Evaluation of Dynamic Changes of Volatile Organic Components for Fishmeal during Storage by HS-SPME-GC-MS with PLS-DA
Abstract
:1. Introduction
2. Materials and Methods
2.1. Fishmeal Sample
2.2. Chemicals and Reagents
2.3. Detection Methods of Freshness Indexes
2.4. Extraction of VOCs by HS-SPME
2.5. VOCs Profiling via GC-MS
2.6. Identification of VOCs
2.7. Statistics Analysis
3. Results and Discussion
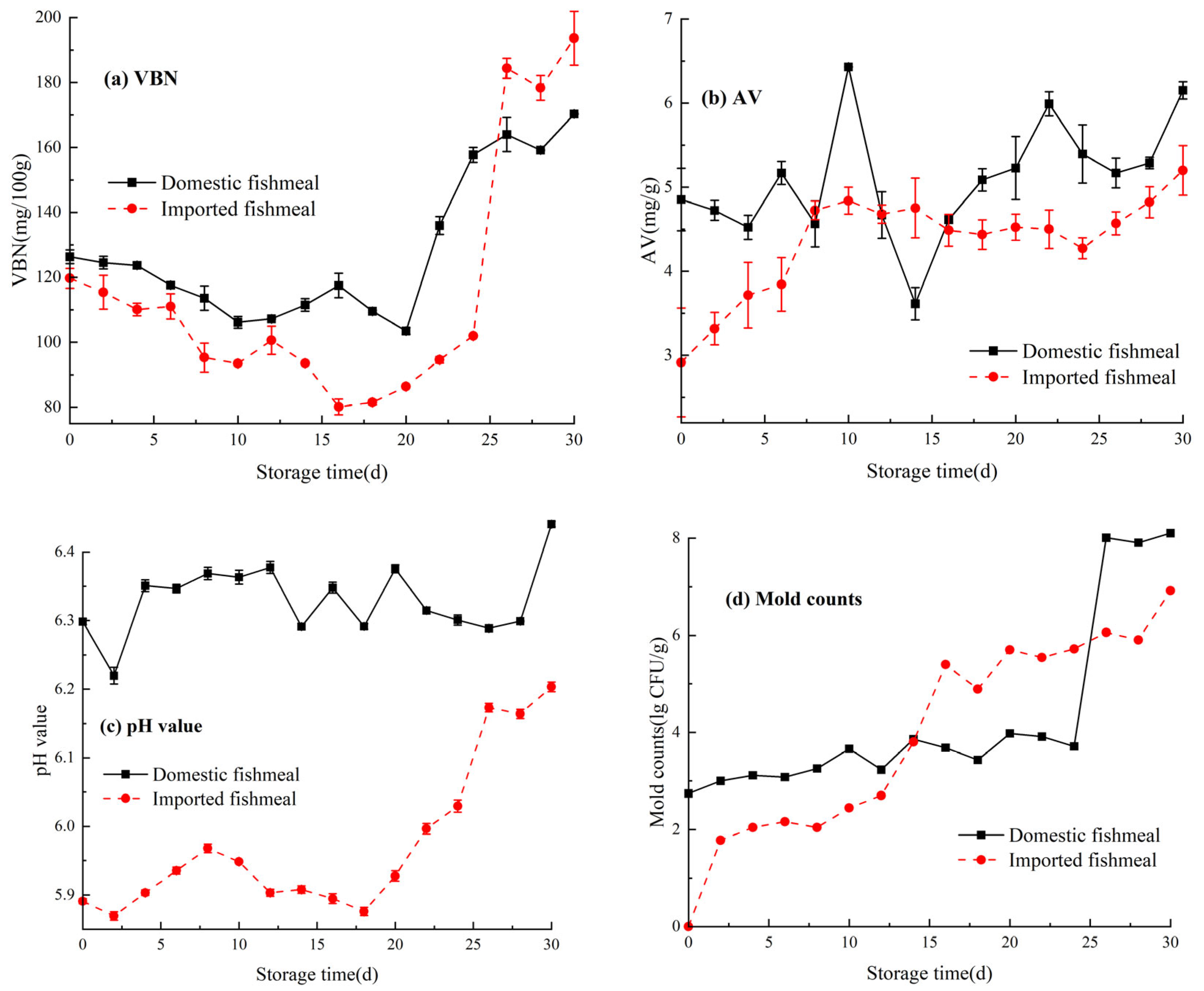
3.1. Regularity of Freshness Indexes during Storage
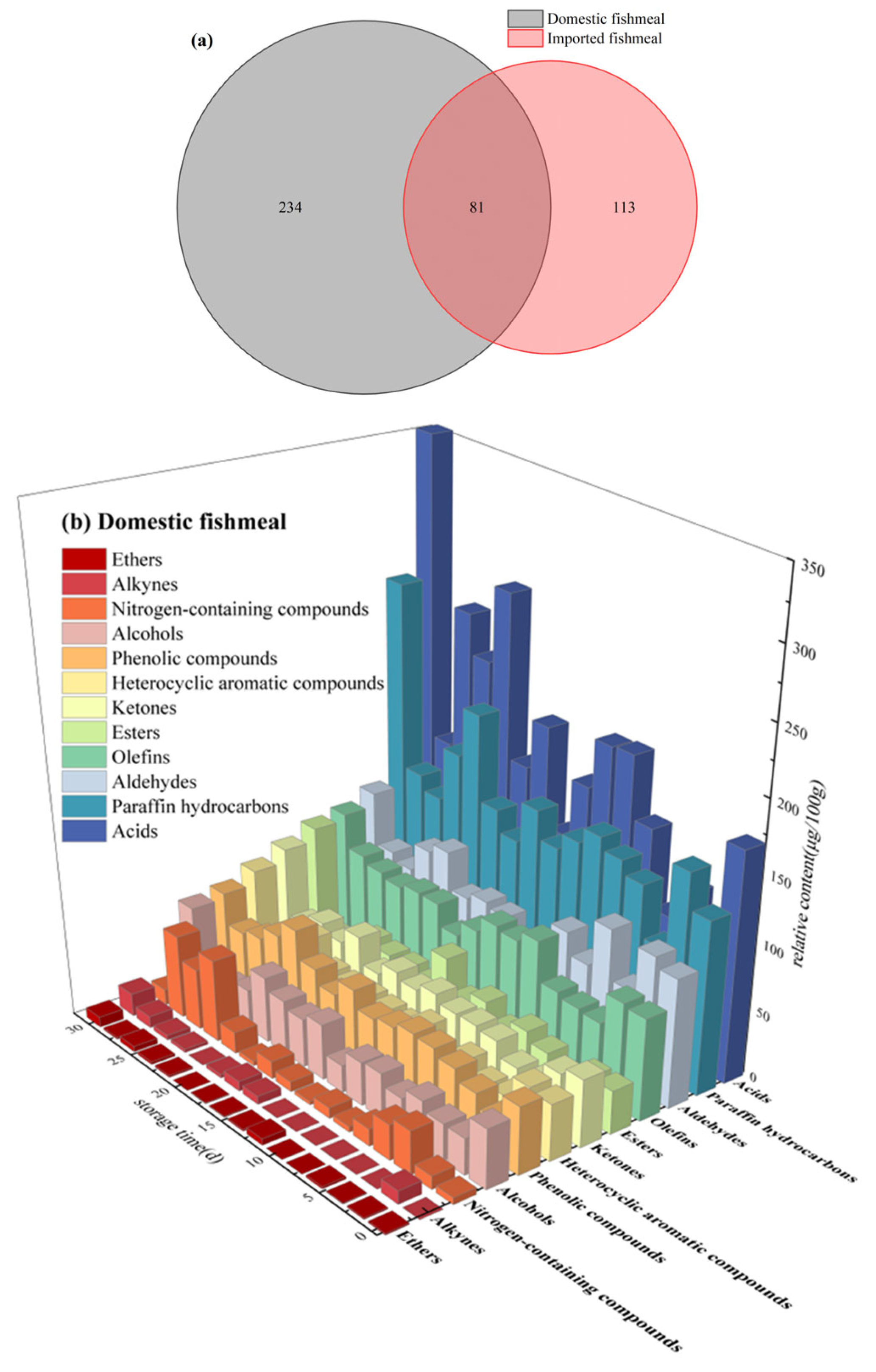
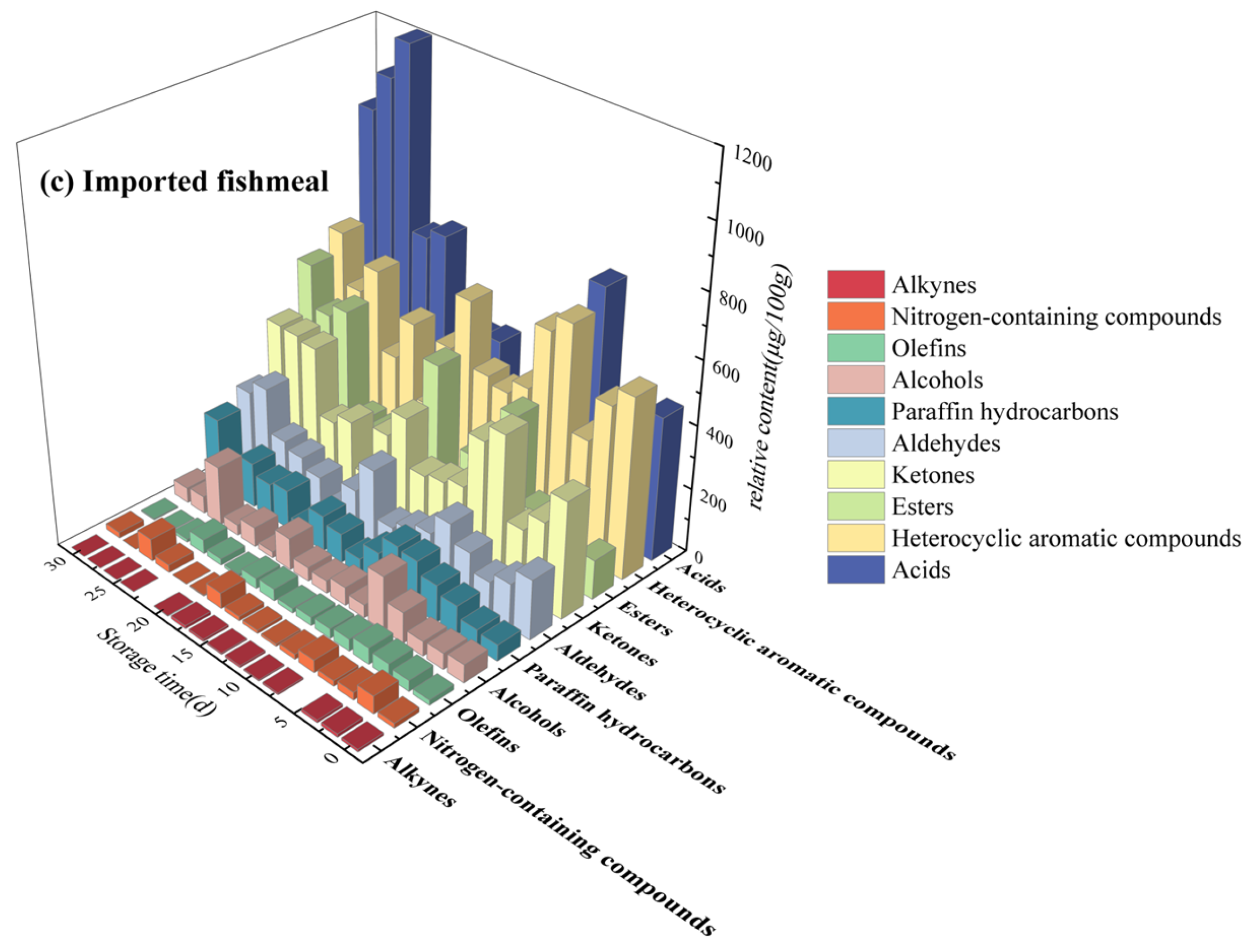
3.2. Regularity of VOCs during Storage
3.2.1. Heterocyclic Aromatic Compounds
3.2.2. Acids and Esters
3.2.3. Ketones, Aldehydes and Alcohols
3.2.4. Others
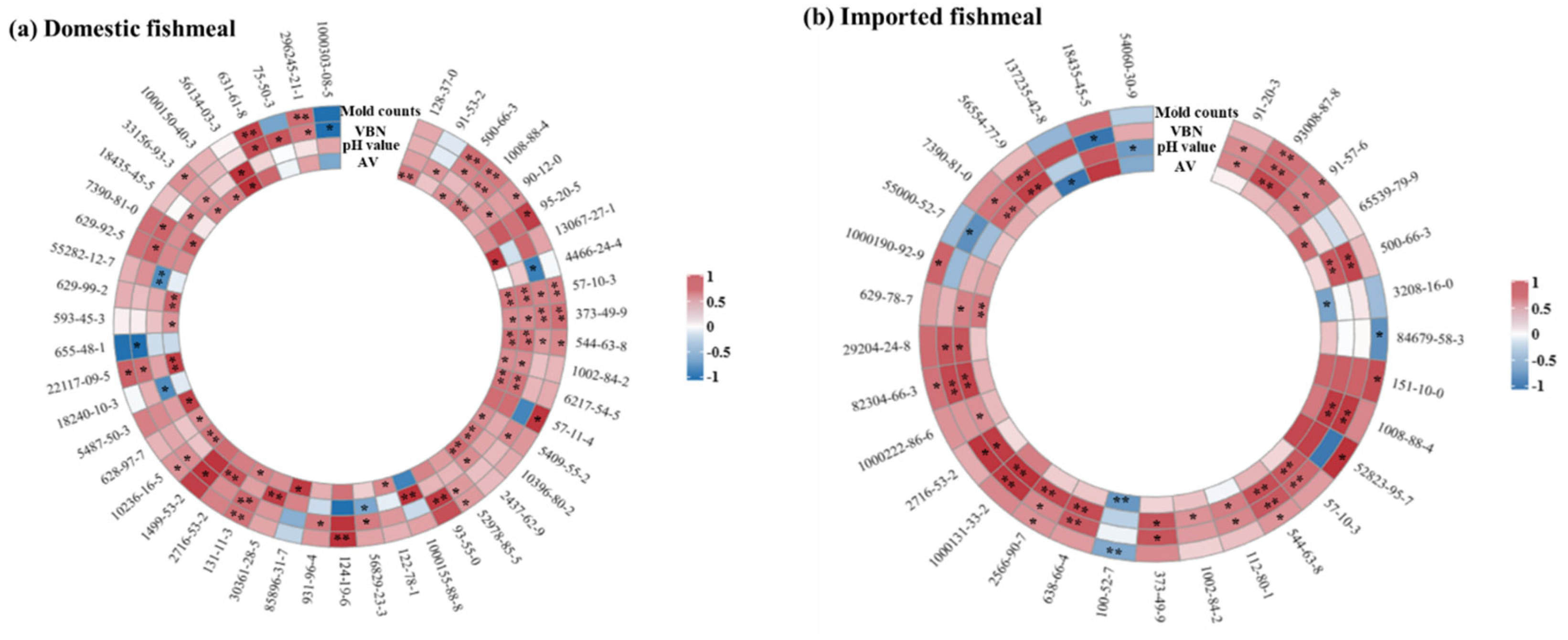
3.3. Correlation Analysis between VOCs and Freshness Indexes
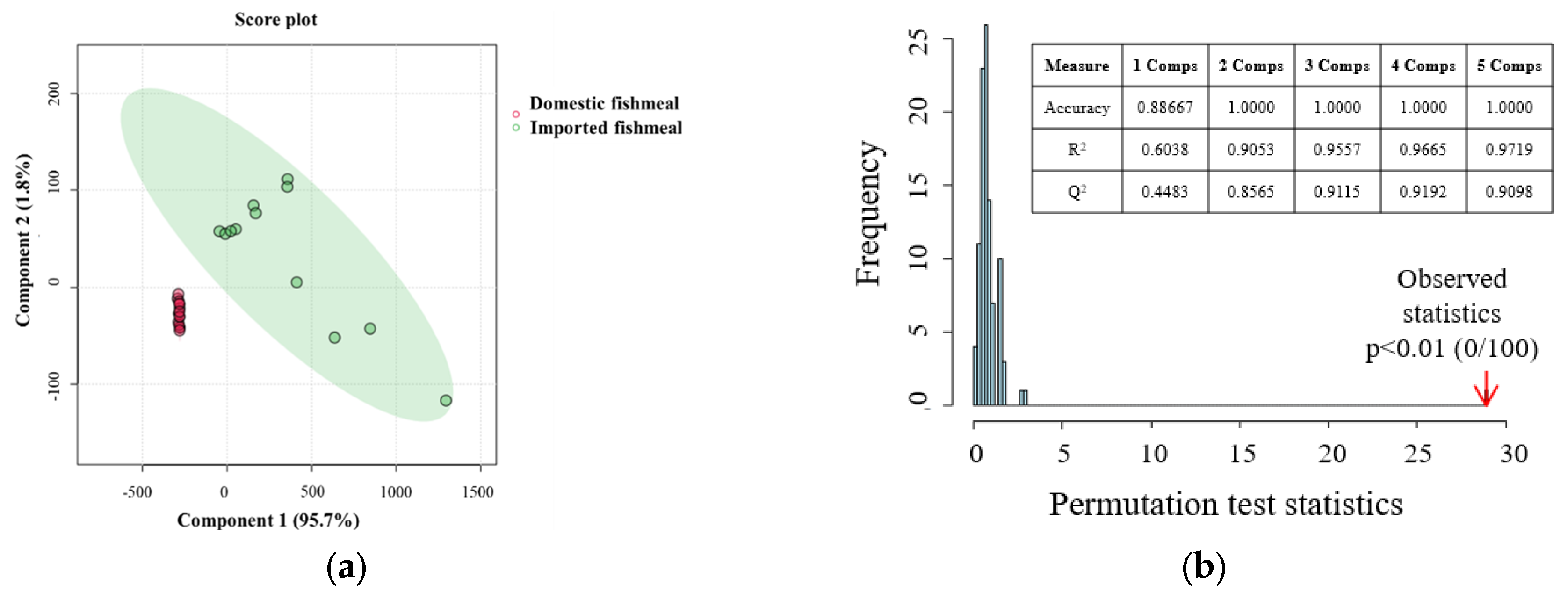
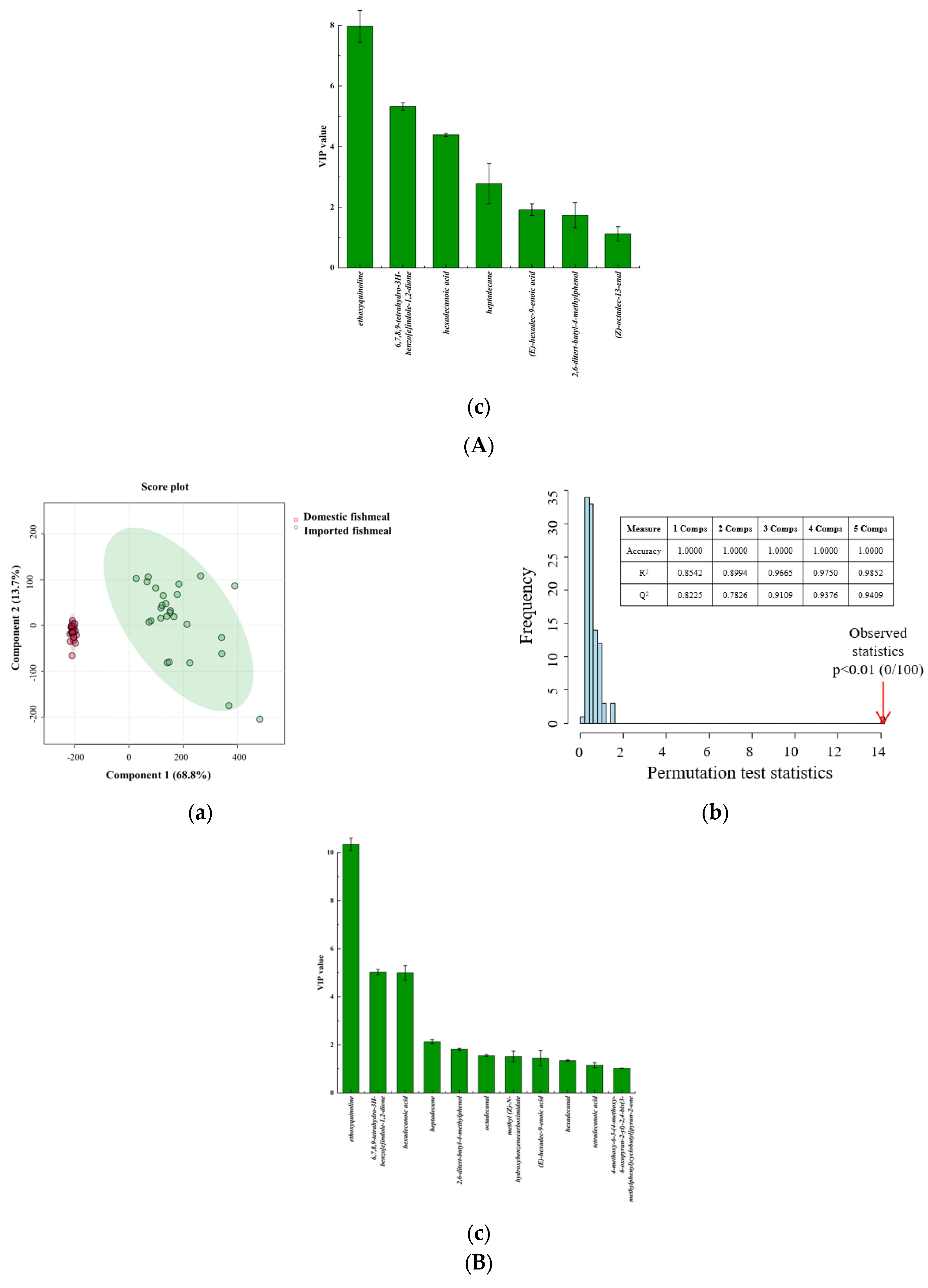
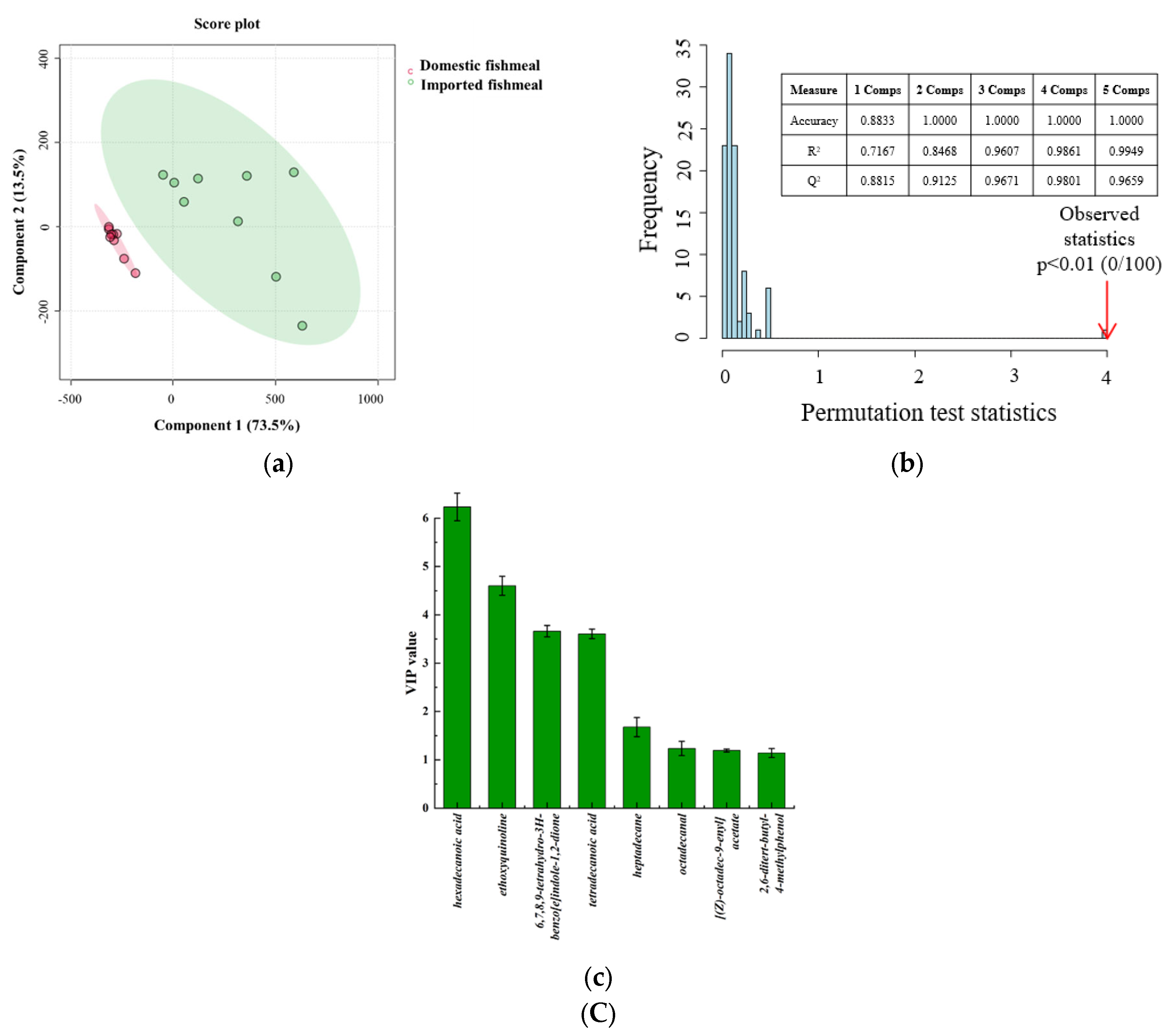
3.4. Determination of the Characteristic VOCs Based on PLS-DA
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Geng, J.; Liu, J.; Kong, X.R.; Shen, B.S.; Niu, Z.Y. The fishmeal adulteration identification based on microscopic image and deep learning. Comput. Electron. Agr. 2022, 198, 106974. [Google Scholar] [CrossRef]
