Prediction of Terpenoid Toxicity Based on a Quantitative Structure–Activity Relationship Model
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. Terpenoids Standards
2.3. Assessment Terpenoid Toxicity
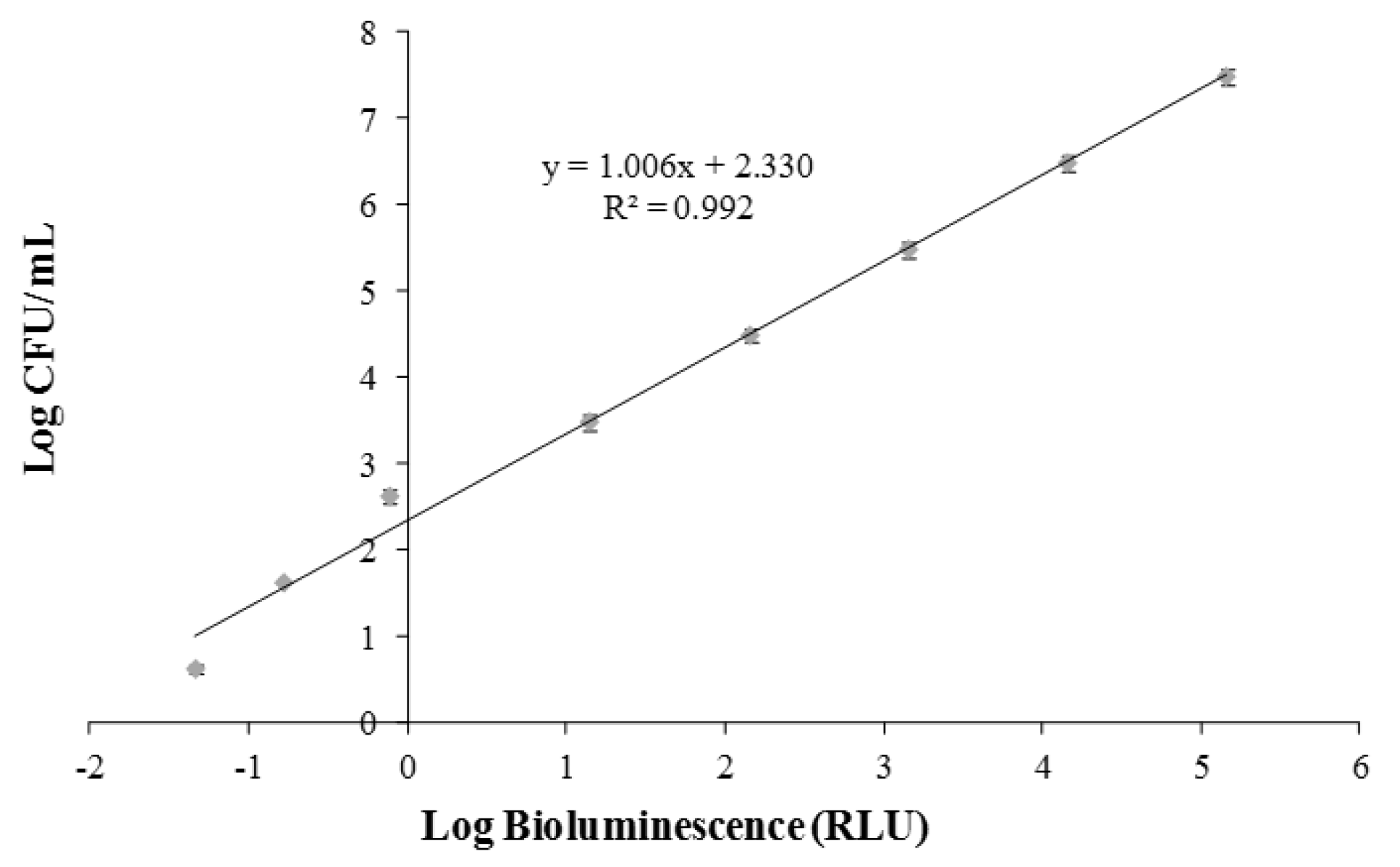
2.4. Calibration of Bioluminescent Signal and Viable Cell Numbers
2.5. QSAR Model Development
2.5.1. Geometry Optimization and Calculation of Molecular Descriptors
2.5.2. Development and Validation of QSAR Models
3. Results and Discussion
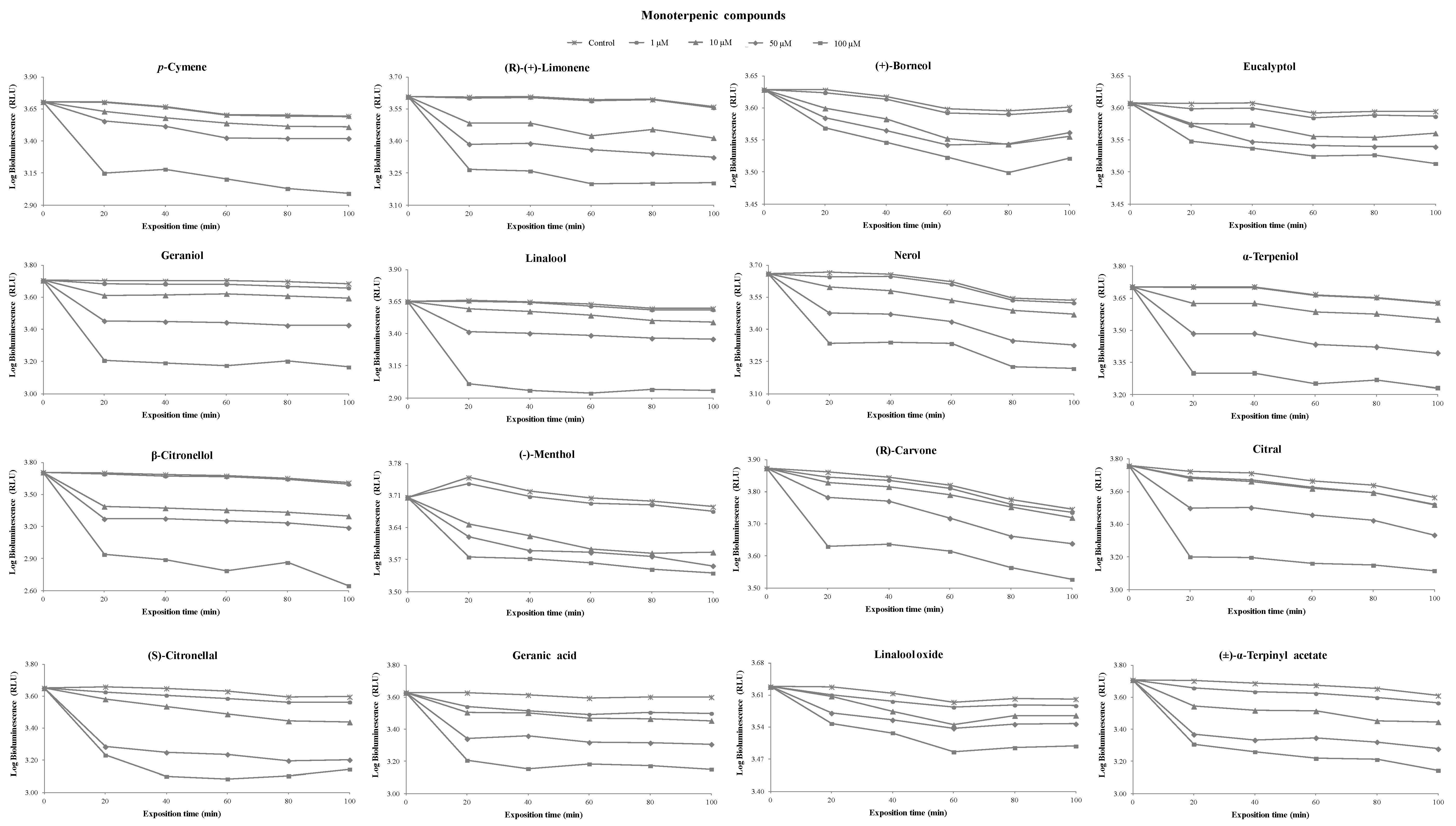
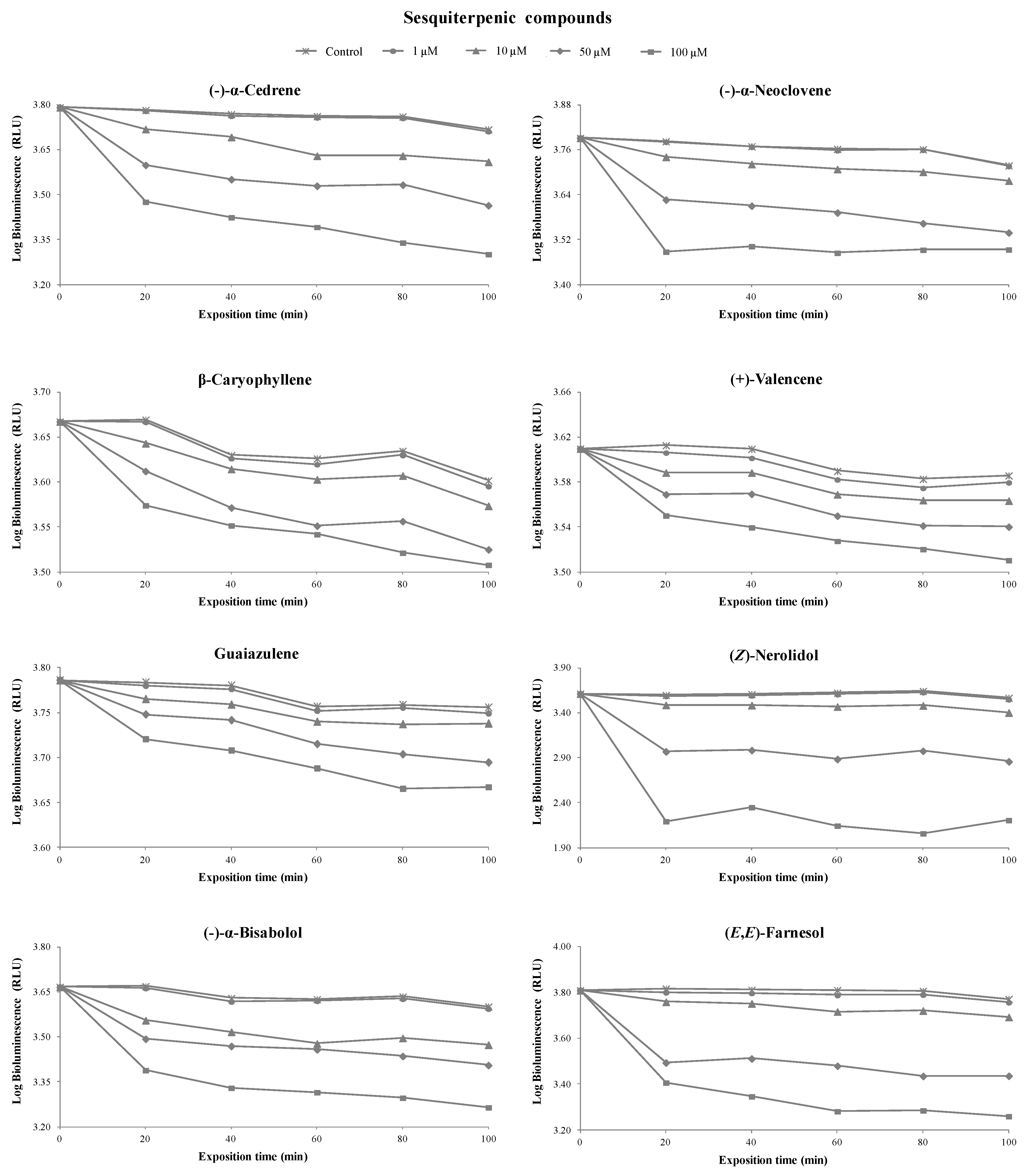
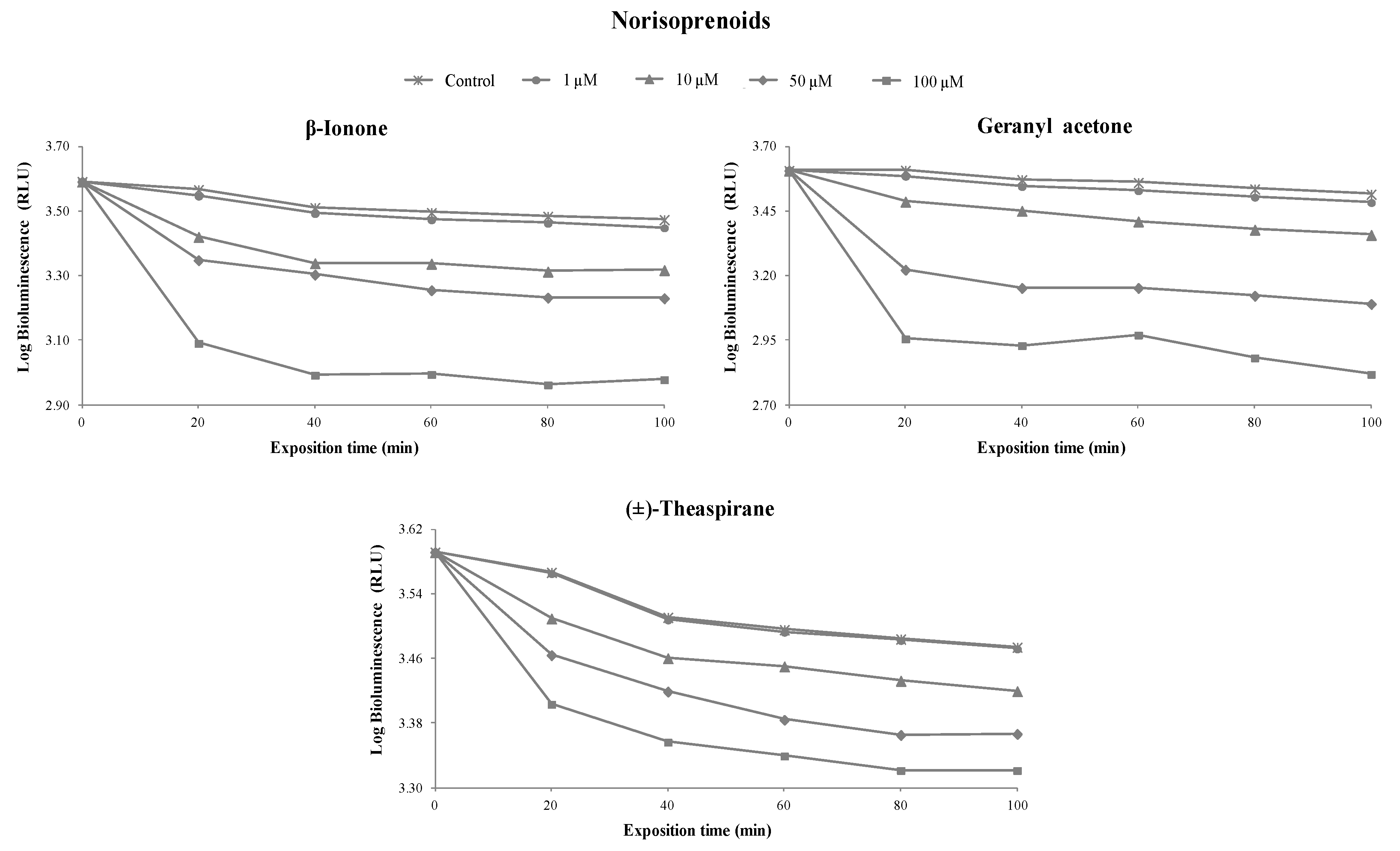
3.1. Toxicity of Terpenoids
3.2. QSAR Models to Predict the Toxicity of Terpenoids
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Vermaas, J.V.; Bentley, G.J.; Beckham, G.T.; Crowley, M.F. Membrane permeability of terpenoids explored with molecular simulation. J. Phys. Chem. B 2018, 122, 10349–10361. [Google Scholar] [CrossRef] [PubMed]
- Ludwiczuk, A.; Skalicka-Woźniak, K.; Georgiev, M.I. Terpenoids. In Pharmacognosy; Academic Press: New York, NY, USA, 2017; pp. 233–266. ISBN 9780128021040. [Google Scholar]
- Mbaveng, A.T.; Hamm, R. Harmful and protective effects of terpenoids from african medicinal plants. In Toxicological Survey of African Medicinal Plants; Elsevier: Amsterdam, The Netherlands, 2014; pp. 557–576. ISBN 9780128000182. [Google Scholar]
- Shakya, A.K. Medicinal plants: Future source of new drugs. Int. J. Herb. Med. 2016, 4, 59–64. [Google Scholar]
- Nevzorova, Y.A.; Grossmann, J.; Trautwein, C. Anti-tumorigenic and anti-angiogenic effects of natural conifer Abies sibirica terpenoids in vivo and in vitro. Biomed. Pharmacother. 2017, 89, 386–395. [Google Scholar] [CrossRef] [PubMed]
