossRef]
- Höckelmann, C.; Jüttner, F. Volatile organic compound (VOC) analysis and sources of limonene, cyclohexanone and straight chain aldehydes in axenic cultures of Calothrix and Plectonema. Water Sci. Technol. 2004, 49, 47–54. [Google Scholar] [CrossRef]
- Dembitsky, V.M.; Dor, I.; Shkrob, I.; Aki, M. Branched Alkanes and Other Apolar Compounds Produced by the Cyanobacterium Microcoleus vaginatusfrom the Negev Desert. Russ. J. Bioorganic Chem. 2001, 27, 110–119. [Google Scholar] [CrossRef]
- Zuo, Z. Why Algae Release Volatile Organic Compounds—The Emission and Roles. Front. Microbiol. 2019, 10, 491. [Google Scholar] [CrossRef]
- Jüttner, F. Nor-Carotenoids as the Major Volatile Excretion Products of Cyanidium. Z. Naturforschung C 1979, 34, 186–191. [Google Scholar] [CrossRef]
- Thiel, V.; Merz-Preiß, M.; Reitner, J.; Michaelis, W. Biomarker studies on microbial carbonates: Extractable lipids of a Calcifying Cyanobacterial mat (Everglades, USA). Facies 1997, 36, 163–172. [Google Scholar] [CrossRef]
- Rzama, A.; Benharref, A.; Arreguy, B.; Dufourc, E.J. Volatile compounds of green microalgae grown on reused waste water. Phytochemistry 1995, 38, 1375–1379. [Google Scholar] [CrossRef]
- Rontani, J.F.; Giral, P.J.P.; Baillet, G.; Raphel, D. “Bound” 6,10,14-trimethylpentadecan-2-one: A useful marker for photodegradation of chlorophylls with a phytol ester group in seawater. Org. Geochem. 1992, 18, 139–142. [Google Scholar] [CrossRef]
- Otles, S.; Pire, R. Fatty acid composition of Chlorella and Spirulina microalgae species. J. AOAC Int. 2001, 84, 1708–1714. [Google Scholar] [CrossRef]
- Isleten Hosoglu, M. Aroma characterization of five microalgae species using solid-phase microextraction and gas chromatography–mass spectrometry/olfactometry. Food Chem. 2018, 240, 1210–1218. [Google Scholar] [CrossRef]
- Czerny, M.; Christlbauer, M.; Christlbauer, M.; Fischer, A.; Granvogl, M.; Hammer, M.; Hartl, C.; Hernandez, N.M.; Schieberle, P. Re-investigation on odour thresholds of key food aroma compounds and development of an aroma language based on odour qualities of defined aqueous odorant solutions. Eur. Food Res. Technol. 2008, 228, 265–273. [Google Scholar] [CrossRef]
- Carrizo, D.; Taborda, G.; Nerín, C.; Bosetti, O. Extension of shelf life of two fatty foods using a new antioxidant multilayer packaging containing green tea extract. Innov. Food Sci. Emerg. Technol. 2016, 33, 534–541. [Google Scholar] [CrossRef]
- Rashash, D.M.C.; Dietrich, A.M.; Hoehn, R.C. FPA of selected odorous compounds. J. AWWA 1997, 89, 131–141. [Google Scholar] [CrossRef]
- Sahin, B.; Hosoglu, M.I.; Guneser, O.; Karagul-Yuceer, Y. Fermented Spirulina products with Saccharomyces and non-Saccharomyces yeasts: Special reference to their microbial, physico-chemical and sensory characterizations. Food Biosci. 2022, 47, 101691. [Google Scholar] [CrossRef]
- Giri, A.; Osako, K.; Okamoto, A.; Ohshima, T. Olfactometric characterization of aroma active compounds in fermented fish paste in comparison with fish sauce, fermented soy paste and sauce products. Food Res. Int. 2010, 43, 1027–1040. [Google Scholar] [CrossRef]
- Masuda, H.; Mihara, S. Olfactive Properties of Alkylpyrazines and 3-Substituted 2-Alkylpyrazines. J. Agric. Food Chem. 1988, 36, 584–587. [Google Scholar] [CrossRef]
- Masuda, H.; Mihara, S. Synthesis of alkoxy-, (alkylthio)-, phenoxy-, and (phenylthio)pyrazines and their olfactive properties. J. Agric. Food Chem. 1986, 34, 377–381. [Google Scholar] [CrossRef]
- Müller, R.; Rappert, S. Pyrazines: Occurrence, formation and biodegradation. Appl. Microbiol. Biotechnol. 2010, 85, 1315–1320. [Google Scholar] [CrossRef]
- Lam, H.S.; Proctor, A. Milled Rice Oxidation Volatiles and Odor Development. J. Food Sci. 2003, 68, 2676–2681. [Google Scholar] [CrossRef]
- Krishnamurthy, R.G.; Smouse, T.H.; Mookherjee, B.D.; Reddy, B.R.; Chang, S.S. Identification of 2-Pentyl Furan in Fats and Oils and Its Relationship to the Reversion Flavor of Soybean Oil. J. Food Sci. 1967, 32, 372–374. [Google Scholar] [CrossRef]
- The Good Scents Company Information System. Available online: http://www.thegoodscentscompany.com (accessed on 9 March 2024).
