Detection and Analysis of VOCs in Cherry Tomato Based on GC-MS and GC×GC-TOF MS Techniques
Abstract
:1. Introduction
2. Materials and Methods
2.1. Test Material and Sampling Treatment
2.2. Sample Preparation and Treatment
2.3. Internal Standard Solution Preparation
2.4. HS-SPME-GC-MS Conditions
2.5. HS-SPME-GC×GC TOFMS Conditions
2.6. Data Processing and Analysis
2.7. Calculating rOAV
2.8. Statistical Analysis
3. Results
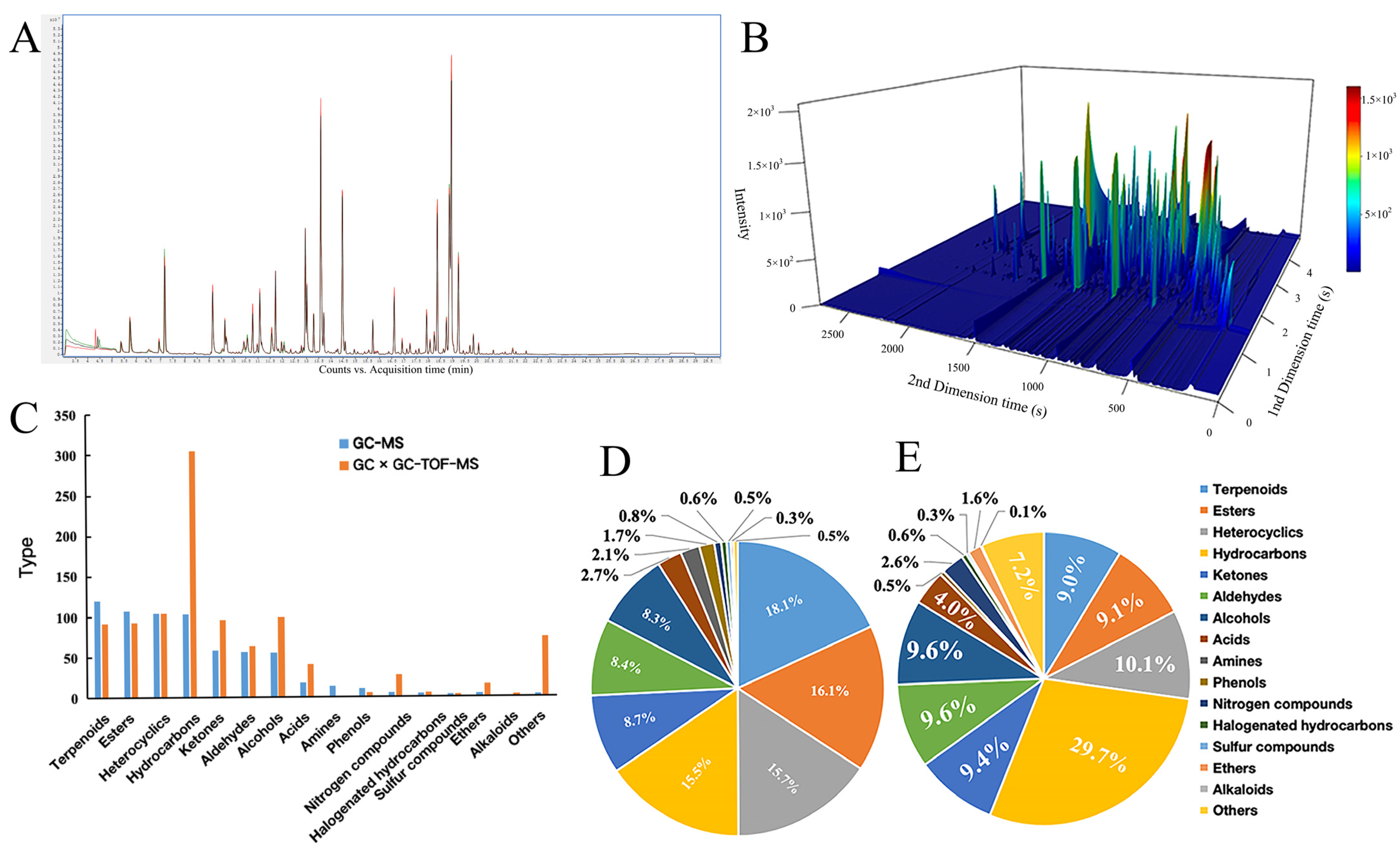
3.1. Comparative Analysis of VOCs Components in Cherry Tomato by Different Techniques
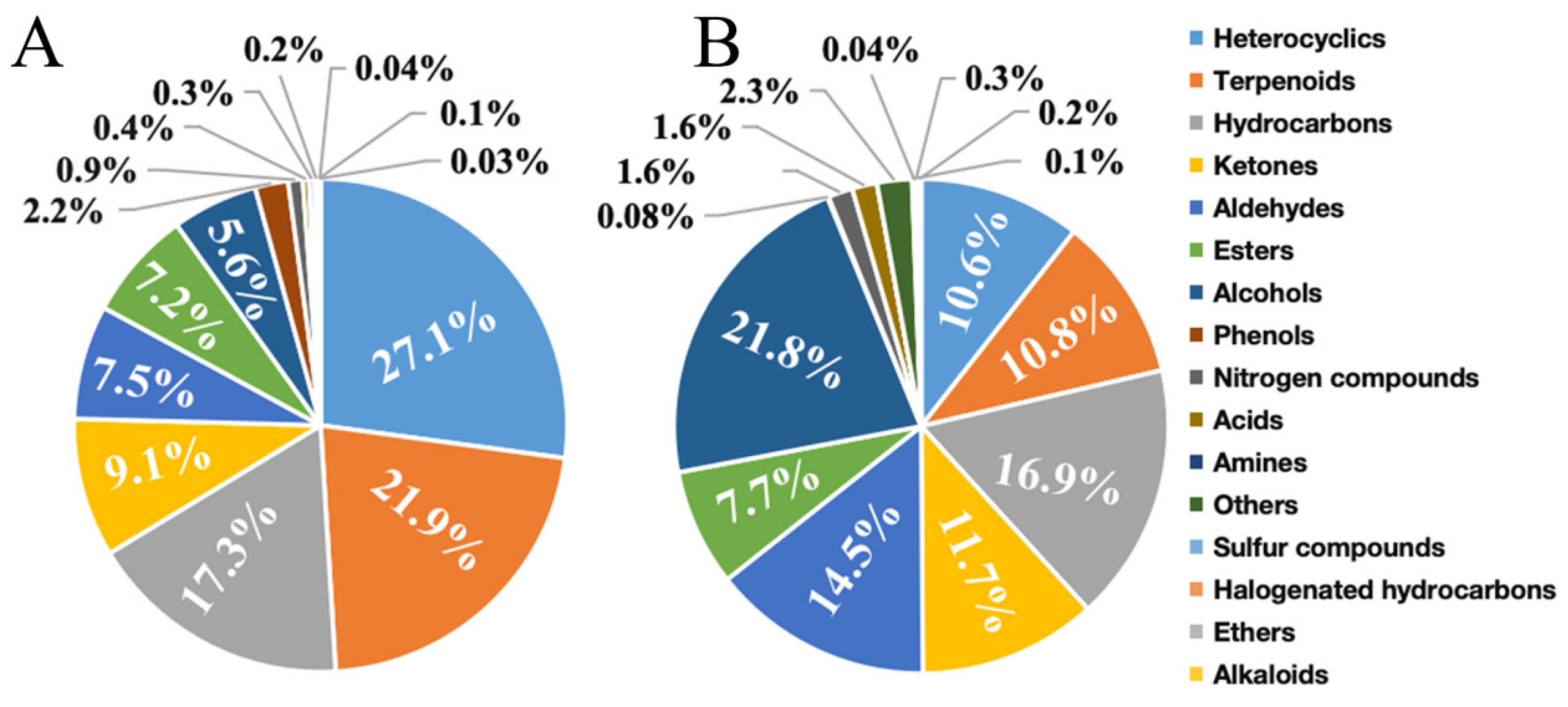
3.2. Comparative Analysis of VOC Content in Cherry Tomato
3.3. Analysis of VOC Components in Cherry Tomato
3.4. The Identification and Sensory Flavor Characterization of Crucial VOCs in Cherry Tomatoes
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Razifard, H.; Ramos, A.; Della Valle, A.L.; Bodary, C.; Goetz, E.; Manser, E.J.; Li, X.; Zhang, L.; Visa, S.; Tieman, D.; et al. Genomic Evidence for Complex Domestication History of the Cultivated Tomato in Latin America. Mol. Biol. Evol. 2020, 37, 1118–1132. [Google Scholar] [CrossRef] [PubMed]
- Zhu, G.; Wang, S.; Huang, Z.; Zhang, S.; Liao, Q.; Zhang, C.; Lin, T.; Qin, M.; Peng, M.; Yang, C.; et al. Rewiring of the Fruit Metabolome in Tomato Breeding. Cell 2018, 172, 249–261.e12. [Google Scholar] [CrossRef] [PubMed]
- Zeng, C.; Tan, P.; Liu, Z. Effect of Exogenous ARA Treatment for Improving Postharvest Quality in Cherry Tomato (Solanum lycopersicum L.) Fruits. Sci. Hortic. 2020, 261, 108959. [Google Scholar] [CrossRef]
- Zhang, J.; Zhao, J.; Xu, Y.; Liang, J.; Chang, P.; Yan, F.; Li, M.; Liang, Y.; Zou, Z. Genome-Wide Association Mapping for Tomato Volatiles Positively Contributing to Tomato Flavor. Front. Plant Sci. 2015, 6, 1042. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Sims, C.A.; Klee, H.J.; Sarnoski, P.J. Sensory and Flavor Characteristics of Tomato Juice from Garden Gem and Roma Tomatoes with Comparison to Commercial Tomato Juice. J. Food Sci. 2018, 83, 153–161. [Google Scholar] [CrossRef] [PubMed]
- Tieman, D.; Zhu, G.; Resende, M.F.R.; Lin, T.; Nguyen, C.; Bies, D.; Rambla, J.L.; Beltran, K.S.O.; Taylor, M.; Zhang, B.; et al. A Chemical Genetic Roadmap to Improved Tomato Flavor. Science 2017, 355, 391–394. [Google Scholar] [CrossRef] [PubMed]
- Casals, J.; Rivera, A.; Sabaté, J.; del Castillo, R.; Simó, J. Cherry and Fresh Market Tomatoes: Differences in Chemical, Morphological, and Sensory Traits and Their Implications for Consumer Acceptance. Agronomy 2019, 9, 9. [Google Scholar] [CrossRef]