- Zhao, J.Y.; Ye, Y.T. Sensory evaluation of fishmeal and statistical analysis of fishmeal index. Feed Ind. 2018, 39, 59–64. [Google Scholar] [CrossRef]
- Li, P.; Niu, Z.Y.; Zhu, M.; Shao, K.Y.; Geng, J.; Li, H.C. Analysis of Volatile Compounds in Fish Meal Based on SPME-GC-MS and Electronic Nose. T Chin. Soc. Agr. Mach. 2020, 51, 397–405. [Google Scholar] [CrossRef]
- Wu, D.W.; Tang, F.; Hu, B.; Ye, Y.T. The impact of different raw materials and dying methods on the quality of fish meal. Feed Ind. 2020, 41, 29–34. [Google Scholar] [CrossRef]
- Yang, S.Y.; Pathak, S.; Tang, H.Y.; Zhang, D.; Chen, Y.Q.; Ntezimana, B.; Ni, D.J.; Yu, Z. Non-Targeted Metabolomics Reveals the Effects of Different Rolling Methods on Black Tea Quality. Foods. 2024, 13, 325. [Google Scholar] [CrossRef] [PubMed]
- Yun, J.; Cui, C.J.; Zhang, S.H.; Zhu, J.J.; Hou, R.Y. Use of Headspace GC/MS Combined with Chemometric Analysis to Identify the Geographic Origins of Black Tea. Food Chem. 2021, 360, 130033. [Google Scholar] [CrossRef] [PubMed]
- Dong, W.J.; Hu, R.S.; Long, Y.Z.; Li, H.H.; Zhang, Y.J.; Zhu, K.X.; Chu, Z. Comparative evaluation of the volatile profiles and taste properties of roasted coffee beans as affected by drying method and detected by electronic nose, electronic tongue, and HS-SPME-GC-MS. Food Chem. 2019, 272, 723–731. [Google Scholar] [CrossRef]
