A Facile Determination of Herbicide Residues and Its Application in On-Site Analysis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Materials
2.2. Instruments
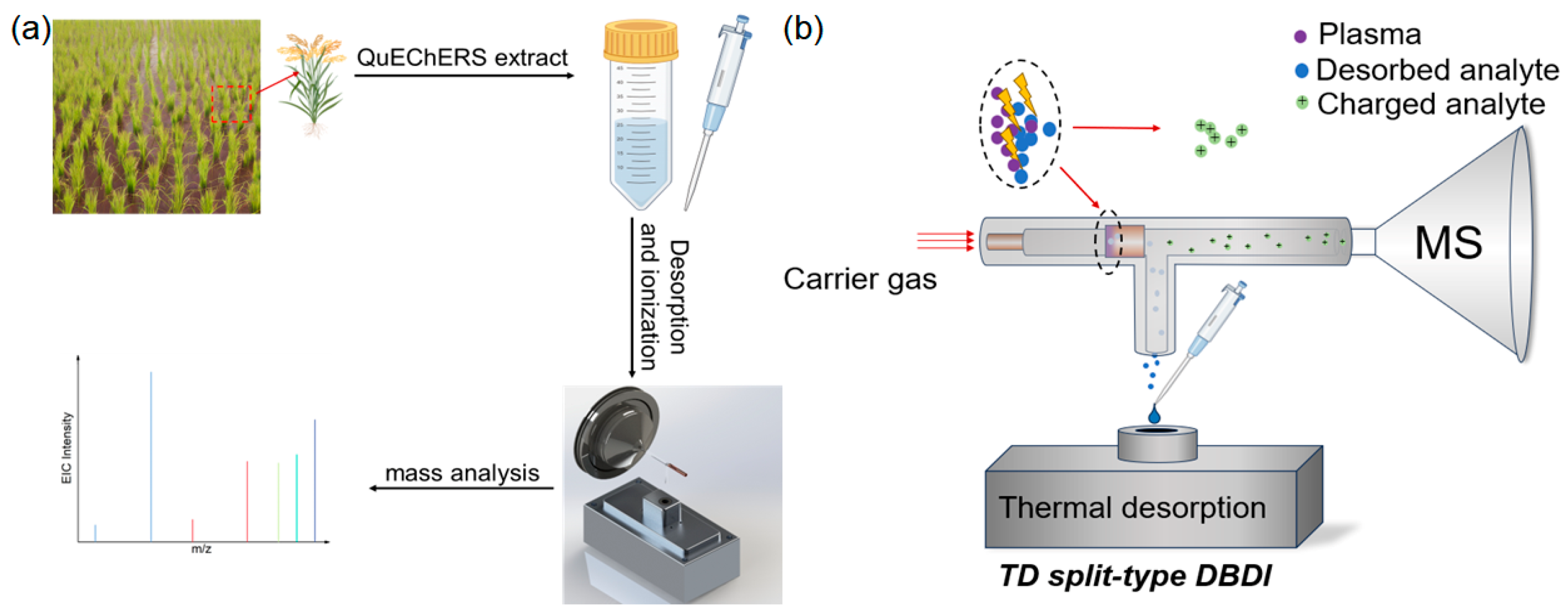
2.3. Sample Preparation and Treatment
3. Results and Discussion
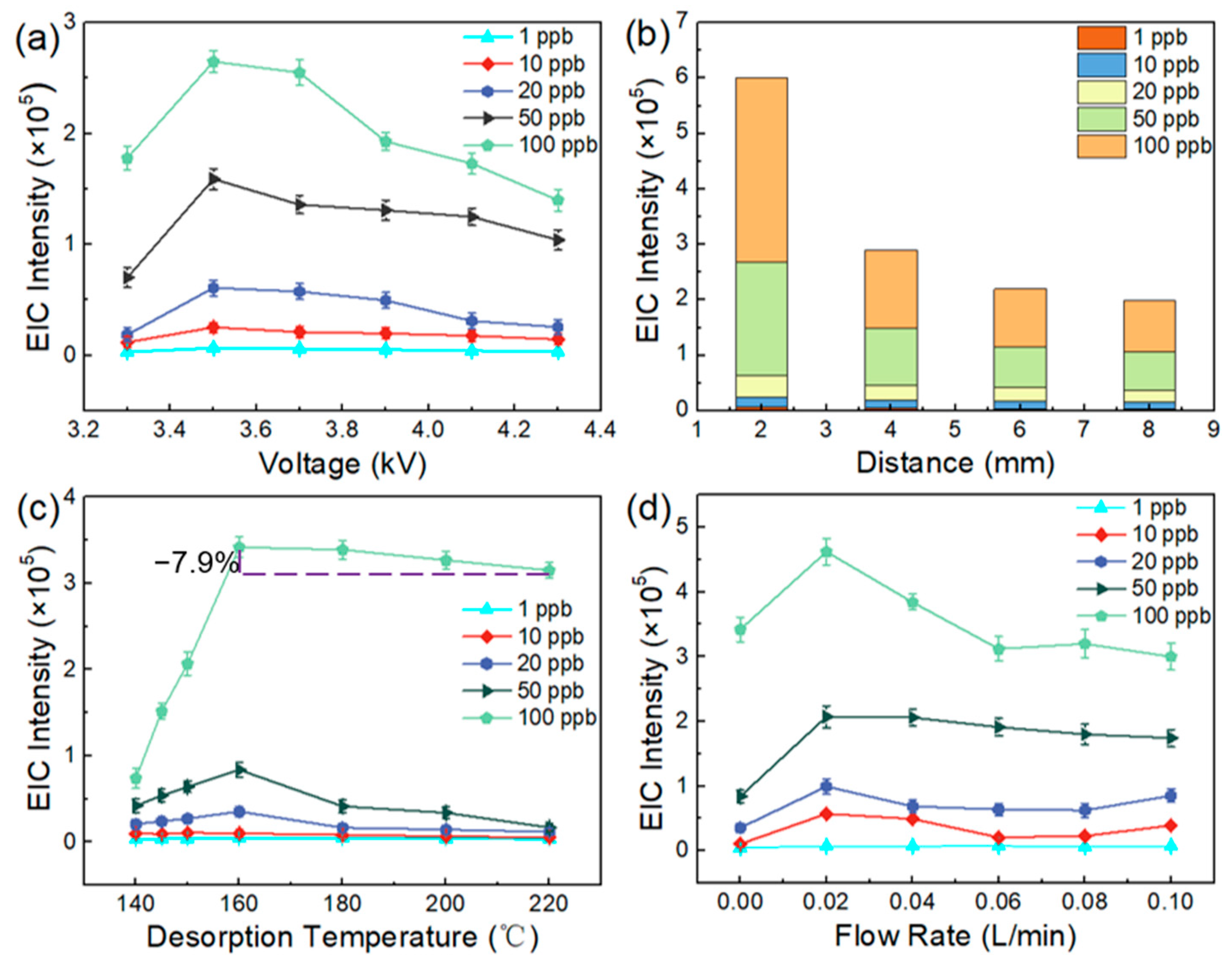
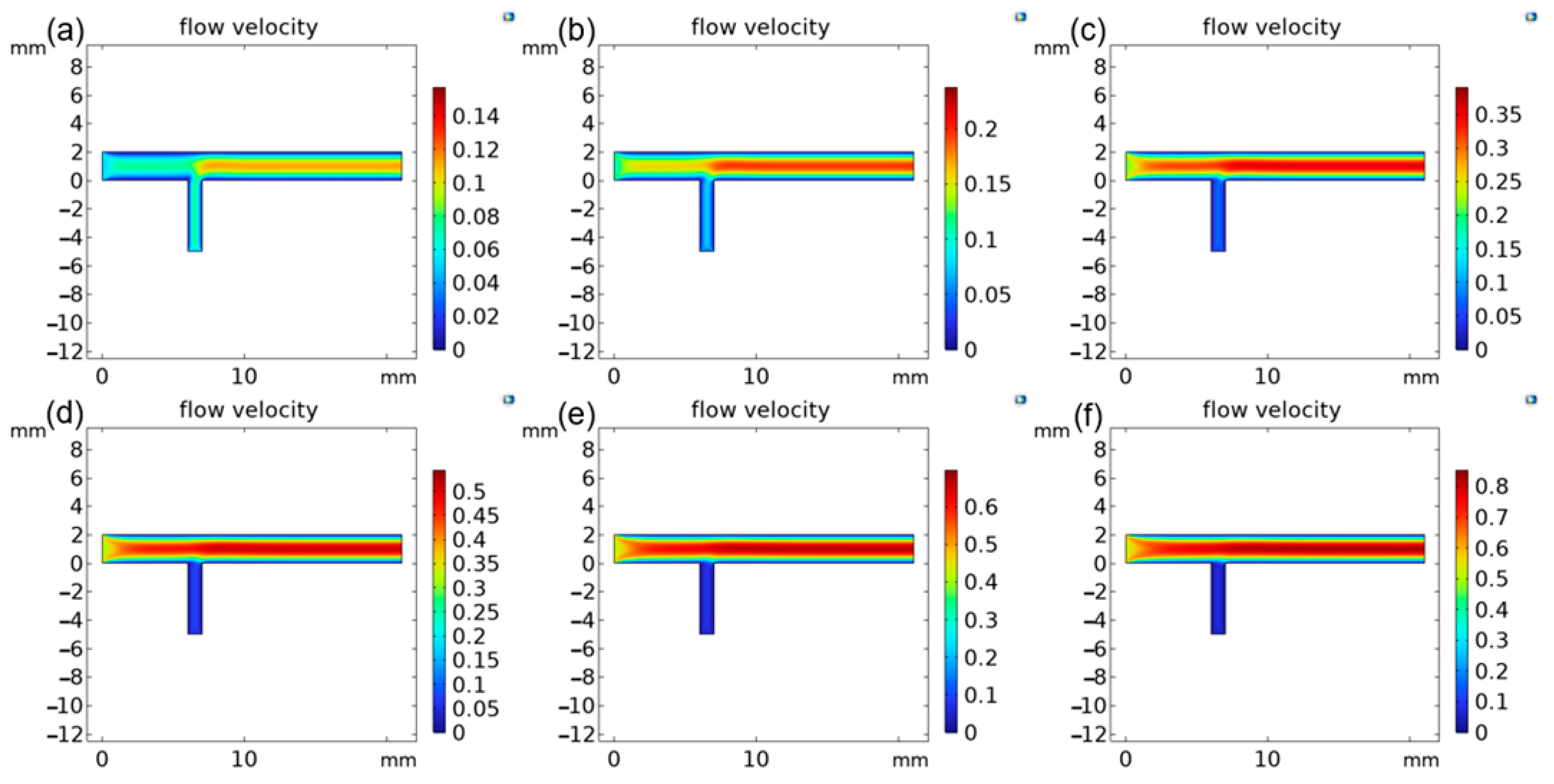
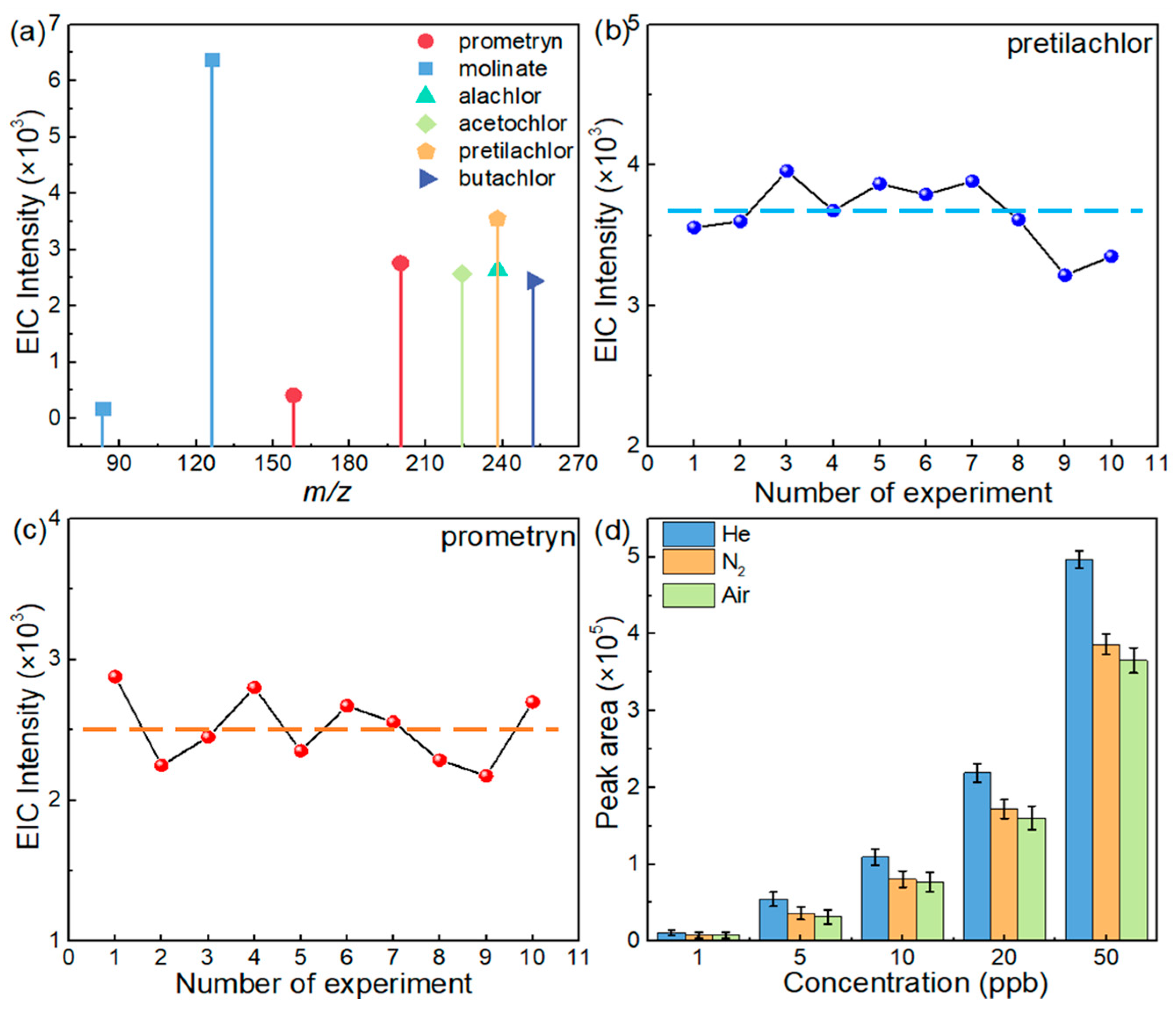
3.1. Optimization of the Parameters
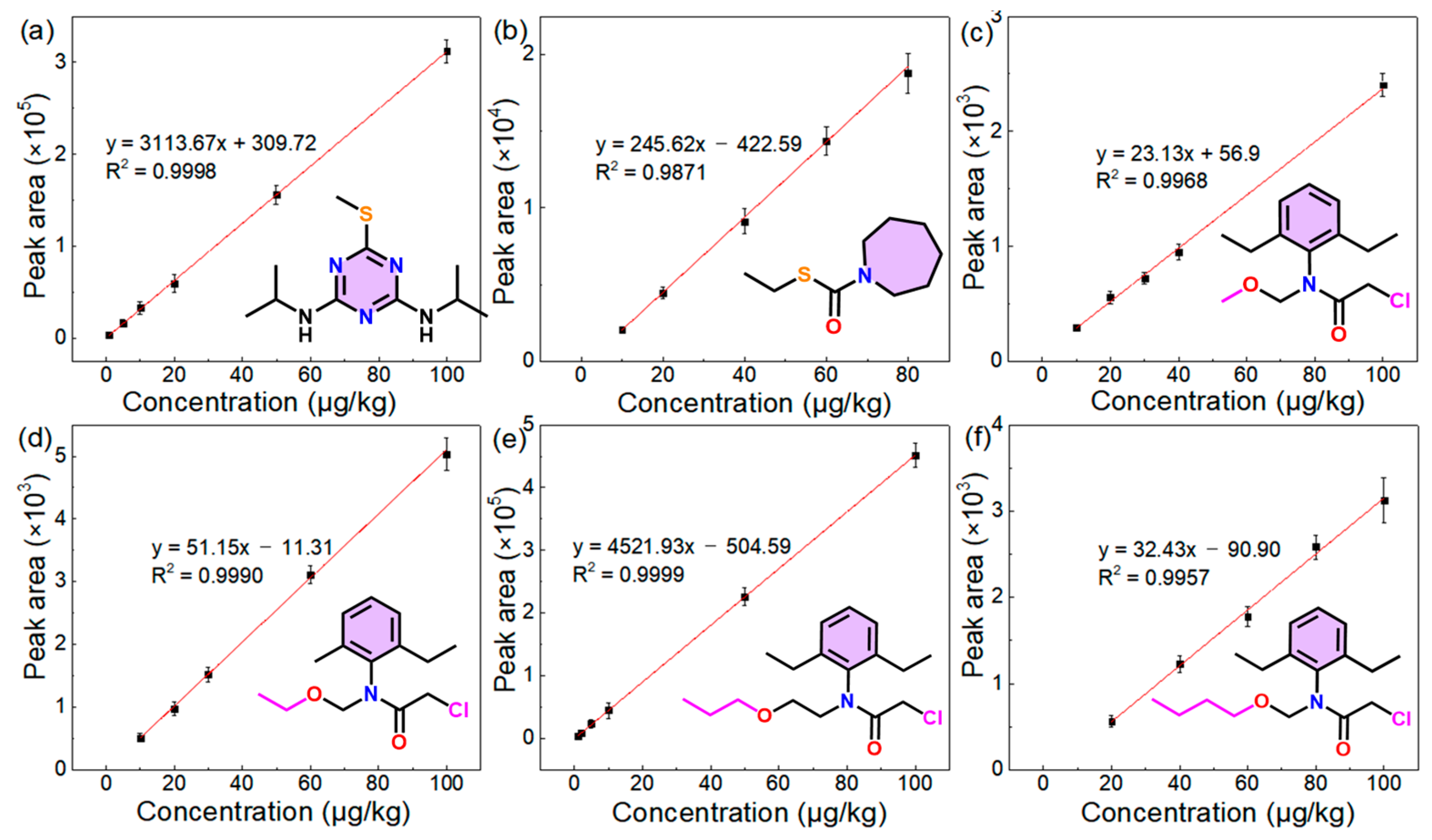
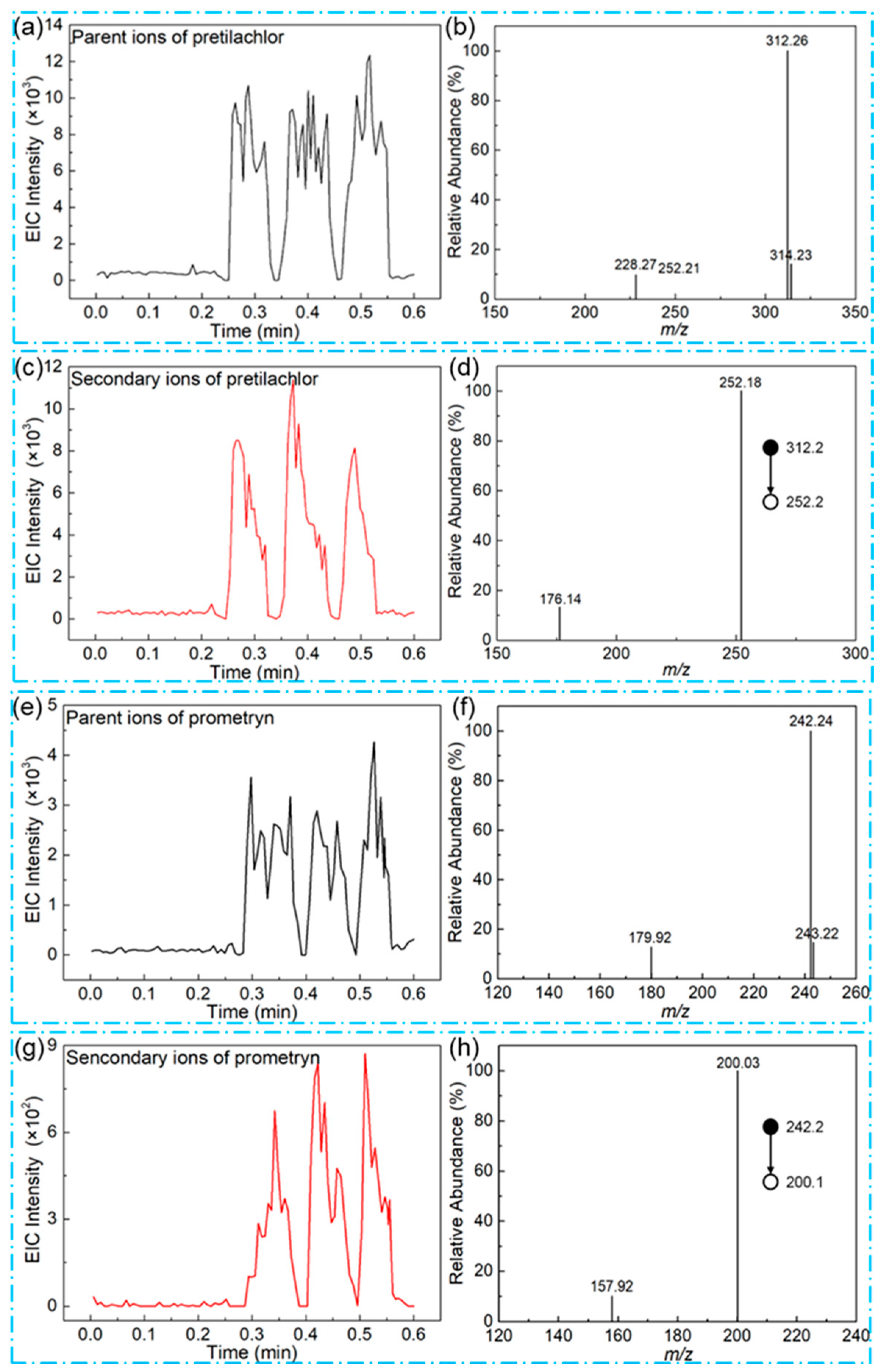
3.2. Validation of the Method in the Detection of Herbicides
3.3. Analytical Performance in Spiked Samples
3.4. Simultaneous Analysis of Herbicides Spiked in Rice Samples
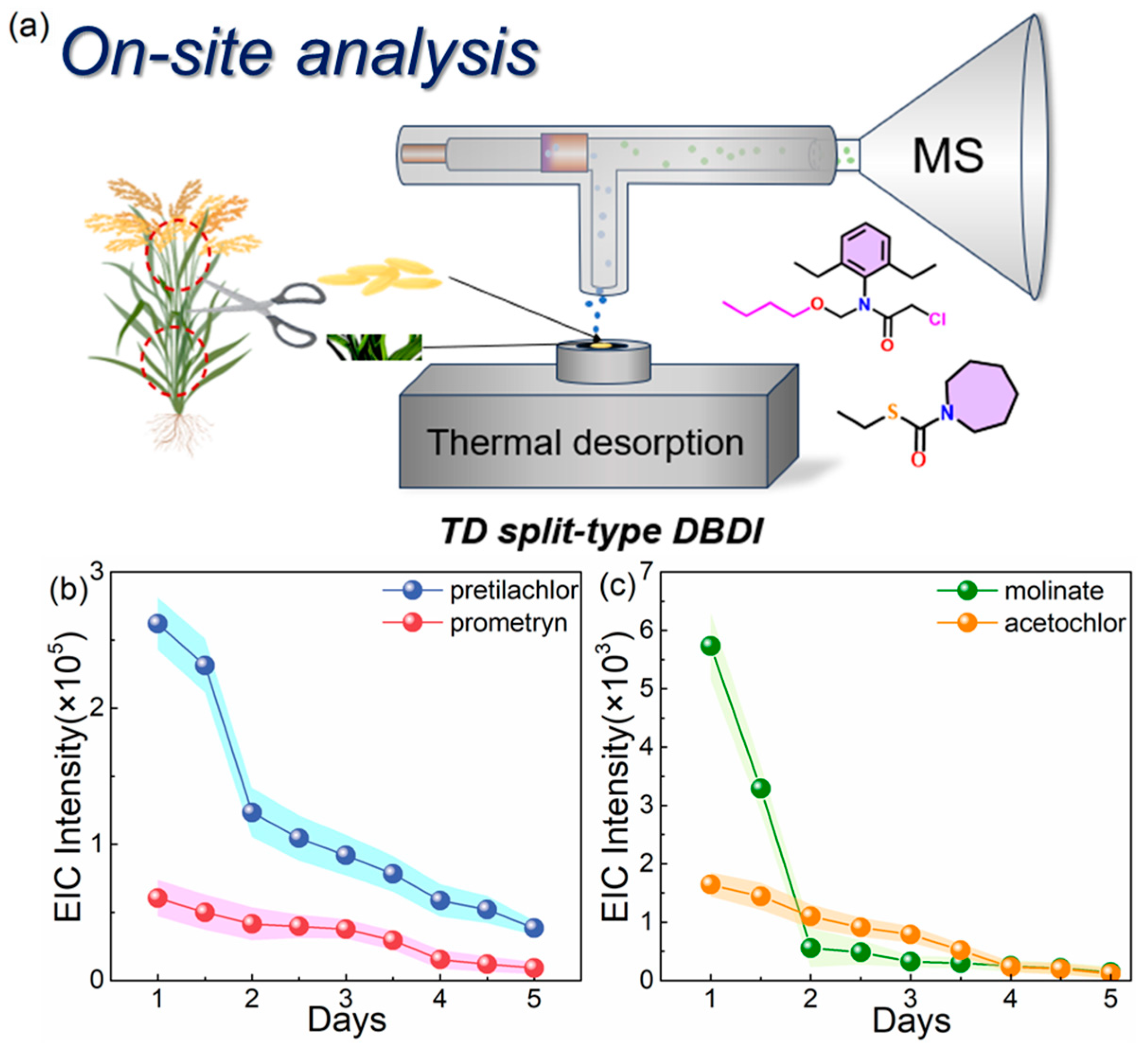
3.5. On-Site Non-Destructive Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mostafalou, S.; Abdollahi, M. Pesticides and human chronic diseases: Evidences, mechanisms, and perspectives. Toxicol. Appl. Pharmacol. 2013, 268, 157–177. [Google Scholar] [CrossRef]
- Barr, D.B.; Panuwet, P.; Nguyen, J.V.; Udunka, S.; Needham, L.L. Assessing Exposure to Atrazine and Its Metabolites Using Biomonitoring. Environ. Health Perspect. 2007, 115, 1474–1478. [Google Scholar] [CrossRef]
