Effect of Ball-Milling Treatment Combined with Glycosylation on the Structure and Functional Properties of Litopenaeus vannamei Protein
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
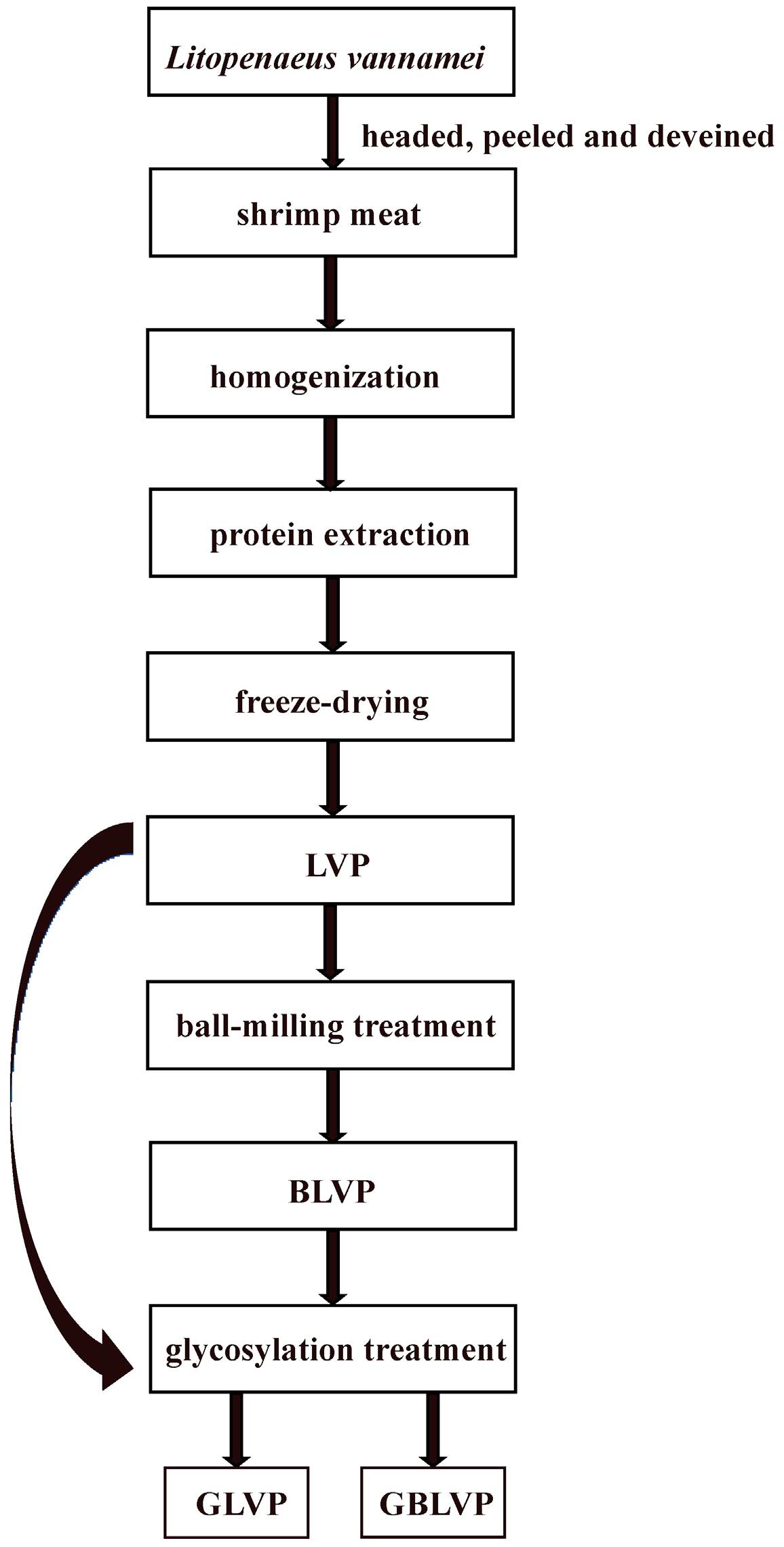
2.2.1. Preparation of LVP
2.2.2. Ball-Milling Treatment of LVP
2.2.3. Glycosylation of LVP and BLVP
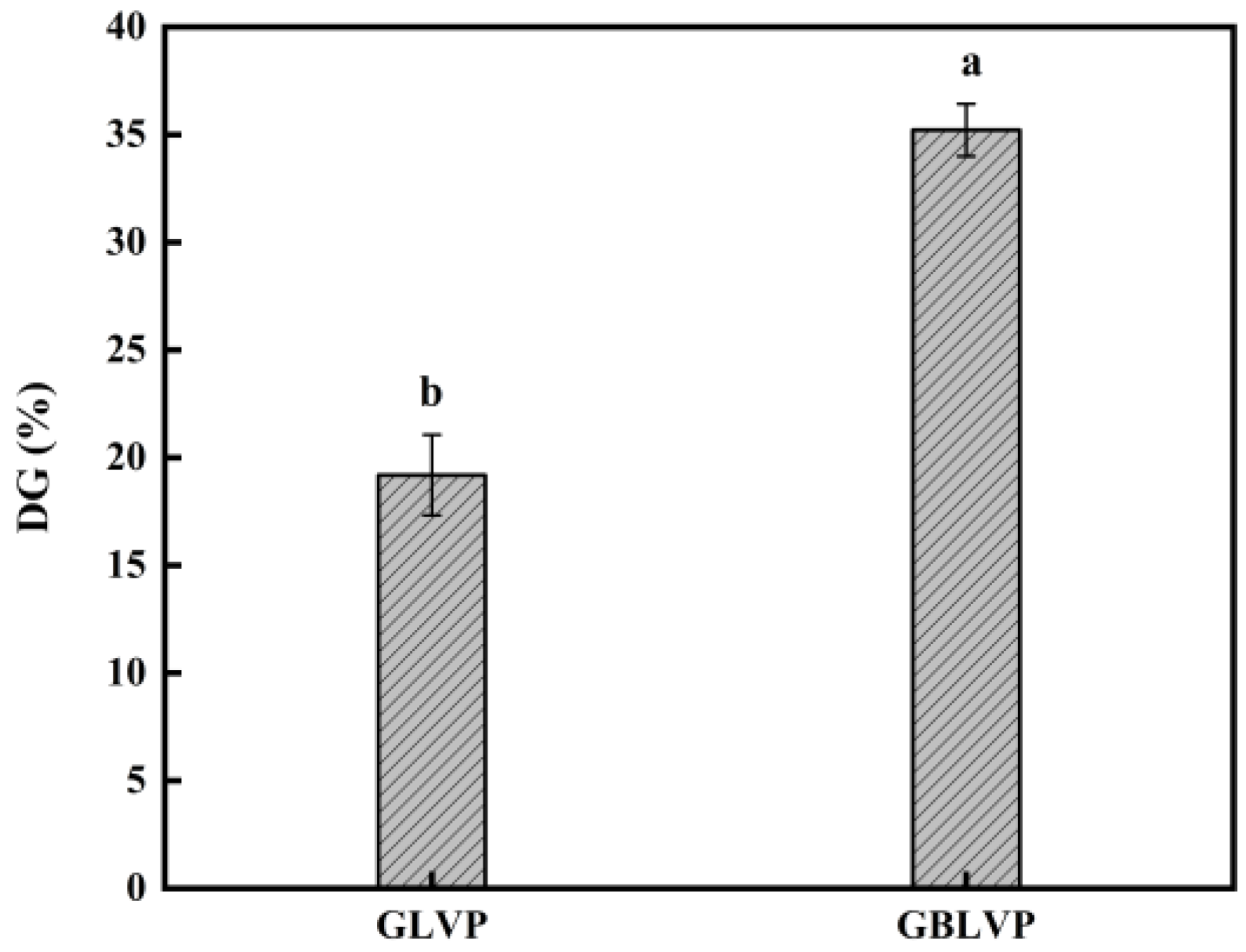
2.2.4. Measurement of Degree of Grafting
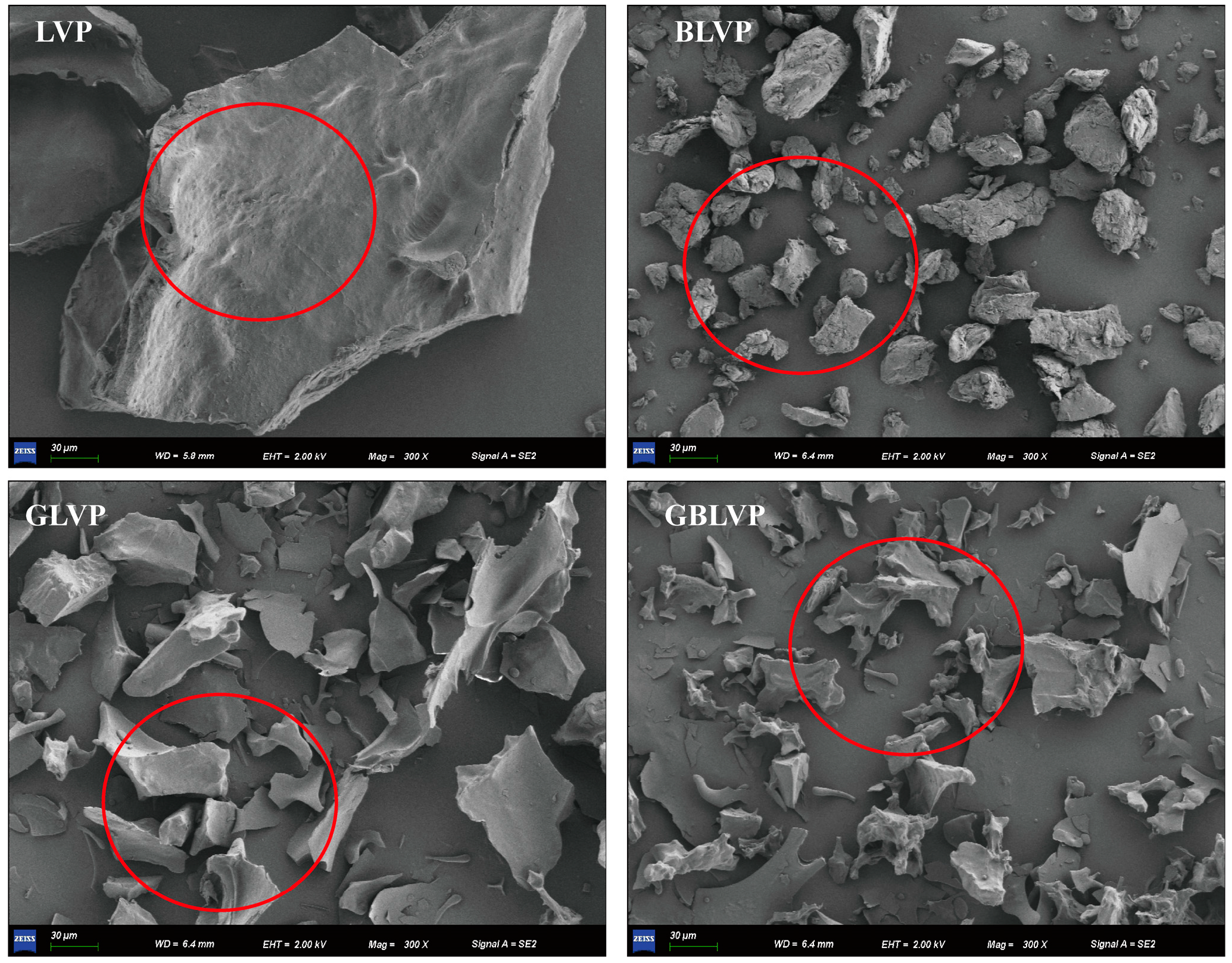
2.2.5. Scanning Electron Microscope Observation
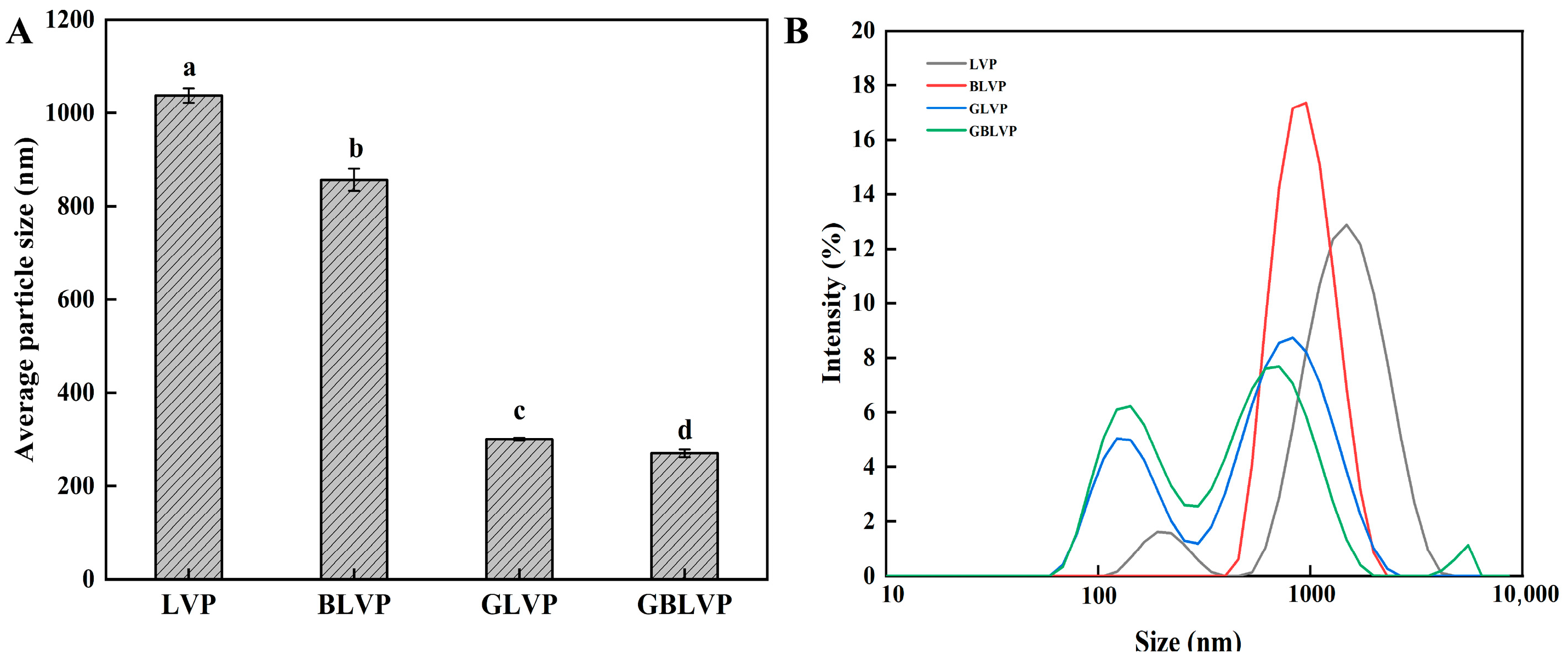
2.2.6. Measurement of Particle Size Distribution
2.2.7. Analysis of Protein Secondary Structure
2.2.8. Measurement of Surface Hydrophobicity
2.2.9. Fluorescence Spectroscopy Analysis
2.2.10. UV Full-Wavelength Scanning
2.2.11. Measurement of Solubility
2.2.12. Measurement of Foaming Capacity and Foaming Stability
2.2.13. Measurement of Water-Holding Capacity and Oil-Holding Capacity
2.2.14. Measurement of In Vitro Digestibility
2.2.15. Measurement of Allergenicity
2.3. Statistical Analysis
3. Results and Discussion
3.1. Effect on the Degree of Grafting of LVP
3.2. Morphology Analysis of LVP after Different Treatments
3.2.1. Microstructure Analysis
3.2.2. Particle Size
3.3. Effects of Different Treatments on Structural Characterizations of LVP
3.3.1. Secondary Structure
3.3.2. Surface Hydrophobicity
3.3.3. Intrinsic Fluorescence Emission Spectra
3.3.4. Ultraviolet Absorption Spectra
3.4. Effects of Different Treatments on Functional Properties of LVP
3.4.1. Solubility
3.4.2. FC and FS
3.4.3. WHC and OHC
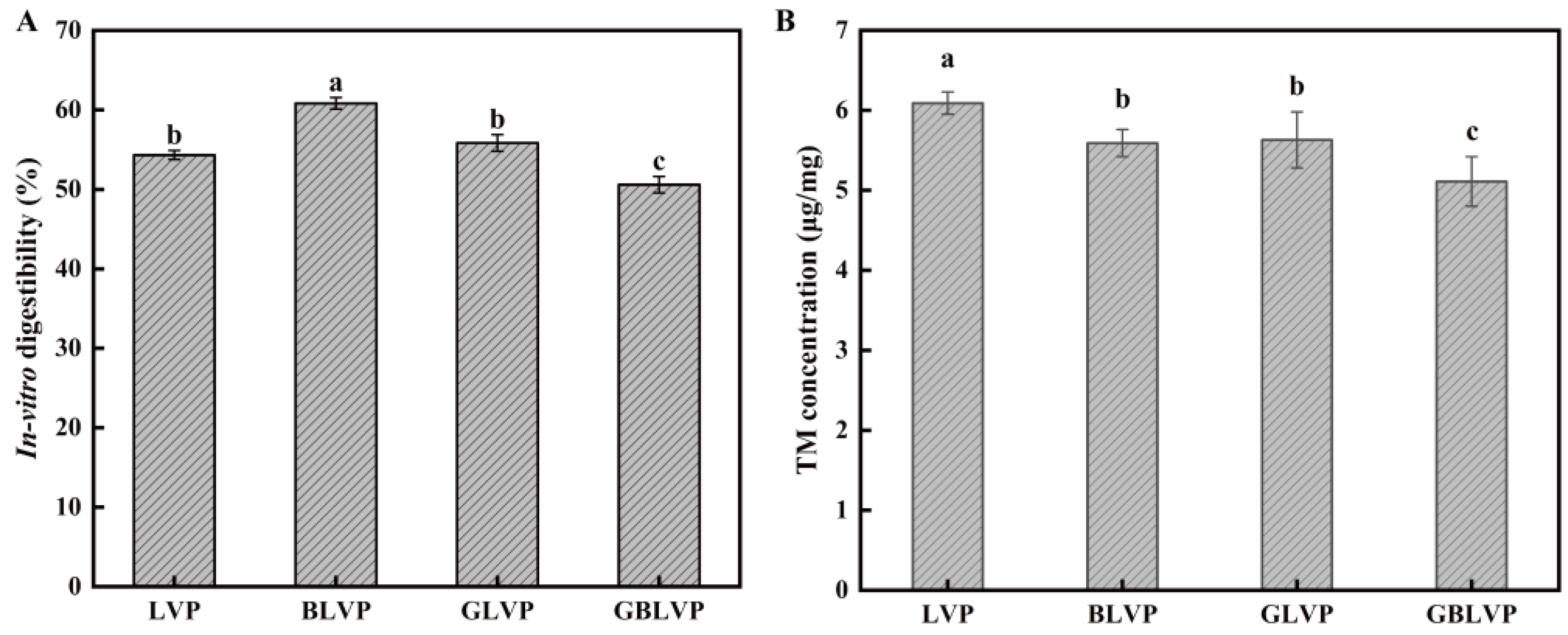
3.5. Effects of Different Treatments on In Vitro Digestibility and Allergenicity of LVP
3.5.1. In Vitro Digestibility
3.5.2. Allergenicity
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hasanthi, M.; Lee, K.-J. Dietary niacin requirement of pacific white shrimp (Litopenaeus vannamei). Aquaculture 2023, 566, 739169. [Google Scholar] [CrossRef]