- Jiang, K.X.; Xu, K.L.; Wang, J.; Meng, F.Y.; Wang, B. Based on HS-SPME-GC-MS combined with GC-O-MS to analyze the changes of aroma compounds in the aging process of Citri Reticulatae Pericarpium. Food Biosci. 2023, 54, 102798. [Google Scholar] [CrossRef]
- Chen, H.F.; Zhang, Y.L.; Wang, X.Y.; Nie, X.; Liu, D.Y.; Zhao, Z.P. The Volatile Flavor Substances, Microbial Diversity, and Their Potential Correlations of Inner and Surface Areas within Chinese Qingcheng Mountain Traditional Bacon. Foods 2023, 12, 3729. [Google Scholar] [CrossRef]
- Kang, M.; Guo, Y.; Ren, Z.Y.; Ma, W.W.; Luo, Y.W.; Zhao, K.; Wang, X.W. Volatile Fingerprint and Differences in Volatile Compounds of Different Foxtail Millet (Setaria italica Beauv.) Varieties. Foods 2023, 12, 4273. [Google Scholar] [CrossRef]
- Pan, Y.Y.; Wang, Y.; Hao, W.J.; Zhou, S.; Duan, C.B.; Li, Q.S.; Wei, J.W.; Liu, G. Exploring the Role of Active Functional Microbiota in Flavor Generation by Integrated Metatranscriptomics and Metabolomics during Niulanshan Baijiu Fermentation. Foods 2023, 12, 4140. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Gao, G.; Tian, L.; Cao, Y.; Dong, X.; Huo, H.; Qi, D.; Zhang, Y.; Xu, J.; Liu, C. Changes of Volatile Organic Compounds of Different Flesh Texture Pears during Shelf Life Based on Headspace Solid-Phase Microextraction with Gas Chromatography–Mass Spectrometry. Foods 2023, 12, 4224. [Google Scholar] [CrossRef]
