Protein Recovery from Rapeseed Press Cake: Varietal and Processing Condition Effects on Yield, Emulsifying Capacity and Antioxidant Activity of the Protein Rich Extract
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Chemicals
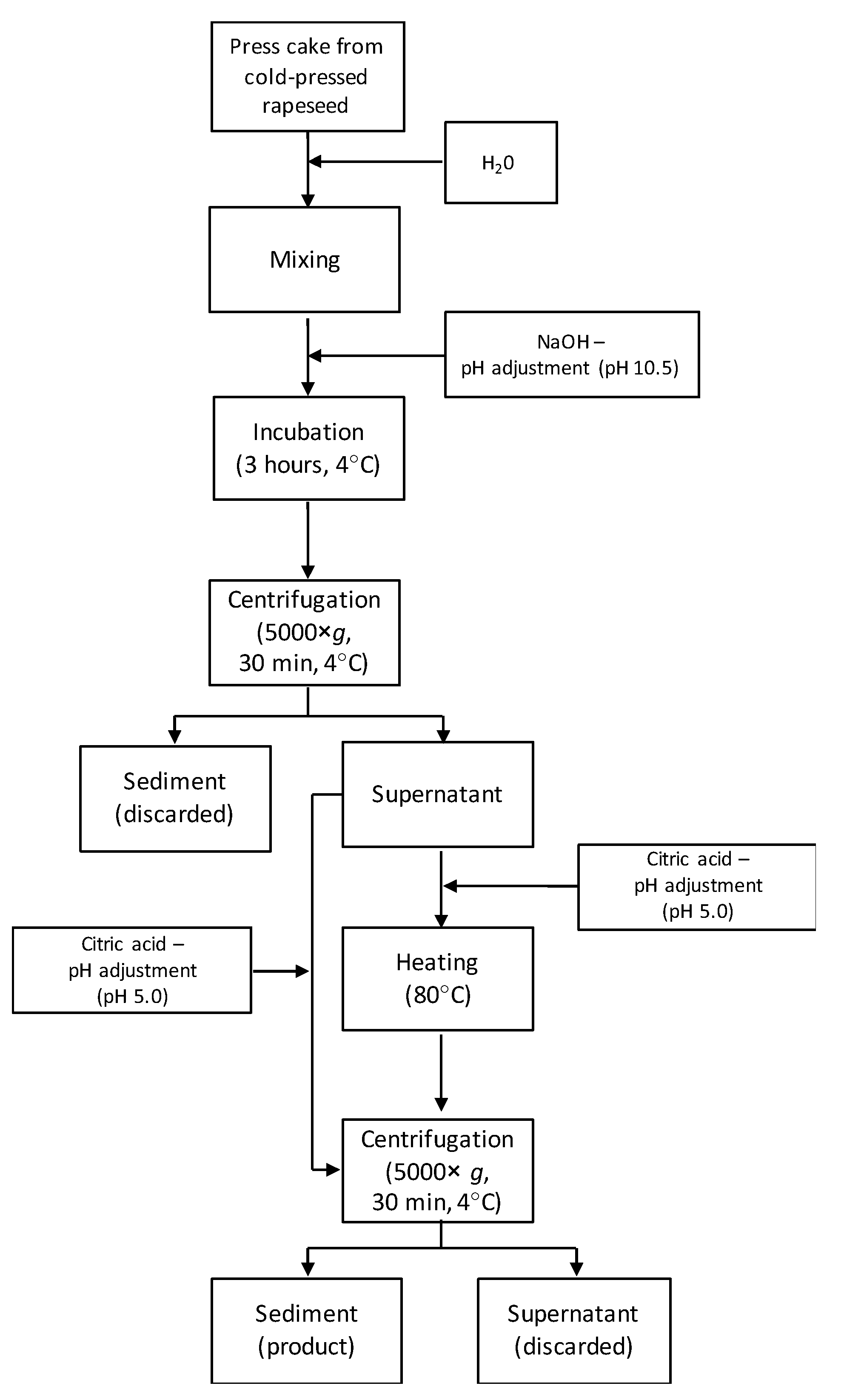
2.2. Protein Recovery from Rapeseed Press Cake
2.3. Protein Quantification and Dry Matter Analysis
2.4. Preparation of Emulsions
2.5. Particle Size Distribution of Emulsions
2.6. Rate of Lipid Oxidation
2.7. Statistical Analysis
3. Results and Discussion
3.1. Effect of Rapeseed Variety on Protein Yield
3.2. Emulsifying Properties
3.3. Emulsion Stability
3.4. Lipid Oxidation
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Wanasundara, J.P.D.; McIntosh, T.C. A Process of Aqueous Protein Extraction from Brassicaceae Oilseeds. U.S. Patent No. 8,55,7963, 2013. [Google Scholar]
- Tzeng, Y.; Diosady, L.; Rubin, L. Preparation of rapeseed protein isolated using ultrafiltration, precipitation and diafiltration. Can. Inst. Food Sci. Technol. 1988, 21, 419–424. [Google Scholar] [CrossRef]
- CCC. Canola Council of Canada. Available online: www.canoalacouncil.org (accessed on 28 August 2019).
