Reducing Postharvest Losses during Storage of Grain Crops to Strengthen Food Security in Developing Countries
Abstract
:1. Introduction
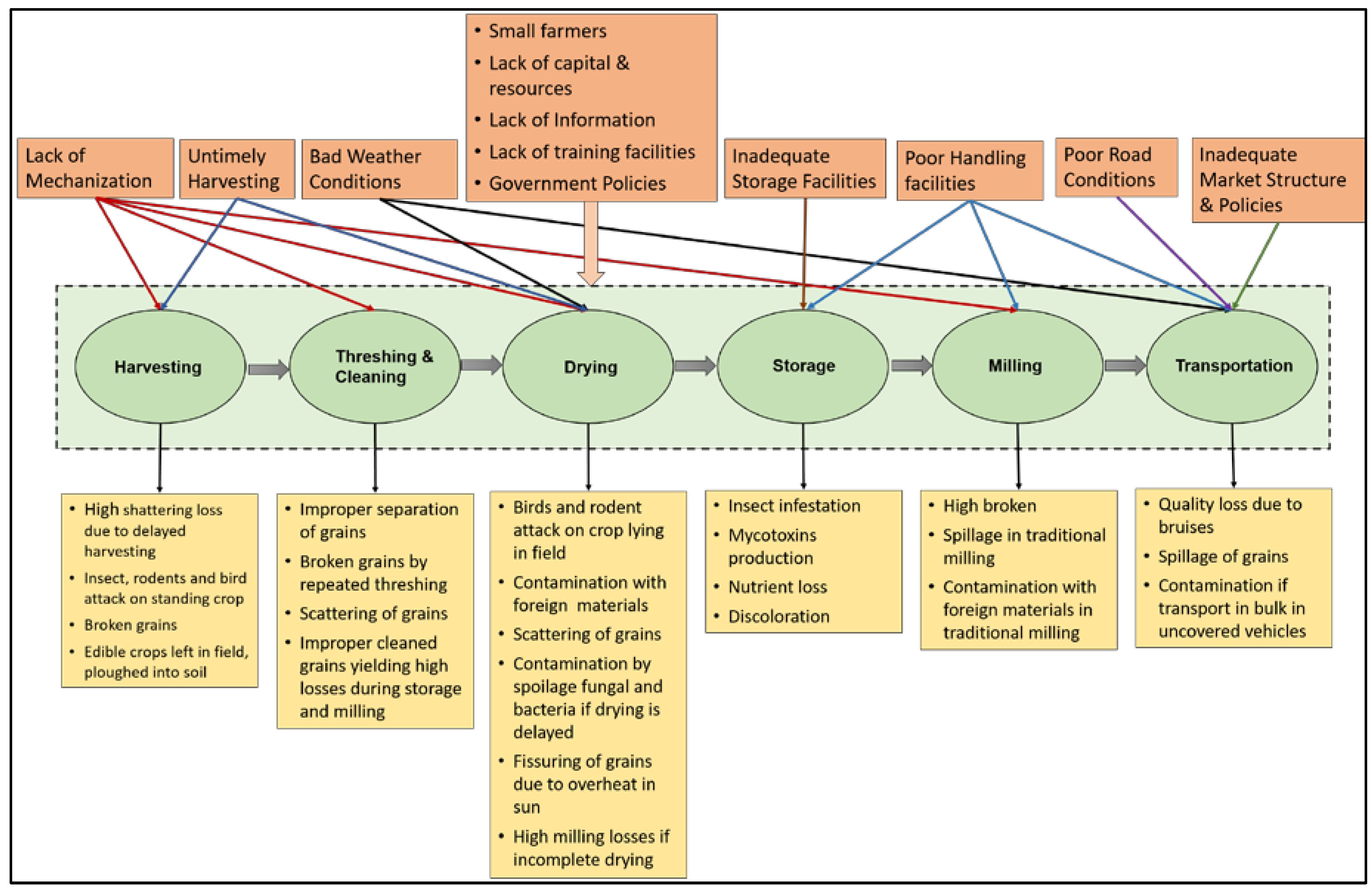
2. Grain Supply Chain
2.1. Harvesting
2.2. Threshing and Cleaning
2.3. Drying
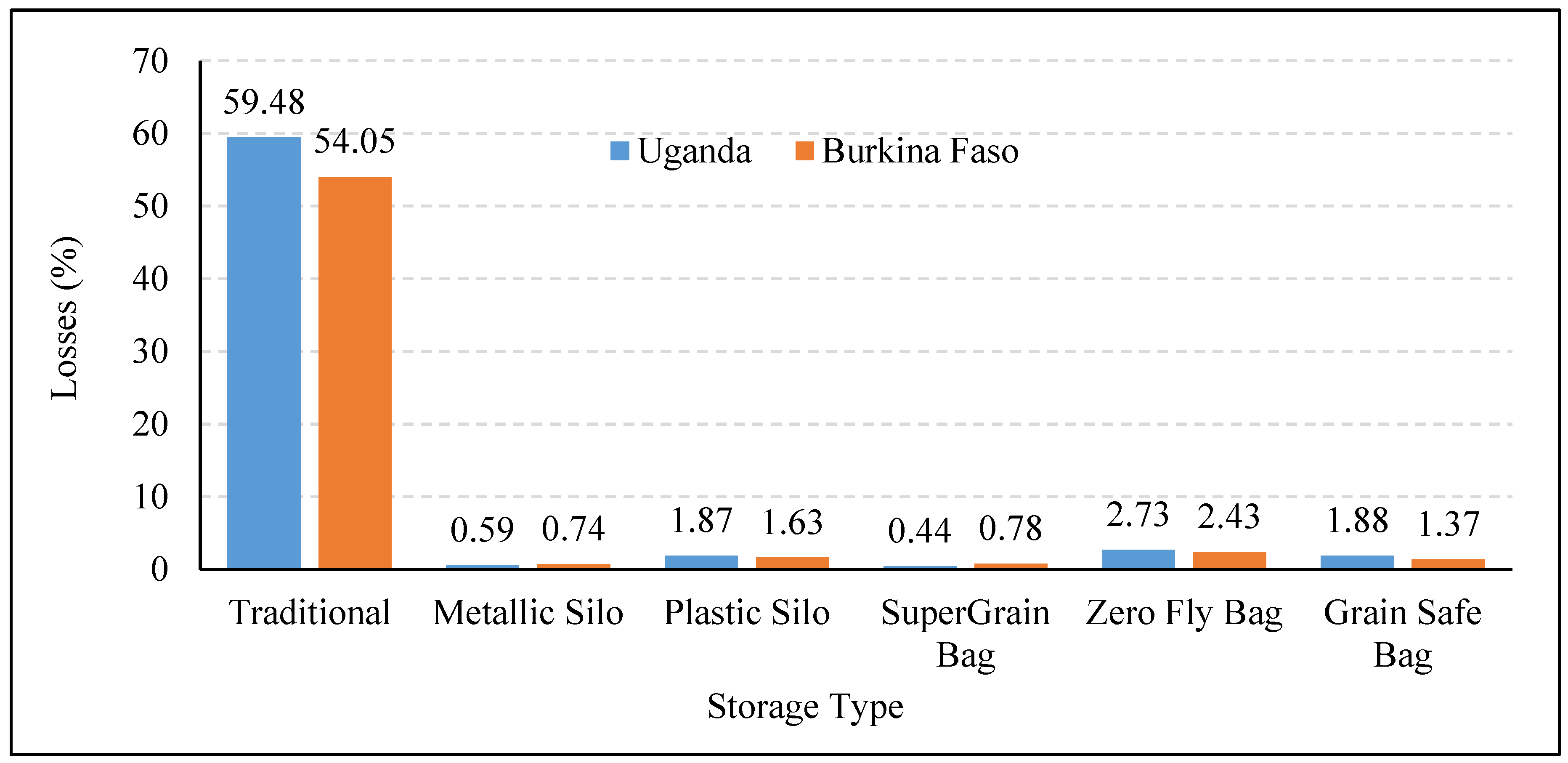
2.4. Storage
2.5. Transportation
2.6. Milling
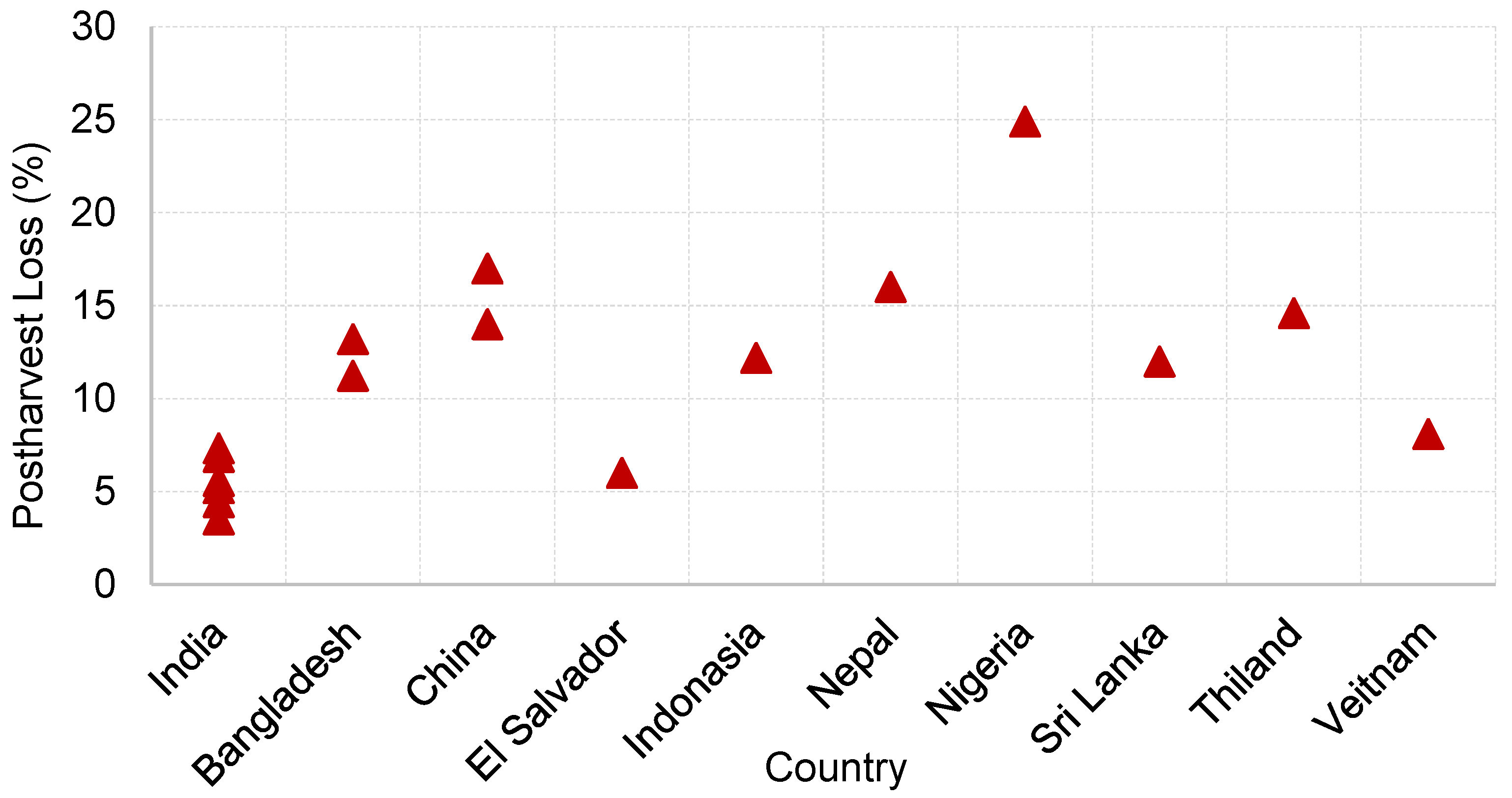
3. Postharvest Losses of Cereal Crops in Developing Countries
3.1. Rice
3.2. Wheat
3.3. Maize
4. Storage Losses in Developing Countries
4.1. Insect Infestation
4.2. Mycotoxins
5. Interventions to Reduce Storage Losses for Smallholders
5.1. Chemical Fumigation
5.2. Natural Insecticides
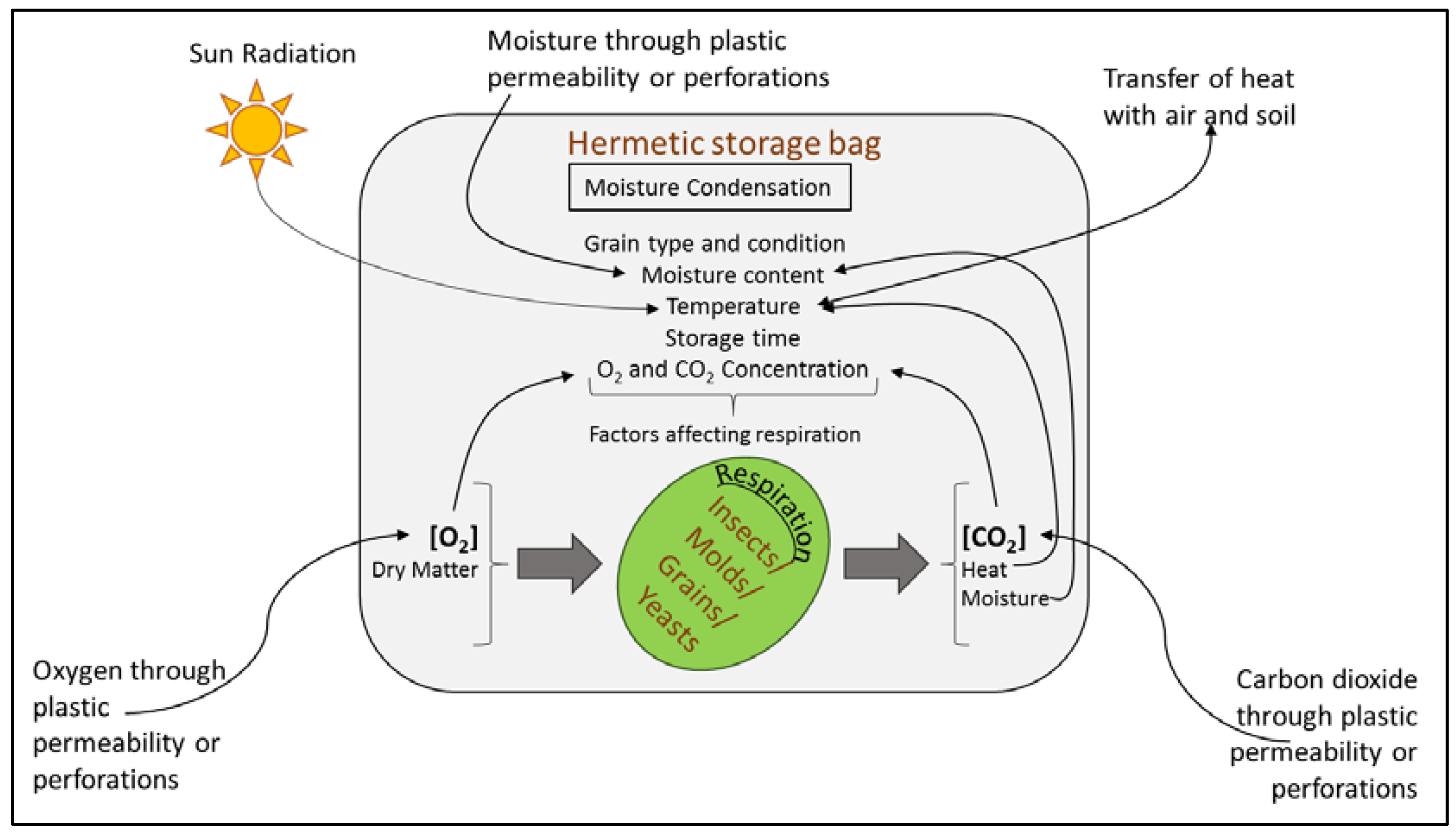
5.3. Hermetic Storage
6. Conclusions
Conflicts of Interest
References
