Impact of Oil Bodies on Structure, Rheology and Function of Acid-Mediated Soy Protein Isolate Gels
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. SPI Dispersion Preparation
2.3. Peanut Oil Body Extraction
2.4. Mixed Gel Solution Preparation
2.5. Preparation of Acid-Induced OB Emulsion-Filled SPI Gel
2.6. Mixed Gel Solution Characteristics
2.6.1. Measurement of Gel Solution Particle Size and ζ-Potential
2.6.2. Measurement of Physical Stability
2.7. Functional Characteristics
2.7.1. Water Holding Capacity
- W2 represents the combined weight of the centrifuged sample (g),
- W0 represents the weight of the empty centrifuge tube (g),
- W1 represents the combined weight of the sample before centrifugation (g).
2.7.2. Measurement of Textural Characteristics
2.7.3. Dynamic Rheological Analysis
2.8. Microstructures
2.8.1. Confocal Laser Scanning Microscopy Analysis
2.8.2. Fourier Transform Infrared (FTIR) Spectrum Analysis
2.8.3. Scanning Electron Microscopy Analysis
2.9. Statistical Analysis
3. Results and Discussion
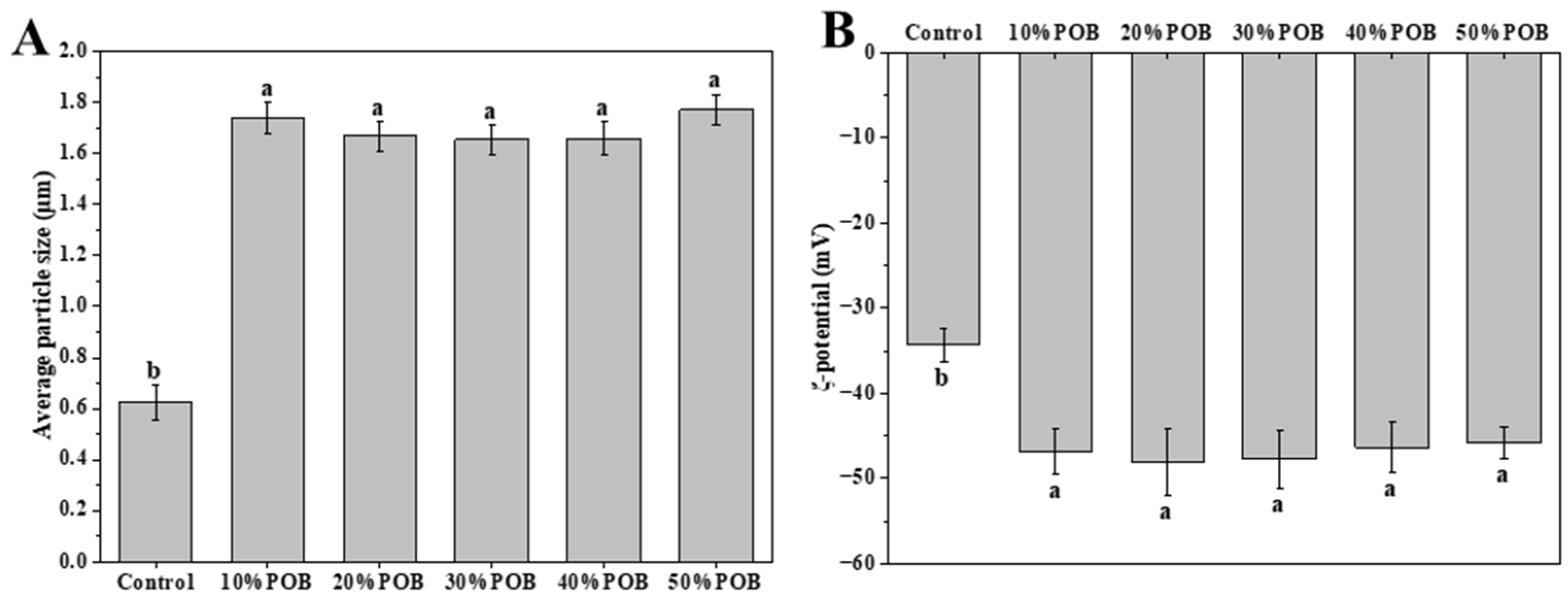
3.1. Effect of Peanut Oil Body Concentration on Particle Size and ζ-Potential of Mixed Gel Solutions
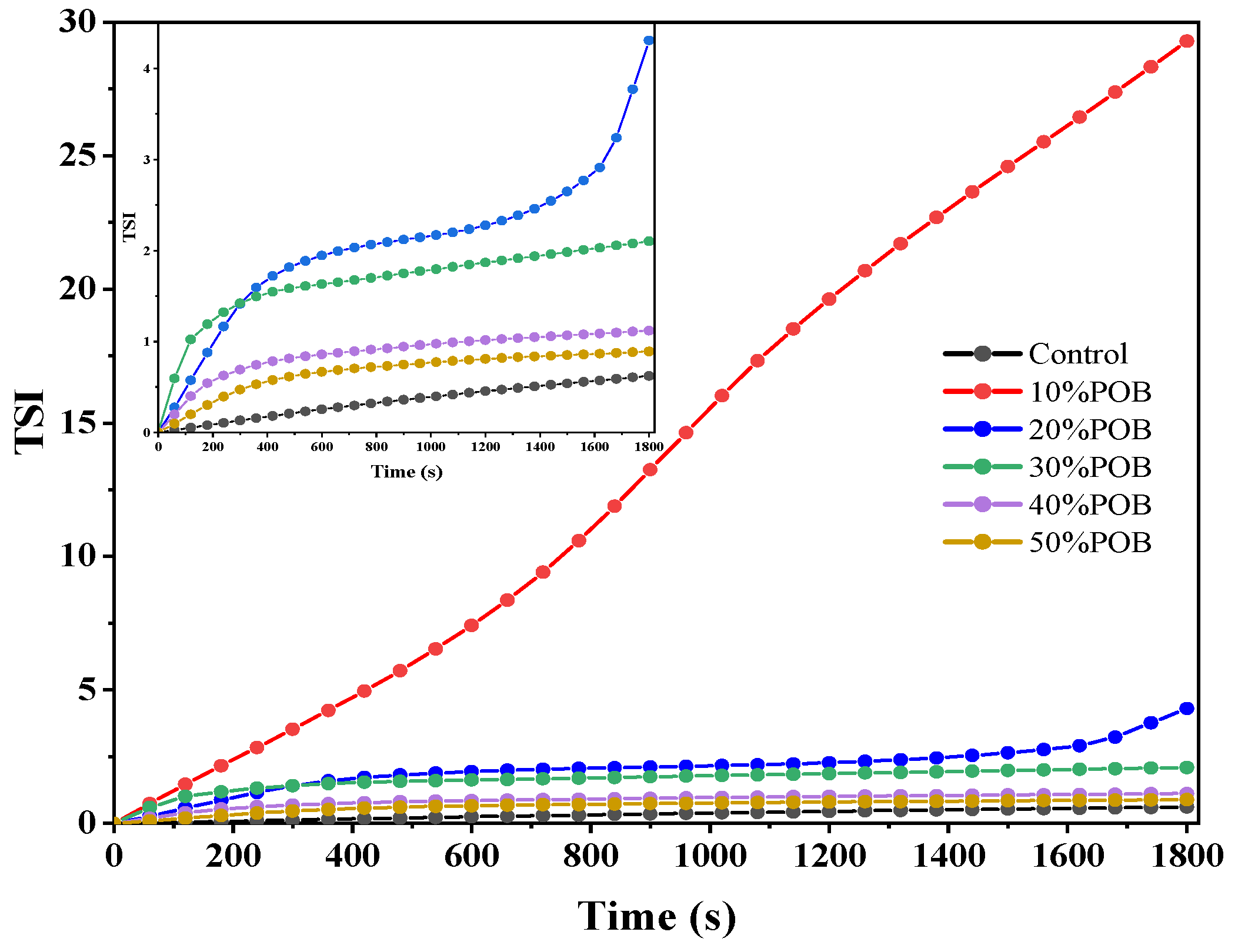
3.2. Stability Analysis of Mixed Gel Solutions
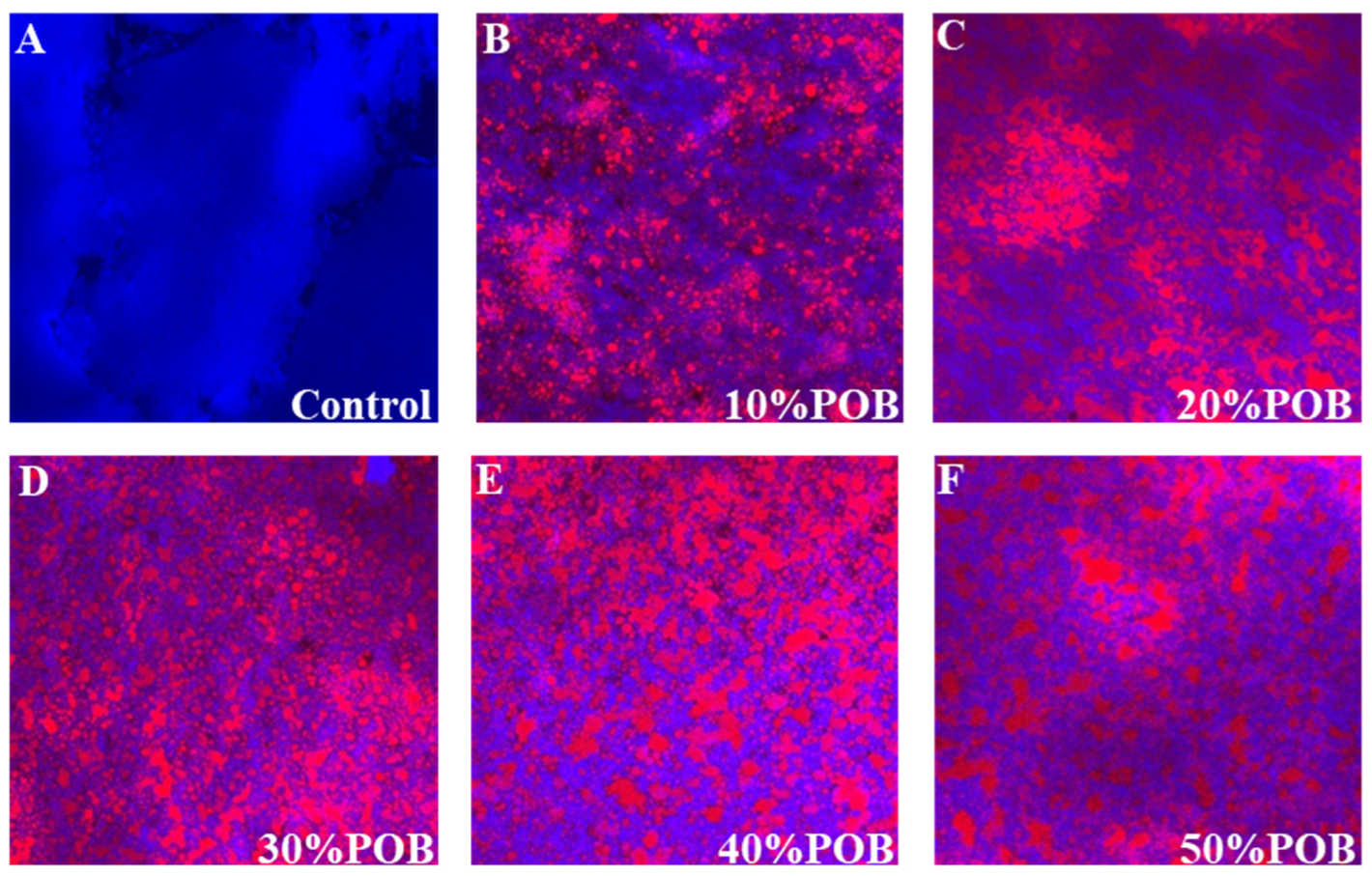
3.3. CLSM Microstructure Observations
3.4. SEM Microstructure Observations
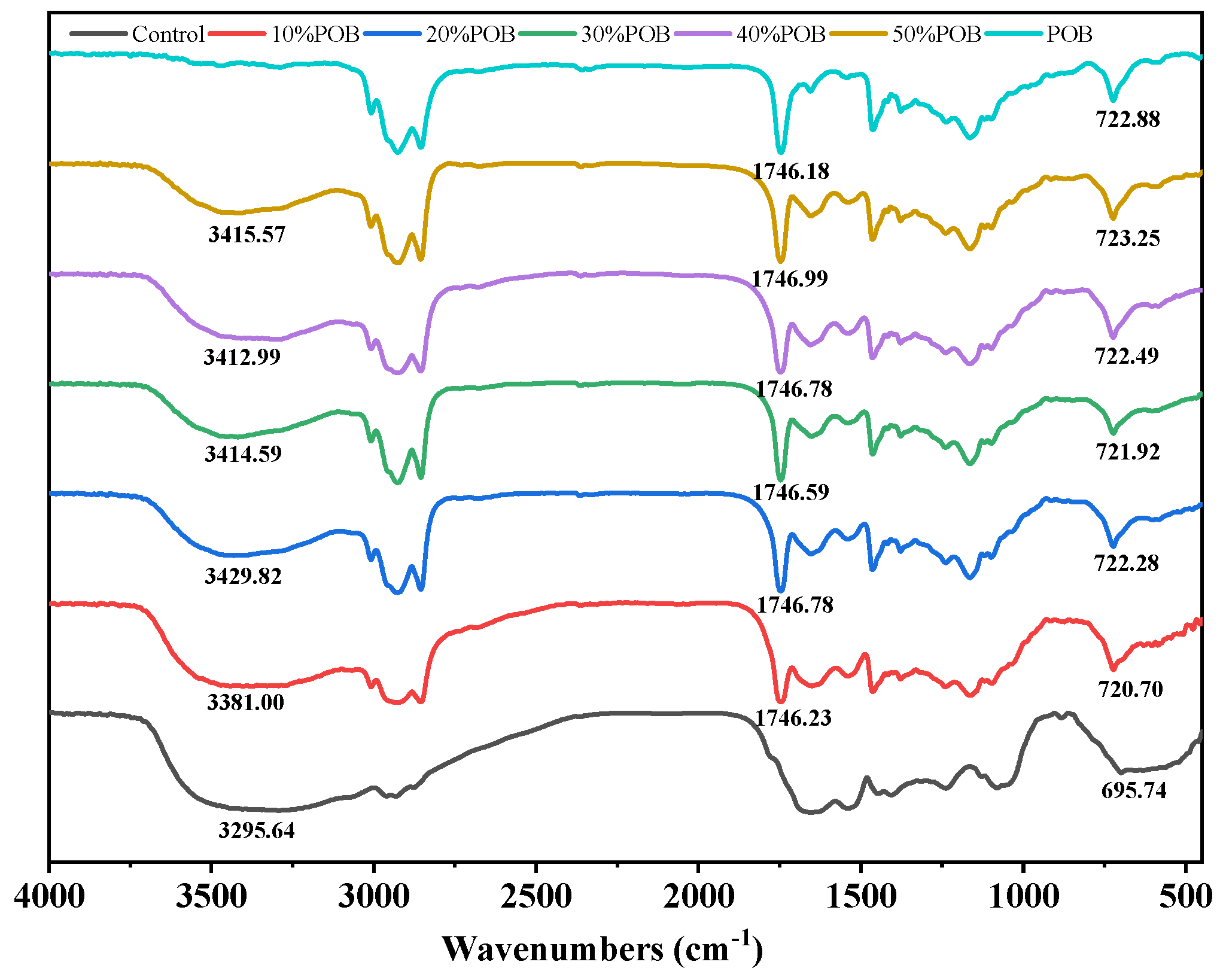
3.5. FTIR Analysis
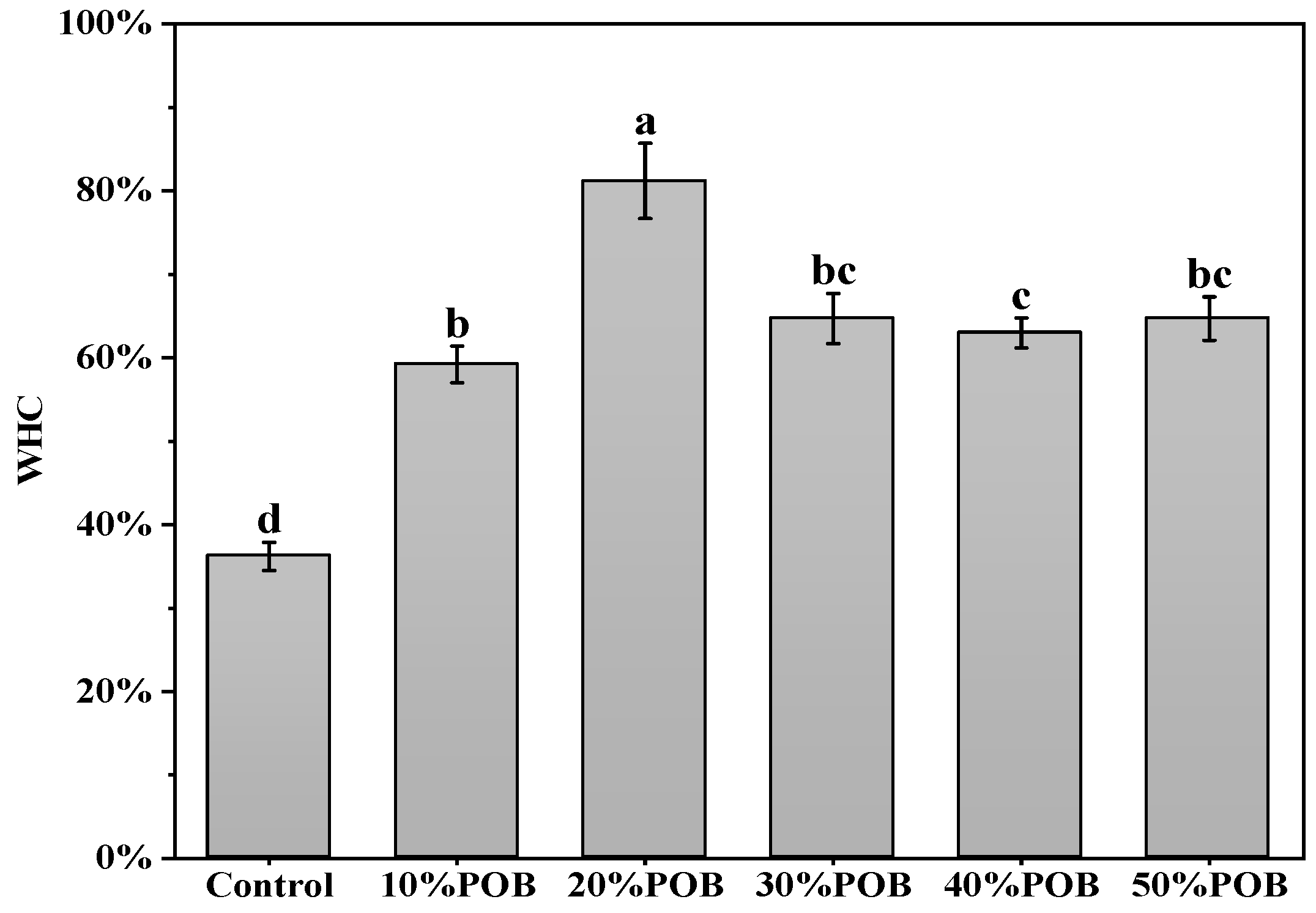
3.6. Water Holding Capacity Analysis
3.7. Texture Profile Analysis
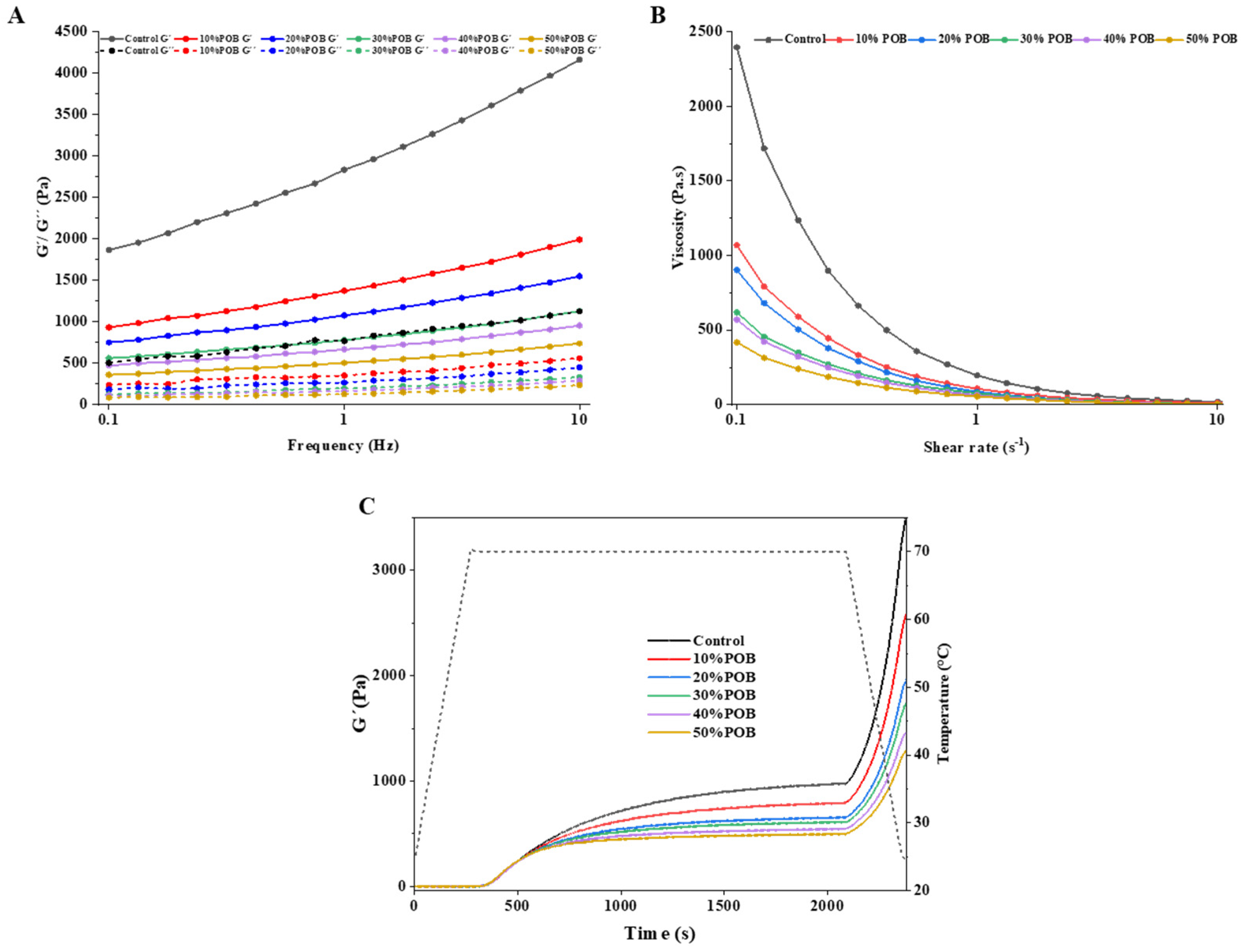
3.8. Rheological Properties