- Von Der Haar, D.; Müller, K.; Bader-Mittermaier, S.; Eisner, P. Rapeseed proteins—Production methods and possible application ranges. OCL 2014, 21, 104. [Google Scholar] [CrossRef]
- Wijesundera, C.; Shen, Z. Mimicking natural oil bodies for stabilising oil-in-water food emulsions. Lip. Technol. 2014, 26, 151–153. [Google Scholar] [CrossRef]
- Tan, S.H.; Mailer, R.J.; Blanchard, C.L.; Agboola, S.O. Extraction and characterization of protein fractions from Australian canola meals. Food Res. Int. 2011, 44, 1075–1082. [Google Scholar] [CrossRef]
- Berton, C.; Ropers, M.H.; Bertrand, D.; Viau, M.; Genot, C. Oxidative stability of oil-in-water emulsions stabilised with protein or surfactant emulsifiers in various conditions. Food Chem. 2012, 131, 1360–1369. [Google Scholar] [CrossRef]
- Alashi, A.M.; Blanchard, C.L.; Mailer, R.J.; Agboola, S.O.; Mawsonm, A.J.; He, R. Antioxidant properties of Australian canola meal protein hydrolysates. Food Chem. 2014, 146, 500–506. [Google Scholar] [CrossRef]
- Ramlan, M.; Maruyama, N.; Adachi, M.; Hontani, N.; Saka, S.; Kato, N. Comparison of protein chemical and physicochemical properties of rapeseed cruciferin with those of soybean glycinin. J. Agric. Food Chem. 2002, 50, 7380–7385. [Google Scholar] [CrossRef]
- Huang, A. Oleosins and oil bodies in seeds and other organs. Plant Physiol. 1996, 110, 1055. [Google Scholar] [CrossRef]
- Rayner, M. Current status on novel ways for stabilizing food dispersions by oleosins, particles and microgels. Curr. Opin. Food Sci. 2015, 3, 94–109. [Google Scholar] [CrossRef]
- Rayner, M.; Östbring, K.; Purhagen, J. Application of Natural Polymers in Food in Natural Polymers; Olatunji, O., Ed.; Springer: Berlin/Heidelberg, Germany, 2016; pp. 115–161. [Google Scholar]
- Chassaing, B.; Koren, O.; Goodrich, J.; Poole, A.; Srinivasan, S.; Ley, R. Dietary emulsifiers impact the mouse gut microbiota promoting colitis and metabolic syndrome. Nature 2015, 519, 92–96. [Google Scholar] [CrossRef] [PubMed]
- Ghnimi, S.; Budilarto, E.; Kamal-Eldin, A. The new paradigm for lipid oxidation and insights to microencapsulation of omega-3 fatty acids. Compr. Rev. Food Sci. Food Saf. 2017, 16, 1206–1218. [Google Scholar] [CrossRef]
- Ries, D.; Ye, A.; Haisman, D.; Singh, H. Antioxidant properties of caseins and whey proteins in model oil-in-water emulsions. Int. Dairy J. 2010, 20, 72–78. [Google Scholar] [CrossRef]