- Godfray, H.C.J.; Beddington, J.R.; Crute, I.R.; Haddad, L.; Lawrence, D.; Muir, J.F.; Pretty, J.; Robinson, S.; Thomas, S.M.; Toulmin, C. Food security: The challenge of feeding 9 billion people. Science 2010, 327, 812–818. [Google Scholar] [CrossRef] [PubMed]
- Hodges, R.J.; Buzby, J.C.; Bennett, B. Postharvest losses and waste in developed and less developed countries: Opportunities to improve resource use. J. Agric. Sci. 2011, 149, 37–45. [Google Scholar] [CrossRef]
- Parfitt, J.; Barthel, M.; Macnaughton, S. Food waste within food supply chains: Quantification and potential for change to 2050. Philos. Trans. R. Soc. B Biol. Sci. 2010, 365, 3065–3081. [Google Scholar] [CrossRef] [PubMed]
- Bourne, M. Post Harvest Food Losses—the Neglected Dimension in Increasing the World Food Supply; Department of Food Science and Technology, Cornell University: Ithaca, NY, USA, 1977. [Google Scholar]
- Greeley, M. Food, Technology and employment: The fram-level post-harvset system in developing countries. J. Agric. Econ. 1986, 37, 333–347. [Google Scholar] [CrossRef]
- Kitinoja, L.; Saran, S.; Roy, S.K.; Kader, A.A. Postharvest technology for developing countries: Challenges and opportunities in research, outreach and advocacy. J. Sci. Food Agric. 2011, 91, 597–603. [Google Scholar] [CrossRef] [PubMed]
- Pantenius, C. Storage losses in traditional maize granaries in Togo. Int. J. Trop. Insect Sci. 1988, 9, 725–735. [Google Scholar] [CrossRef]
- Gustavsson, J.; Cederberg, C.; Sonesson, U.; van Otterdijk, R.; Meybeck, A. Global Food Losses and Food Waste; Food and Agriculture Organization of the United Nations: Rome, Italy, 2011. [Google Scholar]
- Aulakh, J.; Regmi, A.; Fulton, J.R.; Alexander, C. Estimating post-harvest food losses: Developing a consistent global estimation framework. In Proceedings of the Agricultural & Applied Economics Association’s 2013 AAEA & CAES Joint Annual Meeting, Washington, DC, USA, 4–6 August 2013.