- Lim, C.; Park, S.; Park, J.; Ko, J.; Lee, D.W.; Hwang, D.S. Probing nanomechanical interaction at the interface between biological membrane and potentially toxic chemical. J. Hazard. Mater. 2018, 353, 271–279. [Google Scholar] [CrossRef] [PubMed]
- Blaskó, Á.; Gazdag, Z.; Gróf, P.; Máté, G.; Sárosi, S.; Krisch, J.; Vágvölgyi, C.; Makszin, L.; Pesti, M. Effects of clary sage oil and its main components, linalool and linalyl acetate, on the plasma membrane of Candida albicans: An in vivo EPR study. Apoptosis 2017, 22, 175–187. [Google Scholar] [CrossRef] [PubMed]
- Hąc-Wydro, K.; Flasiński, M.F.; Broniatowski, M.; Sołtys, M. Studies on the behavior of eucalyptol and terpinen-4-ol—Natural food additives and ecological pesticides—In model lipid membranes. Langmuir 2017, 33, 6916–6924. [Google Scholar] [CrossRef]
- Mobed, A.; Hasanzadeh, M.; Agazadeh, M.; Mokhtarzadeh, A.; Rezaee, M.A.; Sadeghi, J. Bioassays: The best alternative for conventional methods in detection of Legionella pneumophila. Int. J. Biol. Macromol. 2019, 121, 1295–1307. [Google Scholar] [CrossRef]
- Abbas, M.; Adil, M.; Ehtisham-ul-Haque, S.; Munir, B.; Yameen, M.; Ghaffar, A.; Shar, G.A.; Asif Tahir, M.; Iqbal, M. Vibrio fischeri bioluminescence inhibition assay for ecotoxicity assessment: A review. Sci. Total Environ. 2018, 626, 1295–1309. [Google Scholar] [CrossRef]
- Jarque, S.; Masner, P.; Klánová, J.; Prokeš, R.; Bláha, L. Bioluminescent Vibrio fischeri assays in the assessment of seasonal and spatial patterns in toxicity of xontaminated river sediments. Front. Microbiol. 2016, 7, 1738. [Google Scholar] [CrossRef]
- Parvez, S.; Venkataraman, C.; Mukherji, S. A review on advantages of implementing luminescence inhibition test (Vibrio fischeri) for acute toxicity prediction of chemicals. Environ. Int. 2006, 32, 265–268. [Google Scholar] [CrossRef]