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Dickinson, E. Emulsion Gels: The Structuring of Soft Solids with Protein-Stabilized Oil Droplets. Food Hydrocoll. 2012, 28, 224–241. [Google Scholar] [CrossRef]
- Dickinson, E. Stabilising Emulsion-Based Colloidal Structures with Mixed Food Ingredients. J. Sci. Food Agric. 2013, 93, 710–721. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Mao, L.; Hou, Z.; Yuan, F.; Gao, Y. Roles of Additional Emulsifiers in the Structures of Emulsion Gels and Stability of Vitamin E. Food Hydrocoll. 2020, 99, 105372. [Google Scholar] [CrossRef]
- Lin, D.; Kelly, A.L.; Miao, S. Preparation, Structure-Property Relationships and Applications of Different Emulsion Gels: Bulk Emulsion Gels, Emulsion Gel Particles, and Fluid Emulsion Gels. Trends Food Sci. Technol. 2020, 102, 123–137. [Google Scholar] [CrossRef]
- Dickinson, E. Food Emulsions and Foams: Stabilization by Particles. Curr. Opin. Colloid Interface Sci. 2010, 15, 40–49. [Google Scholar] [CrossRef]
- Chassaing, B.; Koren, O.; Goodrich, J.K.; Poole, A.C.; Srinivasan, S.; Ley, R.E.; Gewirtz, A.T. Promoting Colitis and Metabolic Syndrome. Nature 2015, 519, 92–96. [Google Scholar] [CrossRef] [PubMed]
- Chassaing, B.; Compher, C.; Bonhomme, B.; Liu, Q.; Tian, Y.; Walters, W.; Nessel, L.; Delaroque, C.; Hao, F.; Gershuni, V.; et al. Randomized Controlled-Feeding Study of Dietary Emulsifier Carboxymethylcellulose Reveals Detrimental Impacts on the Gut Microbiota and Metabolome. Gastroenterology 2022, 162, 743–756. [Google Scholar] [CrossRef] [PubMed]
- Tzen, J.T.C.; Huang, A.H.C. Surface Structure and Properties of Plant Seed Oil Bodies. J. Cell Biol. 1992, 117, 327–335. [Google Scholar] [CrossRef]
- Huang, A.H.C. Structure of plant seed oil bodies. Curr. Opin. Struct. Biol. 1994, 4, 493–498. [Google Scholar] [CrossRef]