- Buzby, J.C.; Farah-Wells, H.; Hyman, J. The estimated amount, value, and calories of postharvest food losses at the retail and consumer levels in the United States. Available online: https://papers.ssrn.com/sol3/papers.cfm?abstract_id=2501659 (accessed on 31 May 2015).
- Boxall, R.A. Post-harvest losses to insects—A world review. Int. Biodeterior. Biodegrad. 2001, 48, 137–152. [Google Scholar] [CrossRef]
- Food and Agriculture Organization of United Nations. Global Initiative on Food Losses and Waste Reduction; FAO: Rome, Italy, 2014. [Google Scholar]
- Lipinski, B.; Hanson, C.; Lomax, J.; Kitinoja, L.; Waite, R.; Searchinger, T. Installment 2 of “Creating a Sustainable Food Future” Reducing Food Loss and Waste; Working Paper; World Resource Institute: Washington, DC, USA, 2013. [Google Scholar]
- Fox, T. Global Food: Waste Not, Want Not; Institution of Mechanical Engineers: Westminster, London, UK, 2013. [Google Scholar]
- Abass, A.B.; Ndunguru, G.; Mamiro, P.; Alenkhe, B.; Mlingi, N.; Bekunda, M. Post-harvest food losses in a maize-based farming system of semi-arid savannah area of Tanzania. J. Stored Prod. Res. 2014, 57, 49–57. [Google Scholar] [CrossRef]
- Zorya, S.; Morgan, N.; Diaz Rios, L.; Hodges, R.; Bennett, B.; Stathers, T.; Mwebaze, P.; Lamb, J. Missing Food: The Case of Postharvest Grain Losses in Sub-Saharan Africa; The international bank for reconstruction and development/the world bank: Washington, DC, USA, 2011. [Google Scholar]
- Food and Agriculture Organization of the United Nations. Food Wastage Footprint—Impacts on Natural Resources; FAO: Rome, Italy, 2013. [Google Scholar]
- Gesellschaft für Internationale Zusammenarbeit. Post-Harvest Losses of Rice in Nigeria and Their Ecological Footprint; Deutsche Gesellschaft für Internationale Zusammenarbeit (GIZ) GmbH: Bonn/Eschborn, Germany, 2014. [Google Scholar]
- Food and Agriculture Organization of the United Nations. Toolkit: Reducing the Food Wastage Footprint; FAO: Rome, Italy, 2013. [Google Scholar]
- Khan, M.A. Post Harvest Losses of RICE; Khan, S.L., Ed.; Trade Development Authority of Pakistan: Karachi, Pakistan, 2010. [Google Scholar]
- Baloch, U.K. Wheat: Post-Harvest Operations; Lewis, B., Mejia, D., Eds.; Pakistan Agricultural Research Council: Islamabad, Pakistan, 2010; pp. 1–21. [Google Scholar]
- Grover, D.; Singh, J. Post-harvest losses in wheat crop in Punjab: Past and present. Agric. Econ. Res. Rev. 2013, 26, 293–297. [Google Scholar]
- Kannan, E.; Kumar, P.; Vishnu, K.; Abraham, H. Assessment of Pre and Post Harvest Losses of Rice and Red Gram in Karnataka; Agricultural Development and Rural Transformation Centre, Institute for Social and Economic Change: Banglore, India, 2013. [Google Scholar]
- De Lucia, M.; Assennato, D. Agricultural Engineering in Development: Post-Harvest Operations and Management of Foodgrains; FAO: Rome, Italy, 2006. [Google Scholar]
- Shah, D. Assessment of Pre and Post Harvest Losses in Tur and Soyabean Crops in Maharashtra; Agro-Economic Research Centre Gokhale Institute of Politics and Economics: Pune, India, 2013. [Google Scholar]
- Alavi, H.R.; Htenas, A.; Kopicki, R.; Shepherd, A.W.; Clarete, R. Trusting Trade and the Private Sector for Food Security in Southeast Asia; World Bank Publications: Washington, DC, USA, 2012. [Google Scholar]
- Sarkar, D.; Datta, V.; Chattopadhyay, K.S. Assessment of Pre and Post Harvest Losses in Rice and Wheat in West Bengal; Agro-Economic Research Centre, Visva-Bharati, Santiniketan: Santiniketan, India, 2013. [Google Scholar]
- Calverley, D. A Study of Loss Assessment in Eleven Projects in Asia Concerned With Rice; FAO: Rome, Italy, 1996. [Google Scholar]
- Bala, B.K.; Haque, M.A.; Hossain, M.A.; Majumdar, S. Post Harvest Loss and Technical Efficiency of Rice, Wheat and Maize Production System: Assessment and Measures for Strengthening Food Security; Bangladesh Agricultural University: Mymensingh, Bangladesh, 2010. [Google Scholar]
- Majumder, S.; Bala, B.; Arshad, F.M.; Haque, M.; Hossain, M. Food security through increasing technical efficiency and reducing postharvest losses of rice production systems in Bangladesh. Food Secur. 2016, 8, 361–374. [Google Scholar] [CrossRef]
- Costa, S.J. Reducing Food Losses in Sub-Saharan Africa (Improving Post-Harvest Management and Storage Technologies of Smallholder Farmers); UN World Food Programme: Kampala, Uganda, 2014. [Google Scholar]
- Abedin, M.; Rahman, M.; Mia, M.; Rahman, K. In-store losses of rice and ways of reducing such losses at farmers’ level: An assessment in selected regions of Bangladesh. J. Bangladesh Agric. Univ. 2012, 10, 133–144. [Google Scholar] [CrossRef]
- Basavaraja, H.; Mahajanashetti, S.; Udagatti, N.C. Economic analysis of post-harvest losses in food grains in India: A case study of Karnataka. Agric. Econ. Res. Rev. 2007, 20, 117–126. [Google Scholar]
- Shukla, B.D.; Patil, R.T. Post-harvest management, fight hunger with FAO. India Grains 2002, 4, 20–22. [Google Scholar]
- Nagpal, M.; Kumar, A. Grain losses in India and government policies. Qual. Assur. Saf. Crops Foods 2012, 4, 143. [Google Scholar] [CrossRef]
- Schulten, G. Post-harvest losses in tropical Africa and their prevention. Food Nutr. Bull. 1982, 4, 2–9. [Google Scholar]
- Kannan, E. Assessment of Pre and Post Harvest Losses of Important Crops in India; Agricultural Development and Rural Transformation Centre, Institute for Social and Economic Change: Banglore, India, 2014. [Google Scholar]
- Pathak, O.P.; Gupta, R.A. A study on post-harvest losses of food grains in Rajasthan. Int. J. Appl. Res. Stud. 2015, 4, 1–7. [Google Scholar]
- Sharma, H.O.; Rathi, D. Asseessment of Pre and Post Harvset Losses of Wheat and Soybean in Madhya Pradesh; Jabalpur, M.P., Ed.; Agro-Economic Research Centre for Madhya Pradesh and Chhattisgarh, Jawaharlal Nehru Krishi Vishwa Vidyalaya: Jabalpur, India, 2013. [Google Scholar]
- Inter-American Institute for Cooperation on Agriculture. Post-Harvest Losses in Latin America and the Caribbean: Challenges and Opportunities for Collaboration; IICA: Washington, DC, USA, 2013. [Google Scholar]
- African postharvset loss information system. Available online: http://www.aphlis.net/ (accessed on 15 December 2015).
