Physico-Chemical Properties of Sugar Beet Pectin-Sodium Caseinate Conjugates via Different Interaction Mechanisms
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Dispersion Preperation
2.3. Analysis of Ferulic Acid Concentration and Selection of Experimental Conditions for Laccase-Catalysed Cross-Linking of Sugar Beet Pectin and Sodium Caseinate
2.4. Preparation of Conjugate Dispersions
2.4.1. Laccase-Catalysed SBP Dispersions
2.4.2. Electrostatically-Stabilised SBP-SC Conjugate Dispersions
2.4.3. Laccase-Catalysed SBP-SC Conjugates Dispersions
2.4.4. SBP-SC Maillard Conjugates Dispersions
2.5. Analytical Methods
2.5.1. Conjugate Size
2.5.2. Zeta-Potential Measurement
2.5.3. Shear Viscosity
2.6. Environmental Stress Tests
2.6.1. pH Adjustment
2.6.2. Thermal Stress
2.7. Statistical Analysis
3. Results and Discussion
3.1. Physico-Chemical Properties of the SBP-SC Conjugates
3.1.1. Visual Appearance
3.1.2. Conjugate Size and Zeta Potential
Prior to Heat Treatment
Post Heat Treatment
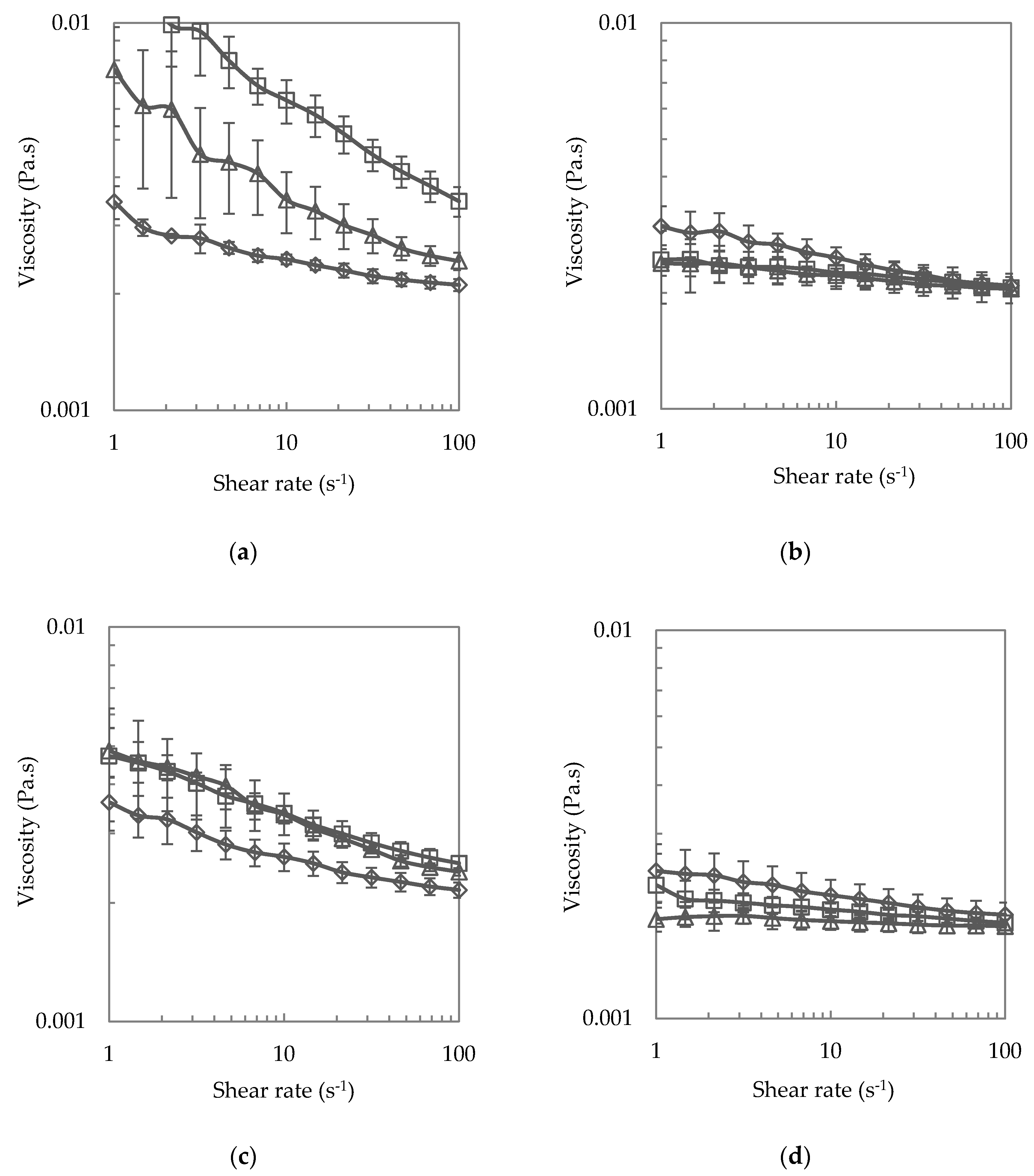
3.1.3. Steady Shear Viscosity
3.2. Microstructure Model
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Schmitt, C.; Sanchez, C.; Desobry-Banon, S.; Hardy, J. Structure and technofunctional properties of protein-polysaccharide complexes: A review. Crit. Rev. Food Sci. Nutr. 1998, 38, 689–753. [Google Scholar] [CrossRef] [PubMed]
- Tolstoguzov, V.B. Functional properties of food proteins and role of protein-polysaccharide interaction. Food Hydrocoll. 1991, 4, 429–468. [Google Scholar] [CrossRef]
- Madene, A.; Jacquot, M.; Scher, J.; Desobry, S. Flavour encapsulation and controlled release—A review. Int. J. Food Sci. Technol. 2006, 41, 1–21. [Google Scholar] [CrossRef]
- Champagne, C.P.; Fustier, P. Microencapsulation for the improved delivery of bioactive compounds into foods. Curr. Opin. Biotechnol. 2007, 18, 184–190. [Google Scholar] [CrossRef] [PubMed]
- Wen-Sherng, C.; Henry George, A.; Gaud Susan, M.; Miller Mark, S.; Kaiser John, M.; Balmadeca Estela, A.; Morgan Ronnie, G.; Baer Cynthia, C.; Borwankar Rajendra, P.; Hellgeth Lorraine, C.; et al. Microfragmented Ionic Polysaccharide/Protein Complex Dispersions. U.S. Patent Application No. US07/548,950, 28 April 1989. [Google Scholar]
- Zolper John, T. Fat Substitutes Based on Carrageenan Gels, Processes for Producing the Same and Food Products Containing the Fat Substitutes. U.S. Patent Application No. US08/139,855, 22 November 1993. [Google Scholar]