- McClements, D.J.; Decker, E.A. Lipid oxidation in oil-in-water emulsions: Impact of molecular environment on chemical reactions in heterogeneous food systems. J. Food Sci. 2000, 65, 1270–1282. [Google Scholar] [CrossRef]
- Hu, M.; McClements, D.J.; Decker, E.A. Lipid oxidation in corn oil-in-water emulsions stabilized by casein, whey protein isolate, and soy protein isolate. J. Agric. Food Chem. 2003, 51, 1696–1700. [Google Scholar] [CrossRef]
- He, R.; Girgih, A.T.; Malomo, S.A.; Ju, X.; Aluko, R.E. Antioxidant activities of enzymatic rapeseed protein hydrolysates and the membrane ultrafiltration fractions. J. Funct. Foods 2013, 5, 219–227. [Google Scholar] [CrossRef]
- Mäkinen, S.; Johannson, T.; Vegarud, G.E.; Pihlava, J.M.; Pihlanto, A. Angiotensin I-converting enzyme inhibitory and antioxidant properties of rapeseed hydrolysates. J. Funct. Foods 2012, 4, 575–583. [Google Scholar] [CrossRef]
- Pan, M.; Jiang, T.S.; Pan, J.L. Antioxidant activities of rapeseed protein hydrolysates. Food Bioprocess Technol. 2011, 4, 1144–1152. [Google Scholar] [CrossRef]
- Ghodsvali, A.; Haddad, K.M.H.; Vosoughi, M.; Diosady, L.L. Preparation of canola protein materials using membrane technology and evaluation of meals functional properties. Food Res. Int. 2005, 38, 223–231. [Google Scholar] [CrossRef]
- Yoshie-Stark, Y.; Wada, Y.; Schott, M.; Wäsche, A. Functional and bioactive properties of rapeseed protein concentrates and sensory analysis of food application with rapeseed protein concentrates. LWT-Food Sci. Technol. 2006, 39, 503–512. [Google Scholar] [CrossRef]
- Manamperi, W.A.; Wiesenborn, D.P.; Chang, S.K.; Pryor, S.W. Effects of protein separation conditions on the functional and thermal properties of canola protein isolates. J. Food Sci. 2011, 76, E266–E273. [Google Scholar] [CrossRef] [PubMed]
- Fetzer, A.; Herfellner, T.; Stäbler, A.; Menner, M.; Eisner, P. Influence of process conditions during aqueous protein extraction upon yield from pre-pressed and cold-pressed press cake. Ind. Crops Prod. 2018, 112, 236–246. [Google Scholar] [CrossRef]
- Wijesundera, C.; Boiteau, T.; Xu, X.; Shen, Z.; Watkins, P.; Logan, A. Stabilization of fish oil-in-water emulsions with oleosin extracted from canola meal. J. Food Sci. 2013, 78, C1340–C1347. [Google Scholar] [CrossRef] [PubMed]
- Rommi, K.; Ercili-Cura, D.; Hakala, T.K.; Nordlund, E.; Poutanen, K.; Lantto, R. Impact of total solid content and extraction pH on enzyme-aided recovery of protein from defatted rapeseed (Brassica rapa L.) press cake and physicochemical properties of the protein fractions. J. Agric. Food Chem. 2015, 63, 2997–3003. [Google Scholar] [CrossRef] [PubMed]
