Bioactive Compounds and Antioxidant Capacity of Camarosa and Selva Strawberries (Fragaria x ananassa Duch.)
Abstract
:1. Introduction
2. Experimental Section
2.1. Plant Material
2.2. Strawberry Sample Preparation
2.3. Apparatus and Software
2.4. Chemicals and Reagents
2.5. Ascorbic Acid and Vitamin C Content
2.6. Phenolic Compounds by HPLC
2.7. Antioxidant Capacity, Total Anthocyanins and Total Phenolics Content
2.7.1. Extracts Preparation
2.7.2. Total Anthocyanins Content
2.7.3. Total Phenolics Content
2.7.4. Antioxidant Capacity by DPPH*
2.7.5. Antioxidant Capacity by FRAP
3. Results and Discussion
3.1. Vitamin C Content
| Cultivar | ||
|---|---|---|
| Camarosa | Selva | |
| Bioactive compounds | ||
| Vit C | 44.5 ± 3.2 a | 39.9 ± 15.8 a |
| AA | 40.2 ± 3.7 a | 27.5 ± 18.0 a |
| DHAA | 5.2 ± 3.7 a | 13.2 ± 1.6 b |
| TP | 295.3 ± 39.7 b | 248.4 ± 62.3 a |
| TA | 38.4 ± 10.2 b | 22.1 ± 4.2 a |
| Antioxidant capacity | ||
| DPPH* (AEAC) | 440.1 ± 8.1 b | 395.7 ± 7.2 a |
| FRAP | 2.4 ± 0.5 b | 1.9 ± 0.9 a |
3.2. Phenolic Compounds Content
| Phenolic compound | Concentration before acid hydrolysis | Concentration after acid hydrolysis | ||