- Li, C.B.; Xin, M.; Li, L.; He, X.M.; Yi, P.; Tang, Y.Y.; Li, J.M.; Zheng, F.J.; Liu, G.M.; Sheng, J.F. Characterization of the aromatic profile of purple passion fruit (Passiflora edulis Sims) during ripening by HS-SPME-GC/MS and RNA sequencing. Food Chem. 2021, 355, 129685. [Google Scholar] [CrossRef]
- Xi, B.N.; Zhang, J.J.; Xu, X.; Li, C.; Shu, Y.; Zhang, Y.; Shi, X.M.; Shen, Y.H. Characterization and metabolism pathway of volatile compounds in walnut oil obtained from various ripening stages via HS-GC-IMS and HS-SPME-GC–MS. Food Chem. 2024, 435, 137547. [Google Scholar] [CrossRef]
- Zhou, M.Z.; Xiong, G.Q.; Qiao, Y.; Liao, L.; Xiang, Y.F.; Wang, L.; Wu, W.J.; Li, X.; Shi, L.; Ding, A.Z. Changes and Correlation of Odor and Freshness of Channel Catfish during Cold Storage. Meat. Res. 2020, 34, 68–74. [Google Scholar] [CrossRef]
- Zhang, J.J. Differences of Odor Characteristics and Influencing Factorsbetween White Croaker and Small Yellow Croaker; Shanghai Ocean University: Shanghai, China, 2020. [Google Scholar] [CrossRef]
- GB/T19164-2021; Feed Material-Fish Meal. General Administration of Quality Supervision. Standards Press of China: Beijing, China, 2021.
- GB5009.237-2016; National Food Safety Standards-Determination of Food pH. Standards Press of China: Beijing, China, 2016.
