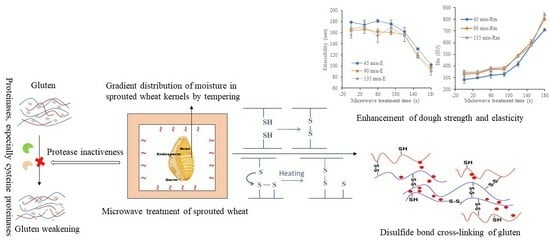
Effect of Microwave Treatment on Protease Activity, Dough Properties and Protein Quality in Sprouted Wheat
Abstract
:1. Introduction
2. Experimental Section
2.1. Preparation of Sprouted Wheat Samples
2.2. Microwave Treatment of Sprouted Wheat Kernels
2.3. Extraction of Protease
2.4. Measurement of Protease Activity
2.5. Measurement of Dough Tensile Properties
2.6. Determination of Dough Mixing Properties and Gluten Quality
2.7. Determination of Protein Solubility
2.8. Size-Exclusion High-Performance Liquid Chromatography (SE-HPLC)
2.9. Sodium Dodecyl Sulfate–Polyacrylamide Gel Electrophoresis (SDS-PAGE) Analysis
2.10. Reversed-Phase High-Performance Liquid Chromatography (RP-HPLC) Analysis
2.11. Statistical Analysis
3. Results and Discussion
3.1. Effect of Microwave Treatment on Protease Activity in Sprouted Wheat
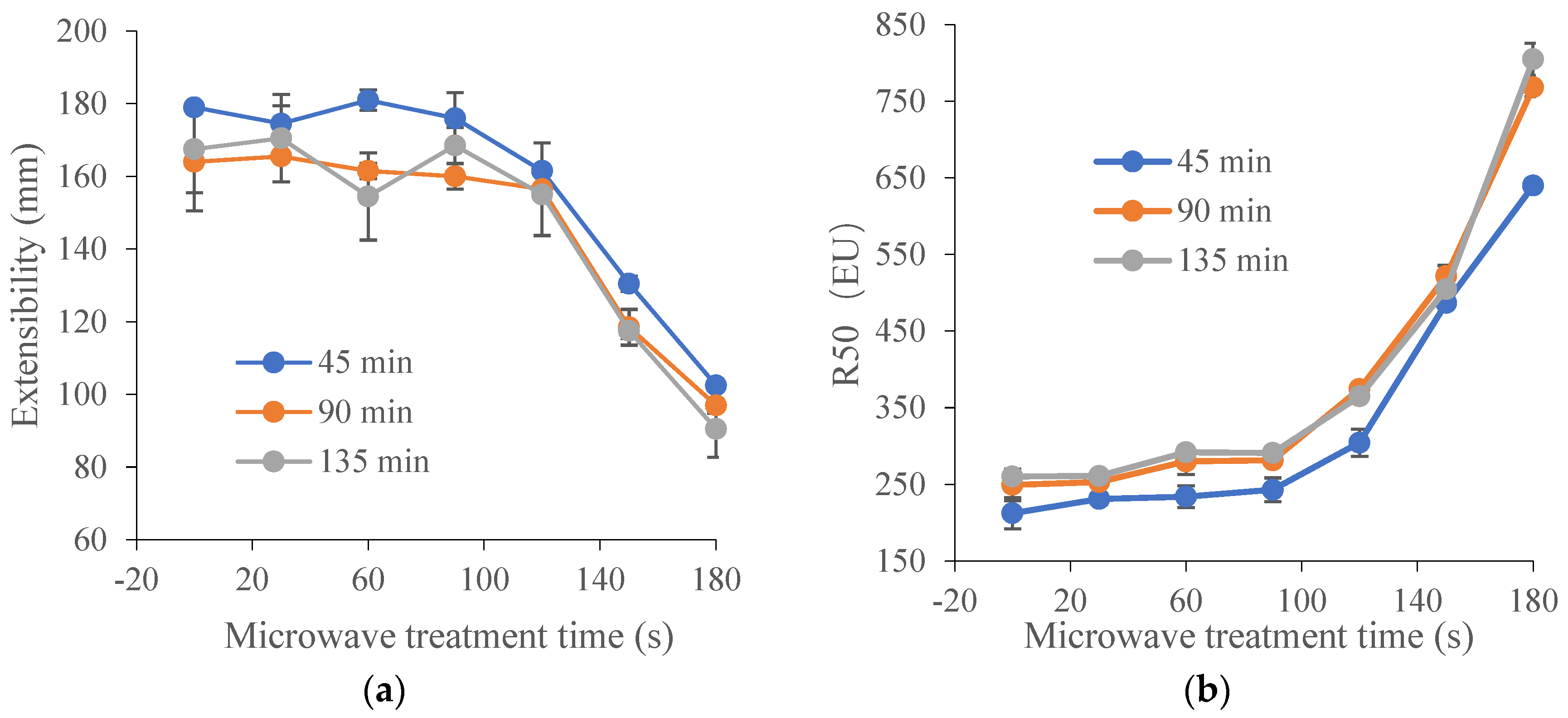
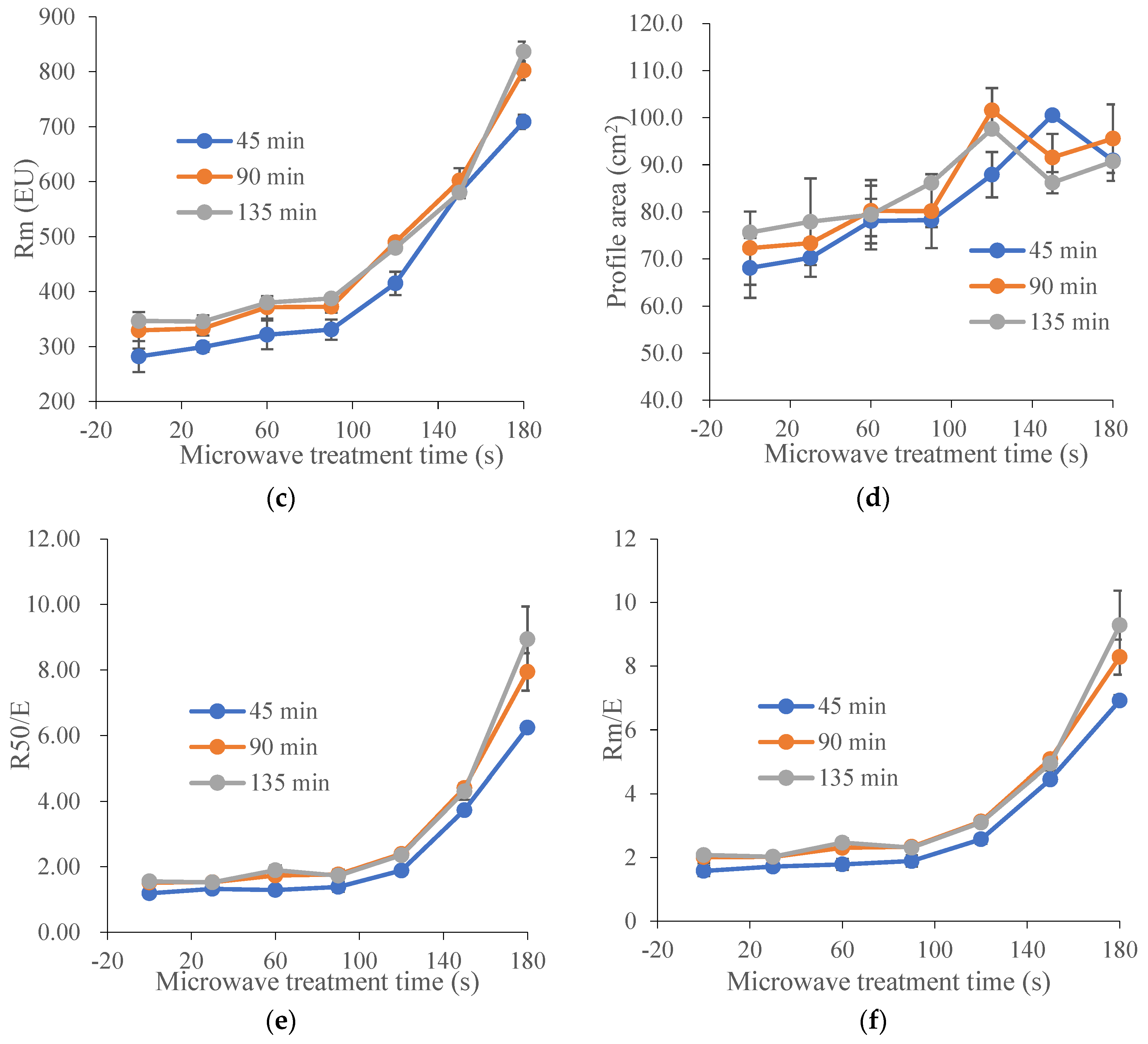
3.2. Effect of Microwave Treatment on Dough Stretching Characteristics, Dough Mixing Properties and Gluten Quality of Sprouted Wheat
3.3. Effect of Microwave Treatment on Protein Solubility
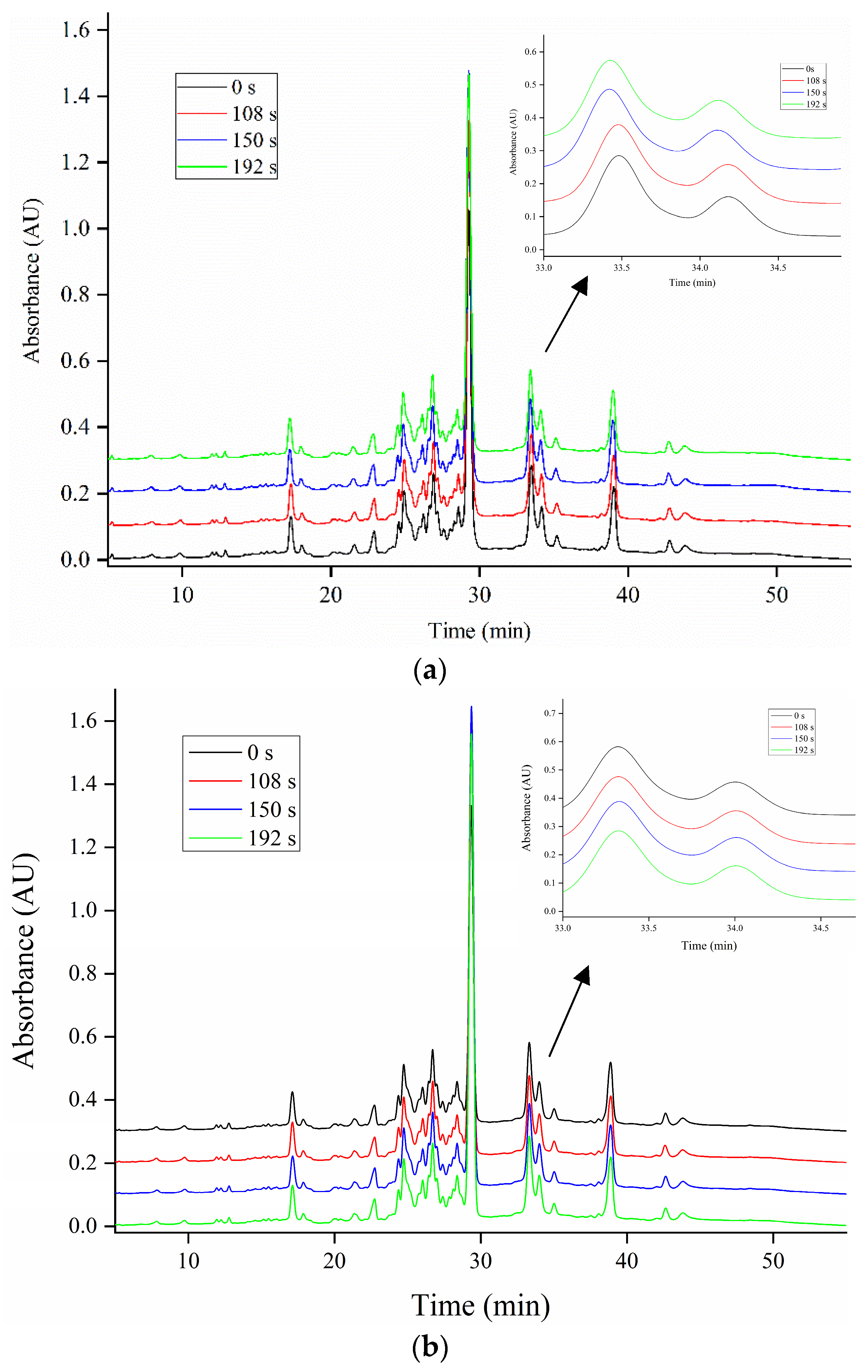
3.4. Analysis of SE-HPLC (Size-Exclusion High-Performance Liquid Chromatography)
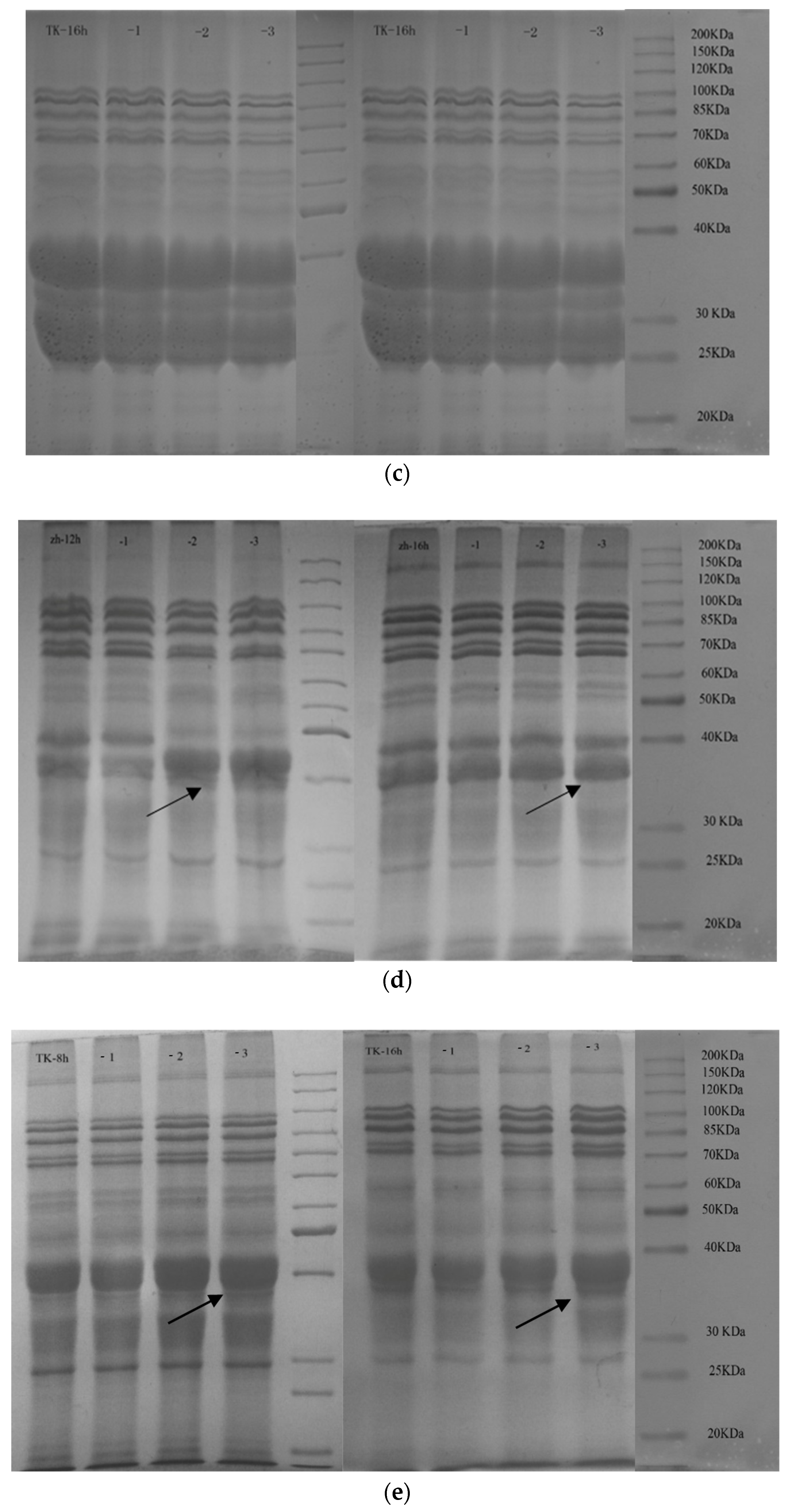
3.5. Analysis of SDS-PAGE (Sodium Dodecyl Sulfate–Polyacrylamide Gel Electrophoresis)
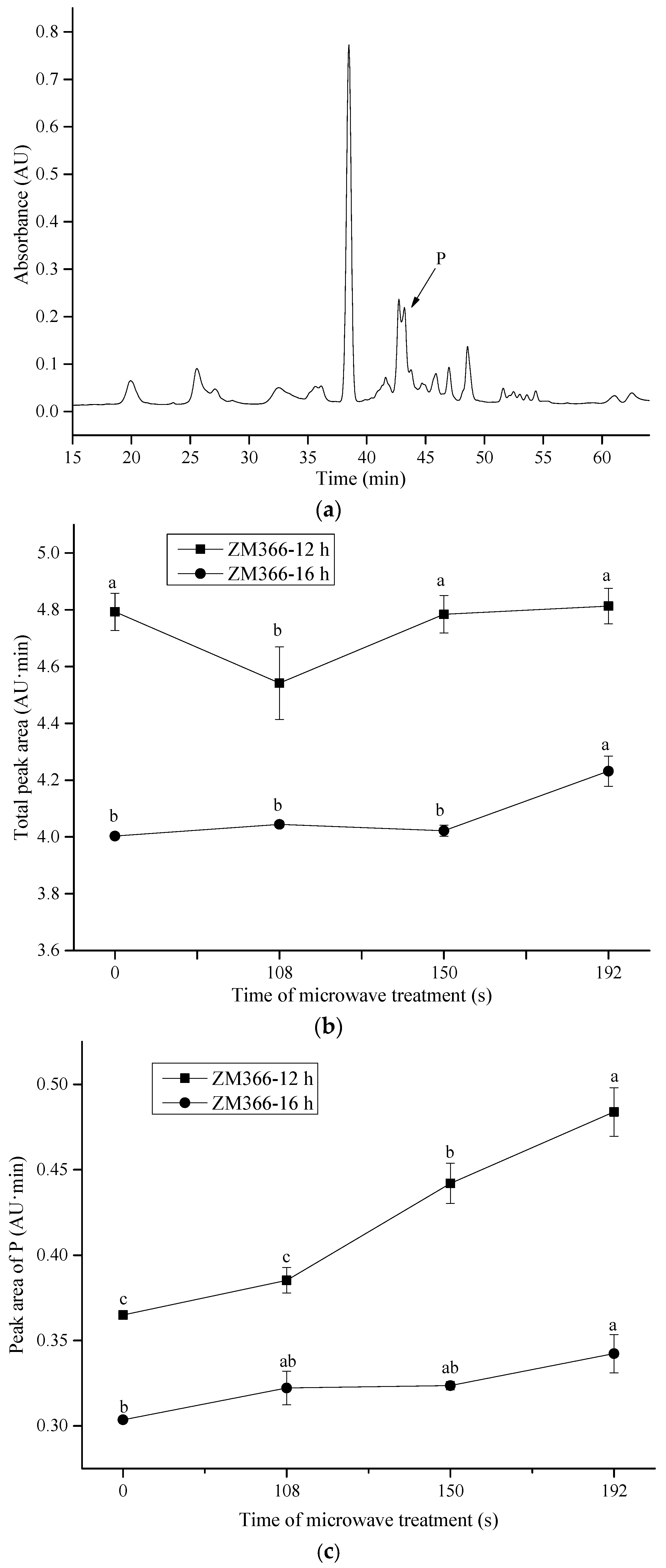
3.6. Analysis of RP-HPLC (Reversed-Phase High-Performance Liquid Chromatography)
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Olaerts, H.; Roye, C.; Derde, L.J.; Sinnaeve, G.; Meza, W.R.; Bodson, B.; Courtin, C.M. Evolution and Distribution of Hydrolytic Enzyme Activities during Preharvest Sprouting of Wheat (Triticum aestivum) in the Field. J. Agric. Food Chem. 2016, 64, 5644–5652. [Google Scholar] [CrossRef]
- Muralikrishna, G.; Nirmala, M. Cereal α-amylases—An overview. Carbohydr. Polym. 2005, 60, 163–173. [Google Scholar]
- Finney, K.F.; Natsuaki, O.; Bolte, L.C.; Mathewson, P.R.; Pomeranz, Y. Alpha-amylase in field-sprouted wheats: Its distribution and effect on Japanese-type sponge cake and related physical and chemical tests. Cereal Chem. 1981, 58, 355–359. [Google Scholar]
- Corder, A.M.; Henry, R.J. Carbohydrate-degrading enzymes in germinating wheat. Cereal Chem. 1989, 66, 435–439. [Google Scholar]
- Chemists, A.A.O.C. Effects of Amylases and Metals on the Pasting Properties of Wheat Flour, Determined by the Amylograph and by Hagberg’s Falling-Number Method. Cereal Chem. 1970, 47, 483–491. [Google Scholar]
- Singh, N.; Sekhon, K.S.; Nagi, H.P.S. Laboratory Sprout Damage and Effect of Heat Treatment on Milling and Baking Properties of Indian Wheats. J. Food Sci. 1987, 52, 176–179. [Google Scholar]
- Strandberg, T.; Kervinen, R.; Linko, P. Extrusion cooking of sprout-damaged wheat. J. Cereal Sci. 1988, 8, 111–123. [Google Scholar]