- Charoenying, P.; Laosinwattana, C.; Chotsaeng, N. The Allelopathic Activity of Extracts and Isolated from Spirulina platensis. Molecules 2022, 27, 3852. [Google Scholar] [CrossRef]
- Henatsch, J.J.; Jüttner, F. Volatile Odorous Excretion Products of Different Strains of Synechococcus (Cyanobacteria). Water Sci. Technol. 1983, 15, 259–266. [Google Scholar] [CrossRef]
- Suyama, K.; Yeow, T.; Nakai, S. Vitamin A oxidation products responsible for haylike flavor production in nonfat dry milk. J. Agric. Food Chem. 1983, 31, 22–26. [Google Scholar] [CrossRef]
- Amrani Zerrifi, S.E.; Khalloufi, F.E.; Mugani, R.; Mahdi, R.E.; Kasrati, A.; Soulaimani, B.; Barros, L.; Ferreira, I.C.F.R.; Amaral, J.S.; Finimundy, T.C.; et al. Seaweed essential oils as a new source of bioactive compounds for cyanobacteria growth control: Innovative ecological biocontrol approach. Toxins 2020, 12, 527. [Google Scholar] [CrossRef]
- Isoe, S.; Be Hyeon, S.; Sakan, T. Photo-oxygenation of carotenoids. I. The formation of dihydroactinidiolide and β-ionone from β-carotene. Tetrahedron Lett. 1969, 10, 279–281. [Google Scholar] [CrossRef]
- World Health Organization. Evaluation of Certain Food Additives: Eighty-Sixth Report of the Joint FAO/WHO Expert Committee on Food Additives (JECFA); WHO technical report series, No. 1014; World Health Organization and Food and Agriculture Organization of the United Nations: Geneva, Switzerland, 2019.
- Takasu, S.; Ishii, Y.; Namiki, M.; Nakamura, K.; Mitsumoto, T.; Takimoto, N.; Nohmi, T.; Ogawa, K. Comprehensive analysis of the general toxicity, genotoxicity, and carcinogenicity of 3-acetyl-2,5-dimethylfuran in male gpt delta rats. Food Chem. Toxicol. 2023, 172, 113544. [Google Scholar] [CrossRef]
- Gai, C.; Zhang, Y.; Chen, W.-T.; Zhang, P.; Dong, Y. An investigation of reaction pathways of hydrothermal liquefaction using Chlorella pyrenoidosa and Spirulina platensis. Energy Convers. Manag. 2015, 96, 330–339. [Google Scholar] [CrossRef]
- Slater, G.P.; Blok, V.C. Volatile Compounds of the Cyanophyceae—A Review. Water Sci. Technol. 1983, 15, 181–190. [Google Scholar] [CrossRef]
- Mofeed, J.; Deyab, M.A.; El-Halim, E. Anticancer activity of some filamentous cyanobacterial isolates against Hep-G2 and MCF-7 cancer cell lines. Int. J. Life Sci. 2018, 8, 10–17. [Google Scholar]
- Sartori, R.B.; Siqueira, S.F.; Maroneze, M.M.; Fagundes, M.B.; Wagner, R.; Zepka, L.Q.; Jacob-Lopes, E. Microalgal secondary metabolites: Effect of climatic variables, seasons, and photocycles on the biogeneration of volatile organic compounds (VOCs). J. Appl. Phycol. 2021, 33, 1457–1472. [Google Scholar] [CrossRef]

| Sample | Product Name/Content According to the Label | Origin | Type | Country of Distributor/Producer |
|---|---|---|---|---|
| S1 | Bio-Spirulina | Greece | tablets | Greece |