- GB/T13092-2006; Enumeration of Molds Count in Feeds. Standards Press of China: Beijing, China, 2006.
- Shi, C.; Cui, J.Y.; Lu, H.; Shen, H.X.; Luo, Y.K. Changes in biogenic amines of silver carp (Hypophthalmichthys molitrix) fillets stored at different temperatures and their relation to total volatile base nitrogen, microbiological and sensory score. J. Sci. Food Agr. 2012, 92, 3079–3084. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Liang, L.; Xia, W.; Regenstein, J.M.; Zhou, P. Biochemical and physical changes of grass carp (Ctenopharyngodon idella) fillets stored at −3 and 0 °C. Food Chem. 2013, 140, 105–114. [Google Scholar] [CrossRef]
- Mehdi, A.; Masoud, R.; Gholamali, F. Influence of chitosan/clay functional bionanocomposite activated with rosemary essential oil on the shelf life of fresh silver carp. Int. J. Food Sci. Technol. 2013, 49, 811–818. [Google Scholar] [CrossRef]
- Yang, Q.Q.; Qiu, Y.; Yu, Y.G.; Xiao, X.L.; Wu, H. Volatile Component Analysis in Fresh and Defatted Deodorized Pseudosciaena crocea. Food Sci. 2012, 33, 206–210. [Google Scholar]
- Sánchez-Sevilla, J.F.; Cruz-Rus, E.; Valpuesta, V.; Botella, M.A.; Amaya, I. Deciphering gamma-decalactone biosynthesis in strawberry fruit using a combination of genetic mapping, RNA-Seq and eQTL analyses. BMC Genom. 2014, 15, 218. [Google Scholar] [CrossRef]