- Soucie William, G.; Chen, W.-S.; Witte Vernon, C.; Henry George, A.; Drehkoff, W.D. Shelf Stable Acid Food Dressings Containing Fibrous Protein Complexes. U.S. Patent Application No. US07/024,507, 1 July 1985. [Google Scholar]
- Bishay Ihab, E.; Clark Deane, R. Carbohydrate/Protein Cream Substitutes. U.S. Patent Application No. US08/438,798, 11 May 1995. [Google Scholar]
- Surh, J.; Decker, E.A.; McClements, D.J. Influence of pH and pectin type on properties and stability of sodium-caseinate stabilized oil-in-water emulsions. Food Hydrocoll. 2006, 20, 607–618. [Google Scholar] [CrossRef]
- Li, X.Y.; Fang, Y.P.; Phillips, G.O.; Al-Assaf, S. Improved Sugar Beet Pectin-Stabilized Emulsions through Complexation with Sodium Caseinate. J. Agric. Food Chem. 2013, 61, 1388–1396. [Google Scholar] [CrossRef]
- Dickinson, E. Hydrocolloids as emulsifiers and emulsion stabilizers. Food Hydrocoll. 2009, 23, 1473–1482. [Google Scholar] [CrossRef]
- Semo, E.; Kesselman, E.; Danino, D.; Livney, Y.D. Casein micelle as a natural nano-capsular vehicle for nutraceuticals. Food Hydrocoll. 2007, 21, 936–942. [Google Scholar] [CrossRef]
- Dickinson, E. Proteins at interfaces and in emulsions—Stability, rheology and interactions. J. Chem. Soc. Faraday Trans. 1998, 94, 1657–1669. [Google Scholar] [CrossRef]
- Dickinson, E. Milk protein interfacial layers and the relationship to emulsion stability and rheology. Colloids Surf. B Biointerfaces 2001, 20, 197–210. [Google Scholar] [CrossRef]
- Livney, Y.D. Milk proteins as vehicles for bioactives. Curr. Opin. Colloid Interface Sci. 2010, 15, 73–83. [Google Scholar] [CrossRef]
- Leroux, J.; Langendorff, V.; Schick, G.; Vaishnav, V.; Mazoyer, J. Emulsion stabilizing properties of pectin. Food Hydrocoll. 2003, 17, 455–462. [Google Scholar] [CrossRef]
- Funami, T.; Zhang, G.Y.; Hiroe, M.; Noda, S.; Nakauma, M.; Asai, I.; Cowman, M.K.; Al-Assaf, S.; Phillips, G.O. Effects of the proteinaceous moiety on the emulsifying properties of sugar beet pectin. Food Hydrocoll. 2007, 21, 1319–1329. [Google Scholar] [CrossRef]
- Siew, C.K.; Williams, P.A. Characterization of the surface-active components of sugar beet pectin and the hydrodynamic thickness of the adsorbed pectin layer. J. Agric. Food Chem. 2008, 56, 8111–8120. [Google Scholar] [CrossRef] [PubMed]
- Siew, C.K.; Williams, P.A. Role of protein and ferulic acid in the emulsification properties of sugar beet pectin. J. Agric. Food Chem. 2008, 56, 4164–4171. [Google Scholar] [CrossRef] [PubMed]