- Kmellár, B.; Pareja, L.; Ferrer, C.; Fodor, P.; Fernández-Alba, A.R. Study of the effects of operational parameters on multiresidue pesticide analysis by LC–MS/MS. Talanta 2011, 84, 262–273. [Google Scholar] [CrossRef]
- Wang, B.-T.; Wu, F.-Q.; Yang, H.-P. Evaluation of inductively coupled plasma tandem mass spectrometry for interference-free determination of metalloids in complex food. Chin. J. Anal. Chem. 2022, 50, 100120–100124. [Google Scholar] [CrossRef]
- Weng, R.; Lou, S.; Pang, X.; Song, Y.; Su, X.; Xiao, Z.; Qiu, J. Multi-residue analysis of 126 pesticides in chicken muscle by ultra-high-performance liquid chromatography coupled to quadrupole time-of-flight mass spectrometry. Food Chem. 2020, 309, 125503–125511. [Google Scholar] [CrossRef]
- Masiáa, A.; Ibáñez, M.; Blascoa, C.; Sanchob, J.V.; Picóa, Y.; Hernándezb, F. Combined use of liquid chromatography triple quadrupole mass spectrometry and liquid chromatography quadrupole time-of-flight mass spectrometry in systematic screening of pesticides and other contaminants in water samples. Anal. Chim. Acta 2013, 761, 117–127. [Google Scholar] [CrossRef]
- Feng, C.; Xu, Q.; Qiu, X.; Jin, Y.e.; Ji, J.; Lin, Y.; Le, S.; Wang, G.; Lu, D. Comprehensive strategy for analysis of pesticide multi-residues in food by GC–MS/MS and UPLC-Q-Orbitrap. Food Chem. 2020, 320, 126576–126585. [Google Scholar] [CrossRef]