- Kaasova, J.; Hubackova, B.; Kadlec, P.; Prihoda, J.; Bubnik, Z. Chemical and biochemical changes during microwave treatment of wheat. Czech J. Food Sci. 2002, 20, 74–78. [Google Scholar]
- Sumnu, G. A review on microwave baking of foods. Int. J. Food Sci. Technol. 2008, 36, 117–127. [Google Scholar]
- Hemis, M.; Choudhary, R.; Watson, D.G. The Effect of Microwave Drying Parameters on the Germination of Wheat Seeds; American Society of Agricultural and Biological Engineers: St. Joseph, MI, USA, 2012. [Google Scholar]
- Wieser, H. Chemistry of gluten proteins. Food Microbiol. 2007, 24, 115–119. [Google Scholar] [CrossRef]
- Preston, K.; Kruger, J. Location and activity of proteolytic enzymes in developing wheat kernel. Can. J. Plant Sci. 1976, 56, 217–223. [Google Scholar]
- Barbeau, W.E.; Griffey, C.A.; Yan, Z. Evidence that Minor Sprout Damage Can Lead to Significant Reductions in Gluten Strength of Winter Wheats. Cereal Chem. 2006, 83, 306–310. [Google Scholar] [CrossRef]
- Redman, D.G. Softening of gluten by wheat proteases. J. Sci. Food Agric. 1971, 22, 75–78. [Google Scholar]
- Gao, K.; Liu, Y.X.; Tan, B.; Tian, X.H.; Zhang, D.Q.; Wang, L.P. An insight into the rheology and texture assessment: The influence of sprouting treatment on the whole wheat flour. Food Hydrocoll. 2022, 125, 107248. [Google Scholar]
- Suliburska, J. The Influence of Pre-harvest Sprouting Grains on the Breadmaking Wheat, Rye, and Triticale Flour. Acta Sci. Pol. Technol. Aliment. 2004, 3, 127–136. [Google Scholar]
- Hara, T.; Sasaki, T.; Tetsuka, T.; Ikoma, H.; Kohyama, K. Effects of Sprouting on Texture of Cooked Buckwheat (Fagopyrum esculentum Moench) Noodles. Plant Prod. Sci. 2015, 12, 492–496. [Google Scholar]
- Fub, X.; Hatcherd, W.; Schlichting, L. Effects of sprout damage on durum wheat milling and pasta processing quality. Can. J. Plant Sci. 2014, 94, 545–553. [Google Scholar]
- Shutov, A.D.; Beltei, N.K.; Vaintraub, I.A. A cysteine proteinase from germinating wheat seeds: Partial purification and hydrolysis of gluten. Biochemistry 1984, 49, 1004–1010. [Google Scholar]
- Dominguez, F.; Cejudo, F.J. Pattern of endoproteolysis following wheat grain germination. Physiol. Plant. 1995, 95, 253–259. [Google Scholar]
- Guo, J.; Qi, X.; Liu, Y.; Guan, E.; Wen, J.; Bian, K. Structure-activity relationship between gluten and dough quality of sprouted wheat flour based on air classification-induced component recombination. J. Sci. Food Agric. 2023, 103, 6905–6911. [Google Scholar]
- Hwang, P.; Bushuk, W. Some changes in the endosperm proteins during sprouting of wheat. Cereal Chem. 1973, 50, 147–160. [Google Scholar]
- Capocchi, A.; Cinollo, M.; Luciano Galleschi, A.; Saviozzi, F.; And, L.C.; Pinzino, C.; Zandomeneghi, M. Degradation of Gluten by Proteases from Dry and Germinating Wheat (Triticum durum) Seeds: An in Vitro Approach to Storage Protein Mobilization. J. Agric. Food Chem. 2000, 48, 6271–6279. [Google Scholar] [PubMed]