- Chae, Y.; Kim, D.; Choi, M.J.; Cho, Y.; An, Y.J. Impact of nano-sized plastic on the nutritional value and gut microbiota of whiteleg shrimp Litopenaeus vannamei via dietary exposure. Environ. Int. 2019, 130, 104848. [Google Scholar] [CrossRef]
- Ding, Q.; Tian, G.; Wang, X.; Deng, W.; Mao, K.; Sang, Y. Effect of ultrasonic treatment on the structure and functional properties of mantle proteins from scallops (Patinopecten yessoensis). Ultrason. Sonochem. 2021, 79, 105770. [Google Scholar] [CrossRef] [PubMed]
- Ma, W.; Wang, J.; Wu, D.; Xu, X.; Du, M.; Wu, C. Effects of preheat treatment on the physicochemical and interfacial properties of cod proteins and its relation to the stability of subsequent emulsions. Food Hydrocoll. 2021, 112, 106338. [Google Scholar] [CrossRef]
- Huyst, A.M.R.; Deleu, L.J.; Luyckx, T.; Buyst, D.; Van Camp, J.; Delcour, J.A.; Van der Meeren, P. Colloidal stability of oil-in-water emulsions prepared from hen egg white submitted to dry and/or wet heating to induce amyloid-like fibril formation. Food Hydrocoll. 2022, 125, 107450. [Google Scholar] [CrossRef]
- Naik, R.R.; Wang, Y.; Selomulya, C. Improvements of plant protein functionalities by maillard conjugation and maillard reaction products. Crit. Rev. Food Sci. Nutr. 2022, 62, 7036–7061. [Google Scholar] [CrossRef] [PubMed]
- Dursun Capar, T.; Yalcin, H. Protein/polysaccharide conjugation via maillard reactions in an aqueous media: Impact of protein type, reaction time and temperature. LWT 2021, 152, 112252. [Google Scholar] [CrossRef]
- Yu, H.; Zhong, Q.; Liu, Y.; Guo, Y.; Xie, Y.; Zhou, W.; Yao, W. Recent advances of ultrasound-assisted maillard reaction. Ultrason. Sonochem. 2020, 64, 104844. [Google Scholar] [CrossRef] [PubMed]
- Dumitrascu, L.; Borda, D.; Aprodu, I. Alternative processing options for improving the proteins functionality by maillard conjugation. Foods 2023, 12, 3588. [Google Scholar] [CrossRef]
- Julakanti, S.; Charles, A.P.R.; Zhao, J.; Bullock, F.; Syed, R.; Myles, Y.; Wu, Y. Hempseed protein (Cannabis sativa L.): Influence of extraction ph and ball milling on physicochemical and functional properties. Food Hydrocoll. 2023, 143, 108835. [Google Scholar] [CrossRef]
