Proteomic Profile of Flaxseed (Linum usitatissimum L.) Products as Influenced by Protein Concentration Method and Cultivar
Abstract
:1. Introduction
2. Materials and Methods
2.1. Flaxseeds
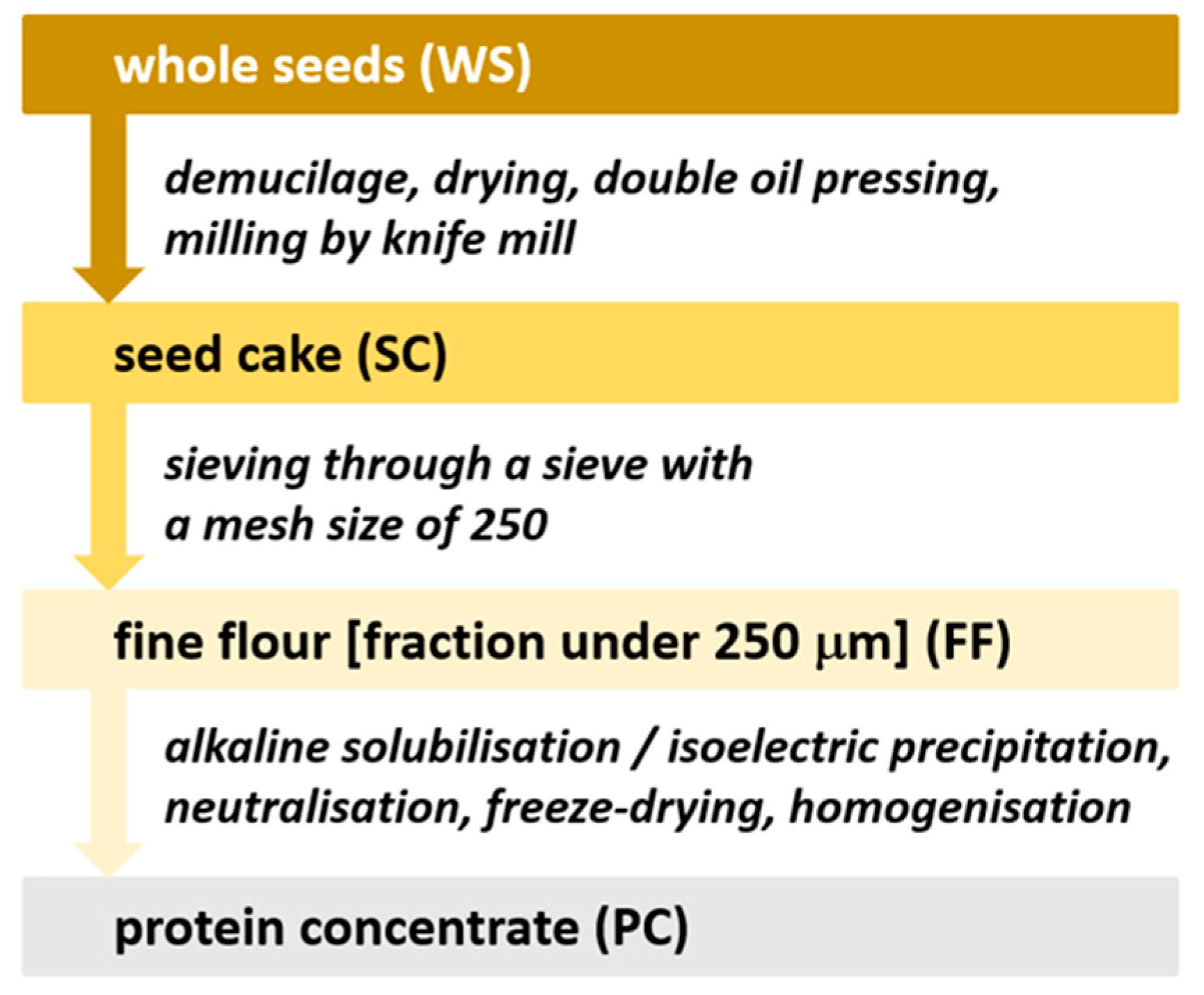
2.2. Preparation of Flaxseed Cake, Flour, and Protein Concentrate
2.3. Proximate Composition Analysis
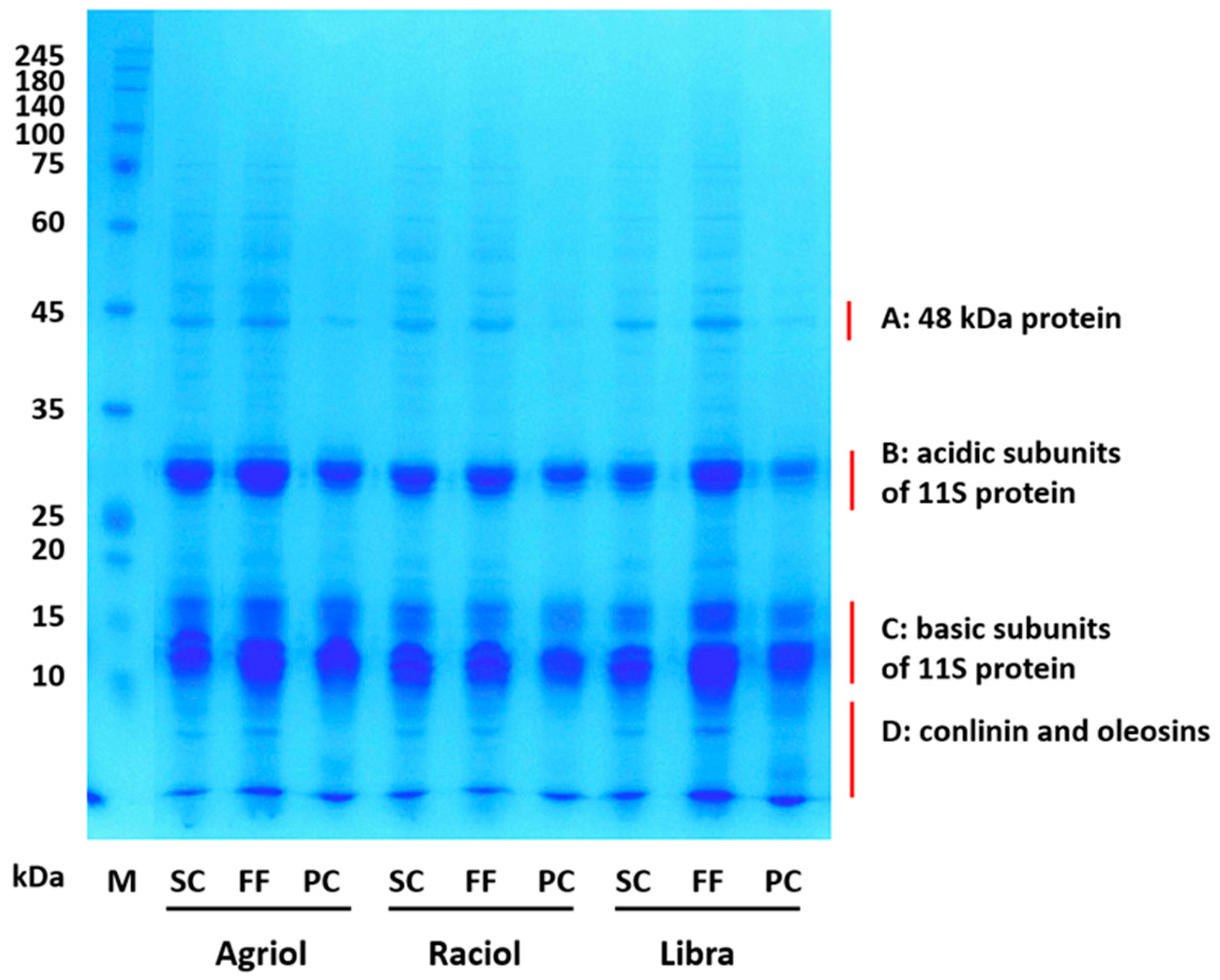
2.4. One-Dimensional Electrophoresis (SDS-PAGE)
2.5. LC-MS/MS Analysis and Proteomic Data Processing
2.6. Statistical Analysis
3. Results and Discussion
3.1. Proximate Composition of Flaxseed Products
3.2. Description of Main Identified Protein Groups in Flaxseed Products
3.2.1. Seed Storage Proteins
3.2.2. Oleosins
3.2.3. Defence- and Stress-Related Proteins
3.2.4. Other Selected Important Proteins
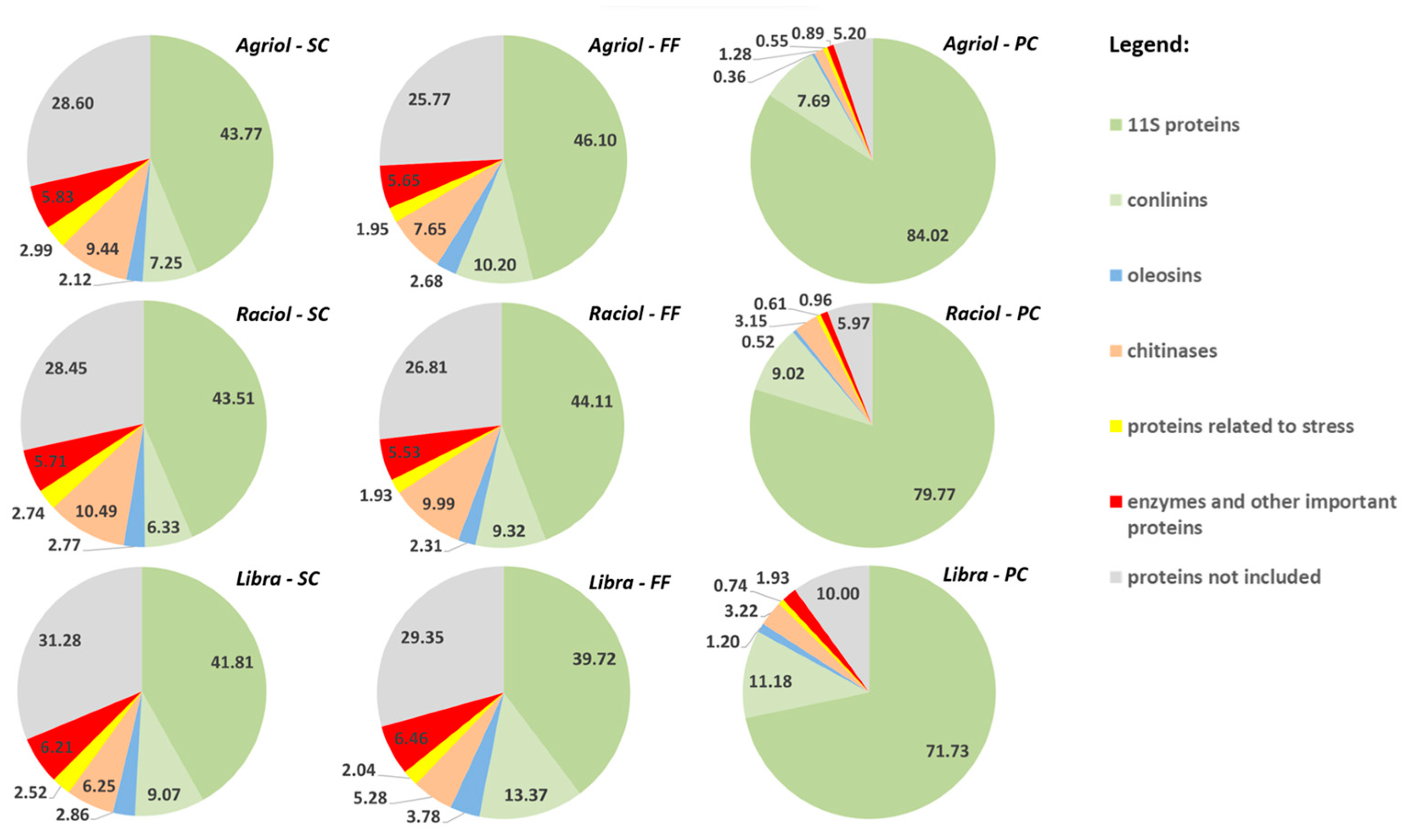
3.3. Influence of Cultivar and Concentration Method on Relative Abundance of Main Protein Groups
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Barvkar, V.T.; Pardeshi, V.C.; Kale, S.M.; Kadoo, N.Y.; Giri, A.P.; Gupta, V.S. Proteome Profiling of Flax (Linum usitatissimum) Seed: Characterization of Functional Metabolic Pathways Operating during Seed Development. J. Proteome Res. 2012, 11, 6264–6276. [Google Scholar] [CrossRef] [PubMed]
- Shim, Y.Y.; Gui, B.; Arnison, P.G.; Wang, Y.; Reaney, M.J.T. Flaxseed (Linum usitatissimum L.) Bioactive Compounds and Peptide Nomenclature: A Review. Trends Food Sci. Technol. 2014, 38, 5–20. [Google Scholar] [CrossRef]
- Marambe, H.K.; Wanasundara, J.P.D. Protein from Flaxseed (Linum usitatissimum L.). In Sustainable Protein Sources; Nadathur, S.R., Wanasundara, J.P.D., Scanlin, L., Eds.; Academic Press: Cambridge, MA, USA, 2017; Chapter 8; pp. 133–144. [Google Scholar]
- Bekhit, A.E.-D.A.; Shavandi, A.; Jodjaja, T.; Birch, J.; Teh, S.; Ahmed, I.A.M.; Al-Juhaimi, F.A.; Saeedi, P.; Bekhit, A.A. Flaxseed: Composition, Detoxification, Utilization, and Opportunities. Biocatal. Agric. Biotechnol. 2018, 13, 129–152. [Google Scholar] [CrossRef]
- Lan, Y.; Ohm, J.-B.; Chen, B.; Rao, J. Physicochemical Properties and Aroma Profiles of Flaxseed Proteins Extracted from Whole Flaxseed and Flaxseed Meal. Food Hydrocoll. 2020, 104, 105731. [Google Scholar] [CrossRef]
- Bueno-Díaz, C.; Biserni, C.; Martín-Pedraza, L.; de las Heras, M.; Blanco, C.; Vázquez-Cortés, S.; Fernández-Rivas, M.; Ba-tanero, E.; Cuesta-Herranz, J.; Villalba, M. Association Between the Seed Storage Proteins 2S Albumin and 11S Globulin and Severe Allergic Reaction after Flaxseed Intake. J. Investig. Allergol. Clin. Immunol. 2022, 32, 375–382. [Google Scholar] [CrossRef] [PubMed]
- Mueed, A.; Shibli, S.; Korma, S.A.; Madjirebaye, P.; Esatbeyoglu, T.; Deng, Z. Flaxseed Bioactive Compounds: Chemical Composition, Functional Properties, Food Applications and Health Benefits-Related Gut Microbes. Foods 2022, 11, 3307. [Google Scholar] [CrossRef] [PubMed]
- Čeh, B.; Štraus, S.; Hladnik, A.; Kušar, A. Impact of Linseed Variety, Location and Production Year on Seed Yield, Oil Content and Its Composition. Agronomy 2020, 10, 1770. [Google Scholar] [CrossRef]
- Nykter, M.; Kymäläinen, H.-R.; Gates, F. Quality Characteristics of Edible Linseed Oil. Agric. Food Sci. 2006, 15, 402–413. [Google Scholar] [CrossRef]
- Rabetafika, H.N.; Van Remoortel, V.; Danthine, S.; Paquot, M.; Blecker, C. Flaxseed Proteins: Food Uses and Health Benefits. Int. J. Food Sci. Technol. 2011, 46, 221–228. [Google Scholar] [CrossRef]