|---|---|---|---|---|
| Camarosa | Selva | Camarosa | Selva | |
| Gallic acid | 1.36 ± 0.05 | - | 14.28 ± 1.35 a | 17.21 ± 1.30 b |
| Catechin | 1.70 ± 0.17 a | 3.35 ± 0.42 b | 0.48 ± 0.09 a | 0.67 ± 0.10 a |
| Epicatechin | - | - | 4.02 ± 0.28 b | 0.84 ± 0.20 a |
| Ferulic acid | - | - | 3.44 ± 0.32 b | 2.75 ± 0.41 a |
| Coumaric acid | - | - | 1.69 ± 0.20 a | 1.96 ± 0.18 a |
| Cyanidin | - | - | 4.46 ± 0.23 a | 5.28 ± 0.29 b |
| Pelargonidin | - | - | 25.20 ± 1.11 b | 18.67 ± 1.09 a |
| Rutin | 1.0 ± 0.10 a | 1.84 ± 0.17 b | 0.09 ± 0.01 a | 0.10 ± 0.02 a |
| Ellagic acid | 0.61 ± 0.09 a | 0.65 ± 0.07 a | 6.67 ± 0.46 a | 11.87 ± 2.00 b |
| Quercetin | - | - | 0.36 ± 0.09 b | 0.18 ± 0.04 a |
| Kaempferol | - | - | 0.09 ± 0.02 a | 0.62 ± 0.09 b |
| Total phenolics 1 | 4.66 ± 0.30 a | 5.82 ± 0.60 b | 60.79 ± 1.90 a | 60.20 ± 2.40 a |
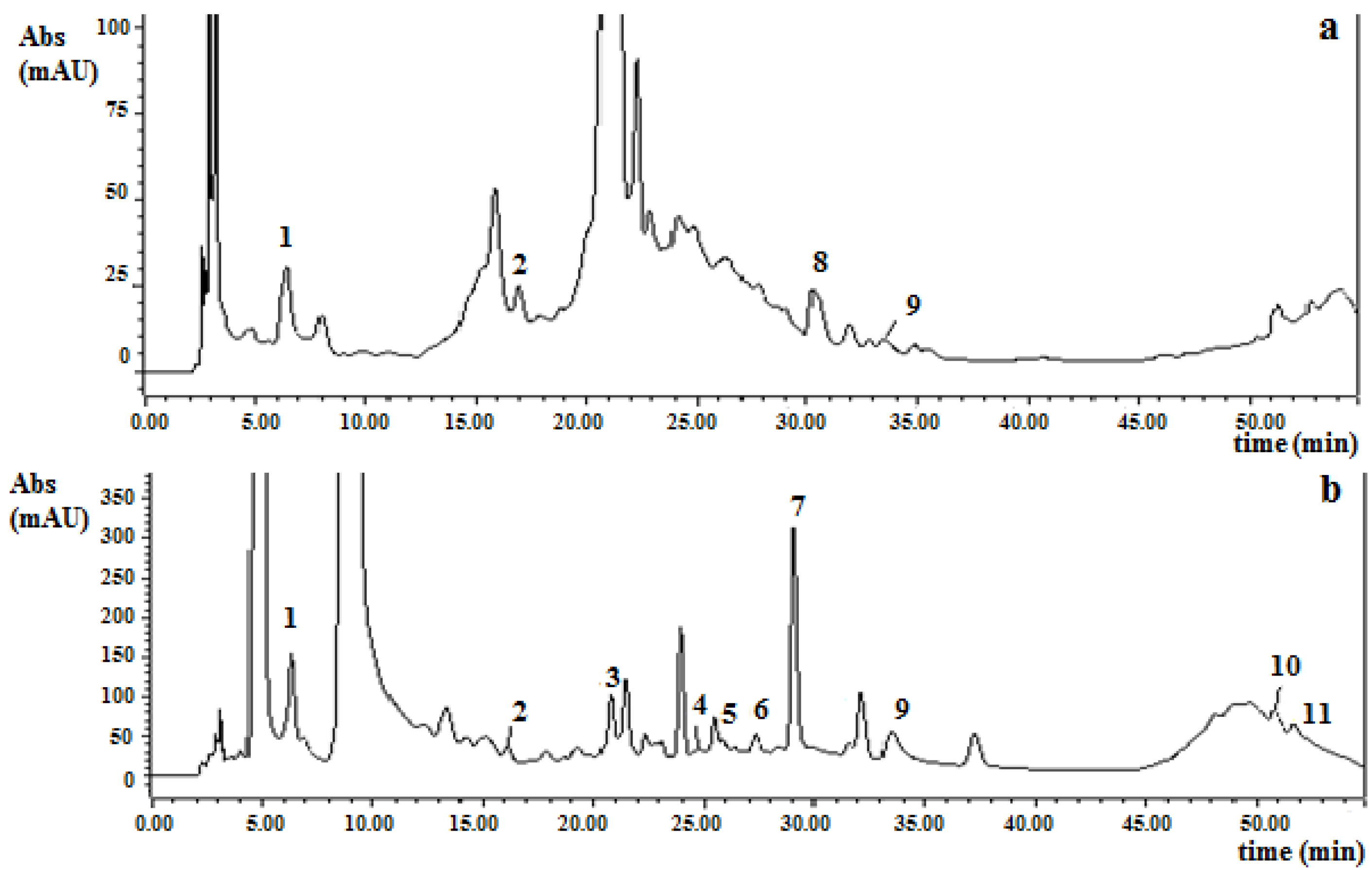
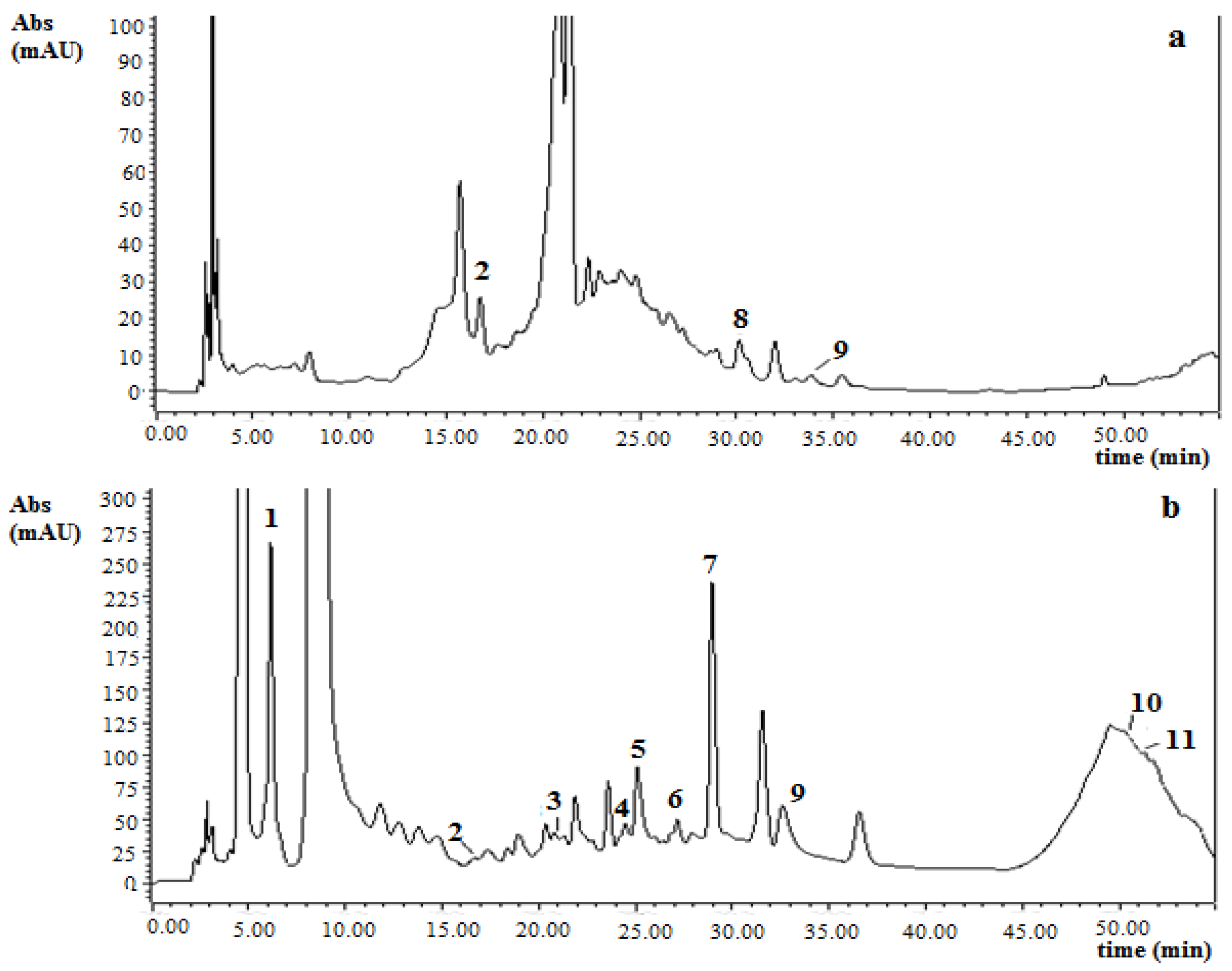
3.3. Antioxidant Capacity
4. Conclusions
Acknowledgments
Conflicts of Interest
References
- Cordenunsi, B.R.; Genovese, M.I.; Oliveira do Nascimento, J.R.; Hassimotto, N.M.A.; dos Santos, R.J.; Lajolo, F.M. Effects of temperature on the chemical composition and antioxidant activity of three strawberry cultivars. Food Chem. 2005, 91, 113–121. [Google Scholar] [CrossRef]