- Kusumahastuti, D.K.A.; Sihtmäe, M.; Kapitanov, I.V.; Karpichev, Y.; Gathergood, N.; Kahru, A. Toxicity profiling of 24 l-phenylalanine derived ionic liquids based on pyridinium, imidazolium and cholinium cations and varying alkyl chains using rapid screening Vibrio fischeri bioassay. Ecotoxicol. Environ. Saf. 2019, 172, 556–565. [Google Scholar] [CrossRef] [PubMed]
- Luis, P.; Garea, A.; Irabien, A. Quantitative structure–activity relationships (QSARs) to estimate ionic liquids ecotoxicity EC50 (Vibrio fischeri). J. Mol. Liq. 2010, 152, 28–33. [Google Scholar] [CrossRef]
- Pandith, A.H.; Giri, S.; Chattaraj, P.K. A comparative study of two quantum chemical descriptors in predicting toxicity of aliphatic xompounds towards Tetrahymena pyriformis. Org. Chem. Int. 2010, 2010, 545087. [Google Scholar] [CrossRef]
- Shahlaei, M. Descriptor selection methods in quantitative structure–activity relationship studies: A review study. Chem. Rev. 2013, 113, 8093–8103. [Google Scholar] [CrossRef]
- Moorthy, N.S.H.N.; Ramos, M.J.; Fernandes, P.A. Topological, hydrophobicity, and other descriptors on α-glucosidase inhibition: A QSAR study on xanthone derivatives. J. Enzyme Inhib. Med. Chem. 2011, 26, 755–766. [Google Scholar] [CrossRef]
- Das, R.N.; Sintra, T.E.; Coutinho, J.A.P.; Ventura, S.P.M.; Roy, K.; Popelier, P.L.A. Development of predictive QSAR models for Vibrio fischeri toxicity of ionic liquids and their true external and experimental validation tests. Toxicol. Res. 2016, 5, 1388–1399. [Google Scholar] [CrossRef]
- Parvez, S.; Venkataraman, C.; Mukherji, S. Toxicity assessment of organic pollutants: Reliability of bioluminescence inhibition assay and univariate QSAR models using freshly prepared Vibrio fischeri. Toxicol. Vitro 2008, 22, 1806–1813. [Google Scholar] [CrossRef]