- Anastasiu, A.-E.; Chira, N.-A.; Banu, I.; Ionescu, N.; Stan, R.; Rosca, S.-I. Oil productivity of seven Romanian linseed varieties as affected by weather conditions. Ind. Crop. Prod. 2016, 86, 219–230. [Google Scholar] [CrossRef]
- Komartin, R.S.; Stroescu, M.; Chira, N.; Stan, R.; Stoica-Guzun, A. Optimization of oil extraction from Lallemantia iberica seeds using ultrasound-assisted extraction. J. Food Meas. Charact. 2021, 15, 2010–2020. [Google Scholar] [CrossRef]
- Gutiérrez, C.; Rubilar, M.; Jara, C.; Verdugo, M.; Sineiro, J.; Shene, C. Flaxseed and Flaxseed Cake as a Source of Compounds for Food Industry. J. Soil Sci. Plant Nutr. 2010, 10, 454–463. [Google Scholar] [CrossRef]
- Kaushik, P.; Dowling, K.; McKnight, S.; Barrow, C.J.; Wang, B.; Adhikari, B. Preparation, Characterization and Functional Properties of Flax Seed Protein Isolate. Food Chem. 2016, 197 Pt A, 212–220. [Google Scholar] [CrossRef]
- Wu, S.; Wang, X.; Qi, W.; Guo, Q. Bioactive Protein/Peptides of Flaxseed: A Review. Trends Food Sci. Technol. 2019, 92, 184–193. [Google Scholar] [CrossRef]
- Bárta, J.; Bártová, V.; Jarošová, M.; Švajner, J. Proteins of Oilseed Cakes, Their Isolation and Usage Possibilities. Chem. Listy 2021, 115, 472–480. (In Czech) [Google Scholar]
- Maghaydah, S.; Alkahlout, A.; Abughoush, M.; Al Khalaileh, N.I.; Olaimat, A.N.; Al-Holy, M.A.; Ajo, R.; Choudhury, I.; Hayajneh, W. Novel Gluten-Free Cinnamon Rolls by Substituting Wheat Flour with Resistant Starch, Lupine and Flaxseed Flour. Foods 2022, 11, 1022. [Google Scholar] [CrossRef] [PubMed]
- Waszkowiak, K.; Mikołajczak, B. The Effect of Roasting on the Protein Profile and Antiradical Capacity of Flaxseed Meal. Foods 2020, 9, 1383. [Google Scholar] [CrossRef] [PubMed]
- Perreault, V.; Hénaux, L.; Bazinet, L.; Doyen, A. Pretreatment of flaxseed protein isolate by high hydrostatic pressure: Impacts on Protein Structure, Enzymatic Hydrolysis and Final Hydrolysate Antioxidant Capacities. Food Chem. 2017, 221, 1805–1812. [Google Scholar] [CrossRef] [PubMed]
- Logarušić, M.; Radošević, K.; Bis, A.; Panić, M.; Slivac, I.; Srček, V.G. Biological Potential of Flaxseed Protein Hydrolysates Obtained by Different Proteases. Plant Foods Hum. Nutr. 2020, 75, 518–524. [Google Scholar] [CrossRef] [PubMed]
- Ye, X.-P.; Xu, M.-F.; Tang, Z.-X.; Chen, H.-J.; Wu, D.-T.; Wang, Z.-Y.; Songzhen, Y.-X.; Hao, J.; Wu, L.-M.; Shi, L.-E. Flaxseed Protein: Extraction, Functionalities and Applications. Food Sci. Technol. 2022, 42, e22021. [Google Scholar] [CrossRef]
- Qin, X.; Li, L.; Yu, X.; Deng, Q.; Xiang, Q.; Zhu, Y. Comparative Composition Structure and Selected Techno-Functional Elucidation of Flaxseed Protein Fractions. Foods 2022, 11, 1820. [Google Scholar] [CrossRef] [PubMed]
- Madhusudhan, K.T.; Singh, N. Isolation and Characterization of a Small Molecular Weight Protein of Linseed Meal. Phytochemistry 1985, 24, 2507–2509. [Google Scholar] [CrossRef]
- Marcone, M.F.; Kakuda, Y.; Yada, R.Y. Salt-soluble Seed Globulins of Various Dicotyledonous and Monocotyledonous Plants—I. Isolation/Purification and Characterization. Food Chem. 1998, 62, 27–47. [Google Scholar] [CrossRef]
- Chung, M.W.Y.; Lei, B.; Li-Chan, E.C.Y. Isolation and Structural Characterization of the Major Protein Fraction from NorMan Flaxseed (Linum usitatissimum L.). Food Chem. 2005, 90, 271–279. [Google Scholar] [CrossRef]
- Lorenc, F.; Jarošová, M.; Bedrníček, J.; Smetana, P.; Bárta, J. Structural Characterization and Functional Properties of Flaxseed Hydrocolloids and Their Application. Foods 2022, 11, 2304. [Google Scholar] [CrossRef] [PubMed]