- Lee, J.H.J.; Jayaprakasha, G.K.; Avila, C.A.; Crosby, K.M.; Patil, B.S. Metabolomic Studies of Volatiles from Tomatoes Grown in Net-House and Open-Field Conditions. Food Chem. 2019, 275, 282–291. [Google Scholar] [CrossRef] [PubMed]
- Tandon, K.S.; Baldwin, E.A.; Shewfelt, R.L. Aroma Perception of Individual Volatile Compounds in Fresh Tomatoes (Lycopersicon esculentum, Mill.) as Affected by the Medium of Evaluation. Postharvest Biol. Technol. 2000, 20, 261–268. [Google Scholar] [CrossRef]
- Engelberth, J.; Engelberth, M. Variability in the Capacity to Produce Damage-Induced Aldehyde Green Leaf Volatiles among Different Plant Species Provides Novel Insights into Biosynthetic Diversity. Plants 2020, 9, 213. [Google Scholar] [CrossRef]
- Aubert, C.; Chalot, G. Chemical Composition, Bioactive Compounds, and Volatiles of Six Table Grape Varieties (Vitis vinifera L.). Food Chem. 2018, 240, 524–533. [Google Scholar] [CrossRef] [PubMed]
- Yuan, H.; Cao, G.; Hou, X.; Huang, M.; Du, P.; Tan, T.; Zhang, Y.; Zhou, H.; Liu, X.; Liu, L.; et al. Development of a Widely Targeted Volatilomics Method for Profiling Volatilomes in Plants. Mol. Plant 2022, 15, 189–202. [Google Scholar] [CrossRef] [PubMed]
- Otify, A.M.; Ibrahim, R.M.; Abib, B.; Laub, A.; Wessjohann, L.A.; Jiang, Y.; Farag, M.A. Unveiling Metabolome Heterogeneity and New Chemicals in 7 Tomato Varieties via Multiplex Approach of UHPLC-MS/MS, GC–MS, and UV–Vis in Relation to Antioxidant Effects as Analyzed Using Molecular Networking and Chemometrics. Food Chem. 2023, 417, 135866. [Google Scholar] [CrossRef] [PubMed]
- López, S.M.Y.; Pastorino, G.N.; Balatti, P.A. Volatile Organic Compounds Profile Synthesized and Released by Endophytes of Tomato (Solanum lycopersici L.) and Their Antagonistic Role. Arch. Microbiol. 2021, 203, 1383–1397. [Google Scholar] [CrossRef] [PubMed]
- Cortina, P.R.; Santiago, A.N.; Sance, M.M.; Peralta, I.E.; Carrari, F.; Asis, R. Neuronal Network Analyses Reveal Novel Associations between Volatile Organic Compounds and Sensory Properties of Tomato Fruits. Metabolomics 2018, 14, 57. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Meng, F.; Miao, H.; Chen, S.; Yin, T.; Hu, S.; Shao, Z.; Liu, Y.; Gao, L.; Zhu, C.; et al. Effects of Postharvest Methyl Jasmonate Treatment on Main Health-Promoting Components and Volatile Organic Compounds in Cherry Tomato Fruits. Food Chem. 2018, 263, 194–200. [Google Scholar] [CrossRef] [PubMed]