- Agrawal, V.K.; Khadikar, P.V. QSAR study on narcotic mechanism of action and toxicity: A molecular connectivity approach to Vibrio fischeri toxicity testing. Bioorg. Med. Chem. 2002, 10, 3517–3522. [Google Scholar] [CrossRef]
- Couling, D.J.; Bernot, R.J.; Docherty, K.M.; Dixon, J.K.; Maginn, E.J. Assessing the factors responsible for ionic liquid toxicity to aquatic organisms via quantitative structure–property relationship modeling. Green Chem. 2006, 8, 82–90. [Google Scholar] [CrossRef]
- Ghanem, O.B.; Mutalib, M.I.A.; Lévêque, J.-M.; El-Harbawi, M. Development of QSAR model to predict the ecotoxicity of Vibrio fischeri using COSMO-RS descriptors. Chemosphere 2017, 170, 242–250. [Google Scholar] [CrossRef]
- Peric, B.; Sierra, J.; Martí, E.; Cruañas, R.; Garau, M.A. Quantitative structure–activity relationship (QSAR) prediction of (eco)toxicity of short aliphatic protic ionic liquids. Ecotoxicol. Environ. Saf. 2015, 115, 257–262. [Google Scholar] [CrossRef] [PubMed]
- Vinholes, J.; Rudnitskaya, A.; Gonçalves, P.; Martel, F.; Coimbra, M.A.; Rocha, S.M. Hepatoprotection of sesquiterpenoids: A quantitative structure-activity relationship (QSAR) approach. Food Chem. 2014, 146, 78–84. [Google Scholar] [CrossRef] [PubMed]
- Grodnitzky, J.A.; Coats, J.R. QSAR evaluation of monoterpenoids’ insecticidal activity. J. Agric. Food Chem. 2002, 50, 4576–4580. [Google Scholar] [CrossRef] [PubMed]
- Chang, H.J.; Kim, H.J.; Chun, H.S. Quantitative structure-activity relationship (QSAR) for neuroprotective activity of terpenoids. Life Sci. 2007, 80, 835–841. [Google Scholar] [CrossRef] [PubMed]