- Chen, J.C.F.; Tzen, J.T.C. An in Vitro System to Examine the Effective Phospholipids and Structural Domain for Protein Targeting to Seed Oil Bodies. Plant Cell Physiol. 2001, 42, 1245–1252. [Google Scholar] [CrossRef]
- Chen, J.C.F.; Tsai, C.C.Y.; Tzen, J.T.C. Cloning and Secondary Structure Analysis of Caleosin, a Unique Calcium-Binding Protein in Oil Bodies of Plant Seeds. Plant Cell Physiol. 1999, 40, 1079–1086. [Google Scholar] [CrossRef]
- Nikiforidis, C.V.; Matsakidou, A.; Kiosseoglou, V. Composition, Properties and Potential Food Applications of Natural Emulsions and Cream Materials Based on Oil Bodies. RSC Adv. 2014, 4, 25067–25078. [Google Scholar] [CrossRef]
- Yang, N.; Feng, Y.; Su, C.; Wang, Q.; Zhang, Y.; Wei, Y.; Zhao, M.; Nishinari, K.; Fang, Y. Structure and Tribology of κ-Carrageenan Gels Filled with Natural Oil Bodies. Food Hydrocoll. 2020, 107, 105945. [Google Scholar] [CrossRef]
- Fu, L.; He, Z.; Zeng, M.; Qin, F.; Chen, J. Effects of Soy Protein Composition in Recombined Soy-Based Cream on the Stability and Physical Properties of Whipping Cream. J. Sci. Food Agric. 2020, 100, 2732–2741. [Google Scholar] [CrossRef] [PubMed]
- Bibat, M.A.D.; Ang, M.J.; Eun, J.B. Impact of Replacing Pork Backfat with Rapeseed Oleosomes—Natural Pre-Emulsified Oil—On Technological Properties of Meat Model Systems. Meat Sci. 2022, 186, 108732. [Google Scholar] [CrossRef]
- Niu, H.; Li, Y.; Han, J.; Liu, Q.; Kong, B. Gelation and Rheological Properties of Myofibrillar Proteins Influenced by the Addition of Soybean Protein Isolates Subjected to an Acidic PH Treatment Combined with a Mild Heating. Food Hydrocoll. 2017, 70, 269–276. [Google Scholar] [CrossRef]