- Dehimeche, N.; Buatois, B.; Bertin, N.; Staudt, M. Insights into the Intraspecific Variability of the above and Belowground Emissions of Volatile Organic Compounds in Tomato. Molecules 2021, 26, 237. [Google Scholar] [CrossRef] [PubMed]
- Distefano, M.; Mauro, R.P.; Page, D.; Giuffrida, F.; Bertin, N.; Leonardi, C. Aroma Volatiles in Tomato Fruits: The Role of Genetic, Preharvest and Postharvest Factors. Agronomy 2022, 12, 376. [Google Scholar] [CrossRef]
- Yue, C.; Cao, H.; Zhang, S.; Hao, Z.; Wu, Z.; Luo, L.; Zeng, L. Aroma Characteristics of Wuyi Rock Tea Prepared from 16 Different Tea Plant Varieties. Food Chem. X 2023, 17, 100586. [Google Scholar] [CrossRef]
- Deng, L.; Yang, X.; Qiu, Y.; Luo, J.; Wu, H.; Liu, X.; Zhao, G.; Zheng, X.; Li, J. Metabolic and Molecular Mechanisms Underlying the Foliar Zn Application Induced Increase of 2-Acetyl-1-Pyrroline Conferring the ‘Taro-like’ Aroma in Pumpkin Leaves. Front. Plant Sci. 2023, 14, 1127032. [Google Scholar] [CrossRef]
- Li, J.; Fu, Y.; Bao, X.; Li, H.; Zuo, J.; Zhang, M.; Wang, J. Comparison and Analysis of Tomato Flavor Compounds Using Different Extraction Methods. Food Meas. 2020, 14, 465–475. [Google Scholar] [CrossRef]
- Sun, Y.; Wu, Y.; Liu, B.; Chen, R.; Qiao, Y.; Zhang, Q.; Li, Q.; Wang, X.; Wang, Z. Analysis for Different Flavor Compounds in Mature Milk from Human and Livestock Animals by GC × GC-TOFMS. Food Chem. X 2023, 19, 100760. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Gao, M.; Liu, Z.; Chen, S.; Xu, Y. Three Extraction Methods in Combination with GC × GC-TOFMS for the Detailed Investigation of Volatiles in Chinese Herbaceous Aroma-Type Baijiu. Molecules 2020, 25, 4429. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Yang, K.; He, Z.; Zhao, D.; Zheng, J.; Qian, M.C. Comparison of Two Data Processing Approaches for Aroma Marker Identification in Different Distilled Liquors Using Comprehensive Two-Dimensional Gas Chromatography-Time-of-Flight Mass Spectrometry Dataset. J. Food Sci. 2023, 88, 2870–2881. [Google Scholar] [CrossRef] [PubMed]
- Xue, J.; Liu, P.; Yin, J.; Wang, W.; Zhang, J.; Wang, W.; Le, T.; Ni, D.; Jiang, H. Dynamic Changes in Volatile Compounds of Shaken Black Tea during Its Manufacture by GC × GC–TOFMS and Multivariate Data Analysis. Foods 2022, 11, 1228. [Google Scholar] [CrossRef] [PubMed]