- Chitrakar, B.; Zhang, M.; Zhang, X.; Devahastin, S. Bioactive dietary fiber powder from asparagus leaf by-product: Effect of low-temperature ball milling on physico-chemical, functional and microstructural characteristics. Powder Technol. 2020, 366, 275–282. [Google Scholar] [CrossRef]
- Wu, Y.; Zhang, Y.; Duan, W.; Wang, Q.; An, F.; Luo, P.; Huang, Q. Ball-milling is an effective pretreatment of glycosylation modified the foaming and gel properties of egg white protein. J. Food Eng. 2022, 319, 110908. [Google Scholar] [CrossRef]
- Li, J.; Dai, Z.; Chen, Z.; Hao, Y.; Wang, S.; Mao, X. Improved gelling and emulsifying properties of myofibrillar protein from frozen shrimp (Litopenaeus vannamei) by high-intensity ultrasound. Food Hydrocoll. 2023, 135, 108188. [Google Scholar] [CrossRef]
- Zhang, K.; Li, N.; Li, J.; Wang, Y.; Liu, C.; Liu, Y.; Liu, X.; Zhou, D.; Li, D. Improving the gelation and digestive properties of myofibrillar protein in litopenaeus vannamei by ultra-high pressure. Food Biosci. 2023, 56, 103402. [Google Scholar] [CrossRef]
- Dong, X.; Wang, J.; Raghavan, V. Impact of microwave processing on the secondary structure, in-vitro protein digestibility and allergenicity of shrimp (Litopenaeus vannamei) proteins. Food Chem. 2021, 337, 127811. [Google Scholar] [CrossRef]
- Duppeti, H.; Manjabhatta, S.N.; Kempaiah, B.B. Physicochemical, structural, functional and flavor adsorption properties of white shrimp (Penaeus vannamei) proteins as affected by processing methods. Food Res. Int. 2023, 163, 112296. [Google Scholar] [CrossRef]
- Long, F.; Yang, X.; Wang, R.; Hu, X.; Chen, F. Effects of combined high pressure and thermal treatments on the allergenic potential of shrimp (Litopenaeus vannamei) tropomyosin in a mouse model of allergy. Innov. Food Sci. Emerg. Technol. 2015, 29, 119–124. [Google Scholar] [CrossRef]