| S2 | Arthrospira monospira & Arthrospira compere | Greece | flakes | Greece |
| S3 | Spirulina | n/a 1 | tablets | USA |
| S4 | Spirulina | n/a | tablets | USA |
| S5 | Spirulina | n/a | tablets | USA |
| S6 | Arthrospira platensis | n/a | tablets | USA |
| S7 | Spirulina platensis | n/a | tablets | Hungary |
| S8 | Spirulina platensis | n/a | tablets | England |
| S9 | Spirulina platensis | n/a | tablets | Canada |
| S10 | Spirulina platensis | Greece | tablets | Greece |
| S11 | Spirulina platensis—Arthrospira platensis | Netherlands | tablets | Netherlands |
| S12 | Spirulina | n/a | capsules | Greece |
| S13 | Bio-Spirulina | Greece | tablets | Greece |
| S14 | Spirulina platensis | n/a | tablets | Cyprus |
| S15 | Spirulina platensis | n/a | capsules | France |
| S16 | Spirulina platensis | n/a | powder | n/a |
| S17 | Bio-Spirulina | China | powder | China |
| Level of Identification | Identification Parameters |
|---|---|
| A | Retention index 1 NET value > 80 using columns A and B Retention time 2 |
| B | Retention index 1 NET value > 80 using column A Retention time 2 |
| C | NET value > 80 using column B Retention time 2 |
| D | Retention index 1 NET value > 80 using columns A and B |
| E | Retention index 1 NET value > 80 using column A |
| F | NET value > 80 using column B (peak annotation) |
| A/A | Chemical Compound | Molecular Formula | Level of Identification 1 | tR (min) Column A/Column B | Number of Samples (n) | Relative Abundance (%) | Odor Descriptor |
|---|---|---|---|---|---|---|---|
| Alkanes | |||||||
| 1 | Tridecane | C13H28 | D | 8.58/31.62 | 4 | 0.05–0.5 | alkane 2 |
| 2 | Tetradecane | C14H30 | B | 9.49/n.d. | 10 | 0.1–4.2 | alkane 2 |
| 3 | Pentadecane | C15H32 | A | 10.31/33.42 | 17 | 1.3–17.0 | alkane 2 |
| 4 | Hexadecane | C16H34 | B | 11.12/n.d. | 17 | 0.4–18.6 | alkane 2 |
| 5 | Heptadecane | C17H36 | A | 11.90/37.80 | 17 | 10.5–48.7 | alkane 2 |
| Alkenes | |||||||
| 6 | Hexadec-7-ene | C16H32 | Ε | 11.01/n.d. | 4 | 0.03–0.4 | n/a |
| 7 | Heptadec-8-ene | C17H34 | Ε | 11.73/n.d. | 14 | 0.1–4.4 | n/a |
| Ketones | |||||||
| 8 | Diphenyl-methanone (benzophenone) | C3H10O | Ε | 11.49/n.d. | 5 | 0.03–1.6 | n/a |
| 9 | Heptan-2-one | C7H14O | A | 4.26/21.92 | 13 | 0.1–0.8 | fruity, soapy 3 |
| 10 | 6-Methylhept-5-en-2-one (sulcatone) | C8H14O | D | 5.28/24.26 | 17 | 0.2–1.1 | n/a |
| 11 | Octan-2-one | C8H16O | E | 5.33/n.d. | 12 | 0.02–0.4 | fruity, soapy 3 |
| 12 | 6-Methylheptan-2-one | C8H16O | D | 4.94/23.54 | 16 | 0.06–0.5 | fruity, sour 3 |
| 13 | 3,5,5-Trimethylcyclohex-2-en-1-one (isophorone) | C9H14O | D | 6.16/26.33 | 11 | 0.4–1.7 | n/a |
| 14 | 3,4,4-Trimethylcyclohex-2-en-1-one | C9H14O | D | 6.41/26.91 | 11 | 0.02–0.3 | n/a |
| 15 | 2,2,6-Trimethylcyclohexan-1-one | C9H16O | D | 5.89/25.77 | 17 | 0.3–2.5 | n/a |
| 16 | 5-methyl-3-propan-2-ylidenehex-4-en-2-one | C10H16O | F | n.d./28.36 | 13 | n/a | |