- Aith Barbará, J.; Primieri Nicolli, K.; Souza-Silva, É.A.; Camarão Telles Biasoto, A.; Welke, J.E.; Alcaraz Zini, C. Volatile Profile and Aroma Potential of Tropical Syrah Wines Elaborated in Different Maturation and Maceration Times Using Comprehensive Two-Dimensional Gas Chromatography and Olfactometry. Food Chem. 2020, 308, 125552. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Zhu, Y.; Zhang, J.; Wang, X.; Shi, W. Characteristic Volatile Compounds in Different Parts of Grass Carp by Comprehensive Two-Dimensional Gas Chromatography/Time-of-Flight Mass Spectrometry. Int. J. Food Prop. 2020, 23, 777–796. [Google Scholar] [CrossRef]
- Huang, W.; Fang, S.; Wang, J.; Zhuo, C.; Luo, Y.; Yu, Y.; Li, L.; Wang, Y.; Deng, W.-W.; Ning, J. Sensomics Analysis of the Effect of the Withering Method on the Aroma Components of Keemun Black Tea. Food Chem. 2022, 395, 133549. [Google Scholar] [CrossRef]
- Zhang, C.; Zhou, C.; Xu, K.; Tian, C.; Zhang, M.; Lu, L.; Zhu, C.; Lai, Z.; Guo, Y. A Comprehensive Investigation of Macro-Composition and Volatile Compounds in Spring-Picked and Autumn-Picked White Tea. Foods 2022, 11, 3628. [Google Scholar] [CrossRef]
- Ko, A.-Y.; Musfiqur Rahman, M.; Abd El-Aty, A.M.; Jang, J.; Choi, J.-H.; Mamun, M.I.R.; Shim, J.-H. Identification of Volatile Organic Compounds Generated from Healthy and Infected Powdered Chili Using Solvent-Free Solid Injection Coupled with GC/MS: Application to Adulteration. Food Chem. 2014, 156, 326–332. [Google Scholar] [CrossRef]
- Reale, S.; Biancolillo, A.; Gasparrini, C.; Di Martino, L.; Di Cecco, V.; Manzi, A.; Di Santo, M.; D’Archivio, A.A. Geographical Discrimination of Bell Pepper (Capsicum annuum) Spices by (HS)-SPME/GC-MS Aroma Profiling and Chemometrics. Molecules 2021, 26, 6177. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, R.; Alqathama, A.; Aldholmi, M.; Riaz, M.; Abdalla, A.N.; Mostafa, A.; Al-Said, H.M.; Alqarni, A.M.; Ullah, R.; Asgher, S.S.; et al. Gas Chromatography-Mass Spectrometry (GC-MS) Metabolites Profiling and Biological Activities of Various Capsicum annum Cultivars. Plants 2022, 11, 1022. [Google Scholar] [CrossRef] [PubMed]
- Garg, N.; Sethupathy, A.; Tuwani, R.; Nk, R.; Dokania, S.; Iyer, A.; Gupta, A.; Agrawal, S.; Singh, N.; Shukla, S.; et al. FlavorDB: A Database of Flavor Molecules. Nucleic Acids Res. 2018, 46, D1210–D1216. [Google Scholar] [CrossRef] [PubMed]
- Soomro, T.; Jordan, M.; Watts, S.; Migicovsky, Z.; Forney, C.F.; Song, J.; Myles, S.; Soomro, T.; Jordan, M.; Watts, S.; et al. Genomic Insights into Apple Aroma Diversity. Fruit Res. 2023, 3, 27. [Google Scholar] [CrossRef]