- Pico, Y.; Alfarhan, A.H.; Barcelo, D. How recent innovations in gas chromatography-mass spectrometry have improved pesticide residue determination: An alternative technique to be in your radar. Trends Anal. Chem. 2020, 122, 115720. [Google Scholar] [CrossRef]
- Mirabelli, M.F.; Wolf, J.-C.; Zenobi, R. Pesticide analysis at ppt concentration levels: Coupling nano-liquid chromatography with dielectric barrier discharge ionization-mass spectrometry. Anal. Bioanal. Chem. 2016, 408, 3425–3434. [Google Scholar] [CrossRef]
- Li, X.; Li, Z.; Wang, X.; Nie, H.; Zhang, Y.; Bai, Y.; Liu, H. Monolith dip-it: A bifunctional device for improving the sensitivity of direct analysis in real time mass spectrometry. Analyst 2016, 141, 4947–4952. [Google Scholar] [CrossRef]
- Cebi, N.; Manav, O.G.; Olgun, E.O. Analysis of pesticide residues in hazelnuts using the QuEChERS method by liquid chromatography–tandem mass spectrometry. Microchem. J. 2021, 166, 106208–106215. [Google Scholar] [CrossRef]
- Lu, H.; Zhang, H.; Chingin, K.; Xiong, J.; Fang, X.; Chen, H. Ambient mass spectrometry for food science and industry. Trends Anal. Chem. 2018, 107, 99–115. [Google Scholar] [CrossRef]