- Alves, E.; Rodrigues, J.M.M.; Faustino, M.A.F.; Neves, M.G.P.M.S.; Cavaleiro, J.A.S.; Lin, Z.; Cunha, Â.; Nadais, M.H.; Tomé, J.P.C.; Almeida, A. A new insight on nanomagnet–porphyrin hybrids for photodynamic inactivation of microorganisms. Dye. Pigment. 2014, 110, 80–88. [Google Scholar] [CrossRef]
- Beheshti, A.; Pourbasheer, E.; Nekoei, M.; Vahdani, S. QSAR modeling of antimalarial activity of urea derivatives using genetic algorithm–multiple linear regressions. J. Saudi Chem. Soc. 2016, 20, 282–290. [Google Scholar] [CrossRef]
- Lather, V.; Kairys, V.; Fernandes, M.X. Quantitative Structure-Activity Relationship models with receptor-dependent descriptors for predicting peroxisome proliferator-activated receptor activities of thiazolidinedione and oxazolidinedione derivatives. Chem. Biol. Drug Des. 2009, 73, 428–441. [Google Scholar] [CrossRef]
- Grover, M.; Gulati, M.; Singh, B.; Singh, S. RP-HPLC determination of lipophilicity of 22 penicillins, their correlation with reported values and establishment of quantitative structure-log Kw relationships. QSAR Comb. Sci. 2005, 24, 639–648. [Google Scholar] [CrossRef]
- Larsson, M.; van den Berg, M.; Brenerová, P.; van Duursen, M.B.M.; van Ede, K.I.; Lohr, C.; Luecke-Johansson, S.; Machala, M.; Neser, S.; Pěnčíková, K.; et al. Consensus toxicity factors for polychlorinated dibenzo- p -dioxins, dibenzofurans, and biphenyls combining in silico models and extensive in Vitro screening of AhR-mediated effects in human and rodent cells. Chem. Res. Toxicol. 2015, 28, 641–650. [Google Scholar] [CrossRef]
- Trombetta, D.; Castelli, F.; Sarpietro, M.G.; Venuti, V.; Cristani, M.; Daniele, C.; Saija, A.; Mazzanti, G.; Bisignano, G. Mechanisms of antibacterial action of three monoterpenes. Antimicrob. Agents Chemother. 2005, 49, 2474–2478. [Google Scholar] [CrossRef]