- Zhang, J.; Gu, X.; Yan, W.; Lou, L.; Xu, X.; Chen, X. Characterization of Differences in the Composition and Content of Volatile Compounds in Cucumber Fruit. Foods 2022, 11, 1101. [Google Scholar] [CrossRef]
- Sirangelo, T.M.; Rogers, H.J.; Spadafora, N.D. Multi-Omic Approaches to Investigate Molecular Mechanisms in Peach Post-Harvest Ripening. Agriculture 2022, 12, 553. [Google Scholar] [CrossRef]
- Amr, A.; Raie, W. Tomato Components and Quality Parameters: A Review. Jordan J. Agric. Sci. 2022, 18, 199–220. [Google Scholar] [CrossRef]
- Baldwin, E.A.; Scott, J.W.; Shewmaker, C.K.; Schuch, W. Flavor Trivia and Tomato Aroma: Biochemistry and Possible Mechanisms for Control of Important Aroma Components. HortScience 2000, 35, 1013–1022. [Google Scholar] [CrossRef]
- Harley, P.C.; Monson, R.K.; Lerdau, M.T. Ecological and Evolutionary Aspects of Isoprene Emission from Plants. Oecologia 1999, 118, 109–123. [Google Scholar] [CrossRef]
- Song, H.; Liu, J. GC-O-MS Technique and Its Applications in Food Flavor Analysis. Food Res. Int. 2018, 114, 187–198. [Google Scholar] [CrossRef]
- Zhang, J.; Liu, S.; Zhu, X.; Chang, Y.; Wang, C.; Ma, N.; Wang, J.; Zhang, X.; Lyu, J.; Xie, J. A Comprehensive Evaluation of Tomato Fruit Quality and Identification of Volatile Compounds. Plants 2023, 12, 2947. [Google Scholar] [CrossRef] [PubMed]
- Cheng, G.; Chang, P.; Shen, Y.; Wu, L.; El-Sappah, A.H.; Zhang, F.; Liang, Y. Comparing the Flavor Characteristics of 71 Tomato (Solanum lycopersicum) Accessions in Central Shaanxi. Front. Plant Sci. 2020, 11, 586834. [Google Scholar] [CrossRef] [PubMed]
- Farneti, B.; Alarcón, A.A.; Papasotiriou, F.G.; Samudrala, D.; Cristescu, S.M.; Costa, G.; Harren, F.J.M.; Woltering, E.J. Chilling-Induced Changes in Aroma Volatile Profiles in Tomato. Food Bioprocess Technol. 2015, 8, 1442–1454. [Google Scholar] [CrossRef] [PubMed]
- Gu, W.; Wei, Y.; Fu, X.; Gu, R.; Chen, J.; Jian, J.; Huang, L.; Yuan, C.; Guan, W.; Hao, X. HS-SPME/GC×GC-TOFMS-Based Flavoromics and Antimicrobial Properties of the Aroma Components of Zanthoxylum motuoense. Foods 2023, 12, 2225. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Xu, R.; Tong, X.; Zeng, J.; Chen, M.; Lin, Z.; Cai, S.; Chen, Y.; Mo, D. Characterization of Different Meat Flavor Compounds in Guangdong Small-Ear Spotted and Yorkshire Pork Using Two-Dimensional Gas Chromatography–Time-of-Flight Mass Spectrometry and Multi-Omics. LWT 2022, 169, 114010. [Google Scholar] [CrossRef]