- Kaminski, J.; Christiaensen, L. Post-harvest loss in sub-Saharan Africa—What do farmers say? Glob. Food Secur. 2014, 3, 149–158. [Google Scholar] [CrossRef]
- Boxall, R. Damage and loss caused by the larger grain borer Prostephanus truncatus. Integr. Pest Manag. Rev. 2002, 7, 105–121. [Google Scholar] [CrossRef]
- Hell, K.; Cardwell, K.; Setamou, M.; Poehling, H.M. The influence of storage practices on aflatoxin contamination in maize in four agroecological zones of Benin, West Africa. J. Stored Prod. Res. 2000, 36, 365–382. [Google Scholar] [CrossRef]
- Wambugu, P.; Mathenge, P.; Auma, E.; van Rheenen, H. Efficacy of traditional maize (Zea mays L.) seed storage methods in western Kenya. Afr. J. Food Agric. Nutr. Dev. 2009, 9, 110–1128. [Google Scholar]
- Tapondjou, L.; Adler, C.; Bouda, H.; Fontem, D. Efficacy of powder and essential oil from Chenopodium ambrosioides leaves as post-harvest grain protectants against six-stored product beetles. J. Stored Prod. Res. 2002, 38, 395–402. [Google Scholar] [CrossRef]
- Compton, J.A.F.; Magrath, P.A.; Addo, S.; Gbedevi, S.R.; Amekupe, S.; Agbo, B.; Penni, H.; Kumi, S.P.; Bokor, G.; Awuku, M.; et al. The influence of insect damage on the market value of maize grain: A comparison of two research methods. In Proceedings of the Premier Colloque International: Lutte Contre les Déprédateurs des Denrées Stockées par les Agriculteurs en Afrique, Lome, Togo, 10–14 February 1997.
- Baoua, I.; Amadou, L.; Ousmane, B.; Baributsa, D.; Murdock, L. PICS bags for post-harvest storage of maize grain in West Africa. J. Stored Prod. Res. 2014, 58, 20–28. [Google Scholar] [CrossRef]
- Kimenju, S.C.; de Groote, H. Economic analysis of alternative maize storage technologies in Kenya. In Proceedings of the Joint 3rd African Association of Agricultural Economists (AAAE) and 48th Agricultural Economists Association of South Africa (AEASA) Conference, Cape Town, South Africa, 19–23 September 2010.
- Meikle, W.; Markham, R.; Nansen, C.; Holst, N.; Degbey, P.; Azoma, K.; Korie, S. Pest management in traditional maize stores in West Africa: A farmer’s perspective. J. Econ. Entomol. 2002, 95, 1079–1088. [Google Scholar] [PubMed]
- Markham, R.; Bosque-Pérez, N.; Borgemeister, C.; Meikle, W. Developing Pest Management Strategies for Sitophilus Zeamais and Prostephanus Truncatus in the Tropics; FAO: Rome, Italy, 1994. [Google Scholar]
- Tefera, T.; Mugo, S.; Likhayo, P. Effects of insect population density and storage time on grain damage and weight loss in maize due to the maize weevil Sitophilus zeamais and the larger grain borer Prostephanus truncatus. Afr. J. Agric. Res. 2011, 6, 2247–2254. [Google Scholar]
- Magrath, P.; Compton, J.; Motte, F.; Awuku, M. Coping With a New Storage Insect Pest: The Impact of the Larger Grain Borer in Eastern Ghana; Natural Resources Institute: Chatham, UK, 1996. [Google Scholar]
- Magrath, P.; Compton, J.; Ofosu, A.; Motte, F. Cost-Benefit Analysis of Client Participation in Agricultural Research: A Case Study From Ghana; Agricultural and Extension Network Paper No. 74b; Overseas Development Institute: London, UK, 1997; pp. 19–39. [Google Scholar]
- Patel, K.; Valand, V.; Patel, S. Powder of neem-seed kernel for control of lesser grainborer (Rhizopertha dominica) in wheat (Triticum aestivum). Indian J. Agric. Sci. 1993, 63, 754–755. [Google Scholar]
- Kumar, R.; Mishra, A.K.; Dubey, N.; Tripathi, Y. Evaluation of Chenopodium ambrosioides oil as a potential source of antifungal, antiaflatoxigenic and antioxidant activity. Int. J. Food Microbiol. 2007, 115, 159–164. [Google Scholar] [CrossRef] [PubMed]
- Magan, N.; Aldred, D. Post-harvest control strategies: Minimizing mycotoxins in the food chain. Int. J. Food Microbiol. 2007, 119, 131–139. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kimanya, M.E.; Meulenaer, B.; Camp, J.; Baert, K.; Kolsteren, P. Strategies to reduce exposure of fumonisins from complementary foods in rural Tanzania. Matern. Child Nutr. 2012, 8, 503–511. [Google Scholar] [CrossRef] [PubMed]
- Suleiman, R.A.; Kurt, R.A. Current maize production, postharvest losses and the risk of mycotoxins contamination in Tanzania. In Proceedings of the American Society of Agricultural and Biological Engineers Annual International Meeting, New Orleans, LA, USA, 26–29 July 2015.