- Zhang, T.; Wang, J.; Feng, J.; Liu, Y.; Suo, R.; Ma, Q.; Sun, J. Effects of ultrasonic-microwave combination treatment on the physicochemical, structure and gel properties of myofibrillar protein in penaeus vannamei (Litopenaeus vannamei) surimi. Ultrason. Sonochem. 2022, 90, 106218. [Google Scholar] [CrossRef]
- Nasrollahzadeh, F.; Varidi, M.; Koocheki, A.; Hadizadeh, F. Effect of microwave and conventional heating on structural, functional and antioxidant properties of bovine serum albumin-maltodextrin conjugates through maillard reaction. Food Res. Int. 2017, 100, 289–297. [Google Scholar] [CrossRef] [PubMed]
- Yu, C.; Cha, Y.; Wu, F.; Fan, W.; Xu, X.; Du, M. Effects of ball-milling treatment on mussel (Mytilus edulis) protein: Structure, functional properties and in vitro digestibility. Int. J. Food Sci. Technol. 2017, 53, 683–691. [Google Scholar] [CrossRef]
- Hadidi, M.; Jafarzadeh, S.; Ibarz, A. Modified mung bean protein: Optimization of microwave-assisted phosphorylation and its functional and structural characterizations. LWT 2021, 151, 112119. [Google Scholar] [CrossRef]
- Chelh, I.; Gatellier, P.; Sante-Lhoutellier, V. Technical note: A simplified procedure for myofibril hydrophobicity determination. Meat Sci. 2006, 74, 681–683. [Google Scholar] [CrossRef] [PubMed]
- Amiratashani, F.; Yarmand, M.S.; Kiani, H.; Askari, G.; Naeini, K.K.; Parandi, E. Comprehensive structural and functional characterization of a new protein-polysaccharide conjugate between grass pea protein (Lathyrus sativus) and xanthan gum produced by wet heating. Int. J. Biol. Macromol. 2024, 254, 127283. [Google Scholar] [CrossRef] [PubMed]
- Kotnala, B.; Panneerselvam, V.; Vijayakumar, A.K. Physicochemical, structural, and functional characterization of guar meal protein isolate (Cyamopsis tetragonoloba). Heliyon 2024, 10, e24925. [Google Scholar] [CrossRef] [PubMed]
- Ortega, M.L.S.; Orellana-Palacios, J.C.; Garcia, S.R.; Rabanal-Ruiz, Y.; Moreno, A.; Hadidi, M. Olive leaf protein: Extraction optimization, in vitro digestibility, structural and techno-functional properties. Int. J. Biol. Macromol. 2024, 256, 128273. [Google Scholar] [CrossRef] [PubMed]