- Fahmy, A.S.; Ali, A.A.; Mohamed, S.A. Characterization of a cysteine protease from wheat Triticum aestivum (cv. Giza 164). Bioresour. Technol. 2004, 91, 297–304. [Google Scholar] [CrossRef] [PubMed]
- Harun, D. Effect of microwaves on technological and rheological properties of suni-bug (Eurygaster spp.) damaged and undamaged wheat flour. Food Sci. Technol. Res. 2010, 16, 313–318. [Google Scholar]
- Lagrain, B.; Thewissen, B.G.; Brijs, K.; Delcour, J.A. Impact of redox agents on the extractability of gluten proteins during bread making. J. Agric. Food Chem. 2007, 55, 5320–5325. [Google Scholar] [PubMed]
- Wang, X.-Y.; Guo, X.-N.; Zhu, K.-X. Polymerization of wheat gluten and the changes of glutenin macropolymer (GMP) during the production of Chinese steamed bread. Food Chem. 2016, 201, 275–283. [Google Scholar] [CrossRef] [PubMed]
- Fischer, T. Effect of extrusion cooking on protein modification in wheat flour. Eur. Food Res. Technol. 2004, 218, 128–132. [Google Scholar] [CrossRef]
- Wagner, M.; Morel, M.H.; Bonicel, J.; Cuq, B. Mechanisms of heat-mediated aggregation of wheat gluten protein upon pasta processing. J. Agric. Food Chem. 2011, 59, 3146–3154. [Google Scholar] [CrossRef]
- Pareyt, B.; Bruneel, C.; Brijs, K.; Goesaert, H.; Delcour, J.A. Flour sodium dodecyl sulfate (SDS)-extractable protein level as a cookie flour quality indicator. J. Agric. Food Chem. 2010, 58, 353–360. [Google Scholar] [CrossRef]
- Lagrain, B.; Brijs, K.; Veraverbeke, W.S.; Delcour, J.A. The impact of heating and cooling on the physico-chemical properties of wheat gluten–water suspensions. J. Cereal Sci. 2005, 42, 327–333. [Google Scholar] [CrossRef]
- Lagrain, B.; Rombouts, I.; Brijs, K.; Delcour, J.A. Kinetics of heat-induced polymerization of gliadin. J. Agric. Food Chem. 2011, 59, 2034–2039. [Google Scholar] [CrossRef] [PubMed]
- Lagrain, B.; Thewissen, B.G.; Brijs, K.; Delcour, J.A. Mechanism of gliadin–glutenin cross-linking during hydrothermal treatment. Food Chem. 2008, 107, 753–760. [Google Scholar] [CrossRef]
- Lagrain, B.; Brijs, K.; Delcour, J.A. Reaction kinetics of gliadin− glutenin cross-linking in model systems and in bread making. J. Agric. Food Chem. 2008, 56, 10660–10666. [Google Scholar] [PubMed]
- Rombouts, I.; Lagrain, B.; Delcour, J.A. Heat-induced cross-linking and degradation of wheat gluten, serum albumin, and mixtures thereof. J. Agric. Food Chem. 2012, 60, 10133–10140. [Google Scholar] [CrossRef] [PubMed]
- Domenek, S.; Morel, M.; Joëlle Bonicel, A.; Guilbert, S. Polymerization Kinetics of Wheat Gluten upon Thermosetting. A Mechanistic Model. J. Agric. Food Chem. 2002, 50, 5947–5954. [Google Scholar] [PubMed]
- Walde, S.G.; Balaswamy, K.; Velu, V.; Rao, D.G. Microwave drying and grinding characteristics of wheat (Triticum aestivum). J. Food Eng. 2002, 55, 271–276. [Google Scholar]