- Zhang, X.L.; Zhang, H.; Wang, X.C.; Huang, K.K.; Wang, D.; Chen, H.W. Advances in Ambient Ionization for Mass Spectrometry. Chin. J. Anal. Chem. 2018, 46, 1703–1713. [Google Scholar] [CrossRef]
- Takats, Z.; Wiseman, J.M.; Gologan, B.; Cooks, R.G. Mass spectrometry sampling under ambient conditions with desorption electrospray ionization. Science 2004, 306, 471–473. [Google Scholar] [CrossRef]
- Roach, P.J.; Laskin, J.; Laskin, A. Nanospray desorption electrospray ionization: An ambient method for liquid-extraction surface sampling in mass spectrometry. Analyst 2010, 135, 2233–2236. [Google Scholar] [CrossRef]
- Wang, Z.; He, B.; Liu, Y.; Huo, M.; Fu, W.; Yang, C.; Wei, J.; Abliz, Z. In situ metabolomics in nephrotoxicity of aristolochic acids based on air flow-assisted desorption electrospray ionization mass spectrometry imaging. Acta Pharm. Sin. B 2020, 10, 1083–1093. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, X.; Nie, H.; Yang, L.; Li, Z.; Bai, Y.; Niu, L.; Song, D.; Liu, H. Interface for Online Coupling of Surface Plasmon Resonance to Direct Analysis in Real Time Mass Spectrometry. Anal. Chem. 2015, 87, 6505–6509. [Google Scholar] [CrossRef]
- Na, N.; Zhao, M.; Zhang, S.; Yang, C.; Zhang, X. Development of a dielectric barrier discharge ion source for ambient mass spectrometry. J. Am. Soc. Mass Spectrom. 2007, 18, 1859–1862. [Google Scholar] [CrossRef]