- Kotecka-Majchrzak, K.; Sumara, A.; Fornal, E.; Montowska, M. Proteomic Analysis of Oilseed Cake: A Comparative Study of Species-Specific Proteins and Peptides Extracted from Ten Seed Species. J. Sci. Food Agric. 2021, 101, 297–306. [Google Scholar] [CrossRef] [PubMed]
- Merkher, Y.; Kontareva, E.; Alexandrova, A.; Javaraiah, R.; Pustovalova, M.; Leonov, S. Anti-Cancer Properties of Flaxseed Proteome. Proteomes 2023, 11, 37. [Google Scholar] [CrossRef] [PubMed]
- Klubicová, K.; Berčák, M.; Danchenko, M.; Skultety, L.; Rashydov, N.M.; Berezhna, V.V.; Miernyk, J.A.; Hajduch, M. Agricultural Recovery of a Formerly Radioactive Area: I. Establishment of High-resolution Quantitative Protein Map of Mature Flax Seeds Harvested from the Remediated Chernobyl Area. Phytochemistry 2011, 72, 1308–1315. [Google Scholar] [CrossRef] [PubMed]
- Bárta, J.; Bártová, V.; Jarošová, M.; Švajner, J.; Smetana, P.; Kadlec, J.; Filip, V.; Kyselka, J.; Berčíková, M.; Zdráhal, Z.; et al. Oilseed Cake Flour Composition, Functional Properties and Antioxidant Potential as Effects of Sieving and Species Differences. Foods 2021, 10, 2766. [Google Scholar] [CrossRef] [PubMed]
- Laemmli, U.K. Cleavage of Structural Proteins during the Assembly of the Head of Bacteriophage T4. Nature 1970, 227, 680–685. [Google Scholar] [CrossRef] [PubMed]
- Bárta, J.; Roudnický, P.; Jarošová, M.; Zdráhal, Z.; Stupková, A.; Bártová, V.; Krejčová, Z.; Kyselka, J.; Filip, V.; Říha, V.; et al. Proteomic Profiles of Whole Seeds, Hulls, and Dehulled Seeds of Two Industrial Hemp (Cannabis sativa L.) Cultivars. Plants 2024, 13, 111. [Google Scholar] [CrossRef]
- Wiśniewski, J.R.; Zougman, A.; Nagaraj, N.; Mann, M. Universal Sample Preparation Method for Proteome Analysis. Nat. Methods 2009, 6, 359–362. [Google Scholar] [CrossRef] [PubMed]
- Demichev, V.; Messner, C.B.; Vernardis, S.I.; Lilley, K.S.; Ralser, M. DIA-NN: Neural Networks and Interference Correction Enable Deep Proteome Coverage in High Throughput. Nat. Methods 2020, 17, 41–44. [Google Scholar] [CrossRef] [PubMed]
- He, B.; Shi, J.; Wang, X.; Jiang, H.; Zhu, J.H. Label-free Absolute Protein Quantification with Data-independent Acquisition. J. Proteom. 2019, 200, 51–59. [Google Scholar] [CrossRef] [PubMed]
- Oomah, B.D.; Mazza, G. Flaxseed Proteins—A Review. Food Chem. 1993, 48, 109–114. [Google Scholar] [CrossRef]
- Wanasundara, P.K.J.P.D.; Shahidi, F. Removal of Flaxseed Mucilage by Chemical and Enzymatic Treatments. Food Chem. 1997, 59, 47–55. [Google Scholar] [CrossRef]
- Waszkowiak, K.; Mikołajczak, B.; Kmiecik, D. Changes in Oxidative Stability and Protein Profile of Flaxseeds Resulting from Thermal Pre-treatment. J. Sci. Food Agric. 2018, 98, 5459–5469. [Google Scholar] [CrossRef] [PubMed]
- Waszkowiak, K.; Mikołajczak, B.; Polanowska, K.; Wieruszewski, M.; Siejak, P.; Smułek, W.; Jarzębski, M. Protein Fractions from Flaxseed: The Effect of Subsequent Extractions on Composition and Antioxidant Capacity. Antioxidants 2023, 12, 675. [Google Scholar] [CrossRef] [PubMed]
- Shewry, P.R. Seed proteins. In Seed Technology and its Biological Basis; Black, M., Bewley, J.D., Eds.; Sheffield Academic Press Ltd.: Sheffield, UK; CRC Press LLC: Boca Raton, FL, USA, 2000; pp. 42–84. [Google Scholar]
- Liu, J.; Shim, Y.Y.; Poth, A.G.; Reaney, M.J.T. Conlinin in Flaxseed (Linum usitatissimum L.) Gum and Its Contribution to Emulsification Properties. Food Hydrocoll. 2016, 52, 963–971. [Google Scholar] [CrossRef]