- Yang, X.; Ke, C.; Li, L. Physicochemical, Rheological and Digestive Characteristics of Soy Protein Isolate Gel Induced by Lactic Acid Bacteria. J. Food Eng. 2021, 292, 110243. [Google Scholar] [CrossRef]
- Zhao, H.; Yu, B.; Hemar, Y.; Chen, J.; Cui, B. Improvement of Calcium Sulfate-Induced Gelation of Soy Protein via Incorporation of Soy Oil before and after Thermal Denaturation. LWT 2020, 117, 108690. [Google Scholar] [CrossRef]
- Kim, K.H.; Renkema, J.M.S.; Van Vliet, T. Rheological Properties of Soybean Protein Isolate Gels Containing Emulsion Droplets. Food Hydrocoll. 2001, 15, 295–302. [Google Scholar] [CrossRef]
- Shun-Tang, G.; Ono, T.; Mikami, M. Incorporation of soy milk lipid into protein coagulum by addition of calcium chloride. J. Agric. Food Chem. 1999, 47, 901–905. [Google Scholar] [CrossRef]
- Jin, I.H.; Kim, J.E.; Seo, J.H.; Lee, S.P. Physicochemical properties of soy protein isolate gels emulsified with various oils using a microbial transglutaminase. Food Sci Biotechnol. 2013, 22, 129–136. [Google Scholar] [CrossRef]
- Gu, X.; Campbell, L.J.; Euston, S.R. Effects of Different Oils on the Properties of Soy Protein Isolate Emulsions and Gels. Food Res. Int. 2009, 42, 925–932. [Google Scholar] [CrossRef]
- Chen, Y.; Chen, Y.; Zhao, L.; Kong, X.; Yang, Z.; Hua, Y. A Two-Chain Aspartic Protease Present in Seeds with High Affinity for Peanut Oil Bodies. Food Chem. 2018, 241, 443–451. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Waardenburg, L.C.; Berton-Carabin, C.C.; Nikiforidis, C.V.; van der Linden, E.; Sagis, L.M.C. Air-Water Interfacial Behaviour of Whey Protein and Rapeseed Oleosome Mixtures. J. Colloid Interface Sci. 2021, 602, 207–221. [Google Scholar] [CrossRef] [PubMed]