| 17 | 4-(2,6,6-Trimethylcyclohex-1-yl)-butan-2-one | E | 9.86/n.d. | 11 | 0.04–0.2 | n/a | |
| 18 | 6,10-Dimethylundeca-5,9-dien-2-one (geranylacetone) * | C13H22O | D | 9.87/33.65 | 17 | 1.0–2.4 | n/a |
| 19 | 6,10,14-Trimethylpentadecan-2-one * | C18H36O | E | 12.92/n.d. | 12 | 0.1–1.2 | n/a |
| 20 | 4-(2,6,6-Trimethylcyclohexa-1,3-dien-1-yl)-but-3-en-2-one (dehydro-β-ionone) | C13H28O | E | 10.18/n.d. | 12 | 0.3–1.5 | violet-like 3 |
| 21 | 4-(2,6,6-Trimethyl-1-cyclohexen-1-yl)-3-buten-2-one, (β-ionone) | C13H20O | A | 10.21/35.02 | 17 | 7.0–21.1 | floral 3 |
| 22 | 4-(2,2,6-Trimethyl-7-oxabicyclo [4.1.0]heptan-1-yl)-but-3-en-2-one, (β-ionone epoxide) | C13H20O | D | 10.24/35.41 | 17 | 3.3–12.1 | fruity 3 |
| 23 | 4-(2,6,6-trimethylcyclohex-2-en-1-yl)but-3-en-2-one (α-ionone) | C13H20O | B | 9.72/n.d. | 17 | 0.6–1.7 | woody, violet 2 |
| Aldehydes | |||||||
| 24 | Pentanal | C5H10O | D | 2.46/16.36 | 13 | 0.02–0.2 | green, fatty 3 |
| 25 | Hexanal | C6H12O | A | 3.28/19.41 | 17 | 1.2–8.7 | green, grassy 3 |
| 26 | Benzaldehyde | C7H6O | A | 5.08/24.36 | 16 | 0.02–1.1 | bitter 3 almond-like 3 |
| 27 | 2-Methylene-hexanal | C7H12O | E | 3.99/n.d. | 6 | 0.02–0.06 | n/a |
| 28 | Hept-2-enal * | C7H12O | D | 4.98/23.93 | 16 | 0.07–1.6 | green apple-like 3 |
| 29 | Cyclohexanecarbaldehyde | C7H12O | F | n.d./23.92 | 12 | n/a | |
| 30 | Heptanal | C7H14O | D | 4.36/22.16 | 17 | 0.1–1.6 | citrus-like 3 |
| 31 | Oct-2-enal | C8H14O | D | 6.10/26.25 | 16 | 0.2–1.2 | fatty, nutty 3 |
| 32 | Octanal * | C8H16O | D | 5.54/24.63 | 9 | 0.08–0.3 | citrus-like, green 3 |
| 33 | 3,4-Dimethylbenzaldehyde | C9H10O | D | 7.40/29.10 | 16 | 0.05–0.7 | n/a |
| 34 | 3-Ethylbenzaldehyde | C9H10O | E | 7.50/n.d. | 10 | 0.06–0.1 | n/a |
| 35 | Nonanal * | C9H18O | Ε | 6.63/n.d. | 7 | 0.1–1.4 | citrus-like, soapy 3 |
| 36 | 2,6,6-Trimethylcyclohexa-1,3-diene-1-carbaldehyde (safranal) | C10H14O | D | 7.65/29.50 | 17 | 0.6–2.4 | herb, sweet 2 |
| 37 | 2,6,6-Trimethylcyclohex-2-ene-1-carbaldehyde (α-cyclocitral) | C10H16O | D | 6.86/27.68 | 12 | 0.02–0.5 | n/a |
| 38 | 2,6,6-Trimethyl-1-cyclohexene-1-carboxaldehyde, (β-cyclocitral) | C10H16O | A | 7.85/29.93 | 17 | 1.2–4.2 | minty 2 |
| 39 | 2-(2,6,6-Trimethyl-1-cyclohexen-1-yl)-acetaldehyde | C11H18O | E | 8.22/n.d. | 12 | 0.06–0.6 | n/a |
| 40 | 2-Butyl-oct-2-enal | C12H22O | E | 9.26/n.d. | 9 | 0.05–0.3 | n/a |
| Alcohols | |||||||
| 41 | Pentan-1-ol | C5H12O | A | 2.70/18.65 | 9 | 0.01–0.2 | fruity, ethereal 3 |
| 42 | Hexan-1-ol | C6H14O | A | 3.99/21.37 | 16 | 0.1–4.3 | grassy, marzipan-like 3 |
| 43 | Heptan-1-ol * | C7H16O | A | 5.11/23.85 | 13 | 0.04–1.5 | fruity, soapy 3 |
| 44 | 4,4-Dimethylcyclohex-2-en-1-ol * | C8H14O | E | 5.58/n.d. | 10 | 0.1–0.8 | n/a |
| 45 | Oct-1-en-3-ol | C8H16O | A | 5.22/23.03 | 17 | 2.2–8.6 | mushroom-like 3 |