- Truksa, M.; MacKenzie, S.L.; Qiu, X. Molecular Analysis of Flax 2S Storage Protein Conlinin and Seed Specific Activity of Its Promoter. Plant Physiol. Biochem. 2003, 41, 141–147. [Google Scholar] [CrossRef]
- Souza, P.F.N. The forgotten 2S albumin proteins: Importance, Structure, and Biotechnological Application in Agriculture and Human Health. Int. J. Biol. Macromol. 2020, 164, 4638–4649. [Google Scholar] [CrossRef] [PubMed]
- Moreno, F.J.; Clemente, A. 2S Albumin Storage Proteins: What Makes them Food Allergens? Open Biochem. J. 2008, 2, 16–28. [Google Scholar] [CrossRef] [PubMed]
- Bhatla, S.C.; Kaushik, V.; Yadav, M.K. Use of Oil bodies and Oleosins in Recombinant Protein Production and Other Biotechnological Applications. Biotechnol. Adv. 2010, 28, 293–300. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Wang, R.; He, S.; Cheng, C.; Ma, Y. The Stability and Gastro-intestinal Digestion of Curcumin Emulsion Stabilized with Soybean Oil Bodies. LWT 2020, 131, 109663. [Google Scholar] [CrossRef]
- Nikiforidis, C.V. Structure and Functions of Oleosomes (Oil Bodies). Adv. Colloid Interface Sci. 2019, 274, 102039. [Google Scholar] [CrossRef] [PubMed]
- Acevedo-Fani, A.; Dave, A.; Singh, H. Nature-Assembled Structures for Delivery of Bioactive Compounds and Their Potential in Functional Foods. Front. Chem. 2020, 8, 564021. [Google Scholar] [CrossRef] [PubMed]
- Kara, H.H.; Araiza-Calahorra, A.; Rigby, N.M.; Sarkar, A. Flaxseed Oleosomes: Responsiveness to Physicochemical Stresses, Tribological Shear and Storage. Food Chem. 2024, 431, 137160. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Chi, H.; Liu, C.; Zhang, T.; Han, L.; Li, L.; Pei, X.; Long, Y. Genome-Wide Identification and Functional Characterization of LEA Genes during Seed Development Process in Linseed Flax (Linum usitatissimum L.). BMC Plant Biol. 2021, 21, 193. [Google Scholar] [CrossRef] [PubMed]
- Carra, S.; Alberti, S.; Benesch, J.L.P.; Boelens, W.; Buchner, J.; Carver, J.A.; Cecconi, C.; Ecroyd, H.; Gusev, N.; Hightower, L.E.; et al. Small Heat Shock Proteins: Multifaceted Proteins with Important Implications for Life. Cell Stress Chaperon. 2019, 24, 295–308. [Google Scholar] [CrossRef] [PubMed]
- Waters, E.R.; Vierling, E. Plant Small Heat Shock Proteins—Evolutionary and Functional Diversity. New Phytol. 2020, 227, 24–37. [Google Scholar] [CrossRef] [PubMed]
- Clemente, M.; Corigliano, M.G.; Pariani, S.A.; Sánchez-López, E.F.; Sander, V.A.; Ramos-Duarte, V.A. Plant Serine Protease Inhibitors: Biotechnology Application in Agriculture and Molecular Farming. Int. J. Mol. Sci. 2019, 20, 1345. [Google Scholar] [CrossRef] [PubMed]
- Anaya, K.; Cruz, A.C.B.; Cunha, D.C.S.; Monteiro, S.M.N.; dos Santos, E.A. Growth Impairment Caused by Raw Linseed Consumption: Can Trypsin Inhibitors Be Harmful for Health? Plant Foods Hum. Nutr. 2015, 70, 338–343. [Google Scholar] [CrossRef] [PubMed]
- Shao, Q.; Liu, X.; Su, T.; Ma, C.; Wang, P. New Insights Into the Role of Seed Oil Body Proteins in Metabolism and Plant Development. Front. Plant Sci. 2019, 10, 1568. [Google Scholar] [CrossRef] [PubMed]
- Contreras del Mar, M.; Lama-Muñoz, A.; Gutiérrez-Pérez, J.M.; Espínola, F.; Moya, M.; Castro, E. Protein Extraction from Agri-food Residues for Integration in Biorefinery: Potential Techniques and Current Status. Bioresour. Technol. 2019, 280, 459–477. [Google Scholar] [CrossRef]
- Rodrigues, I.M.; Coelho, J.F.J.; Graça, M.; Carvalho, V.S. Isolation and Valorisation of Vegetable Proteins from Oilseed Plants: Methods, Limitations and Potential. J. Food Eng. 2012, 109, 337–346. [Google Scholar] [CrossRef]