- Dong, X.; Wang, J.; Raghavan, V. Effects of high-intensity ultrasound processing on the physiochemical and allergenic properties of shrimp. Innov. Food Sci. Emerg. Technol. 2020, 65, 102441. [Google Scholar] [CrossRef]
- Pirestani, S.; Nasirpour, A.; Keramat, J.; Desobry, S. Preparation of chemically modified canola protein isolate with gum arabic by means of maillard reaction under wet-heating conditions. Carbohydr. Polym. 2017, 155, 201–207. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Zhao, H.; Wang, H.; Xu, P.; Chen, M.; Xu, Z.; Wen, L.; Cui, B.; Yu, B.; Zhao, H.; et al. Preparation of high-solubility rice protein using an ultrasound-assisted glycation reaction. Food Res. Int. 2022, 161, 111737. [Google Scholar] [CrossRef] [PubMed]
- Yousefi, N.; Abbasi, S. Food proteins: Solubility & thermal stability improvement techniques. Food Chem. Adv. 2022, 1, 100090. [Google Scholar] [CrossRef]
- Li, C.; Li, W.; Zhang, X.; Wang, G.; Liu, X.; Wang, Y.; Sun, L. The changed structures of cyperus esculentus protein decide its modified physicochemical characters: Effects of ball-milling, high pressure homogenization and cold plasma treatments on structural and functional properties of the protein. Food Chem. 2024, 430, 137042. [Google Scholar] [CrossRef] [PubMed]
- Kosaraju, S.L.; Weerakkody, R.; Augustin, M.A. Chitosan–lucose conjugates: Influence of extent of maillard reaction on antioxidant properties. J. Agric. Food Chem. 2010, 58, 12449–12455. [Google Scholar] [CrossRef] [PubMed]
- Karbasi, M.; Askari, G. Modification of whey protein microgel particles with mono- oligo- and polysaccharides through the maillard reaction: Effects on structural and techno-functional properties. Food Struct. 2021, 28, 100184. [Google Scholar] [CrossRef]