- Muñoz-González, C.; Brule, M.; Martin, C.; Feron, G.; Canon, F. Molecular Mechanisms of Aroma Persistence: From Noncovalent Interactions between Aroma Compounds and the Oral Mucosa to Metabolization of Aroma Compounds by Saliva and Oral Cells. Food Chem. 2022, 373, 131467. [Google Scholar] [CrossRef] [PubMed]
- D’Angelo, M.; Zanor, M.I.; Sance, M.; Cortina, P.R.; Boggio, S.B.; Asprelli, P.; Carrari, F.; Santiago, A.N.; Asís, R.; Peralta, I.E.; et al. Contrasting Metabolic Profiles of Tasty Andean Varieties of Tomato Fruit in Comparison with Commercial Ones. J. Sci. Food Agric. 2018, 98, 4128–4134. [Google Scholar] [CrossRef] [PubMed]
- d’Acampora Zellner, B.; Dugo, P.; Dugo, G.; Mondello, L. Gas Chromatography–Olfactometry in Food Flavour Analysis. J. Chromatogr. A 2008, 1186, 123–143. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Yu, X.; Li, M.; Chen, J.; Wang, X. Monitoring Oxidative Stability and Changes in Key Volatile Compounds in Edible Oils during Ambient Storage through HS-SPME/GC–MS. Int. J. Food Prop. 2017, 20, S2926–S2938. [Google Scholar] [CrossRef]
- Zhu, Y.; Chen, J.; Chen, X.; Chen, D.; Deng, S. Use of Relative Odor Activity Value (ROAV) to Link Aroma Profiles to Volatile Compounds: Application to Fresh and Dried Eel (Muraenesox cinereus). Int. J. Food Prop. 2020, 23, 2257–2270. [Google Scholar] [CrossRef]
- Rambla, J.L.; Tikunov, Y.M.; Monforte, A.J.; Bovy, A.G.; Granell, A. The Expanded Tomato Fruit Volatile Landscape. J. Exp. Bot. 2013, 65, 4613–4623. [Google Scholar] [CrossRef] [PubMed]
- Martina, M.; Tikunov, Y.; Portis, E.; Bovy, A.G. The Genetic Basis of Tomato Aroma. Genes 2021, 12, 226. [Google Scholar] [CrossRef] [PubMed]
- Comparison of SPME Versus SAFE Processes for the Analysis of Flavor Compounds in Watermelon Juice|Food Analytical Methods. Available online: https://link.springer.com/article/10.1007/s12161-018-1153-x (accessed on 30 March 2024).
- Liu, Y.; Yang, C.; Wang, Q.; Zhang, J.; Zhang, L. Identification and Confirmation of Key Compounds Causing Cooked Off-Flavor in Heat-Treated Tomato Juice. J. Food Sci. 2022, 87, 2515–2526. [Google Scholar] [CrossRef] [PubMed]
- Trichoderma Atroviride LZ42 Releases Volatile Organic Compounds Promoting Plant Growth and Suppressing Fusarium Wilt Disease in Tomato Seedlings|BMC Microbiology|Full Text. Available online: https://bmcmicrobiol.biomedcentral.com/articles/10.1186/s12866-022-02511-3 (accessed on 30 March 2024).