- Liao, Y.; Sun, Y.; Wang, Z.; Zhong, M.; Li, R.; Yan, S.; Qi, B.; Li, Y. Structure, Rheology, and Functionality of Emulsion-Filled Gels: Effect of Various Oil Body Concentrations and Interfacial Compositions. Food Chem. X 2022, 16, 100509. [Google Scholar] [CrossRef] [PubMed]
- Sukhotu, R.; Shi, X.; Hu, Q.; Nishinari, K.; Fang, Y.; Guo, S. Aggregation Behaviour and Stability of Maize Germ Oil Body Suspension. Food Chem. 2014, 164, 1–6. [Google Scholar] [CrossRef]
- Liu, C.; Wang, R.; He, S.; Cheng, C.; Ma, Y. The Stability and Gastro-Intestinal Digestion of Curcumin Emulsion Stabilized with Soybean Oil Bodies. LWT 2020, 131, 109663. [Google Scholar] [CrossRef]
- Li, M.; Yang, R.; Feng, X.; Fan, X.; Liu, Y.; Xu, X.; Zhou, G.; Zhu, B.; Ullah, N.; Chen, L. Effects of Low-Frequency and High-Intensity Ultrasonic Treatment Combined with Curdlan Gels on the Thermal Gelling Properties and Structural Properties of Soy Protein Isolate. Food Hydrocoll. 2022, 127, 107506. [Google Scholar] [CrossRef]
- Hou, W.; Long, J.; Hua, Y.; Chen, Y.; Kong, X.; Zhang, C.; Li, X. Formation and Characterization of Solid Fat Mimetic Based on Pea Protein Isolate/Polysaccharide Emulsion Gels. Front. Nutr. 2022, 9, 1053469. [Google Scholar] [CrossRef]
- Han, C.; Yang, X.; Li, L. Physicochemical Properties and Microstructure of Soybean Protein Isolate-Vegetable Oil Complex Gels Induced by Lactic Acid Bacteria: Effects of Vegetable Oil Types and Concentrations. Food Hydrocoll. 2023, 145, 109109. [Google Scholar] [CrossRef]
- Yu, J.; Wang, X.; Li, D.; Wang, L.; Wang, Y. Development of Soy Protein Isolate Emulsion Gels as Extrusion-Based 3D Food Printing Inks: Effect of Polysaccharides Incorporation. Food Hydrocoll. 2022, 131, 107824. [Google Scholar] [CrossRef]