- Cristani, M.; D’Arrigo, M.; Mandalari, G.; Castelli, F.; Sarpietro, M.G.; Micieli, D.; Venuti, V.; Bisignano, G.; Saija, A.; Trombetta, D. Interaction of four monoterpenes contained in essential oils with model membranes: Implications for their antibacterial activity. J. Agric. Food Chem. 2007, 55, 6300–6308. [Google Scholar] [CrossRef] [PubMed]
- Group, M.P.; Ayrshire, S.; Estadual, U.; Julio, P.; Filho, D.M.; Safety, F. Antimicrobial agents from plants: Antibacterial activity of plant volatile oils. J. Appl. Microbiol. 2006, 88, 25–27. [Google Scholar]
- Mahizan, N.A.; Yang, S.K.; Moo, C.L.; Song, A.A.L.; Chong, C.M.; Chong, C.W.; Abushelaibi, A.; Erin Lim, S.H.; Lai, K.S. Terpene derivatives as a potential agent against antimicrobial resistance (AMR) pathogens. Molecules 2019, 24, 2631. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Viljoen, A.M. Geraniol—A review of a commercially important fragrance material. S. Afr. J. Bot. 2010, 76, 643–651. [Google Scholar] [CrossRef]
- Dambolena, J.S.; López, A.G.; Cánepa, M.C.; Theumer, M.G.; Zygadlo, J.A.; Rubinstein, H.R. Inhibitory effect of cyclic terpenes (limonene, menthol, menthone and thymol) on Fusarium verticillioides MRC 826 growth and fumonisin B1 biosynthesis. Toxicon 2008, 51, 37–44. [Google Scholar] [CrossRef] [PubMed]
- Maran, U.; Sild, S. QSAR modeling of mutagenicity on non-congeneric sets of organic compounds. In Artificial Intelligence Methods and Tools for Systems Biology; Springer: Dordrecht, The Netherlands, 2004; pp. 19–35. [Google Scholar]
- Bruzzone, S.; Chiappe, C.; Focardi, S.E.; Pretti, C.; Renzi, M. Theoretical descriptor for the correlation of aquatic toxicity of ionic liquids by quantitative structure–toxicity relationships. Chem. Eng. J. 2011, 175, 17–23. [Google Scholar] [CrossRef]