| 46 | Oct-2-en-1-ol * | C8H16O | D | 6.18/26.18 | 17 | 0.2–2.0 | soapy 3 |
| 47 | 2,3-Dimethylcyclohexan-1-ol | C8H16O | F | n.d./27.8 | 11 | n/a | |
| 48 | Octan-1-ol | C8H18O | A | 6.21/26.09 | 4 | 0.2–0.6 | soapy, citrus-like 3 |
| 49 | 2-Ethylhexan-1-ol | C8H18O | D | 5.98/25.22 | 10 | 0.03–1.2 | ethereal, fruity 3 |
| 50 | Νonan-1-ol * | C9H20O | E | 7.27/n.d. | 9 | 0.05–0.5 | soapy, fruity 3 |
| 51 | 3,7-Dimethylocta-1,6-dien-3-ol (linalool) | C10H18O | D | 6.54/26.72 | 9 | 0.03–0.6 | citrus-like, flowery 3 |
| 52 | 2,4-Di-tert-butylphenol | C14H22O | Β | 10.38/n.d. | 7 | 0.01–0.09 | phenolic-like 3 |
| 53 | Tetradeca-11,13-dien-1-ol | C14H26O | E | 11.89/n.d. | 4 | 0.03–1.3 | n/a |
| 54 | Tetradec-9-en-1-ol * | C14H28O | E | 11.68/n.d. | 9 | 0.08–1.3 | n/a |
| Pyrazines | |||||||
| 55 | 2,6-Diethylpyrazine * | C8H12N2 | E | 6.31/n.d. | 10 | 0.03–1.5 | sweet 3 |
| 56 | 2,5-Dimethyl-3-(3-methylbutyl) pyrazine | C11H18N2 | E | 8.6/n.d. | 4 | 0.3–1.9 | n/a |
| Furans—Furanones | |||||||
| 57 | 1-(2,5-Dimethylfuran-3-yl)ethanone * | C8H10O2 | E | 5.99/n.d. | 17 | 0.04–1.3 | n/a |
| 58 | 2-Pentylfuran | C9H14O | D | 5.36/23.79 | 14 | 0.05–1.3 | n/a |
| 59 | 4,4,7a-Trimethyl-6,7-dihydro-5H-1-benzofuran-2-one | C11H16O2 | E | 10.71/n.d. | 17 | 1.4–4.3 | n/a |
| Esters | |||||||
| 60 | Ethenyl hexanoate (n-caproic acid vinyl ester) | C6H12O2 | E | 5.26/n.d. | 7 | 0.05–0.6 | sweaty 3 |
| 61 | Methyl hexanoate | C7H14O2 | E | 4.60/n.d. | 6 | 0.01–0.05 | n/a |
| 62 | Methyl (11Z)-hexadec-11-enoate | C17H32O2 | E | 13.38/n.d. | 4 | 0.08–0.3 | n/a |
| 63 | Methyl hexadecanoate (methyl palmitate) | C17H34O2 | E | 13.48/n.d. | 15 | 0.3–2.5 | n/a |
| Other | |||||||
| 64 | 2-Pentylpyridine | C10H15N | E | 7.54/n.d. | 4 | 0.2–1.1 | fatty, tallow-like 3 |
| 65 | 2-Methyl-6-pentylpyridine | C11H17N | E | 8.02/n.d. | 6 | 0.02–0.2 | n/a |
| 66 | 1-Octoxyoctane (di-n-octyl ether) | C16H34O | E | 11.6/n.d. | 17 | 0.02–0.9 | n/a |
| 67 | Propylcyclopropane | C6H12 | F | n.d./21.37 | 5 | n/a | |
| 68 | 5,5-dimethyl-4-propan-2-ylidene-1H-pyrazole | C8H14N2 | F | n.d./26.32 | 4 | n/a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Paraskevopoulou, A.; Kaloudis, T.; Hiskia, A.; Steinhaus, M.; Dimotikali, D.; Triantis, T.M. Volatile Profiling of Spirulina Food Supplements. Foods 2024, 13, 1257. https://doi.org/10.3390/foods13081257
Paraskevopoulou A, Kaloudis T, Hiskia A, Steinhaus M, Dimotikali D, Triantis TM. Volatile Profiling of Spirulina Food Supplements. Foods. 2024; 13(8):1257. https://doi.org/10.3390/foods13081257
Chicago/Turabian StyleParaskevopoulou, Aikaterina, Triantafyllos Kaloudis, Anastasia Hiskia, Martin Steinhaus, Dimitra Dimotikali, and Theodoros M. Triantis. 2024. "Volatile Profiling of Spirulina Food Supplements" Foods 13, no. 8: 1257. https://doi.org/10.3390/foods13081257