- Andrade, F.J.; Shelley, J.T.; Wetzel, W.C.; Webb, M.R.; Gamez, G.; Ray, S.J.; Hieftje, G.M. Atmospheric Pressure Chemical Ionization Source. 2. Desorption–Ionization for the Direct Analysis of Solid Compounds. Anal. Chem. 2008, 80, 2654–2663. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Venter, A.; Cooks, R.G. Extractive electrospray ionization for direct analysis of undiluted urine, milk and other complex mixtures without sample preparation. Chem. Commun. 2006, 2042–2044. [Google Scholar] [CrossRef]
- Hagenhoff, S.; Franzke, J.; Hayen, H. Determination of peroxide explosive TATP and related compounds by dielectric barrier discharge ionization-mass spectrometry (DBDI-MS). Anal. Chem. 2017, 89, 4210–4215. [Google Scholar] [CrossRef] [PubMed]
- Klute, F.D.; Brandt, S.; Vogel, P.; Biskup, B.; Reininger, C.; Horvatic, V.; Vadla, C.; Farnsworth, P.B.; Franzke, J. Systematic Comparison between Half and Full Dielectric Barrier Discharges Based on the Low Temperature Plasma Probe (LTP) and Dielectric Barrier Discharge for Soft Ionization (DBDI) Configurations. Anal. Chem. 2017, 89, 9368–9374. [Google Scholar] [CrossRef]