- Cao, C.C.; Xie, J.C.; Hou, L.; Zhao, J.; Chen, F.; Xiao, Q.F.; Zhao, M.Y.; Fan, M.D. Effect of glycine on reaction of cysteine-xylose: Insights on initial Maillard stage intermediates to develop meat flavor. Food Res. Int. 2016, 444, 99. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.W.; Zhang, M. Analysis of Volatile Compounds in Chinese Mitten Crab (Eriocheir Sinensis). J. Food Drug Anal. 2006, 14, 11. [Google Scholar] [CrossRef]
- Drumm, T.D.; Spanier, A.M. Changes in the content of lipid autoxidation and sulfur-containing compounds in cooked beef during storage. J. Agr. Food Chem. 1991, 39, 336–343. [Google Scholar] [CrossRef]
- Piveteau, F.; Le Guen, S.; Gandemer, G.; Baud, J.P.; Prost, C.; Demaimay, M. Aroma of Fresh Oysters Crassostrea gigas: Composition and Aroma Notes. J. Agr. Food Chem. 2000, 48, 4851. [Google Scholar] [CrossRef] [PubMed]
- Turchini, G.M.; Ivan, G.; Fabio, C.; Moretti, V.M.; Franco, V. Discrimination of origin of farmed trout by means of biometrical parameters, fillet composition and flavor volatile compounds. Ital. J. Anim. Sci. 2010, 3, 123–140. [Google Scholar] [CrossRef]
- Iglesias, J.; Medina, I. Solid-phase microextraction method for the determination of volatile compounds associated to oxidation of fish muscle. J. Chromatogr. A 2008, 1192, 9–16. [Google Scholar] [CrossRef] [PubMed]