- Li, L.; He, H.; Wu, D.; Lin, D.; Qin, W.; Meng, D.; Yang, R.; Zhang, Q. Rheological and Textural Properties of Acid-Induced Soybean Protein Isolate Gel in the Presence of Soybean Protein Isolate Hydrolysates or Their Glycosylated Products. Food Chem. 2021, 360, 129991. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Zeng, M.; Qin, F.; Adhikari, B.; He, Z.; Chen, J. Enhanced CaSO4-Induced Gelation Properties of Soy Protein Isolate Emulsion by Pre-Aggregation. Food Chem. 2018, 242, 459–465. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Chen, W.; Hao, J.; Xu, D. Construction of Different Properties Single and Double Cross-Linked Binary Emulsion Filled Gels Based on Rice Bran Oil Body Emulsion. Colloids Surfaces A Physicochem. Eng. Asp. 2023, 665, 131238. [Google Scholar] [CrossRef]
- Szczesniak, A.S. Texture Is a Sensory Property. Food Qual. Prefer. 2002, 13, 215–225. [Google Scholar] [CrossRef]
- Singh, V.; Guizani, N.; Al-Alawi, A.; Claereboudt, M.; Rahman, M.S. Instrumental Texture Profile Analysis (TPA) of Date Fruits as a Function of Its Physico-Chemical Properties. Ind. Crops Prod. 2013, 50, 866–873. [Google Scholar] [CrossRef]
- Wang, X.; He, Z.; Zeng, M.; Qin, F.; Adhikari, B.; Chen, J. Effects of the Size and Content of Protein Aggregates on the Rheological and Structural Properties of Soy Protein Isolate Emulsion Gels Induced by CaSO4. Food Chem. 2017, 221, 130–138. [Google Scholar] [CrossRef]
- Huang, Z.; Wang, X.; Zhang, J.; Liu, Y.; Zhou, T.; Chi, S.; Gao, F.; Li, J.; Tian, B.; Shi, W.; et al. Effect of Heat Treatment on the Nonlinear Rheological Properties of Acid-Induced Soy Protein Isolate Gels Modified by High-Pressure Homogenization. LWT 2022, 157, 113094. [Google Scholar] [CrossRef]