- Da Silva Pinto, M.; Lajolo, F.; Genovese, M. Bioactive compounds and quantification of ellagic acid in strawberries (Fragaria x ananassa Duch.). Food Chem. 2008, 107, 1629–1635. [Google Scholar] [CrossRef]
- Giampieri, F.; Tulipani, S.; Alvarez-Suarez, A.M.; Quiles, J.L.; Mezzetti, B.; Battino, M. The strawberry: Composition, nutritional quality, and impact on human health. Nutrition 2012, 28, 9–19. [Google Scholar] [CrossRef]
- Lopes Da Silva, F.; Escribano-Bailón, M.; Pérez Alonso, J.; Rivas-Gonzalo, J.C.; Santos-Buelga, C. Anthocyanin pigments in strawberry. LWT Food Sci. Technol. 2007, 40, 374–382. [Google Scholar] [CrossRef]
- Molina, N.; Giménez, L.; Richieri, C. Horticultural Sector Economy of Corrientes. Regional Strawberry Production and Its Relationship to the National Supply and MERCOSUR. 2007. (in Spanish). Available online: http://inta.gob.ar/documentos/economia-del-sector-horticola-de-corrientes.-produccion-frutillera-regional-y-su-relacion-con-la-oferta-nacional-y-del-mercosur (acceded on 14 March 2013).