- Aziznia, S.; Askari, G.; Emamdjomeh, Z.; Salami, M. Effect of ultrasonic assisted grafting on the structural and functional properties of mung bean protein isolate conjugated with maltodextrin through maillard reaction. Int. J. Biol. Macromol. 2024, 254, 127616. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.-Q.; Sheng, H.-B.; Zhou, J.-Y.; Yuan, P.-P.; Zhang, X.-F.; Lu, M.-L.; Gu, R.-X. The effect of a variable initial pH on the structure and rheological properties of whey protein and monosaccharide gelation via the maillard reaction. Int. Dairy J. 2021, 113, 104896. [Google Scholar] [CrossRef]
- Dnyaneshwar Patil, N.; Bains, A.; Kaur, S.; Yadav, R.; Ali, N.; Patil, S.; Goksen, G.; Chawla, P. Influence of dual succinylation and ultrasonication modification on the amino acid content, structural and functional properties of chickpea (Cicer arietinum L.) protein concentrate. Food Chem. 2024, 445, 138671. [Google Scholar] [CrossRef] [PubMed]
- Chailangka, A.; Seesuriyachan, P.; Wangtueai, S.; Ruksiriwanich, W.; Jantanasakulwong, K.; Rachtanapun, P.; Sommano, S.R.; Leksawasdi, N.; Barba, F.J.; Phimolsiripol, Y. Cricket protein conjugated with different degrees of polymerization saccharides by maillard reaction as a novel functional ingredient. Food Chem. 2022, 395, 133594. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Cui, Q.; Wang, G.; Liu, J.; Chen, S.; Wang, X.; Wang, X.; Jiang, L. Relationship between surface functional properties and flexibility of soy protein isolate-glucose conjugates. Food Hydrocoll. 2019, 95, 349–357. [Google Scholar] [CrossRef]
- Dev, M.J.; Pandit, A.B.; Singhal, R.S. Ultrasound assisted vis-à-vis classical heating for the conjugation of whey protein isolate-gellan gum: Process optimization, structural characterization and physico-functional evaluation. Innov. Food Sci. Emerg. Technol. 2021, 72, 102724. [Google Scholar] [CrossRef]
- Parandi, E.; Mousavi, M.; Assadpour, E.; Kiani, H.; Jafari, S.M. Sesame protein hydrolysate-gum arabic maillard conjugates for loading natural anthocyanins: Characterization, in vitro gastrointestinal digestion and storage stability. Food Hydrocoll. 2024, 148, 109490. [Google Scholar] [CrossRef]
- Li, C.; Xue, H.; Chen, Z.; Ding, Q.; Wang, X. Comparative studies on the physicochemical properties of peanut protein isolate-polysaccharide conjugates prepared by ultrasonic treatment or classical heating. Food Res. Int. 2014, 57, 1–7. [Google Scholar] [CrossRef]