- Wanasundara, J.P.D.; Tan, S.H.; Alashi, A.M.; Pudel, F.; Blanchard, C. Proteins from Canola/Rapeseed: Current status. In Sustainable Protein Sources, 1st ed.; Nadathur, S., Wanasundara, J.P.D., Scanlin, L., Eds.; Elsevier Science: Amsterdam, The Netherlands, 2016; pp. 285–304. [Google Scholar]
- Totosaus, A.; Montejano, J.; Salazar, J.; Guerrero, I. A review of physical and chemical protein-gel induction. Int. J. Food Sci. Technol. 2002, 37, 589–601. [Google Scholar] [CrossRef]
- Hermansson, A. Physico-chemical aspects of soy proteins structure formation. J. Texture Stud. 1978, 9, 33–58. [Google Scholar] [CrossRef]
- Tan, S.; Mailer, R.; Blanchard, C.; Agboola, S. Emulsifying properties of proteins extracted from Australian canola meal. LWT—Food Sci. Technol. 2014, 57, 376–382. [Google Scholar] [CrossRef]
- Aluko, R.E.; McIntosh, T. Polypeptide profile and functional properties of defatted meals and protein isolates of canola seeds. J. Sci. Food Agric. 2001, 81, 391–396. [Google Scholar] [CrossRef]
- Elias, R.J.; Kellerby, S.S.; Decker, E.A. Antioxidant activity of proteins and peptides. Critic. Rev. Food Sci. Nutr. 2008, 48, 430–441. [Google Scholar] [CrossRef]
- Donnelly, J.L.; Decker, E.A.; McClements, D.J. Iron-catalysed oxidation of Menhaden oils as affected by emulsifiers. J. Food Sci. 1998, 63, 997–1000. [Google Scholar] [CrossRef]
- Mackie, A.R.; Ridout, M.J.; Moates, G.; Husband, F.A.; Wilde, P.J. Effect of the interfacial layer composition of the properties of emulsion creams. J. Agric. Food Chem. 2007, 55, 5611–5619. [Google Scholar] [CrossRef] [PubMed]
- Wilde, P.J.; Mackie, A.R.; Husband, F.A.; Gunning, P.; Morris, V. Proteins and emulsifiers at liquid interfaces. Adv. Colloid Interface Sci. 2004, 108, 63–71. [Google Scholar] [CrossRef] [PubMed]




| Rapeseed Cultivar | Heat Treatment During Protein Recovery | Mass of Sediment (g)/100 g RSPC | Dry Matter (%) | Protein Concentration on Dry Basis (%) | Protein Recovery Yield (%) |
|---|---|---|---|---|---|
| Mixed | no | 30 ± 2 a | 32 ± 0.4 a | 70 ± 3 a | 29 ± 2 a |
| Alegria | no | 43 ± 5 a,b,c | 33 ± 0.9 a,b | 64 ± 5 a,b,c | 40 ± 1 b |
| Epure | no | 45 ± 2 a,b,c | 35 ± 1 b | 62 ± 1 a,b,c | 41 ± 1 b |
| Festivo | no | 38 ± 3 a,b,c | 35 ± 1 b | 70 ± 2 a,b | 37 ± 2 c |
| Lyside | no | 48 ± 3 c | 33 ± 0.4 a,b | 57 ± 2 c | 36 ± 1 c |