- de Pomerai, D.I.; Smith, B.; Dawe, A.; North, K.; Smith, T.; Archer, D.B.; Duce, I.R.; Jones, D.; Candido, E.P.M. Microwave radiation can alter protein conformation without bulk heating. FEBS Lett. 2003, 543, 93–97. [Google Scholar] [CrossRef] [PubMed]
- Singh, H.; Singh, N.; Kaur, L.; Saxena, S. Effect of sprouting conditions on functional and dynamic rheological properties of wheat. J. Food Eng. 2001, 47, 23–29. [Google Scholar]
- Rani, K.U.; Prasada Rao, U.J.S.; Leelavathi, K.; Haridas Rao, P. Distribution of Enzymes in Wheat Flour Mill Streams. J. Cereal Sci. 2001, 34, 233–242. [Google Scholar] [CrossRef]
- Yang, K.; Yue, Q.; Kong, J.; Zhao, P.; Gao, Y.; Fu, K.; Gao, B. Microbial diversity in combined UAF–UBAF system with novel sludge and coal cinder ceramic fillers for tetracycline wastewater treatment. Chem. Eng. J. 2016, 285, 319–330. [Google Scholar] [CrossRef]
- Osborne, T.B. The proteins of the Wheat Kernel; Carnegie Institution of Washington Publications: Washington, DC, USA, 1907; Volume 84, pp. 1–119. [Google Scholar]
- Bean, S.R.; Lyne, R.K.; Tilley, K.A.; Chung, O.K.; Lookhart, G.L. A rapid method for quantitation of insoluble polymeric proteins in flour. Cereal Chem. 1998, 75, 374–379. [Google Scholar]
- Morel, M.H.; Dehlon, P.; Autran, J.C.; Leygue, J.P.; Barl’Helgouac’H, C. Effects of temperature, sonication time, and power settings on size distribution and extractability of total wheat flour proteins as determined by size-exclusion high-performance liquid chromatography. Cereal Chem. 2000, 77, 685–691. [Google Scholar]
- Bottari, A.; Capocchi, A.; Fontanini, D.; Galleschi, L. Major proteinase hydrolysing gliadin during wheat germination. Phytochemistry 1996, 43, 39–44. [Google Scholar]
- Gänzle, M.G.; Loponen, J.; Gobbetti, M. Proteolysis in sourdough fermentations: Mechanisms and potential for improved bread quality. Trends Food Sci. Technol. 2008, 19, 513–521. [Google Scholar] [CrossRef]
- Bigiarini, L.; Pieri, N.; Grilli, I.; Galleschi, L.; Capocchi, A.; Fontanini, D. Hydrolysis of Gliadin during Germination of Wheat Seeds. J. Plant Physiol. 1995, 147, 161–167. [Google Scholar] [CrossRef]
- Mohammed, I.; Ahmed, A.R.; Senge, B. Dough rheology and bread quality of wheat–chickpea flour blends. Ind. Crops Prod. 2012, 36, 196–202. [Google Scholar] [CrossRef]
- Miś, A.; Dziki, D. Extensograph curve profile model used for characterising the impact of dietary fibre on wheat dough. J. Cereal Sci. 2013, 57, 471–479. [Google Scholar] [CrossRef]
- Li, M.; Sun, Q.J.; Zhu, K.X. Delineating the quality and component changes of whole-wheat flour and storage stability of fresh noodles induced by microwave treatment. LWT Food Sci. Technol. 2017, 84, 378–384. [Google Scholar]
- Sharma, B.; Gujral, H.S. Characterization of thermo-mechanical behavior of dough and starch digestibility profile of minor millet flat breads. J. Cereal Sci. 2019, 90, 102842. [Google Scholar]