- Jia, X.; Zhou, Q.; Wang, J.; Liu, C.; Huang, F.; Huang, Y. Identification of Key Aroma-Active Compounds in Sesame Oil from Microwaved Seeds Using E-Nose and HS-SPME-GC×GC-TOF/MS. J. Food Biochem. 2019, 43, e12786. [Google Scholar] [CrossRef] [PubMed]
- Hua, J.; Li, J.; Ouyang, W.; Wang, J.; Yuan, H.; Jiang, Y. Effect of Strobilanthes tonkinensis Lindau Addition on Black Tea Flavor Quality and Volatile Metabolite Content. Foods 2022, 11, 1678. [Google Scholar] [CrossRef]
- Kazachkova, Y.; Zemach, I.; Panda, S.; Bocobza, S.; Vainer, A.; Rogachev, I.; Dong, Y.; Ben-Dor, S.; Veres, D.; Kanstrup, C.; et al. The GORKY Glycoalkaloid Transporter Is Indispensable for Preventing Tomato Bitterness. Nat. Plants 2021, 7, 468–480. [Google Scholar] [CrossRef]


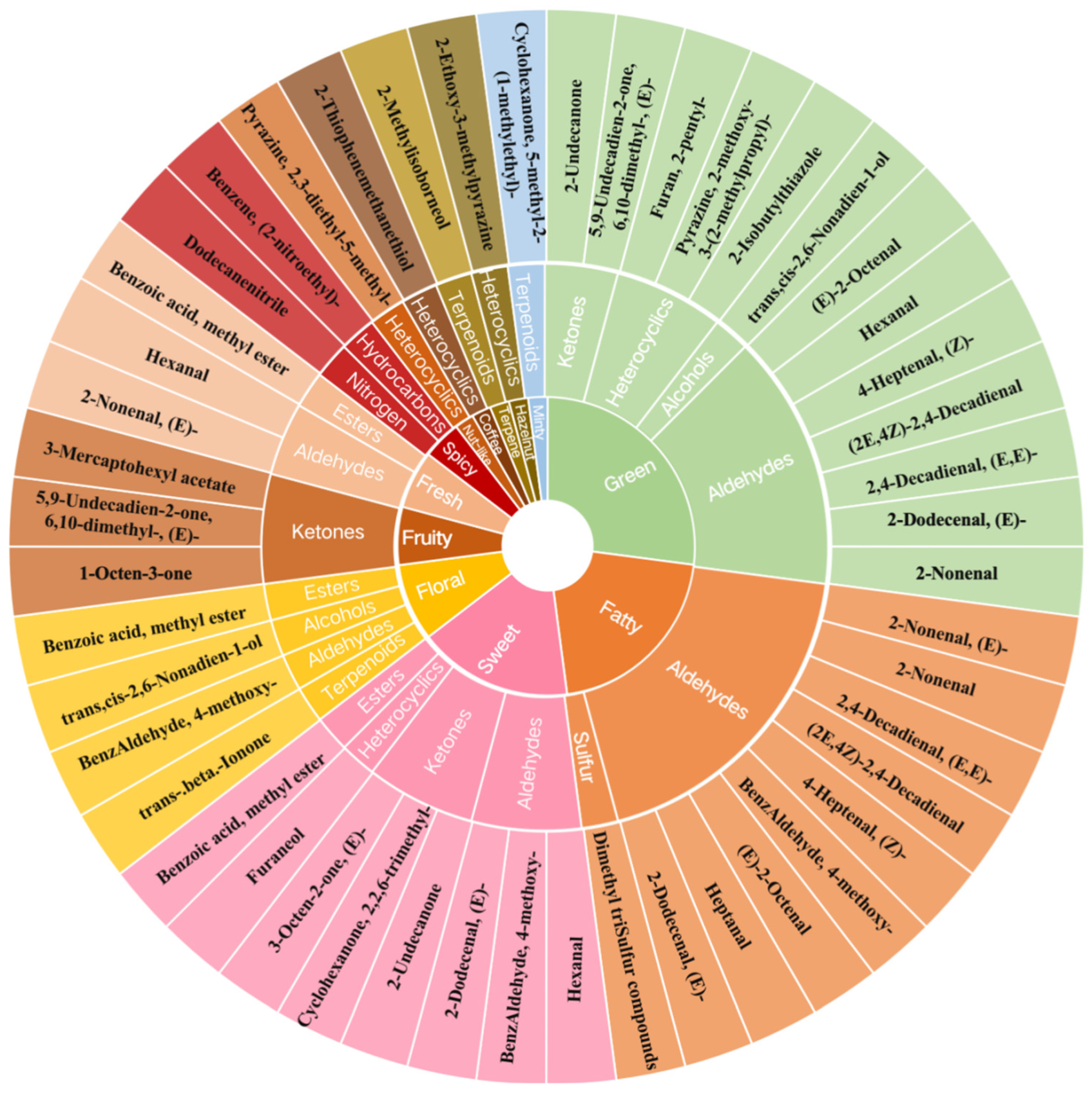

| Name | Type | Sensory Flavor Characteristics | rOAV |
|---|---|---|---|
| HS-SPME-GC-MS | |||
| β-ionone | Terpenoids | Floral | 100.00 |
| 2-Nonenal, (E)- | Aldehydes | Green/fatty/fresh | 67.79 |
| Dimethyl triSulfur compounds | Sulfur compounds | Fatty | 54.06 |
| 2-Thiophenemethanethiol | Heterocyclics | Coffee | 44.62 |
| Pyrazine, 2-methoxy-3-(2-methylpropyl)- | Heterocyclics | Green | 39.60 |
| 2,4-Decadienal, (E,E)- | Aldehydes | Green/fatty | 16.58 |
| Furaneol | Heterocyclics | Sweet | 13.84 |
| Pyrazine, 2,3-diethyl-5-methyl- | Heterocyclics | Nut-like | 13.04 |
| Dodecanenitrile | Nitrogen compounds | Spicy | 11.92 |
| (2E,4Z)-2,4-Decadienal | Aldehydes | Green/fatty | 8.57 |
| 2-Methylisoborneol | Terpenoids | Terpene | 5.90 |
| 3-Octen-2-one | Ketones | Sweet | 4.91 |
| 4-Heptenal, (Z)- | Aldehydes | Green/fatty | 4.85 |
| 2-Nonenal | Aldehydes | Green/fatty | 4.67 |
| Benzene, (2-nitroethyl)- | Hydrocarbons | Spicy | 4.29 |
| Hexanal | Aldehydes | Green/sweet/fresh | 3.13 |