- Saccone, K. Characterization of Coronda Area as a Producer of Strawberries. Secretary for Production and Environment, Municipality of Coronda, Santa Fe, Argentina. 2006. (in Spainsh). Available online: http://64.76.123.202/SAGPYA/economias_regionales/_frutas/_informes/Produccion_de_frutillas_en_coronda.pdf (acceded on 14 March 2013).
- Deutsch, M.J. Official Methods of Analysis; Association of Official Agricultural Chemists International: Arlington, VA, USA, 1995. [Google Scholar]
- Van De Velde, F.; Pirovani, M.E.; Cámara, M.S.; Güemes, D.R.; Bernardi, C. Optimization and validation of a UV-HPLC method for vitamin C determination in strawberries (Fragaria ananassa Duch.), using experimental designs. Food Anal. Methods 2012, 5, 1097–1104. [Google Scholar] [CrossRef]
- Tarola, A.M.; van de Velde, F.; Salvagni, L.; Pretti, R. Determination of phenolic compounds in strawberries (Fragaria ananassa Duch) by high performance liquid chromatography with diode array detection. Food Anal. Methods 2013, 6, 227–237. [Google Scholar] [CrossRef]
- Jin-Heo, H.; Yong-Lee, C. Strawberry and its anthocyanins reduce oxidative stress-induced apoptosis in PC12 cells. J. Agric. Food Chem. 2005, 53, 1984–1989. [Google Scholar] [CrossRef]
- Singleton, V.L.; Rossi, J.A. Colorimetry of total phenolics with phosphomolybdic-phosphotungstic acid reagents. Am. J. Enol. Vitic. 1965, 16, 144–158. [Google Scholar]
- Sánchez-Moreno, C.; Plaza, L.; de Ancos, B.; Cano, M.P. Quantitative bioactive compounds assessment and their relative contribution to the antioxidant capacity of commercial orange juices. J. Sci. Food Agric. 2003, 83, 430–439. [Google Scholar] [CrossRef]
- Lim, Y.Y.; Lim, T.T.; Tee, J.J. Antioxidant properties of several tropical fruits: A comparative study. Food Chem. 2007, 103, 1003–1008. [Google Scholar] [CrossRef]
- Benzie, I.F.F.; Strain, J.J. Simultaneous automated measurement of the total antioxidant (reducing) capacity and ascorbic acid concentration. Redox. Rep. 1997, 3, 233–238. [Google Scholar]
- Tulipani, S.; Mezzetti, B.; Capocasa, F.; Bompadre, S.; Beekwilder, J.; Ric de Vos, C.H.; Capanoglu, E.; Bovy, A.; Battino, M. Antioxidants, phenolic compounds, and nutritional quality of different strawberry genotypes. J. Agric. Food Chem. 2008, 56, 696–704. [Google Scholar] [CrossRef]
- Pincemail, J.; Kevers, C.; Tabart, J.; Defraigne, J.O.; Dommes, J. Cultivars, culture conditions, and harvest time influence phenolic and ascorbic acid contents and antioxidant capacity of strawberry (Fragaria x ananassa). J. Food Sci. 2012, 77, 205–210. [Google Scholar] [CrossRef]
- Davey, M.W.; van Montagu, M.; Inzé, D.; Sanmartin, M.; Kanellis, A.; Smirnoff, N.; Benzie, I.J.J.; Strain, J.J.; Favell, D.; Fletcher, J. Rview: Plant l-ascorbic acid: chemistry, function, metabolism, bioavailability and effects of processing. J. Sci. Food Agric. 2000, 80, 825–860. [Google Scholar] [CrossRef]