- Jin, Y.; Bu, T.T.; Li, M.; Zheng, L.; Song, Z.G.; Li, H.S. Analysis of the Volatile Components of Dried Cuttlefish by Electronic Nose Combined with HS-SPME-GC-MS. Food Sci. 2016, 37, 75–80. [Google Scholar] [CrossRef]
- Song, Z. The Study of Boiled Beef Flavor and the Research on Formation Mechanism of Maillard Reaction; Shanghai Institute of Technology: Shanghai, China, 2019. [Google Scholar] [CrossRef]
- Gou, Y.; Han, Y.; Li, J.; Niu, X.; Ma, G.; Xu, Q. Discriminant Analysis of Aroma Differences between Cow Milk Powder and Special Milk Powder (Donkey, Camel, and Horse Milk Powder) in Xinjiang Based on GC-IMS and Multivariate Statistical Methods. Foods 2023, 12, 4036. [Google Scholar] [CrossRef]
- Liu, H.C.; Yu, Y.S.; Zou, B.; Yu, Y.Y.; Yang, J.G.; Xu, Y.J.; Chen, X.W.; Yang, F. Evaluation of Dynamic Changes and Regularity of Volatile Flavor Compounds for Different Green Plum (Prunus mume Sieb. et Zucc) Varieties during the Ripening Process by HS-GC–IMS with PLS-DA. Foods 2023, 12, 551. [Google Scholar] [CrossRef]
- Li, P.; Geng, J.; Li, H.C.; Niu, Z.Y. Fish meal freshness detection by GBDT based on a portable electronic nose system and HS-SPME–GC–MS. Eur. Food Res. Technol. 2020, 246, 1129–1140. [Google Scholar] [CrossRef]