- Vera, A.; Valenzuela, M.A.; Yazdani-Pedram, M.; Tapia, C.; Abugoch, L. Conformational and physicochemical properties of quinoa proteins affected by different conditions of high-intensity ultrasound treatments. Ultrason. Sonochem. 2019, 51, 186–196. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.-J.; Ji, H.; Chen, Y.; Zhang, Y.-F.; Zheng, X.-C.; Li, S.-H.; Chen, Y. Analysis of the glycosylation products of peanut protein and lactose by cold plasma treatment: Solubility and structural characteristics. Int. J. Biol. Macromol. 2020, 158, 1194–1203. [Google Scholar] [CrossRef] [PubMed]
- Fu, J.-J.; Yu, J.-X.; He, F.-Y.; Huang, Y.-N.; Wu, Z.-P.; Chen, Y.-W. Physicochemical and functional characteristics of glycated collagen protein from giant salamander skin induced by ultrasound maillard reaction. Int. J. Biol. Macromol. 2024, 254, 127558. [Google Scholar] [CrossRef]
- Singh, T.P.; Sogi, D.S. Comparative study of structural and functional characterization of bran protein concentrates from superfine, fine and coarse rice cultivars. Int. J. Biol. Macromol. 2018, 111, 281–288. [Google Scholar] [CrossRef] [PubMed]
- Fu, X.; Liu, Q.; Tang, C.; Luo, J.; Wu, X.; Lu, L.; Cai, Z. Study on structural, rheological and foaming properties of ovalbumin by ultrasound-assisted glycation with xylose. Ultrason. Sonochem. 2019, 58, 104644. [Google Scholar] [CrossRef] [PubMed]
- Mitra, B.; Kristensen, L.; Lametsch, R.; Ruiz-Carrascal, J. Cooking affects pork proteins in vitro rate of digestion due to different structural and chemical modifications. Meat Sci. 2022, 192, 108924. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Wan, Y.; Ren, L.; Li, M.; Lv, Y.; Guo, S.; Waqar, K. Physical-chemical properties and in vitro digestibility of phosphorylated and glycosylated soy protein isolate. LWT 2021, 152, 112380. [Google Scholar] [CrossRef]