- Hadidi, M.; Aghababaei, F.; McClements, D.J. Enhanced Alkaline Extraction Techniques for Isolating and Modifying Plant-Based Proteins. Food Hydrocoll. 2023, 145, 109132. [Google Scholar] [CrossRef]
- Hou, F.; Ding, W.; Qu, W.; Oladejo, A.O.; Xiong, F.; Zhang, W.; He, R.; Ma, H. Alkali Solution Extraction of Rice Residue Protein Isolates: Influence of Alkali Concentration on Protein Functional, Structural Properties and Lysinoalanine Formation. Food Chem. 2017, 218, 207–215. [Google Scholar] [CrossRef] [PubMed]
| Component | Cultivar | WS | SC | FF | PC |
|---|---|---|---|---|---|
| Protein (% FM) | Agriol | 18.02 ± 0.58 Ad | 30.19 ± 1.24 Ac | 35.81 ± 0.50 Ab | 78.34 ± 0.52 Aa |
| Raciol | 17.06 ± 0.66 Ad | 29.27 ± 0.59 Ac | 33.87 ± 0.89 Ab | 70.09 ± 1.63 Ba | |
| Libra | 17.84 ± 1.04 Ad | 30.42 ± 1.04 Ac | 36.02 ± 0.22 Ab | 70.87 ± 1.28 Ba | |
| Fat (% FM) | Agriol | 37.76 ± 0.61 Ba | 8.53 ± 0.47 Ac | 10.94 ± 0.04 Ab | 2.41 ± 0.77 Bd |
| Raciol | 38.24 ± 0.08 Ba | 9.46 ± 0.16 Ac | 12.04 ± 1.09 Ab | 2.42 ± 0.36 Bd | |
| Libra | 40.76 ± 0.54 Aa | 9.22 ±1.20 Ac | 12.21 ± 1.22 Ab | 5.04 ± 1.27 Ad | |
| Carbohydrates (% FM) | Agriol | 34.27 ± 0.47 Ac | 47.28 ± 1.23 Aa | 38.75 ± 0.66 Ab | 9.78 ± 0.54 Cd |
| Raciol | 34.86 ± 0.67 Ac | 48.01 ± 0.31 Aa | 39.68 ± 0.70 Ab | 19.41 ± 2.15 Ad | |
| Libra | 31.90 ± 1.69 Ac | 46.29 ± 0.91 Aa | 36.87 ± 0.82 Ab | 15.99 ± 0.32 Bd | |
| Ash (% FM) | Agriol | 3.43 ± 0.12 Ac | 5.36 ± 0.12 Bb | 6.28 ± 0.03 Ba | 2.71 ± 0.12 Ad |
| Raciol | 3.52 ± 0.09 Ac | 5.27 ± 0.08 Bb | 6.28 ± 0.02 Ba | 2.72 ± 0.08 Ad | |
| Libra | 3.52 ± 0.09 Ac | 5.78 ± 0.13 Ab | 7.01 ± 0.04 Aa | 2.82 ± 0.15 Ad | |
| Moisture (% FM) | Agriol | 6.52 ± 0.04 Ab | 8.63 ± 0.24 Aa | 8.22 ± 0.21 Aa | 6.76 ± 0.86 Ab |
| Raciol | 6.32 ± 0.05 Ab | 7.99 ± 0.32 Aa | 8.12 ± 0.13 Aa | 5.36 ± 1.03 Bb | |
| Libra | 5.98 ± 0.08 Ab | 8.29 ± 0.14 Aa | 7.89 ± 0.24 Aa | 5.27 ± 0.15 Bb |
| Accession | Name of Protein Groups | Description from UniProt KB/(Available Information from NCBI) |
|---|---|---|
| Seed storage proteins | ||
| CAI0558954.1 | unnamed protein product; LINTE | (cupin 11S legumin N/11S legumin seed storage globulin/N-terminal cupin) |
| CAI0432378.1 | unnamed protein product; LINTE | (11S globulin-like/11S globulin seed storage protein) |
| CAI0558846.1 | unnamed protein product; LINTE | (cupin_RmlC-like protein) |
| CAI0434421.1 | unnamed protein product; LINTE | (cupin_RmlC-like protein) 11S globulin |
| CAI0418748.1 | unnamed protein product; LINTE | (cupin_RmlC-like protein) |
| CAC94011.1 | conlinin; LINUS | 2S seed storage albumins family; nutrient reservoir activity |
| CAC94010.1 | conlinin; LINUS | 2S seed storage albumins family; nutrient reservoir activity |
| Oleosins | ||
| ABB01617.1 | oleosin low molecular weight isoform; LINUS | oleosin family; membrane; monolayer-surrounded lipid storage body |
| ABB01620.1 | oleosin low molecular weight isoform, partial; LINUS | oleosin family; membrane; monolayer-surrounded lipid storage body |
| ABB01616.1 | oleosin high molecular weight isoform; LINUS | oleosin family; membrane; monolayer-surrounded lipid storage body |
| ABB01624.1 | oleosin high molecular weight isoform; LINUS | oleosin family; membrane; monolayer-surrounded lipid storage body |
| ABB01618.1 | oleosin low molecular weight isoform; LINUS | oleosin family; membrane; monolayer-surrounded lipid storage body |
| ABB01619.1 | oleosin low molecular weight isoform, partial; LINUS | oleosin family; membrane; monolayer-surrounded lipid storage body |