| V316OL | no | 37 ± 2 a,b,c | 34 ± 0.3 b | 61 ± 0.5 b,c | 32 ± 2 c |
| Mixed | yes | 78 ± 8 d | 19 ± 0.6 c | 41 ± 2 d | 26 ± 1 a |
| Alegria | yes | 110 ± 2 d | 21 ± 0.9 c | 42 ± 5 d,e | 41 ± 4 b |
| Epure | yes | 91 ± 1 d | 20 ± 0.4 c | 54 ± 2 f | 41 ± 1 b |
| Festivo | yes | 84 ± 2 d | 19 ± 1 c | 51 ± 3 e,f | 33 ± 1 c |
| Lyside | yes | 100 ± 7 d | 21 ± 0.7 c | 42 ± 3 d,e | 34 ± 3 c |
| V316OL | yes | 83 ± 2 d | 19 ± 0.2 c | 50 ± 1 d,e,f | 33 ± 1 c |
| Rapeseed Variety | Protein Concentration (mg protein/mL oil) | d43 (μm) | d32 (μm) | Span | Mode (μm) |
|---|---|---|---|---|---|
| (a) | |||||
| Mixed | 2 | 90 ± 11 | 81 ± 10 | 0.84 ± 0.1 | 85 ± 10 |
| 4 | 67 ± 4 | 56 ± 7 | 1.1 ± 0.1 | 62 ± 4 | |
| 8 | 56 ± 2 | 45 ± 4 | 1.2 ± 0.1 | 50 ± 1 | |
| 16 | 45 ± 2 | 29 ± 1 | 1.4 ± 0.1 | 41 ± 1 | |
| 32 | 38 ± 3 | 23 ± 1 | 1.6 ± 0.1 | 36 ± 4 | |
| Alegria | 2 | 86 ± 14 | 74 ± 19 | 1.0 ± 0.2 | 81 ± 15 |
| 4 | 59 ± 2 | 48 ± 3 | 1.2 ± 0.1 | 52 ± 3 | |
| 8 | 47 ± 3 | 36 ± 3 | 1.4 ± 0.1 | 39 ± 3 | |
| 16 | 49 ± 13 | 32 ± 5 | 1.4 ± 0.2 | 44 ± 17 | |
| 32 | 43 ± 10 | 23 ±5 | 1.7 ± 0.1 | 38 ± 15 | |
| Epure | 2 | 78 ± 8 | 68 ± 10 | 0.88 ± 0.2 | 75 ± 8 |
| 4 | 57 ± 5 | 50 ± 5 | 0.92 ± 0.1 | 53 ± 4 | |
| 8 | 46 ± 3 | 39 ± 2 | 1.1 ± 0.1 | 42 ± 3 | |
| 16 | 40 ± 2 | 32 ± 2 | 1.2 ± 0.04 | 36 ± 1 | |
| 32 | 38 ± 1 | 26 ± 0.3 | 1.3 ± 0.1 | 36 ± 1 | |
| Festivo | 2 | 68 ± 8 | 63 ± 6 | 0.96 ± 0.1 | 70 ± 5 |
| 4 | 63 ± 14 | 53 ± 10 | 1.2 ± 0.2 | 62 ± 10 | |
| 8 | 46 ± 6 | 39 ± 5 | 1.3 ± 0.2 | 46 ± 4 | |
| 16 | 39 ± 6 | 28 ± 4 | 1.3 ± 0.1 | 37 ± 3 | |
| 32 | 36 ± 4 | 23 ± 1 | 1.6 ± 0.1 | 34 ± 2 | |
| Lyside | 2 | 70 ± 5 | 57 ± 5 | 1.1 ± 0.2 | 66 ± 7 |
| 4 | 53 ± 3 | 43 ± 6 | 1.2 ± 0.2 | 47 ± 5 | |
| 8 | 42 ± 2 | 34 ± 3 | 1.3 ± 0.2 | 36 ± 3 | |
| 16 | 38 ± 3 | 28 ± 3 | 1.4 ± 0.1 | 31 ± 3 | |
| 32 | 32 ± 3 | 22 ± 2 | 1.5 ± 0.1 | 27 ± 3 | |
| V316OL | 2 | 80 ± 6 | 73 ± 5 | 0.8 ± 0.1 | 76 ± 5 |
| 4 | 58 ± 5 | 51 ± 4 | 1.0 ± 0.1 | 54 ± 5 | |
| 8 | 51 ± 4 | 44 ± 4 | 1.0 ± 0.1 | 48 ± 3 | |
| 16 | 50 ± 1 | 43 ± 1 | 1.0 ± 0.1 | 47 ± 1 | |
| 32 | 48 ± 2 | 31 ± 1 | 1.0 ± 0.1 | 48 ± 2 | |
| (b) | |||||
| Mixed | 1 | 140 ± 9 | 110 ± 10 | 1.0 ± 0.1 | 123 ± 8 |
| 2 | 110 ± 6 | 96 ± 5 | 0.99 ± 0.1 | 102 ± 4 | |
| 4 | 100 ± 6 | 78 ± 8 | 0.93 ± 0.1 | 95 ± 6 | |
| 8 | 81 ± 10 | 65 ± 10 | 1.0 ± 0.1 | 77 ± 12 | |
| 16 | 63 ± 8 | 45 ± 10 | 1.0 ± 0.1 | 62 ± 8 | |
| 32 | 44 ± 4 | 29 ± 5 | 1.0 ± 0.1 | 42 ± 4 | |
| Alegria | 1 | 140 ± 10 | 120 ± 9 | 1.0 ± 0.1 | 131 ± 12 |