- Almasian, M.R.; Yang, C.; Xing, Z.; Zhang, S.; Zhang, X. Development of a graphite low-temperature plasma source with dual-mode in-source fragmentation for ambient mass spectrometry. Rapid Commun. Mass Spectrom. 2010, 24, 742–748. [Google Scholar] [CrossRef]
- Guo, C.; Tang, F.; Chen, J.; Wang, X.; Zhang, S.; Zhang, X. Development of dielectric-barrier-discharge ionization. Anal. Bioanal. Chem. 2015, 407, 2345–2364. [Google Scholar] [CrossRef]
- Pape, A.; Schmitz, O.J. Dielectric barrier discharge in mass spectrometry–An overview over plasma investigations and ion sources applications. Trends Anal. Chem. 2024, 170, 117420–117438. [Google Scholar] [CrossRef]
- Lu, Q.; Lin, R.; Du, C.; Meng, Y.; Yang, M.; Zenobi, R.; Hang, W. Metal Probe Microextraction Coupled to Dielectric Barrier Discharge Ionization-Mass Spectrometry for Detecting Drug Residues in Organisms. Anal. Chem. 2020, 92, 5921–5928. [Google Scholar] [CrossRef] [PubMed]
- Nudnova, M.M.; Zhu, L.; Zenobi, R. Active capillary plasma source for ambient mass spectrometry. Rapid Commun. Mass Spectrom. 2012, 26, 1447–1452. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Jarquin, S.; Winkler, R. Design of a low-temperature plasma (LTP) probe with adjustable output temperature and variable beam diameter for the direct detection of organic molecules. Rapid Commun. Mass Spectrom. 2013, 27, 629–634. [Google Scholar] [CrossRef]