- Van Vliet, T. Rheological Properties of Filled Gels. Influence of Filler Matrix Interaction. Colloid Polym. Sci. 1988, 266, 518–524. [Google Scholar] [CrossRef]
- Chen, J.; Dickinson, E. Effect of Surface Character of Filler Particles on Rheology of Heat-Set Whey Protein Emulsion Gels. Colloids Surf. B Biointerfaces 1999, 12, 373–381. [Google Scholar] [CrossRef]
- Dickinson, E. Milk protein interfacial layers and the relationship to emulsion stability and rheology. Colloids Surf. B Biointerfaces 2001, 20, 197–210. [Google Scholar] [CrossRef] [PubMed]
- Sheikh, F.; Hasani, M.; Kiani, H.; Asadollahzadeh, M.J.; Sabbagh, F. Enhancing Rheological and Textural Properties of Gelatin-Based Composite Gels through Incorporation of Sesame Seed Oleosome-Protein Fillers. Gels 2023, 9, 774. [Google Scholar] [CrossRef] [PubMed]
- Nicole, M.; Caimeng, Z.; Joseph, H.; Eric, K.; Yufei, H. Soybean Oil Volume Fraction Effects on the Rheology Characteristics and Gelation Behavior of Glucono-δ-Lactone and Calcium Sulfate-Induced Tofu Gels. J. Texture Stud. 2016, 47, 112–130. [Google Scholar] [CrossRef]
- Ningtyas, D.W.; Tam, B.; Bhandari, B.; Prakash, S. Effect of Different Types and Concentrations of Fat on the Physico-Chemical Properties of Soy Protein Isolate Gel. Food Hydrocoll. 2021, 111, 106226. [Google Scholar] [CrossRef]
- Jaros, D.; Pätzold, J.; Schwarzenbolz, U.; Rohm, H. Small and Large Deformation Rheology of Acid Gels from Transglutaminase Treated Milks. Food Biophys. 2006, 1, 124–132. [Google Scholar] [CrossRef]