- Goudarzi, N.; Fatemi, M.H.; Samadi-Maybodi, A. Quantitative structure–properties relationship study of the 29 Si-NMR chemical shifts of some silicate species. Spectrosc. Lett. 2009, 42, 186–193. [Google Scholar] [CrossRef]
- Liu, H.X.; Yao, X.J.; Zhang, R.S.; Liu, M.C.; Hu, Z.D.; Fan, B.T. Prediction of the tissue/blood partition coefficients of organic compounds based on the molecular structure using least-squares support vector machines. J. Comput. Aided Mol. Des. 2005, 19, 499–508. [Google Scholar] [CrossRef]
- Andrade-Ochoa, S.; Nevárez-Moorillón, G.V.; Sánchez-Torres, L.E.; Villanueva-García, M.; Sánchez-Ramírez, B.E.; Rodríguez-Valdez, L.M.; Rivera-Chavira, B.E. Quantitative structure-activity relationship of molecules constituent of different essential oils with antimycobacterial activity against Mycobacterium tuberculosis and Mycobacterium bovis. BMC Complement. Altern. Med. 2015, 15, 332. [Google Scholar] [CrossRef]

| (Terpenoids) (µM) | Nº | B | t-Test | Molecular Descriptors | Statistical Parameters |
|---|---|---|---|---|---|
| 1 | 0 | 18.36 | 8.56 | Intercept | r2training = 0.952 |
| 1 | 209.81 | 14.73 | Maximum partial charge for a C atom (Zefirov’s PC) | r2test = 0.923 | |
| 2 | −10.28 | −8.28 | Maximum atomic orbital electronic population | F = 84.14 | |
| 3 | 0.93 | 6.70 | Kier shape index (3rd order) | s2 = 1.05 | |
| 4 | −0.11 | −3.90 | WNSA-1 weighted PNSA (PNSA1×TMSA/1000) (Zefirov’s PC) | Q2 = 0.900 | |
| 10a | 0 | 31.85 | 7.68 | Intercept | r2training = 0.873 |
| 1 | 42.92 | 8.06 | Asphericity | r2test = 0.6987 | |
| 2 | −0.30 | −6.50 | PNSA-1 partial negative surface area (Zefirov’s PC) | F = 27.57 | |