- Rosell, C.M.; Collar, C.; Haros, M. Assessment of hydrocolloid effects on the thermo-mechanical properties of wheat using the Mixolab. Food Hydrocoll. 2007, 21, 452–462. [Google Scholar] [CrossRef]
- Han, L.; Zhou, Y.; Tatsumi, E.; Shen, Q.; Cheng, Y.; Li, L. Thermomechanical Properties of Dough and Quality of Noodles Made from Wheat Flour Supplemented with Different Grades of Tartary Buckwheat (Fagopyrum tataricum Gaertn.) Flour. Food Bioprocess Technol. 2012, 6, 1953–1962. [Google Scholar] [CrossRef]
- Yalcin, E.; Sakiyan, O.; Sumnu, G.; Celik, S.; Koksel, H. Functional properties of microwave-treated wheat gluten. Eur. Food Res. Technol. 2008, 227, 1411–1417. [Google Scholar] [CrossRef]
- Chakraborty, K.; Khan, K. Biochemical and breadmaking properties of wheat protein components. II. Reconstitution baking studies of protein fractions from various isolation procedures. Cereal Chem. 1988, 65, 340–344. [Google Scholar]
- Wang, P.; Chen, H.; Mohanad, B.; Xu, L.; Ning, Y.; Xu, J.; Wu, F.; Yang, N.; Jin, Z.; Xu, X. Effect of frozen storage on physico-chemistry of wheat gluten proteins: Studies on gluten-, glutenin- and gliadin-rich fractions. Food Hydrocoll. 2014, 39, 187–194. [Google Scholar] [CrossRef]
- Bruneel, C.; Pareyt, B.; Brijs, K.; Delcour, J.A. The impact of the protein network on the pasting and cooking properties of dry pasta products. Food Chem. 2010, 120, 371–378. [Google Scholar] [CrossRef]
- Singh, H.; MacRitchie, F. Changes in proteins induced by heating gluten dispersions at high temperature. J. Cereal Sci. 2004, 39, 297–301. [Google Scholar] [CrossRef]
- Singh, N.K.; Donovan, G.R.; Batey, I.L.; Macritchie, F. Use of sonication and size-exclusion high-performance liquid chromatography in the study of wheat flour proteins. I. Dissolution of total proteins in the absence of reducing agents. Jew. Q. Rev. 1990, 70, 96–116. [Google Scholar]
- Hayta, M.; Schofield, J.D. Heat and additive induced biochemical transitions in gluten from good and poor breadmaking quality wheats. J. Cereal Sci. 2004, 40, 245–256. [Google Scholar] [CrossRef]
- Náthia-Neves, G.; Calix-Rivera, C.S.; Villanueva, M.; Ronda, F. Microwave radiation induces modifications in the protein fractions of tef flours and modulates their derived techno-functional properties. Int. J. Biol. Macromol. 2023, 253, 126908. [Google Scholar] [CrossRef]
- Tosi, P.; Masci, S.; Giovangrossi, A.; D’Ovidio, R.; Bekes, F.; Larroque, O.; Napier, J.; Shewry, P. Modification of the Low Molecular Weight (LMW) Glutenin Composition of Transgenic Durum Wheat: Effects on Glutenin Polymer Size and Gluten Functionality. Mol. Breed. 2005, 16, 113–126. [Google Scholar] [CrossRef]
- Lamacchia, C.; Baiano, A.; Lamparelli, S.; Notte, E.L.; Luccia, A.D. Changes in durum wheat kernel and pasta proteins induced by toasting and drying processes. Food Chem. 2010, 118, 191–198. [Google Scholar] [CrossRef]