- Evans, M.; Ratcliffe, I.; Williams, P.A. Emulsion stabilisation using polysaccharide-protein complexes. Curr. Opin. Colloid Interface Sci. 2013, 18, 272–282. [Google Scholar] [CrossRef]
- Jones, O.G.; McClements, D.J. Functional Biopolymer Particles: Design, Fabrication, and Applications. Compr. Rev. Food Sci. Food Saf. 2010, 9, 374–397. [Google Scholar] [CrossRef]
- Ridley, B.L.; O’Neill, M.A.; Mohnen, D.A. Pectins: Structure, biosynthesis, and oligogalacturonide-related signaling. Phytochemistry 2001, 57, 929–967. [Google Scholar] [CrossRef]
- Mattinen, M.L.; Kruus, K.; Buchert, J.; Nielsen, J.H.; Andersen, H.J.; Steffensen, C.L. Laccase-catalyzed polymerization of tyrosine-containing peptides. FEBS J. 2005, 272, 3640–3650. [Google Scholar] [CrossRef] [PubMed]
- Zeeb, B.; Fischer, L.; Weiss, J. Stabilization of food dispersions by enzymes. Food Funct. 2014, 5, 198–213. [Google Scholar] [CrossRef] [PubMed]
- Mattinen, M.L.; Hellman, M.; Permi, P.; Autio, K.; Kalkkinen, N.; Buchert, J. Effect of protein structure on laccase-catalyzed protein oligomerization. J. Agric. Food Chem. 2006, 54, 8883–8890. [Google Scholar] [CrossRef] [PubMed]
- Jung, J.Y.; Wicker, L. Laccase mediated conjugation of sugar beet pectin and the effect on emulsion stability. Food Hydrocoll. 2012, 28, 168–173. [Google Scholar] [CrossRef]
- Zaidel, D.N.A.; Chronakis, I.S.; Meyer, A.S. Stabilization of oil-in-water emulsions by enzyme catalyzed oxidative gelation of sugar beet pectin. Food Hydrocoll. 2013, 30, 19–25. [Google Scholar] [CrossRef]
- Chen, B.C.; Li, H.J.; Ding, Y.P.; Suo, H.Y. Formation and microstructural characterization of whey protein isolate/beet pectin coacervations by laccase catalyzed cross-linking. LWT Food Sci. Technol. 2012, 47, 31–38. [Google Scholar] [CrossRef]
- Gazme, B.; Madadlou, A. Fabrication of whey protein-pectin conjugate particles through laccase-induced gelation of microemulsified nanodroplets. Food Hydrocoll. 2014, 40, 189–195. [Google Scholar] [CrossRef]
- Zeeb, B.; Gibis, M.; Fischer, L.; Weiss, J. Crosslinking of interfacial layers in multilayered oil-in-water emulsions using laccase: Characterization and pH-stability. Food Hydrocoll. 2012, 27, 126–136. [Google Scholar] [CrossRef]
- Zeeb, B.; Lopez-Pena, C.L.; Weiss, J.; McClements, D.J. Controlling lipid digestion using enzyme-induced crosslinking of biopolymer interfacial layers in multilayer emulsions. Food Hydrocoll. 2015, 46, 125–133. [Google Scholar] [CrossRef]
- Jung, J.; Wicker, L. beta-Lactoglobulin conformation and mixed sugar beet pectin gel matrix is changed by laccase. LWT Food Sci. Technol. 2014, 55, 9–15. [Google Scholar] [CrossRef]