| 2 | 120 ± 9 | 100 ± 10 | 1.1 ± 0.1 | 112 ± 7 | |
| 4 | 110 ± 8 | 79 ± 20 | 1.1 ± 0.1 | 104 ± 6 | |
| 8 | 88 ± 7 | 53 ± 8 | 1.1 ± 0.1 | 87 ± 6 | |
| 16 | 69 ± 10 | 39 ± 5 | 0.93 ± 0.1 | 71 ± 10 | |
| 32 | 46 ± 2 | 27 ± 1 | 0.99 ± 0.1 | 47 ± 3 | |
| Epure | 1 | 150 ± 7 | 120 ± 10 | 1.0 ± 0.1 | 138 ± 6 |
| 2 | 130 ± 4 | 110 ± 4 | 1.1 ± 0.1 | 110 ± 4 | |
| 4 | 90 ± 4 | 90 ± 8 | 1.1 ± 0.1 | 106 ± 2 | |
| 8 | 98 ± 4 | 66 ± 10 | 1.0 ± 0.1 | 95 ± 2 | |
| 16 | 76 ± 3 | 43 ± 2 | 0.97 ± 0.1 | 77 ± 3 | |
| 32 | 55 ± 2 | 30 ± 2 | 1.1 ± 0.2 | 56 ± 2 | |
| Festivo | 1 | 150 ± 20 | 120 ± 10 | 1.0 ± 0.1 | 136 ± 16 |
| 2 | 120 ± 4 | 97 ± 10 | 1.1 ± 0.1 | 111 ± 3 | |
| 4 | 110 ± 4 | 75 ± 10 | 1.2 ± 0.2 | 102 ± 2 | |
| 8 | 92 ± 5 | 51 ± 7 | 1.1 ± 0.1 | 91 ± 4 | |
| 16 | 77 ± 8 | 43 ± 4 | 1.1 ± 0.2 | 77 ± 7 | |
| 32 | 54 ± 7 | 30 ± 3 | 1.0 ± 0.1 | 54 ± 7 | |
| Lyside | 1 | 150 ± 6 | 130 ± 10 | 0.98 ± 0.1 | 140 ± 5 |
| 2 | 120 ± 5 | 110 ± 6 | 1.0 ± 0.1 | 110 ± 4 | |
| 4 | 110 ± 6 | 86 ± 20 | 1.1 ± 0.1 | 100 ± 4 | |
| 8 | 95 ± 3 | 60 ± 9 | 1.1 ± 0.2 | 92 ± 3 | |
| 16 | 75 ± 2 | 50 ± 9 | 0.94 ± 0.1 | 73 ± 2 | |
| 32 | 51 ± 2 | 31 ± 1 | 1.0 ± 0.1 | 51 ± 2 | |
| V316OL | 1 | 140 ± 10 | 120 ± 10 | 1.0 ± 0.1 | 130 ± 10 |
| 2 | 120 ± 4 | 110 ± 4 | 1.1 ± 0.1 | 110 ± 3 | |
| 4 | 110 ± 5 | 91 ± 4 | 1.1 ± 0.1 | 98 ± 4 | |
| 8 | 91 ± 6 | 68 ± 10 | 1.0 ± 0.1 | 87 ± 5 | |
| 16 | 70 ± 2 | 49 ± 10 | 0.97 ± 0.1 | 68 ± 3 | |
| 32 | 50 ± 3 | 30 ± 1 | 1.0 ± 0.1 | 49 ± 4 | |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Östbring, K.; Tullberg, C.; Burri, S.; Malmqvist, E.; Rayner, M. Protein Recovery from Rapeseed Press Cake: Varietal and Processing Condition Effects on Yield, Emulsifying Capacity and Antioxidant Activity of the Protein Rich Extract. Foods 2019, 8, 627. https://doi.org/10.3390/foods8120627
Östbring K, Tullberg C, Burri S, Malmqvist E, Rayner M. Protein Recovery from Rapeseed Press Cake: Varietal and Processing Condition Effects on Yield, Emulsifying Capacity and Antioxidant Activity of the Protein Rich Extract. Foods. 2019; 8(12):627. https://doi.org/10.3390/foods8120627
Chicago/Turabian StyleÖstbring, Karolina, Cecilia Tullberg, Stina Burri, Emma Malmqvist, and Marilyn Rayner. 2019. "Protein Recovery from Rapeseed Press Cake: Varietal and Processing Condition Effects on Yield, Emulsifying Capacity and Antioxidant Activity of the Protein Rich Extract" Foods 8, no. 12: 627. https://doi.org/10.3390/foods8120627