- Bruneel, C.; Lagrain, B.; Brijs, K.; Delcour, J.A. Redox agents and N-ethylmaleimide affect the extractability of gluten proteins during fresh pasta processing. Food Chem. 2011, 127, 905–911. [Google Scholar] [CrossRef] [PubMed]
- D’Ovidio, R.; Masci, S. The low-molecular-weight glutenin subunits of wheat gluten. J. Cereal Sci. 2004, 39, 321–339. [Google Scholar] [CrossRef]

| pH 7.5 | pH 4.4 | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ST | TK6# (u/g) | DR | ZM366 (u/g) | DR | TK6# (u/g) | DR | ZM366 (u/g) | DR | ||||
| (h) | SW A | SWMT B | (%) | SW A | SWMT B | (%) | SW A | SWMT B | (%) | SW A | SWMT B | (%) |
| 0 | 10.40 ± 4.01 d | - | - | 3.78 ± 1.78 c | - | - | 199.40 ± 1.34 g | - | - | 190.89 ± 0.89 f | - | - |
| 8 | 13.23 ± 2.67 d | 6.62 ± 1.34 b | 50.00 | - | - | - | 296.73 ± 0.00 f | 40.64 ± 1.34 e | 86.30 | - | - | - |
| 10 | - | - | - | 7.56 ± 1.78 c | 0 ± 0 b | 100 | - | - | - | 389.97 ± 2.67 e | - | - |
| 12 | 16.07 ± 1.34 cd | 6.62 ± 1.34 b | 58.81 | 8.19 ± 2.67 c | 0 ± 0 b | 100 | 348.71 ± 4.01 e | 100.17 ± 5.35 d | 71.27 | 415.17 ± 0.89 d | 42.84 ± 1.78 d | 89.68 |
| 16 | 18.90 ± 2.67 cd | 8.51 ± 1.34 b | 54.97 | 14.49 ± 0.89 b | 8.82 ± 1.78 a | 39.13 | 378.95 ± 1.34 d | 193.73 ± 1.34 c | 48.88 | 437.22 ± 5.35 c | 54.18 ± 1.78 c | 87.61 |
| 20 | 23.63 ± 6.68 bc | 10.40 ± 4.01 ab | 55.99 | 15.75 ± 0.89 ab | 9.45 ± 0.89 a | 40.00 | 440.37 ± 0.00 c | - | - | 456.12 ± 1.78 b | 124.11 ± 6.24 b | 72.79 |
| 24 | 30.24 ± 2.67 ab | 14.18 ± 1.34 a | 53.11 | 19.53 ± 2.67 a | 10.08 ± 0.00 a | 44.70 | 477.23 ± 4.01 b | 232.47 ± 8.02 b | 51.29 | 535.51 ± 3.56 a | 363.51 ± 0.89 a | 32.12 |
| 48 | 35.91 ± 2.67 a | 15.15 ± 0.00 a | 57.81 | - | - | - | 493.29 ± 5.35 a | 433.76 ± 1.34 a | 12.07 | - | - | - |
| pH 7.5 | pH 4.4 | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| MTT | STWK | TK6#, 8 h | TK6#, 16 h | ZM366, 12 h | ZM366, 16 h | TK6#, 8 h | TK6#, 16 h | ZM366, 12 h | ZM366, 16 h |
| (s) | (°C) | (u/g) | (u/g) | (u/g) | (u/g) | (u/g) | (u/g) | (u/g) | (u/g) |
| 0 | - | 13.23 ± 2.67 a | 18.90 ± 2.67 a | 8.19 ± 2.67 a | 14.49 ± 0.89 a | 296.73 ± 0.00 a | 378.95 ± 1.34 a | 415.17 ± 0.89 a | 437.22 ± 5.35 a |
| 108 | 65 c | 13.23 ± 0.00 a | 17.96 ± 1.34 a | 8.19 ± 0.89 a | 14.49 ± 2.67 a | 243.81 ± 2.67 b | 286.34 ± 1.34 b | 301.77 ± 0.89 b | 268.38 ± 1.78 b |
| 150 | 75 b | 7.56 ± 2.67 b | 8.5 ± 1.34 b | 0 ± 0 b | 10.08 ± 1.78 a | 18.90 ± 2.67 c | 106.79 ± 6.68 c | 44.73 ± 0.89 c | 112.14 ± 0.00 c |
| 192 | 85 a | 0 ± 0 c | 0 ± 0 c | 0 ± 0 b | 0 ± 0 b | 0 ± 0 d | 0 ± 0 d | 0 ± 0 d | 0 ± 0 d |
| Samples | MTT | WA | Stability | C2 | WG | GI |
|---|---|---|---|---|---|---|
| (s) | (%) | (min) | (Nm) | (%) | (%) | |
| TK6#, 8 h | 0 | 51.20 ± 0.14 a | 3.99 ± 0.09 c | 0.33 ± 0.00 d | 29.00 ± 0.14 a | 54.00 ± 1.41 c |
| 108 | 50.80 ± 0.00 b | 5.35 ± 0.45 b | 0.36 ± 0.01 c | 28.66 ± 0.07 a | 53.50 ± 0.71 c | |