- Littoz, F.; McClements, D.J. Bio-mimetic approach to improving emulsion stability: Cross-linking adsorbed beet pectin layers using laccase. Food Hydrocoll. 2008, 22, 1203–1211. [Google Scholar] [CrossRef]
- Jones, O.G.; Decker, E.A.; McClements, D.J. Comparison of protein-polysaccharide nanoparticle fabrication methods: Impact of biopolymer complexation before or after particle formation. J. Colloid Interface Sci. 2010, 344, 21–29. [Google Scholar] [CrossRef]
- Juvonen, H.; Smolander, M.; Boer, H.; Pere, J.; Buchert, J.; Peltonen, J. Film Formation and Surface Properties of Enzymatically Crosslinked Casein Films. J. Appl. Polym. Sci. 2011, 119, 2205–2213. [Google Scholar] [CrossRef]
- Al-Hakkak, M.; Kavale, S. Improvement of emulsification properties of sodium caseinate by conjugating to pectin through the Maillard reaction. Mail. React. Food Chem. Med Sci. Update Postgenomic Era 2002, 1245, 491–499. [Google Scholar] [CrossRef]
- Jaeger, H.; Janositz, A.; Knorr, D. The Maillard reaction and its control during food processing. The potential of emerging technologies. Pathol. Biol. 2010, 58, 207–213. [Google Scholar] [CrossRef] [PubMed]
- Tamnak, S.; Mirhosseini, H.; Tan, C.P.; Ghazali, H.M.; Muhammad, K. Physicochemical properties, rheological behavior and morphology of pectin-pea protein isolate mixtures and conjugates in aqueous system and oil in water emulsion. Food Hydrocoll. 2016, 56, 405–416. [Google Scholar] [CrossRef]
- Bi, B.W.; Yang, H.; Fang, Y.P.; Nishinari, K.; Phillips, G.O. Characterization and emulsifying properties of beta-lactoglobulin-gum Acacia Seyal conjugates prepared via the Maillard reaction. Food Chem. 2017, 214, 614–621. [Google Scholar] [CrossRef] [PubMed]
- Jones, O.; Decker, E.A.; McClements, D.J. Thermal analysis of beta-lactoglobulin complexes with pectins or carrageenan for production of stable biopolymer particles. Food Hydrocoll. 2010, 24, 239–248. [Google Scholar] [CrossRef]
- Jones, O.G.; Decker, E.A.; McClements, D.J. Formation of biopolymer particles by thermal treatment of beta-lactoglobulin-pectin complexes. Food Hydrocoll. 2009, 23, 1312–1321. [Google Scholar] [CrossRef]
- Jones, O.G.; Lesmes, U.; Dubin, P.; McClements, D.J. Effect of polysaccharide charge on formation and properties of biopolymer nanoparticles created by heat treatment of beta-lactoglobulin-pectin complexes. Food Hydrocoll. 2010, 24, 374–383. [Google Scholar] [CrossRef]