- Häkkinen, S.; Törrönen, A. Content of flavonols and selected phenolic acids in strawberries and Vaccinium species: Infuence of cultivar, cultivation site and technique. Food Res. Int. 2000, 33, 517–524. [Google Scholar] [CrossRef]
- Mattila, P.; Kumpulainen, J. Determination of free and total phenolic acids in plant-derived foods by HPLC with diode-array detection. J. Agric. Food Chem. 2002, 50, 3660–3667. [Google Scholar] [CrossRef]
- Baettie, J.; Crozier, A.; Duthie, G.G. Potential health benefits of berries. Curr. Nutr. Food Sci. 2005, 1, 71–86. [Google Scholar] [CrossRef]
- Williner, M.R.; Pirovani, M.E.; Güemes, D.R. Ellagic acid content in strawberries of different cultivars and ripening stages. J. Sci. Food Agric. 2003, 83, 842–845. [Google Scholar] [CrossRef]
- Giné Bordonaba, J.; Crespo, P.; Terry, L. A new acetonitrile-free mobile phase for HPLC-DAD determination of individual anthocyanins in blackcurrant and strawberry fruits: A comparison and validation study. Food Chem. 2011, 129, 1265–1273. [Google Scholar] [CrossRef]
- Castro, I.; Gonçalves, J.A.; Vicente, A.A. Comparative study of Selva and Camarosa strawberries for the comercial market. J. Food Sci. 2002, 67, 2132–2136. [Google Scholar] [CrossRef] [Green Version]
- Prior, R.L.; Wu, X.; Schaich, K. Standardized methods for the determination of antioxidant capacity and phenolics in food and dietary supplements. J. Agric. Food Chem. 2005, 5, 4290–4302. [Google Scholar] [CrossRef]
- Halvorsen, B.L.; Holte, K.; Myhrstad, M.C.W.; Barikmo, I.; Hvattum, E.; Remberg, S.F.; Wold, A.B.; Haffner, K.; Baugerød, H.; Andersen, L.F.; et al. A systematic screening of total antioxidants in dietary plants. J. Nutr. 2002, 132, 461–471. [Google Scholar]
- Leong, L.P.; Shui, G. An investigation of antioxidant capacity of fruits in Singapore markets. Food Chem. 2007, 79, 69–75. [Google Scholar]
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Van De Velde, F.; Tarola, A.M.; Güemes, D.; Pirovani, M.E. Bioactive Compounds and Antioxidant Capacity of Camarosa and Selva Strawberries (Fragaria x ananassa Duch.). Foods 2013, 2, 120-131. https://doi.org/10.3390/foods2020120
Van De Velde F, Tarola AM, Güemes D, Pirovani ME. Bioactive Compounds and Antioxidant Capacity of Camarosa and Selva Strawberries (Fragaria x ananassa Duch.). Foods. 2013; 2(2):120-131. https://doi.org/10.3390/foods2020120
Chicago/Turabian StyleVan De Velde, Franco, Anna M. Tarola, Daniel Güemes, and María E. Pirovani. 2013. "Bioactive Compounds and Antioxidant Capacity of Camarosa and Selva Strawberries (Fragaria x ananassa Duch.)" Foods 2, no. 2: 120-131. https://doi.org/10.3390/foods2020120
APA StyleVan De Velde, F., Tarola, A. M., Güemes, D., & Pirovani, M. E. (2013). Bioactive Compounds and Antioxidant Capacity of Camarosa and Selva Strawberries (Fragaria x ananassa Duch.). Foods, 2(2), 120-131. https://doi.org/10.3390/foods2020120