| Defence and stress-related proteins | ||
| WMZ41542.1 | chitinase; LINUS | (chitinase; involved in somatic embryogenesis in flax) |
| AAW31878.1 | chitinase, partial; LINUS | chitinase activity; chitin binding; cell wall macromolecule catabolic process; defense response |
| AMY26620.1 | late embryogenesis abundant (lea) group 1-embryo development; LINUS | LEA type 1 family; embryo development ending in seed dormancy |
| AVY09180.1 | Sequence 3353 from patent US 9878004; UNKNOWN | (17.3 kDa class I heat shock protein) |
| CAI0408810.1 | unnamed protein product; LINTE | (luminal-binding protein 5; heat shock 70 kDa protein) |
| 1DWM_A | Chain A, Linum usitatissimum trypsin inhibitor; LINUS | (Serine proteinase inhibitor class) |
| Other selected important proteins | ||
| AVY09175.1 | Sequence 3348 from patent US 9878004; UNKNOWN | (11-beta-hydroxysteroid dehydrogenase) |
| CAI0407981.1 | unnamed protein product; LINTE | (glucose and ribitol dehydrogenase/NADPH-dependent aldehyde reductase 1) |
| CAF22093.1 | glyceraldehyde 3-phosphate dehydrogenase, partial; LINUS | glyceraldehyde-3-phosphate dehydrogenase family; oxidoreductase |
| BAL41455.1 | fructose-bisphosphate aldolase 1, partial; LINGR | class I fructose-bisphosphate aldolase family; fructose-bisphosphate aldolase activity; glycolytic process |
| P48417.1 | Allene oxide synthase, chloroplastic; LINUS | allene oxide synthase; activity; fatty acid biosynthetic process |
| ABM64783.1 | cell wall glycosidase, partial; LINUS | hydrolase activity, hydrolyzing O-glycosyl compounds; carbohydrate metabolic process |
| CAI0384355.1 | unnamed protein product; LINTE | (copper/zinc superoxide dismutase) |
| CAI0431450.1 | unnamed protein product; LINTE | (manganese superoxide dismutase) |
| CAI0412769.1 | unnamed protein product; LINTE | (linoleate 13S-lipoxygenase 2-1, chloroplastic; Lipoxygenase) |
| AVY09204.1 | Sequence 3377 from patent US 9878004; UNKNOWN | (hypothetical protein DKX38_015183; malate dehydrogenase) |
| CAI0440995.1 | unnamed protein product; LINTE | (putative mitochondrial malate dehydrogenase; malate dehydrogenase, mitochondrial) |
| CAI0411675.1 | unnamed protein product; LINTE | (elongation factor 1-alpha; translation elongation factor) |
| AGN56421.1 | non-specific lipid transfer protein 1; LINUS | lipid binding; lipid transport; plant LTP family |
| CAI0414382.1 | unnamed protein product; LINTE | (thaumatin like protein) |
| Accession | Protein Name | Agriol | Raciol | Libra | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| SC | FF | PC | SC | FF | PC | SC | FF | PC | ||
| Seed storage proteins | ||||||||||
| CAI0558954.1 | unnamed protein product; LINTE | 29.13 c ± 1.30 | 30.44 c ± 0.32 | 53.37 a ± 0.45 | 31.09 bc ± 6.62 | 29.37 c ± 0.43 | 51.71 a ± 1.63 | 26.34 c ± 1.10 | 23.34 c ± 0.28 | 38.83 b ± 4.24 |
| CAI0432378.1 | unnamed protein product; LINTE | 7.12 cd ± 0.14 | 8.06 cd ± 0.70 | 16.08 ab ± 0.83 | 6.39 d ± 0.77 | 7.94 cd ± 0.44 | 14.57 b ± 0.30 | 8.19 cd ± 0.93 | 8.89 c ± 0.19 | 16.88 a ± 0.95 |
| CAI0558846.1 | unnamed protein product; LINTE | 6.69 b ± 0.15 | 6.42 b ± 0.29 | 11.86 a ± 0.55 | 5.21 b ± 0.43 | 5.69 b ± 0.26 | 10.91 a ± 0.28 | 6.37 b ± 0.68 | 6.30 b ± 0.10 | 13.23 a ± 0.20 |
| CAI0434421.1 | unnamed protein product; LINTE | 0.82 bc ± 0.02 | 0.97 bc ± 0.05 | 2.44 a ± 0.10 | 0.65 c ± 0.15 | 0.92 bc ± 0.02 | 2.41 a ± 0.09 | 0.68 c ± 0.03 | 1.06 b ± 0.07 | 2.59 a ± 0.29 |
| CAI0418748.1 | unnamed protein product; LINTE | 0.01 a ± 0.01 | 0.21 a ± 0.02 | 0.27 a ± 0.01 | 0.17 a ± 0.02 | 0.19 a ± 0.03 | 0.17 a ± 0.15 | 0.23 a ± 0.01 | 0.13 a ± 0.11 | 0.20 a ± 0.21 |