- Jones, O.G.; McClements, D.J. Stability of biopolymer particles formed by heat treatment of beta-lactoglobulin/beet pectin electrostatic complexes. Food Biophys. 2008, 3, 191–197. [Google Scholar] [CrossRef]
- Jones, O.G.; McClements, D.J. Recent progress in biopolymer nanoparticle and microparticle formation by heat-treating electrostatic protein-polysaccharide complexes. Adv. Colloid Interface Sci. 2011, 167, 49–62. [Google Scholar] [CrossRef]
- Sauer, A.; Moraru, C.I. Heat stability of micellar casein concentrates as affected by temperature and pH. J. Dairy Sci. 2012, 95, 6339–6350. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, M.; Zhang, W.B.; Wen, P.C.; Zhang, Y.; Liang, Q. Heat stability of yak micellar casein as affected by heat treatment temperature and duration. Dairy Sci. Technol. 2014, 94, 469–481. [Google Scholar] [CrossRef]
- Li, X.B.; Fang, Y.P.; Al-Assaf, S.; Phillips, G.O.; Nishinari, K.; Zhang, H.B. Rheological study of gum arabic solutions: Interpretation based on molecular self-association. Food Hydrocoll. 2009, 23, 2394–2402. [Google Scholar] [CrossRef]
- Dickinson, E. An Introduction to Food Colloids; Oxford University Press: Oxford, UK, 1992. [Google Scholar]
- Mueller, S.; Llewellin, E.W.; Mader, H.M. The rheology of suspensions of solid particles. Proc. R. Soc. A Math. Phys. Eng. Sci. 2010, 466, 1201–1228. [Google Scholar] [CrossRef]
- De Oliveira, F.C.; Coimbra, J.S.D.; de Oliveira, E.B.; Zuniga, A.D.G.; Rojas, E.E.G. Food Protein-polysaccharide Conjugates Obtained via the Maillard Reaction: A Review. Crit. Rev. Food Sci. Nutr. 2016, 56, 1108–1125. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.G.; Ma, C.C.; Gao, Y.X.; McClements, D.J. Food-Grade Covalent Complexes and Their Application as Nutraceutical Delivery Systems: A Review. Compr. Rev. Food Sci. Food Saf. 2017, 16, 76–95. [Google Scholar] [CrossRef]



© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, J.; Wolf, B. Physico-Chemical Properties of Sugar Beet Pectin-Sodium Caseinate Conjugates via Different Interaction Mechanisms. Foods 2019, 8, 192. https://doi.org/10.3390/foods8060192
Zhang J, Wolf B. Physico-Chemical Properties of Sugar Beet Pectin-Sodium Caseinate Conjugates via Different Interaction Mechanisms. Foods. 2019; 8(6):192. https://doi.org/10.3390/foods8060192
Chicago/Turabian StyleZhang, Juyang, and Bettina Wolf. 2019. "Physico-Chemical Properties of Sugar Beet Pectin-Sodium Caseinate Conjugates via Different Interaction Mechanisms" Foods 8, no. 6: 192. https://doi.org/10.3390/foods8060192