- Lu, Q.; Xu, Z.; You, X.; Ma, S.; Zenobi, R. Atmospheric Pressure Mass Spectrometry Imaging Using Laser Ablation, Followed by Dielectric Barrier Discharge Ionization. Anal. Chem. 2021, 93, 6232–6238. [Google Scholar] [CrossRef]
- Smoluch, M.; Babij, M.; Zuba, D.; Schroeder, G.; Gotszalk, T.; Silberring, J. Heat assisted sample introduction and determination of cannabinoids by dielectric barrier discharge ionization mass spectrometry. Int. J. Mass Spectrom. 2015, 386, 32–36. [Google Scholar] [CrossRef]
- Wolf, J.C.; Etter, R.; Schaer, M.; Siegenthaler, P.; Zenobi, R. Direct and Sensitive Detection of CWA Simulants by Active Capillary Plasma Ionization Coupled to a Handheld Ion Trap Mass Spectrometer. J. Am. Soc. Mass Spectrom. 2016, 27, 1197–1202. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, E.; Xiao, D.; Dumlao, M.C.; Steel, C.C.; Schmidtke, L.M.; Fletcher, J.; Donald, W.A. Nanosecond Pulsed Dielectric Barrier Discharge Ionization Mass Spectrometry. Anal. Chem. 2020, 92, 4468–4474. [Google Scholar] [CrossRef] [PubMed]
- EN 15662; Foods of Plant Origin—Determination of Pesticide Residues Using GC–MS and/or LC–MS/MS Following Acetonitrile Extraction/Partitioning and Clean-Up by Dispersive SPE–Quechers–Method. British Standard: London, UK, 2008.