| 3 | 148.82 | 4.50 | Maximum partial charge for a C atom (Zefirov’s PC) | s2 = 11.25 | |
| 4 | −3.92 | −4.48 | Log P | Q2 = 0.794 | |
| 50 | 0 | 39.71 | 1.07 | Intercept | r2training = 0.810 |
| 1 | 110.40 | 7.40 | Asphericity | r2test = 0.535 | |
| 2 | 7.53 | 3.71 | Kier and Hall index (2nd order) | F = 18.17 | |
| 3 | −0.28 | −2.81 | PNSA-1 partial negative surface area (Zefirov’s PC) | s2 = 62.05 | |
| 4 | −77.99 | −1.95 | Minimum atomic orbital electronic population | Q2 = 0.689 | |
| 100 | 0 | 19.48 | 0.94 | Intercept | r2training = 0.846 |
| 1 | 195.98 | 8.58 | Asphericity | r2test = 0.676 | |
| 2 | 21.01 | 0.21 | Kier and Hall index (2nd order) | F = 23.39 | |
| 3 | −1.17 | −4.54 | XY shadow | s2 = 103.69 | |
| 4 | −72.04 | 3.96 | Relative number of single bonds | Q2 = 0.734 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Perestrelo, R.; Silva, C.; Fernandes, M.X.; Câmara, J.S. Prediction of Terpenoid Toxicity Based on a Quantitative Structure–Activity Relationship Model. Foods 2019, 8, 628. https://doi.org/10.3390/foods8120628
Perestrelo R, Silva C, Fernandes MX, Câmara JS. Prediction of Terpenoid Toxicity Based on a Quantitative Structure–Activity Relationship Model. Foods. 2019; 8(12):628. https://doi.org/10.3390/foods8120628
Chicago/Turabian StylePerestrelo, Rosa, Catarina Silva, Miguel X. Fernandes, and José S. Câmara. 2019. "Prediction of Terpenoid Toxicity Based on a Quantitative Structure–Activity Relationship Model" Foods 8, no. 12: 628. https://doi.org/10.3390/foods8120628
APA StylePerestrelo, R., Silva, C., Fernandes, M. X., & Câmara, J. S. (2019). Prediction of Terpenoid Toxicity Based on a Quantitative Structure–Activity Relationship Model. Foods, 8(12), 628. https://doi.org/10.3390/foods8120628