| 150 | 50.10 ± 0.00 c | 8.69 ± 0.44 a | 0.41 ± 0.01 b | 25.57 ± 0.00 b | 74.50 ± 0.71 b | |
| 192 | 50.05 ± 0.07 c | 4.70 ± 0.61 bc | 0.46 ± 0.01 a | 19.00 ± 0.28 c | 98.50 ± 0.71 a | |
| TK6#, 16 h | 0 | 51.85 ± 0.07 a | 3.58 ± 0.08 b | 0.28 ± 0.00 c | 28.90 ± 0.14 a | 43.50 ± 0.71 c |
| 108 | 50.50 ± 0.00 b | 3.77 ± 0.23 b | 0.29 ± 0.00 c | 28.90 ± 0.14 a | 44.50 ± 0.71 c | |
| 150 | 50.45 ± 0.07 b | 6.13 ± 0.21 a | 0.34 ± 0.01 b | 25.53 ± 0.14 b | 62.5 ± 2.12 b | |
| 192 | 50.05 ± 0.07 c | 3.34 ± 0.45 b | 0.39 ± 0.01 a | 18.50 ± 0.14 c | 89.50 ± 0.71 a | |
| ZM366, 12 h | 0 | 61.70 ± 0.57 a | 7.26 ± 0.32 d | 0.34 ± 0.01 c | 32.61 ± 0.29 a | 84.45 ± 4.97 c |
| 108 | 59.60 ± 0.00 b | 8.56 ± 0.08 c | 0.41 ± 0.00 b | 32.10 ± 0.07 a | 91.21 ± 0.91 b | |
| 150 | 58.75 ± 0.64 bc | 10.06 ± 0.13 b | 0.45 ± 0.03 b | 28.80 ± 0.36 b | 98.78 ± 0.23 a | |
| 192 | 57.60 ± 0.00 c | 10.88 ± 0.18 a | 0.54 ± 0.00 a | 22.30 ± 0.14 c | 100.00 ± 0.00 a | |
| ZM366, 16 h | 0 | 60.90 ± 0.00 a | 5.79 ± 0.02 d | 0.28 ± 0.01 c | 33.23 ± 0.00 a | 90.40 ± 3.66 c |
| 108 | 59.00 ± 0.00 b | 7.09 ± 0.05 b | 0.31 ± 0.02 c | 32.33 ± 0.29 b | 92.81 ± 0.38 bc | |
| 150 | 58.30 ± 0.14 c | 9.54 ± 0.05 a | 0.35 ± 0.01 b | 29.22 ± 0.36 c | 97.07 ± 1.19 ab | |
| 192 | 58.10 ± 0.00 d | 6.02 ± 0.10 c | 0.41 ± 0.01 a | 22.15 ± 0.07 d | 100.00 ± 0.00 a |
| Samples | MTT | SSP | ASP | AASG | AAIG |
|---|---|---|---|---|---|
| (s) | (%) | (%) | (%) | (%) | |
| TK6#, 8 h | 0 | 23.14 ± 0.03 b | 36.63 ± 0.15 a | 6.75 ± 0.19 b | 33.49 ± 0.14 d |
| 108 | 23.66 ± 0.05 a | 35.56 ± 0.11 b | 4.03 ± 0.05 d | 36.75 ± 0.12 c | |
| 150 | 23.69 ± 0.15 a | 33.24 ± 0.33 c | 5.45 ± 0.16 c | 37.61 ± 0.01 b | |
| 192 | 19.93 ± 0.01 c | 30.89 ± 0.01 d | 7.64 ± 0.16 a | 41.54 ± 0.17 a | |
| TK6#, 16 h | 0 | 23.16 ± 0.16 a | 37.23 ± 0.06 a | 6.71 ± 0.25 b | 32.90 ± 0.12 d |
| 108 | 21.95 ± 0.13 b | 35.25 ± 0.16 b | 7.67 ± 0.22 a | 35.13 ± 0.07 c | |
| 150 | 21.43 ± 0.08 c | 33.96 ± 0.01 c | 6.53 ± 0.05 b | 38.08 ± 0.13 b | |
| 192 | 19.20 ± 0.22 d | 33.46 ± 0.17 d | 7.75 ± 0.31 a | 39.60 ± 0.19 a | |
| ZM366, 12 h | 0 | 23.46 ± 0.18 b | 36.20 ± 0.11 b | 6.42 ± 0.22 a | 33.92 ± 0.33 d |
| 108 | 24.53 ± 0.26 a | 34.37 ± 0.45 d | 5.86 ± 0.24 b | 35.24 ± 0.17 c | |
| 150 | 21.03 ± 0.05 c | 35.56 ± 0.98 c | 5.09 ± 0.26 c | 38.31 ± 0.76 b | |
| 192 | 19.25 ± 0.11 d | 36.55 ± 0.17 a | 5.09 ± 0.35 c | 39.11 ± 0.15 a | |
| ZM366, 16 h | 0 | 23.56 ± 0.10 a | 36.26 ± 0.14 a | 6.46 ± 0.33 ab | 33.72 ± 0.23 c |
| 108 | 20.08 ± 0.18 b | 34.76 ± 0.24 b | 6.73 ± 0.19 a | 38.43 ± 0.11 b | |
| 150 | 20.09 ± 0.20 b | 33.59 ± 0.18 c | 6.51 ± 0.24 ab | 39.82 ± 0.19 a | |
| 192 | 19.76 ± 0.27 c | 33.90 ± 0.18 c | 6.22 ± 0.32 b | 40.12 ± 0.32 a |
| Traditional Extraction | Ultrasonic-Assisted Extraction | ||||||
|---|---|---|---|---|---|---|---|
| Samples | MTT | Glutenin | Gliadin | Gluten | Glutenin | Gliadin | Gluten |