| 3-Mercaptohexyl acetate | Esters | Fruity | 2.91 |
| BenzAldehyde, 4-methoxy- | Aldehydes | Fatty/sweet/floral | 2.74 |
| trans,cis-2,6-Nonadien-1-ol | Alcohols | Green/floral | 2.22 |
| 2-Isobutylthiazole | Heterocyclics | Green | 2.02 |
| Cyclohexanone, 5-methyl-2-(1-methylethyl)- | Terpenoids | Minty-like | 1.90 |
| Benzoic acid, methyl ester | Esters | Sweet/floral/fresh | 1.29 |
| 2-Ethoxy-3-methylpyrazine | Heterocyclics | Hazelnut-like | 1.14 |
| 5,9-Undecadien-2-one, 6,10-dimethyl-, (E)- | Ketones | Fruity | 1.13 |
| HS-SPME-GC×GC-TOF-MS | |||
| 2-Nonenal, (E)- | Aldehydes | Green/fatty/fresh | 100 |
| 2-Octenal, (E)- | Aldehydes | Green/fatty | 24.52 |
| Furan, 2-pentyl- | Heterocyclics | Green | 20.02 |
| Heptanal | Aldehydes | Fatty | 3.31 |
| 2-Dodecenal, (E)- | Aldehydes | Green/fatty/sweet | 1.64 |
| 1-Octen-3-one | Ketones | Fruity | 1.30 |
| 2-Undecanone | Ketones | Green/sweet | 1.01 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guan, S.; Liu, C.; Yao, Z.; Wan, H.; Ruan, M.; Wang, R.; Ye, Q.; Li, Z.; Zhou, G.; Cheng, Y. Detection and Analysis of VOCs in Cherry Tomato Based on GC-MS and GC×GC-TOF MS Techniques. Foods 2024, 13, 1279. https://doi.org/10.3390/foods13081279
Guan S, Liu C, Yao Z, Wan H, Ruan M, Wang R, Ye Q, Li Z, Zhou G, Cheng Y. Detection and Analysis of VOCs in Cherry Tomato Based on GC-MS and GC×GC-TOF MS Techniques. Foods. 2024; 13(8):1279. https://doi.org/10.3390/foods13081279
Chicago/Turabian StyleGuan, Sihui, Chenxu Liu, Zhuping Yao, Hongjian Wan, Meiying Ruan, Rongqing Wang, Qingjing Ye, Zhimiao Li, Guozhi Zhou, and Yuan Cheng. 2024. "Detection and Analysis of VOCs in Cherry Tomato Based on GC-MS and GC×GC-TOF MS Techniques" Foods 13, no. 8: 1279. https://doi.org/10.3390/foods13081279