- Suleiman, R.A.; Rosentrater, K.A.; Bern, C.J. Effects of deterioration parameters on storage of maize: A review. J. Nat. Sci. Res. 2013, 3, 147. [Google Scholar]
- Turner, P.; Sylla, A.; Gong, Y.; Diallo, M.; Sutcliffe, A.; Hall, A.; Wild, C. Reduction in exposure to carcinogenic aflatoxins by postharvest intervention measures in west Africa: A community-based intervention study. Lancet 2005, 365, 1950–1956. [Google Scholar] [CrossRef]
- Williams, J.H.; Phillips, T.D.; Jolly, P.E.; Stiles, J.K.; Jolly, C.M.; Aggarwal, D. Human aflatoxicosis in developing countries: A review of toxicology, exposure, potential health consequences, and interventions. Am. J. Clin. Nutr. 2004, 80, 1106–1122. [Google Scholar] [PubMed]
- Tefera, T.; Kanampiu, F.; de Groote, H.; Hellin, J.; Mugo, S.; Kimenju, S.; Beyene, Y.; Boddupalli, P.M.; Shiferaw, B.; Banziger, M. The metal silo: An effective grain storage technology for reducing post-harvest insect and pathogen losses in maize while improving smallholder farmers’ food security in developing countries. Crop Prot. 2011, 30, 240–245. [Google Scholar] [CrossRef]
- WeiFen, Q.; ZuXun, J. Paddy and rice storage in China. In Advances in Stored Product Protection, Proceedings of the 8th International Working Conference on Stored Product Protection; CAB International: Wallingford, UK, 2003; pp. 26–39. [Google Scholar]
- Shaaya, E.; Kostjukovski, M.; Eilberg, J.; Sukprakarn, C. Plant oils as fumigants and contact insecticides for the control of stored-product insects. J. Stored Prod. Res. 1997, 33, 7–15. [Google Scholar] [CrossRef]
- De Groote, H.; Kimenju, S.C.; Likhayo, P.; Kanampiu, F.; Tefera, T.; Hellin, J. Effectiveness of hermetic systems in controlling maize storage pests in Kenya. J. Stored Prod. Res. 2013, 53, 27–36. [Google Scholar] [CrossRef]
- Mutungi, C.; Affognon, H.; Njoroge, A.; Baributsa, D.; Murdock, L. Storage of mung bean (Vigna radiata [L.] Wilczek) and pigeonpea grains (Cajanus cajan [L.] Millsp) in hermetic triple-layer bags stops losses caused by Callosobruchus maculatus (F.) (Coleoptera: Bruchidae). J. Stored Prod. Res. 2014, 58, 39–47. [Google Scholar]
- Savvidou, N.; Mills, K.A.; Pennington, A. Phosphine resistance in Lasioderma serricorne (F.) (Coleoptera: Anobiidae). In Advances in Stored Product Protection, Proceedings of the 8th International Working Conference on Stored Product Protection, York, UK, 22–26 July 2002; Credland, P.F.A., Armitage, D.M., Bell, C.H., Cogan, P.M., Highley, E., Eds.; CAB International: Wallingford, UK, 2003; pp. 702–712. [Google Scholar]
- Villers, P.; Navarro, S.; de Bruin, T. New applications of hermetic storage for grain storage and transport. In Proceedings of the 10th International Working Conference on Stored Product Protection, Estoril, Portugal, 27 June–2 July 2010.
- Adler, C.; Corinth, H.G.; Reichmuth, C. Modified atmospheres. In Alternatives to Pesticides in Stored-Product IPM; Springer: New York, NK, USA, 2000; pp. 105–146. [Google Scholar]
- Global Harvest Initiative. Global Agricultural Productivity Report—Global Revolutions in Agriculture: The Challenges and Promise of 2050; GHI: Washington, DC, USA, 2014. [Google Scholar]
- Cardoso, M.L.; Bartosik, R.E.; Rodríguez, J.C.; Ochandio, D. Factors affecting carbon dioxide concentration in interstitial air of soybean stored in hermetic plastic bags (silo-bag). In Proceedings of the 8th International Conference on Controlled Atmosphere and Fumigation in Stored Products, Chengdu, China, 21–26 September 2008.
- Bartosik, R.; Rodríguez, J.; Cardoso, L. Storage of corn, wheat soybean and sunflower in hermetic plastic bags. In Proceedings of the International Grain Quality and Technology Congress, Chicago, IL, USA, 15–18 July 2008.
- Zeigler, M.; Truitt Nakata, G. The next global breadbasket: How Latin America can feed the world: A Call to Action for Addressing Challenges & Developing Solutions; Inter-American Development Bank: Washington, DC, USA, 2014. [Google Scholar]
- Food and Agriculture Organization of the United Nations. Household MetalSilo: Key Allies in FAO’s Fight against Hunger; Agricultural and Food Engineering Technologies Service, FAO: Rome, Italy, 2008. [Google Scholar]
- Yusuf, B.L.; He, Y. Design, development and techniques for controlling grains post-harvest losses with metal silo for small and medium scale farmers. Afr. J. Biotechnol. 2013, 10, 14552–14561. [Google Scholar]
- Baoua, I.; Amadou, L.; Lowenberg-DeBoer, J.; Murdock, L. Side by side comparison of GrainPro and PICS bags for postharvest preservation of cowpea grain in Niger. J. Stored Prod. Res. 2013, 54, 13–16. [Google Scholar] [CrossRef]
- Somavat, P.; Huang, H.; Kumar, S.; Garg, M.; Danao, M.; Singh, V.; Paulsen, M.; Rausch, K.D. Hermetic wheat storage for small holder farmers in India. In Proceedings of the First International Congress on Postharvest Loss Prevention, Rome, Italy, 4–7 October 2015.