| (s) | (AU·min) | (AU·min) | (AU·min) | (AU·min) | (AU·min) | (AU·min) | |
| TK6#, 8 h | 0 | 0.6830 ± 0.0014 a | 1.5652 ± 0.0008 a | 2.2482 ± 0.0022 a | 1.0195 ± 0.0089 a | 1.7392 ± 0.0033 a | 2.7587 ± 0.0057 a |
| 108 | 0.6425 ± 0.0001 b | 1.5420 ± 0.0038 b | 2.1845 ± 0.0038 b | 0.9378 ± 0.0167 b | 1.6806 ± 0.0382 b | 2.6184 ± 0.0549 b | |
| 150 | 0.5447 ± 0.0003 c | 1.5146 ± 0.0009 c | 2.0593 ± 0.0012 c | 0.8804 ± 0.0043 c | 1.6798 ± 0.0156 b | 2.5601 ± 0.0199 bc | |
| 192 | 0.4326 ± 0.0020 d | 1.4466 ± 0.0020 d | 1.8792 ± 0.0040 d | 0.8521 ± 0.0059 d | 1.6697 ± 0.0001 b | 2.5218 ± 0.0059 c | |
| TK6#, 16 h | 0 | 0.7069 ± 0.0063 a | 1.5103 ± 0.0046 a | 2.2172 ± 0.0016 a | 1.0822 ± 0.0238 a | 1.7134 ± 0.0156 a | 2.7956 ± 0.0394 a |
| 108 | 0.6914 ± 0.0031 b | 1.5039 ± 0.0066 a | 2.1953 ± 0.0097 b | 1.0618 ± 0.0124 a | 1.6910 ± 0.0090 a | 2.7527 ± 0.0214 a | |
| 150 | 0.5846 ± 0.0015 c | 1.4812 ± 0.0009 b | 2.0658 ± 0.0023 c | 0.9556 ± 0.0052 b | 1.6500 ± 0.0009 b | 2.6056 ± 0.0043 b | |
| 192 | 0.4573 ± 0.0030 d | 1.4113 ± 0.0019 c | 1.8686 ± 0.0049 d | 0.9267 ± 0.0050 b | 1.6515 ± 0.0030 b | 2.5781 ± 0.0081 b | |
| ZM366, 12 h | 0 | 0.6441 ± 0.0006 a | 1.8249 ± 0.0033 a | 2.4690 ± 0.0039 a | 1.0908 ± 0.0076 b | 1.7134 ± 0.0156 a | 2.8042 ± 0.0080 a |
| 108 | 0.6254 ± 0.0008 b | 1.8108 ± 0.0003 b | 2.4362 ± 0.0011 b | 1.1519 ± 0.0081 a | 1.6910 ± 0.0090 a | 2.8429 ± 0.0170 b | |
| 150 | 0.5565 ± 0.0004 c | 1.7941 ± 0.0006 c | 2.3506 ± 0.0010 c | 1.0542 ± 0.0021 c | 1.6950 ± 0.0062 a | 2.7492 ± 0.0083 c | |
| 192 | 0.4719 ± 0.0036 d | 1.7376 ± 0.0063 d | 2.2095 ± 0.0099 d | 0.9411 ± 0.0046 d | 1.6515 ± 0.0030 b | 2.5925 ± 0.0076 d | |
| ZM366, 16 h | 0 | 0.6596 ± 0.0012 a | 1.7994 ± 0.0017 a | 2.4590 ± 0.0029 a | 1.1888 ± 0.0152 a | 1.9616 ± 0.0024 a | 3.1504 ± 0.0128 ab |
| 108 | 0.6408 ± 0.0002 b | 1.7979 ± 0.0003 a | 2.4387 ± 0.0005 b | 1.1777 ± 0.0186 a | 1.9854 ± 0.0021 a | 3.1630 ± 0.0399 a | |
| 150 | 0.5706 ± 0.0012 c | 1.7751 ± 0.0052 b | 2.3457 ± 0.0064 c | 1.1408 ± 0.0030 b | 1.9620 ± 0.0069 a | 3.1028 ± 0.0100 ab | |
| 192 | 0.4964 ± 0.0004 d | 1.7438 ± 0.0001 c | 2.2402 ± 0.0003 d | 1.1246 ± 0.0107 b | 1.9759 ± 0.0134 a | 3.1005 ± 0.0026 b | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, X.; Zhao, M.; Shang, P.; Liu, J.; Zhao, R. Effect of Microwave Treatment on Protease Activity, Dough Properties and Protein Quality in Sprouted Wheat. Foods 2024, 13, 1277. https://doi.org/10.3390/foods13081277
Wang X, Zhao M, Shang P, Liu J, Zhao R. Effect of Microwave Treatment on Protease Activity, Dough Properties and Protein Quality in Sprouted Wheat. Foods. 2024; 13(8):1277. https://doi.org/10.3390/foods13081277
Chicago/Turabian StyleWang, Xiangyu, Mengyuan Zhao, Panpan Shang, Jing Liu, and Renyong Zhao. 2024. "Effect of Microwave Treatment on Protease Activity, Dough Properties and Protein Quality in Sprouted Wheat" Foods 13, no. 8: 1277. https://doi.org/10.3390/foods13081277