| CAC94011.1 | conlinin; LINUS | 7.01 ab ± 1.09 | 9.62 ab ± 1.13 | 7.43 ab ± 1.41 | 5.90 b ± 1.29 | 9.01 ab ± 1.55 | 8.56 ab ± 0.93 | 7.72 ab ± 2.31 | 11.32 a ± 1.01 | 9.72 ab ± 2.51 |
| CAC94010.1 | conlinin; LINUS | 0.24 c ± 0.07 | 0.58 bc ± 0.30 | 0.26 c ± 0.04 | 0.43 c ± 0.16 | 0.31 c ± 0.16 | 0.46 c ± 0.27 | 1.35 ab ± 0.60 | 2.05 a ± 0.25 | 1.46 a ± 0.28 |
| Oleosins | ||||||||||
| ABB01617.1 | oleosin low molecular weight isoform; LINUS | 0.12 b ± 0.00 | 0.24 ab ± 0.23 | 0.03 b ± 0.02 | 0.23 ab ± 0.15 | 0.15 ab ± 0.01 | 0.05 b ± 0.03 | 0.17 ab ± 0.02 | 0.51 a ± 0.29 | 0.11 b ± 0.07 |
| ABB01620.1 | oleosin low molecular weight isoform, partial; LINUS | 0.17 a ± 0.01 | 0.29 a ± 0.20 | 0.03 a ± 0.01 | 0.30 a ± 0.12 | 0.17 a ± 0.01 | 0.06 a ± 0.03 | 0.26 a ± 0.11 | 0.38 a ± 0.26 | 0.12 a ± 0.07 |
| ABB01616.1 | oleosin high molecular weight isoform; LINUS | 0.98 b ± 0.60 | 1.11 ab ± 0.46 | 0.12 c ± 0.03 | 1.77 a ± 0.11 | 0.60 bc ± 0.01 | 0.16 c ± 0.03 | 0.95 b ± 0.19 | 1.09 ab ± 0.11 | 0.41 bc ± 0.10 |
| ABB01624.1 | oleosin high molecular weight isoform; LINUS | 0.64 abc ± 0.45 | 0.73 abc ± 0.55 | 0.14 c ± 0.01 | 0.18 c ± 0.01 | 1.11 ab ± 0.16 | 0.19 c ± 0.02 | 1.17 ab ± 0.25 | 1.39 a ± 0.32 | 0.44 bc ± 0.18 |
| ABB01618.1 | oleosin low molecular weight isoform; LINUS | 0.14 d ± 0.003 | 0.18 bc ± 0.01 | 0.03 f ± 0.01 | 0.15 cd ± 0.01 | 0.19 b ± 0.01 | 0.04 ef ± 0.01 | 0.17 bc ± 0.01 | 0.22 a ± 0.01 | 0.06 e ± 0.004 |
| ABB01619.1 | oleosin low molecular weight isoform, partial; LINUS | 0.07 ab ± 0.01 | 0.13 ab ± 0.09 | 0.01 b ± 0.005 | 0.14 ab ± 0.05 | 0.09 ab ± 0.01 | 0.02 a ± 0.01 | 0.14 ab ± 0.06 | 0.19 a ± 0.12 | 0.06 ab ± 0.04 |
| Defence and stress-related proteins | ||||||||||
| WMZ41542.1 | chitinase; LINUS | 9.38 a ± 0.15 | 7.63 b ± 0.06 | 1.28 e ± 0.05 | 10.39 e ± 0.58 | 9.94 a ± 0.25 | 3.14 d ± 0.59 | 6.18 c ± 0.56 | 5.25 c ± 0.12 | 3.21 d ± 0.31 |
| AAW31878.1 | chitinase, partial; LINUS | 0.06 b ± 0.01 | 0.02 de ± 0.002 | 0.004 e ± 0.00004 | 0.10 a ± 0.0004 | 0.05 c ± 0.0003 | 0.01 e ± 0.002 | 0.07 b ± 0.01 | 0.03 d ± 0.003 | 0.01 e ± 0.0003 |
| AMY26620.1 | late embryogenesis abundant (lea) group 1-embryo development; LINUS | 0.39 a ± 0.03 | 0.22 bc ± 0.01 | 0.01 d ± 0.003 | 0.38 a ± 0.07 | 0.16 c ± 0.03 | 0.03 d ± 0.005 | 0.32 ab ± 0.10 | 0.21 bc ± 0.02 | 0.02 d ± 0.01 |
| AVY09180.1 | Sequence 3353 from patent US 9878004; UNKNOWN | 0.86 a ± 0.01 | 0.91 a ± 0.05 | 0.29 c ± 0.01 | 0.89 a ± 0.08 | 0.95 a ± 0.04 | 0.35 bc ± 0.05 | 0.85 a ± 0.03 | 0.96 a ± 0.02 | 0.45 b ± 0.06 |
| CAI0408810.1 | unnamed protein product; LINTE | 0.46 a ± 0.01 | 0.22 c ± 0.01 | 0.04 d ± 0.01 | 0.35 b ± 0.03 | 0.20 c ± 0.01 | 0.04 d ± 0.004 | 0.38 b ± 0.03 | 0.18 c ± 0.003 | 0.06 d ± 0.01 |
| 1DWM_A | Chain A, Linum usitatissimum trypsin inhibitor; LINUS | 1.28 a ± 0.21 | 0.60 bc ± 0.07 | 0.21 cd ± 0.01 | 1.12 a ± 0.29 | 0.62 b ± 0.09 | 0.19 d ± 0.04 | 0.97 ab ± 0.16 | 0.69 b ± 0.05 | 0.21 cd ± 0.05 |
| Other selected important proteins | ||||||||||
| AVY09175.1 | Sequence 3348 from patent US 9878004; UNKNOWN | 1.32 b ± 0.04 | 1.32 b ± 0.04 | 0.24 d ± 0.05 | 1.29 b ± 0.11 | 1.25 b ± 0.03 | 0.22 d ± 0.02 | 1.37 ab ± 0.05 | 1.48 a ± 0.01 | 0.55 c ± 0.04 |
| CAI0407981.1 | unnamed protein product; LINTE | 0.88 c ± 0.02 | 0.85 c ± 0.01 | 0.25 e ± 0.08 | 0.97 bc ± 0.11 | 0.94 bc ± 0.05 | 0.29 e ± 0.04 | 1.11 ab ± 0.07 | 1.18 a ± 0.12 | 0.57 d ± 0.05 |