- Liu, Y.; Lin, S.; Liu, K.; Wang, S.; Sun, N. Gastrointestinal digestion products of shrimp (Penaeus vannamei) proteins retain an allergenic potential. Food Res. Int. 2022, 162, 111916. [Google Scholar] [CrossRef] [PubMed]
- Arena, S.; Salzano, A.M.; Renzone, G.; D’Ambrosio, C.; Scaloni, A. Non-enzymatic glycation and glycoxidation protein products in foods and diseases: An interconnected, complex scenario fully open to innovative proteomic studies. Mass Spectrom. Rev. 2013, 33, 49–77. [Google Scholar] [CrossRef]
- Zhang, Z.; Xiao, H.; Zhou, P. Allergenicity suppression of tropomyosin from exopalaemon modestus by glycation with saccharides of different molecular sizes. Food Chem. 2019, 288, 268–275. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Huan, F.; Han, T.-J.; Liu, S.-H.; Li, M.-S.; Yang, Y.; Wu, Y.-H.; Chen, G.-X.; Cao, M.-J.; Liu, G.-M. Combination processing method reduced ige-binding activity of litopenaeus vannamei by modifying lysine, arginine, and cysteine on multiple allergen epitopes. J. Agric. Food Chem. 2021, 69, 4865–4873. [Google Scholar] [CrossRef] [PubMed]
- Teodorowicz, M.; van Neerven, J.; Savelkoul, H. Food processing: The influence of the maillard reaction on immunogenicity and allergenicity of food proteins. Nutrients 2017, 9, 835. [Google Scholar] [CrossRef] [PubMed]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, D.; Liu, Y.; Guo, M.; Sun, J. Effect of Ball-Milling Treatment Combined with Glycosylation on the Structure and Functional Properties of Litopenaeus vannamei Protein. Foods 2024, 13, 1284. https://doi.org/10.3390/foods13091284
Wang D, Liu Y, Guo M, Sun J. Effect of Ball-Milling Treatment Combined with Glycosylation on the Structure and Functional Properties of Litopenaeus vannamei Protein. Foods. 2024; 13(9):1284. https://doi.org/10.3390/foods13091284
Chicago/Turabian StyleWang, Dan, Yangliu Liu, Mingzhu Guo, and Jilu Sun. 2024. "Effect of Ball-Milling Treatment Combined with Glycosylation on the Structure and Functional Properties of Litopenaeus vannamei Protein" Foods 13, no. 9: 1284. https://doi.org/10.3390/foods13091284