- Zhao, H.; Chen, J.; Hemar, Y.; Cui, B. Improvement of the Rheological and Textural Properties of Calcium Sulfate-Induced Soy Protein Isolate Gels by the Incorporation of Different Polysaccharides. Food Chem. 2020, 310, 125983. [Google Scholar] [CrossRef]

| Sample | Hardness/g | Springiness | Cohesiveness | Gumminess/g | Chewiness/g |
|---|---|---|---|---|---|
| Control | 101.60 ± 5.28 A | 0.89 ± 0.04 C | 0.27 ± 0.01 C | 27.13 ± 1.66 A | 24.17 ± 1.53 A |
| 10%POB | 59.15 ± 1.62 B | 0.94 ± 0.015 A | 0.39 ± 0.01 A | 22.92 ± 0.9 B | 21.62 ± 0.95 B |
| 20%POB | 43.86 ± 1.20 C | 0.94 ± 0.02 AB | 0.39 ± 0.01 A | 16.95 ± 0.52 C | 15.87 ± 0.66 C |
| 30%POB | 41.68 ± 1.94 CD | 0.94 ± 0.02 AB | 0.36 ± 0.01 B | 14.97 ± 0.64 D | 14.03 ± 0.62 CD |
| 40%POB | 39.66 ± 1.19 CD | 0.94 ± 0.02 A | 0.36 ± 0.01 B | 14.17 ± 0.08 DE | 13.32 ± 0.28 CD |
| 50%POB | 37.62 ± 0.61 D | 0.90 ± 0.02 CB | 0.35 ± 0.01 B | 13.10 ± 0.46 E | 11.81 ± 0.55 D |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, S.; Zhao, Z.; Zhou, P.; Deng, Y.; Liu, G.; Li, P.; Zeng, J.; Zhang, Y.; Zhang, M. Impact of Oil Bodies on Structure, Rheology and Function of Acid-Mediated Soy Protein Isolate Gels. Foods 2024, 13, 1289. https://doi.org/10.3390/foods13091289
Liu S, Zhao Z, Zhou P, Deng Y, Liu G, Li P, Zeng J, Zhang Y, Zhang M. Impact of Oil Bodies on Structure, Rheology and Function of Acid-Mediated Soy Protein Isolate Gels. Foods. 2024; 13(9):1289. https://doi.org/10.3390/foods13091289
Chicago/Turabian StyleLiu, Songbin, Zhihao Zhao, Pengfei Zhou, Yuanyuan Deng, Guang Liu, Ping Li, Jiarui Zeng, Yi Zhang, and Mingwei Zhang. 2024. "Impact of Oil Bodies on Structure, Rheology and Function of Acid-Mediated Soy Protein Isolate Gels" Foods 13, no. 9: 1289. https://doi.org/10.3390/foods13091289