- Somavat, P.; Huang, H.; Kumar, S.; Garg, M.K.; Danao, M.G.C.; Singh, V.; Rausch, K.D.; Paulsen, M.R. Comparison of hermetic storage of wheat with traditional storage methods in India. In Proceedings of the American Society of Agricultural and Biological Engineers (ASABE) and the Canadian Society for Bioengineering (CSBE/SCGAB) Annual International Meeting, Montreal, QC, Canada, 13–16 July 2014.
- Baoua, I.; Amadou, L.; Baributsa, D.; Murdock, L. Triple bag hermetic technology for post-harvest preservation of Bambara groundnut (Vigna subterranea (L.) Verdc.). J. Stored Prod. Res. 2014, 58, 48–52. [Google Scholar] [CrossRef]
- Njoroge, A.; Affognon, H.; Mutungi, C.; Manono, J.; Lamuka, P.; Murdock, L. Triple bag hermetic storage delivers a lethal punch to Prostephanus truncatus (Horn) (Coleoptera: Bostrichidae) in stored maize. J. Stored Prod. Res. 2014, 58, 12–19. [Google Scholar] [CrossRef]
- Vales, M.; Rao, C.R.; Sudini, H.; Patil, S.; Murdock, L. Effective and economic storage of pigeonpea seed in triple layer plastic bags. J. Stored Prod. Res. 2014, 58, 29–38. [Google Scholar] [CrossRef]
- Ognakossan, K.E.; Tounou, A.K.; Lamboni, Y.; Hell, K. Post-harvest insect infestation in maize grain stored in woven polypropylene and in hermetic bags. Int. J. Trop. Insect Sci. 2013, 33, 71–81. [Google Scholar] [CrossRef]
- Awal, M.A.; Hossain, M.A.; Ali, M.R.; Alam, M.M. Effective Rice Storage Technologies for Smallholding Farmers of Bangladesh. In Proceedings of the First International Congress on Postharvest Loss Prevention, Rome, Italy, 4–7 October 2015.
- Sudini, H.; Rao, G.R.; Gowda, C.; Chandrika, R.; Margam, V.; Rathore, A.; Murdock, L. Purdue Improved Crop Storage (PICS) bags for safe storage of groundnuts. J. Stored Prod. Res. 2015, 64, 133–138. [Google Scholar] [CrossRef]
- Ben, D.C.; van Liem, P.; Dao, N.T.; Gummert, M.; Rickman, J.F. Effect of Hermetic Storage in the Super Bag on Seed Quality and Milled Rice Quality of Different Varieties in Bac Lieu, Vietnam. Int. Rice Res. Notes 2009. [Google Scholar] [CrossRef]
- Kitinoja, L. Innovative small-scale postharvest technologies for reducing losses in horticultural crops. Ethiop. J. Appl. Sci. Technol. 2013, 1, 9–15. [Google Scholar]
| Crop | Maturity Moisture Content | Crop | Maturity Moisture Content |
|---|---|---|---|
| Paddy | 22–28 | Beans | 30–40 |
| Maize | 23–28 | Groundnut | 30–35 |
| Sorghum | 20–25 | Sunflower | 9–10 |
| Country | Year | Losses (%) | Comments | Reference |
|---|---|---|---|---|
| Bangladesh | 2010 | 3.62 |
| [29] |
| India | 2013 | 1.84 |
| [22] |
| 2013 | 2.74 |
| [37] | |
| 2004 | 4.32 |
| [33] | |
| 2012 | 4.32 |
| [38] | |
| 2012 | 8.61 |
| [39] | |
| 2013 | 7.22 |
| [37] | |
| 2013 | 11.71 |
| [37] | |
| Peru | 2012 | 15–25 | - | [40] |
| Sub-Saharan Africa | 2013 | 15.2 | - | [41] |
| Country | Year | Losses (%) | Comments | Reference |
|---|---|---|---|---|
| Bangladesh | 2010 | 4.07 |
| [29] |
| Ecuador | 2012 | 10–30 |
| [40] |
| Guatemala | 2012 | 50 | - | [40] |
| Malawai | 2010 | 1.4 |
| [42] |
| Panama | 2012 | 20 |
| [40] |
| Peru | 2012 | 15–25 | - | [40] |
| Sub-Saharan Africa | 2013 | 17.8 | - | [41] |
| Tanzania | 2008 | 4.4 |
| [42] |
| 2010 | 2.9 | |||
| Uganda | 2009 | 5.9 |
| [42] |
| Type of Storage | Crop | Country | Duration of Storage | Investigations | Findings | Reference |
|---|---|---|---|---|---|---|
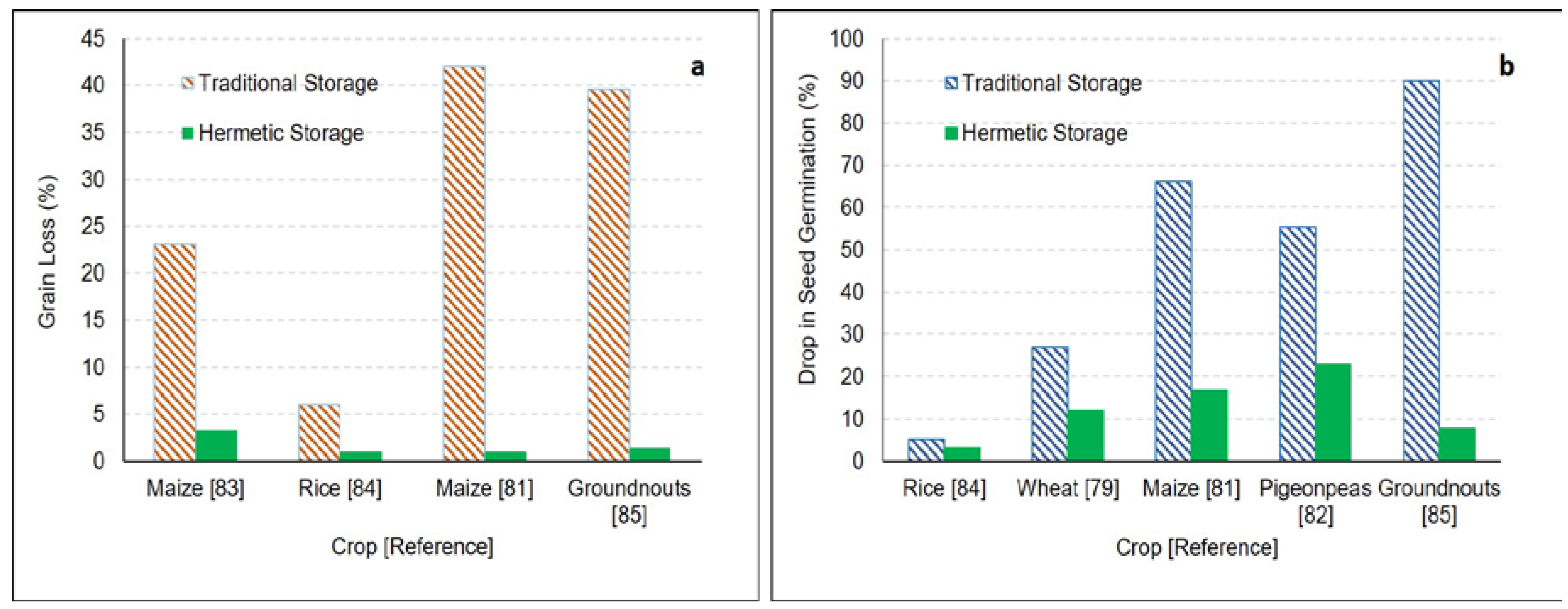
| SuperGrain Bags | Maize | Kenya | 6 months | Evaluated performance of hermetic storage (metal silos and super grain bags) and polypropylene bags to control infestation of pests. | Metal silo was the most effective option in controlling pest infestation. Metal silo was equally effective in controlling pest infestation even without any insecticide use. Supergrain bags were effective in controlling the infestation, however, the insect mortality was not complete. Bags were perforated by a larger grain borer. | [66] |