- Feng, X.Y.; Wang, H.W.; Wang, Z.R.; Huang, P.M.; Kan, J.Q. Discrimination and characterization of the volatile organic compounds in eight kinds of huajiao with geographical indication of China using electronic nose, HS-GC-IMS and HS-SPME-GC–MS. Food Chem. 2022, 375, 131671. [Google Scholar] [CrossRef] [PubMed]
| Composition | Domestic Fishmeal | Imported Fishmeal |
|---|---|---|
| Protein | ≥63% | ≥68% |
| Fat | ≤12% | ≤10% |
| Moisture | ≤10% | ≤10% |
| Salt and sand | ≤5% | ≤4% |
| Sand only | ≤2% | ≤1% |
| Ash | ≤17% | ≤18% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Geng, J.; Cao, Q.; Jiang, S.; Huangfu, J.; Wang, W.; Niu, Z. Evaluation of Dynamic Changes of Volatile Organic Components for Fishmeal during Storage by HS-SPME-GC-MS with PLS-DA. Foods 2024, 13, 1290. https://doi.org/10.3390/foods13091290
Geng J, Cao Q, Jiang S, Huangfu J, Wang W, Niu Z. Evaluation of Dynamic Changes of Volatile Organic Components for Fishmeal during Storage by HS-SPME-GC-MS with PLS-DA. Foods. 2024; 13(9):1290. https://doi.org/10.3390/foods13091290
Chicago/Turabian StyleGeng, Jie, Qing Cao, Shanchen Jiang, Jixuan Huangfu, Weixia Wang, and Zhiyou Niu. 2024. "Evaluation of Dynamic Changes of Volatile Organic Components for Fishmeal during Storage by HS-SPME-GC-MS with PLS-DA" Foods 13, no. 9: 1290. https://doi.org/10.3390/foods13091290