- Li, X.; Li, Y.; Zhang, P.; Jia, P.; Dong, L. Improved performance of a barrier-discharge plasma jet biased by a direct-current voltage. Sci. Rep. 2016, 6, 35653–35660. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, F.; Mushyam, A.; Pascoa, J.; Trancossi, M. A new plasma actuator configuration for improved efficiency: The stair-shaped dielectric barrier discharge actuator. Phys. D Appl. Phys. 2019, 52, 385201–385214. [Google Scholar] [CrossRef]
- Yang, Z.; Ren, Z.; Cheng, Y.; Sun, W.; Xi, Z.; Jia, W.; Li, G.; Wang, Y.; Guo, M.; Li, D. Review and prospect on portable mass spectrometer for recent applications. Vacuum 2022, 199, 110889–110906. [Google Scholar] [CrossRef]
- Saha, S.; Chen, L.C.; Mandal, M.K.; Hiraoka, K. Leidenfrost Phenomenon-assisted Thermal Desorption (LPTD) and Its Application to Open Ion Sources at Atmospheric Pressure Mass Spectrometry. J. Am. Soc. Mass Spectrom. 2013, 24, 341–347. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Shu, J.; Yang, B.; Guo, Y.; Zhang, Z.; Jiang, K.; Li, Z. Ultrasensitive detection of trace chemical warfare agent-related compounds by thermal desorption associative ionization time-of-flight mass spectrometry. Talanta 2021, 235, 122788–122795. [Google Scholar] [CrossRef] [PubMed]
- He, J.; Tang, F.; Luo, Z.; Chen, Y.; Xu, J.; Zhang, R.; Wang, X.; Abliz, Z. Air flow assisted ionization for remote sampling of ambient mass spectrometry and its application. Rapid Commun. Mass Spectrom. 2011, 25, 843–850. [Google Scholar] [CrossRef] [PubMed]
- Tang, F.; Chen, Y.; He, J.-M.; Luo, Z.-G.; Abliz, Z.; Wang, X.-H. Design and performance of air flow-assisted ionization imaging mass spectrometry system. Chin. Chem. Lett. 2014, 25, 687–692. [Google Scholar] [CrossRef]
- Waraksa, E.; Perycz, U.; Namieśnik, J.; Sillanpää, M.; Dymerski, T.; Wójtowicz, M.; Puton, J. Dopants and gas modifiers in ion mobility spectrometry. Trends Anal. Chem. 2016, 82, 237–249. [Google Scholar] [CrossRef]
- Yang, Z.; Lin, B.; Huang, X.; Shi, B.; Ma, Q.; Han, C.; Shen, Y. Determination of eight herbicide residues in foodstuffs of plant origin by gas chromatography–atmospheric pressure chemical ionization–tandem mass spectrometry. Microchem. J. 2023, 193, 109130–109139. [Google Scholar] [CrossRef]
- Mihailova, A.; Kelly, S.D.; Chevallier, O.P.; Elliott, C.T.; Maestroni, B.M.; Cannavan, A. High-resolution mass spectrometry-based metabolomics for the discrimination between organic and conventional crops: A review. Trends Food Sci. Technol. 2021, 110, 142–154. [Google Scholar] [CrossRef]
- Bouza, M.; García-Martínez, J.; Gilbert-López, B.; Brandt, S.; García-Reyes, J.F.; Molina-Díaz, A.; Franzke, J. Dielectric Barrier Discharge Ionization Mechanisms: Polycyclic Aromatic Hydrocarbons as a Case of Study. Anal. Chem. 2022, 95, 854–861. [Google Scholar] [CrossRef] [PubMed]
- Zhou, C.; Wu, H.; Zhang, X.; Zhang, Y.; Xie, W.; Xu, W. High-Throughput and Direct Sample Screening Using a Laser Spray Ionization Miniature Mass Spectrometer. Anal. Chem. 2019, 91, 8808–8813. [Google Scholar] [CrossRef] [PubMed]
- Lawton, Z.E.; Traub, A.; Fatigante, W.L.; Mancias, J.; O’Leary, A.E.; Hall, S.E.; Wieland, J.R.; Oberacher, H.; Gizzi, M.C.; Mulligan, C.C. Analytical Validation of a Portable Mass Spectrometer Featuring Interchangeable, Ambient Ionization Sources for High Throughput Forensic Evidence Screening. J. Am. Soc. Mass Spectrom. 2016, 28, 1048–1059. [Google Scholar] [CrossRef]
- Wang, W.; Wang, S.; Xu, C.; Li, H.; Xing, Y.; Hou, K.; Li, H. Rapid Screening of Trace Volatile and Nonvolatile Illegal Drugs by Miniature Ion Trap Mass Spectrometry: Synchronized Flash-Thermal-Desorption Purging and Ion Injection. Anal. Chem. 2019, 91, 10212–10220. [Google Scholar] [CrossRef]
| Analyte | L.R. (μg/kg) | Regression Equation | R2 | MRLs (μg/kg) | LOQ (μg/kg) | LOD (μg/kg) | RSD |
|---|---|---|---|---|---|---|---|
| Prometryn | 1–100 | y = 3113.67x + 309.72 | 0.9998 | 20 | 1 | 0.3 | 14.2% |
| Molinate | 10–80 | y = 246.62x − 422.59 | 0.9871 | 100 | 10 | 3 | 9.8% |
| Alachlor | 10–100 | y = 23.13x + 56.9 | 0.9968 | 50 | 10 | 3 | 14.8% |
| Acetochlor | 10–100 | y = 51.51x − 11.31 | 0.9990 | 50 | 10 | 3 | 8.9% |
| Pretilachlor | 1–100 | y = 4521.93x − 504.59 | 0.9999 | 10 | 1 | 0.3 | 11.9% |
| Butachlor | 20–100 | y = 32.43x − 90.90 | 0.9957 | 100 | 20 | 6 | 7.6% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sun, Y.; Tang, Y.; Chen, Z.; Ge, M.; Xiong, W.; Wen, L. A Facile Determination of Herbicide Residues and Its Application in On-Site Analysis. Foods 2024, 13, 1280. https://doi.org/10.3390/foods13081280
Sun Y, Tang Y, Chen Z, Ge M, Xiong W, Wen L. A Facile Determination of Herbicide Residues and Its Application in On-Site Analysis. Foods. 2024; 13(8):1280. https://doi.org/10.3390/foods13081280
Chicago/Turabian StyleSun, Yifei, Yan Tang, Zetao Chen, Miaoxiu Ge, Wei Xiong, and Luhong Wen. 2024. "A Facile Determination of Herbicide Residues and Its Application in On-Site Analysis" Foods 13, no. 8: 1280. https://doi.org/10.3390/foods13081280