| Maize | Benin | 150 days | Compared performance of hermetic bags and woven polypropylene bags for storage of maize infested with Prostephanus truncatus (Horn) and Sitophilus zeamaiswas (Motschulsky). | Moisture levels remained unchanged in hermetic bags. Growth of insects (Prostephanus truncatus and Sitophilus zeamaiswas) was significantly less in hermetic bags. There were 0.5%–6% losses at end of storage compared to 19.2%–27.1% losses in woven bags. | [83] | |
| Rice | Bangladesh | 4 months | Compared performance of hermetic bags and traditional structures for storage of rice. | Moisture content of grains remained unchanged in hermetic bags. A total of 97% seed germination in hermetic bags vs. 95% in traditional storage. A total of 1% damaged grains in hermetic bags in contrary to 6% in traditional storage. | [84] | |
| PICS bags | Mung bean, pigeonpea | Kenya | 6 months | Evaluated performance of hermetic bags for naturally and artificially infested (Callosobruchus maculatus (F.)) grains. | One hundred grain weight, infestation, and grain damage remain unchanged in hermetic bags. There was 60.3 to 76.9% damage in mung beans and 75.8%–95.7% grain damage for pigeonpeas stored in woven polypropylene. | [67] |
| Maize | Benin, Burkina Faso and Ghana | 6.5 months | Evaluated performance of hermetic bags for preserving maize quality during storage. | There was 95%–100% insect mortality in hermetic bags. PICS bags maintained the seed viability and germination. | [48] | |
| Maize | Kenya | 6 months | Evaluated performance of hermetic bags for naturally and artificially infested (Prostephanus truncates) grains. | There was 0%–2% weight loss in PICS bags compared to 36.3%–47.7% weight loss in woven polypropylene bags. There was a 13%–20.1% reduction in germination for grains stored in PICS bags compared to a 54.1%–78.4% drop for grains stored in wove bags. | [81] | |
| Bambara groundnut | Maradi, Niger | 7 months | Evaluated performance of hermetic bags for preserving naturally infested Bambara groundnut quality during storage. | For highly infested grains, oxygen concentrations decreased significantly in hermetic bags contrary to unchanged in woven bags. Infestation level of C. maculatus in woven bags was 128 times higher than that of hermetic bags. There was a 34.8%–89.3% decrease in seed viability in woven bags, whereas, there was no change in grains stored in PICS bags. Abrasions were observed in inner HDPE bags. | [80] | |
| Pigeonpeas | India | 8 months | Compared performance of hermetic bags vs. gunny bags for storage of pigeonpeas. | Germination of infested grains in gunny bags dropped to 44.5% compared to high germination (77%) for grains stored for 8 months in hermetic bags. | [82] | |
| Groundnuts | India | 4 months | Evaluated performance of hermetic bags for preserving the quality of natural and artificial infested groundnuts. | There was a 0.8% decrease in seed weight for groundnut stored in hermetic bags compared to 7.2% in cloth bags. Only 1.4% weight loss for artificially infested groundnut stored in hermetic bags compared to 39.6% in cloth bag. There was 92.3% germination for artificially infested seeds stored in hermetic bags compared to only 10% in the case of cloth bags. | [85] |
© 2017 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kumar, D.; Kalita, P. Reducing Postharvest Losses during Storage of Grain Crops to Strengthen Food Security in Developing Countries. Foods 2017, 6, 8. https://doi.org/10.3390/foods6010008
Kumar D, Kalita P. Reducing Postharvest Losses during Storage of Grain Crops to Strengthen Food Security in Developing Countries. Foods. 2017; 6(1):8. https://doi.org/10.3390/foods6010008
Chicago/Turabian StyleKumar, Deepak, and Prasanta Kalita. 2017. "Reducing Postharvest Losses during Storage of Grain Crops to Strengthen Food Security in Developing Countries" Foods 6, no. 1: 8. https://doi.org/10.3390/foods6010008
APA StyleKumar, D., & Kalita, P. (2017). Reducing Postharvest Losses during Storage of Grain Crops to Strengthen Food Security in Developing Countries. Foods, 6(1), 8. https://doi.org/10.3390/foods6010008