| CAF22093.1 | glyceraldehyde 3-phosphate dehydrogenase, partial; LINUS | 0.38 ab ± 0.03 | 0.34 b ± 0.003 | 0.05 c ± 0.02 | 0.36 b ± 0.03 | 0.35 b ± 0.02 | 0.05 c ± 0.004 | 0.43 a ± 0.02 | 0.39 ab ± 0.01 | 0.10 c ± 0.02 |
| BAL41455.1 | fructose-bisphosphate aldolase 1, partial; LINGR | 0.33 b ± 0.02 | 0.33 b ± 0.01 | 0.06 c ± 0.01 | 0.33 b ± 0.02 | 0.32 b ± 0.02 | 0.08 c ± 0.005 | 0.39 a ± 0.03 | 0.40 a ± 0.01 | 0.10 c ± 0.02 |
| P48417.1 | Allene oxide synthase, chloroplastic; LINUS | 0.67 b ± 0.03 | 0.58 cd ± 0.01 | 0.02 e ± 0.01 | 0.58 cd ± 0.05 | 0.56 d ± 0.02 | 0.02 e ± 0.004 | 0.66 bc ± 0.04 | 0.75 a ± 0.03 | 0.07 e ± 0.01 |
| ABM64783.1 | cell wall glycosidase, partial; LINUS | 0.04 bc ± 0.003 | 0.04 cd ± 0.003 | 0.03 d ± 0.004 | 0.05 b ± 0.01 | 0.06 a ± 0.002 | 0.04 c ± 0.001 | 0.01 e ± 0.0004 | 0.01 e ± 0.001 | 0.01 e ± 0.001 |
| CAI0384355.1 | unnamed protein product; LINTE | 0.04 a ± 0.002 | 0.03 bc ± 0.002 | 0.01 d ± 0.002 | 0.04 a ± 0.01 | 0.03 c ± 0.001 | 0.01 d ± 0.001 | 0.04 a ± 0.003 | 0.04 ab ± 0.002 | 0.01 d ± 0.002 |
| CAI0431450.1 | unnamed protein product; LINTE | 0.10 ab ± 0.002 | 0.09 b ± 0.002 | 0.02 d ± 0.004 | 0.09 ab ± 0.012 | 0.09 b ± 0.003 | 0.03 d ± 0.003 | 0.11 a ± 0.006 | 0.11 a ± 0.003 | 0.06 c ± 0.005 |
| CAI0412769.1 | unnamed protein product; LINTE | 0.06 ab ± 0.005 | 0.05 b ± 0.003 | 0.003 c ± 0.001 | 0.07 a ± 0.012 | 0.06 ab ± 0.003 | 0.004 c ± 0.002 | 0.05 b ± 0.003 | 0.06 ab ± 0.004 | 0.01 c ± 0.003 |
| AVY09204.1 | Sequence 3377 from patent US 9878004; UNKNOWN | 0.08 b ± 0.004 | 0.08 b ± 0.003 | 0.01 d ± 0.002 | 0.08 b ± 0.009 | 0.07 b ± 0.002 | 0.01 d ± 0.001 | 0.09 a ± 0.003 | 0.09 ab ± 0.003 | 0.03 c ± 0.001 |
| CAI0440995.1 | unnamed protein product; LINTE | 0.12 ab ± 0.007 | 0.10 b ± 0.006 | 0.01 c ± 0.003 | 0.11 ab ± 0.012 | 0.10 b ± 0.007 | 0.01 c ± 0.002 | 0.13 a ± 0.009 | 0.11 ab ± 0.010 | 0.02 c ± 0.002 |
| CAI0411675.1 | unnamed protein product; LINTE | 1.01 ab ± 0.09 | 1.10 a ± 0.03 | 0.11 c ± 0.03 | 0.95 b ± 0.05 | 1.06 ab ± 0.04 | 0.10 c ± 0.01 | 1.09 a ± 0.05 | 1.12 a ± 0.04 | 0.22 c ± 0.02 |
| AGN56421.1 | non-specific lipid transfer protein 1; LINUS | 0.54 a ± 0.03 | 0.49 ab ± 0.01 | 0.05 d ± 0.003 | 0.47 abc ± 0.03 | 0.43 bc ± 0.04 | 0.06 d ± 0.01 | 0.49 abc ± 0.05 | 0.42 c ± 0.02 | 0.05 d ± 0.01 |
| CAI0414382.1 | unnamed protein product; LINTE | 0.26 a ± 0.02 | 0.25 ab ± 0.07 | 0.03 c ± 0.002 | 0.32 a ± 0.06 | 0.21 ab ± 0.03 | 0.04 c ± 0.01 | 0.24 ab ± 0.03 | 0.30 a ± 0.07 | 0.13 bc ± 0.04 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jarošová, M.; Roudnický, P.; Bárta, J.; Zdráhal, Z.; Bártová, V.; Stupková, A.; Lorenc, F.; Bjelková, M.; Kyselka, J.; Jarošová, E.; et al. Proteomic Profile of Flaxseed (Linum usitatissimum L.) Products as Influenced by Protein Concentration Method and Cultivar. Foods 2024, 13, 1288. https://doi.org/10.3390/foods13091288
Jarošová M, Roudnický P, Bárta J, Zdráhal Z, Bártová V, Stupková A, Lorenc F, Bjelková M, Kyselka J, Jarošová E, et al. Proteomic Profile of Flaxseed (Linum usitatissimum L.) Products as Influenced by Protein Concentration Method and Cultivar. Foods. 2024; 13(9):1288. https://doi.org/10.3390/foods13091288
Chicago/Turabian StyleJarošová, Markéta, Pavel Roudnický, Jan Bárta, Zbyněk Zdráhal, Veronika Bártová, Adéla Stupková, František Lorenc, Marie Bjelková, Jan Kyselka, Eva Jarošová, and et al. 2024. "Proteomic Profile of Flaxseed (Linum usitatissimum L.) Products as Influenced by Protein Concentration Method and Cultivar" Foods 13, no. 9: 1288. https://doi.org/10.3390/foods13091288






